Abstract
Rationale
Experimental evidence suggests that the differential behavioral effects of benzodiazepines depend on their relative actions at γ-aminobutyric acid type A (GABAA) receptors that contain either an α1, α2, α3 or α5 subunit.
Objectives
The present study was aimed at understanding the role of α3 subunit-containing GABAA (α3GABAA) receptors by examining the behavioral pharmacology of TP003 (4,2’-difluoro-5’-[8-fluoro-7-(1-hydroxy-1-methylethyl)imidazo[1,2-a]pyridine-3-yl]biphenyl-2-carbonitrile), which shows functional selectivity for α3GABAA receptors.
Methods
First, a conflict procedure was used to assess the anxiolytic-like effects of TP003 and a representative clinically available benzodiazepine. TP003 was also administered before daily periods of sucrose pellet availability to evaluate potential hyperphagic effects. In separate experiments, observable behavioral effects were used to assess the motor and sedative effects of TP003.
Results
Administration of TP003 produced robust anti-conflict effects without the rate-decreasing effects that were observed with the representative benzodiazepine. Unlike reported effects of benzodiazepines, TP003 did not enhance palatable food consumption. However, increases in observable sleep-associated posture were induced by TP003, as were decreases in some species-typical behaviors (vocalization, locomotion, and environment-directed behaviors). When evaluated for its ability to induce a procumbent posture, TP003 failed to produce an effect.
Conclusions
Based on conflict and observation tests in monkeys, our results suggest that TP003 may have anxiolytic properties but lacks ataxic, hyperphagic, and pronounced sedative effects characteristic of classical benzodiazepines. TP003 did induce myorelaxant-like effects and had relatively mild sedative effects. Collectively, these results suggest that α3GABAA receptors play an important role in the anxiolytic-like and motor effects of benzodiazepine-type drugs.
Keywords: GABAA receptors, α3 subunit, benzodiazepine, anxiety, sedation, ataxia, myorelaxation, hyperphagia
INTRODUCTION
The γ-aminobutyric acid type A (GABAA) receptors are the primary sites of action for benzodiazepines and related drugs used to treat anxiety and sleep disorders. The therapeutic use of benzodiazepine-type drugs for the treatment of these disorders is constrained, however, by the occurrence of other characteristic effects that are mediated through GABAA receptors. In addition to their therapeutic effects, benzodiazepines produce unwanted side-effects including daytime drowsiness, impairment of motor coordination, and increases in food consumption.
Benzodiazepine-type drugs act by binding allosterically to a distinct site on GABAA receptors which produces changes that enhance the ability of GABA to increase chloride conductance. Research during the past two decades has revealed the existence of multiple subtypes of the GABAA receptor (e.g., Pritchett et al. 1989; Rudolph et al. 2001). Subsequent reports have postulated that the diverse behavioral effects of benzodiazepine-type drugs may reflect actions at different subtypes of GABAA receptors (Rudolph et al. 1999; McKernan et al. 2000; Löw et al. 2000; Rowlett et al. 2005).
GABAA receptors in the central nervous system are pentamers constituted from structurally distinct proteins, with each protein family consisting of different subunits (for review, see Rudolph et al. 2001). The majority of GABAA receptors are composed of α, β, and γ subunits and benzodiazepines bind to a site on the native GABAA receptor that is located at the interface of the γ2 subunit and one of the α1, α2, α3, or α5 subunits. Benzodiazepines do not bind to the corresponding α4- and α6-subunit containing receptors. Approximately 75% of the GABAA receptors in the brain contain α1, α2, and α3 subunits (McKernan and Whiting, 1996), and GABAA receptors containing α1 subunits (α1GABAA receptors) recently have been implicated in the sedative effects of benzodiazepines, whereas GABAA receptors containing α2 and α3 subunits (α2GABAA and α3GABAA receptors) are associated with the anxiolytic and myorelaxant effects of benzodiazepines (McKernan et al. 2000; Löw et al. 2000; Rowlett et al. 2005, Morris et al. 2006). GABAA receptors containing α5 subunits (α5GABAA receptors), in contrast, are a relatively minor population in the brain as a whole but are preferentially expressed within the hippocampus and play a role in certain memory processes, but are not responsible for the anxiolytic or motor effects associated with benzodiazepines (Collinson et al. 2002; Crestani et al. 2002; Atack et al. 2006; but see Savic et al. 2008).
Pharmacological efforts to attribute the contribution of GABAA receptor subtypes to the multiple effects of benzodiazepines have been enhanced in recent years by the increasing availability of compounds with selectivity for the individual receptor subtypes. In this regard, Dias et al. (2005) described an imidazopyridine compound, TP003, which exhibits “functional selectivity” rather than binding selectivity for GABAA receptor subtypes containing α3 subunits. That is, in vitro TP003 has comparatively high agonist efficacy at α3GABAA receptors, but essentially no efficacy at α1GABAA, α2GABAA, and α5GABAA receptors (Dias et al. 2005). Because TP003 exhibits appreciable efficacy only at α3GABAA receptors, the extent to which this compound induces an effect characteristic of conventional benzodiazepines can be interpreted as evidence for a specific role of α3GABAA receptors in that particular effect.
Using this approach in the present study, we evaluated the ability of TP003 to engender characteristic anxiolytic-like, hyperphagic, motor and sedative effects in relevant non-human primate models of the therapeutic and side effects of benzodiazepines (Platt et al. 2002; Licata et al. 2005; Duke et al. 2006; Rowlett et al. 2006). The anxiolytic-like effects of TP003 were first assessed in a conflict procedure in which behavior was maintained under a fixed-ratio schedule of food delivery in the absence (non-suppressed responding) and presence (suppressed responding) of response-contingent electric shock. In addition, hyperphagic effects were assessed by administering TP003 before 10-min periods of sucrose availability. Finally, previously validated observational techniques were used to assess the motor and sedative effects of TP003. The behavioral effects of TP003 are discussed in relation to previous studies assessing both conventional benzodiazepines and subtype-selective GABAA receptor agonists.
MATERIALS AND METHODS
Animals
Subjects were adult rhesus monkeys (Macaca mulatta) for the conflict studies, and adult squirrel monkeys (Saimiri sciureus) for the sucrose pellet consumption and observation studies. Separate groups of monkeys were used fo each procedure. Monkeys in the conflict studies were maintained at 90–95% of their free-feeding weights, the other monkeys were not food restricted. Monkeys were housed individually and maintained on a 12-hr lights-on/12-hr lights-off cycle (lights on at 0600 hr), with water available continuously. All testing occurred during the lights-on phase of the cycle. Rhesus monkeys were prepared with a chronic indwelling venous catheter according to the procedures described by Platt et al. (2005). Animals in this study were maintained in accordance with the “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003) and the principles of laboratory animal care were adhered to.
Conflict Procedure
Four rhesus monkeys (2 males, 2 females) were trained as described in detail by Rowlett et al. (2006). A daily session consisted of 4 cycles, each preceded by a 10-min time out period in which all lights in the chamber were off and responding had no programmed consequences. Each cycle consisted of two components. The first component was signaled by red stimulus lights and consisted of a fixed ratio (FR) 18 schedule of food pellet delivery (Bioserve, Frenchtown, NJ) followed by a 10 s time out. The second component, signaled by green stimulus lights, consisted of the FR 18 schedule of food delivery combined with a FR 20 schedule of foot shock delivery (1.5 – 3.0 mA, adjusted for each monkey based on individual performance, 0.25 s duration). Both components were 5 min in duration, or ended after the monkey obtained 5 food pellets or received 3 foot shocks, whichever occurred first.
Test sessions were conducted once or twice per week. Here, i.v. injections of vehicle or drug were administered in the 5th minute of each time out. In successive cycles, increasing doses of the test drug were administered using a cumulative dosing procedure. The dependent measure was the average rates of responding (responses/s), calculated by dividing responses by time during components 1 and 2, excluding responding during time outs or reinforcer delivery.
Sucrose pellet consumption
Sucrose pellet consumption was evaluated as described in Duke et al. (2006). Briefly, male squirrel monkeys (N=5) were placed in the observation area once a week with access to a dish containing 100 sucrose pellets (P.J. Noyes, Lancaster, NH) for 10 min. Drugs or vehicle were administered intramuscularly (i.m.) 15 min prior to each test session. Sucrose pellet consumption was measured by subtracting the number of pellets remaining in the food dish or elsewhere in the observation chamber from 100.
Observation
Five male squirrel monkeys were habituated to an observation arena and injection procedures (described in Platt et al. 2002) for approximately one month. Following habituation, 30-min observational sessions were conducted following a 15 min pretreatment of drug or vehicle. During the sixth, eighteenth and thirtieth min of each 30-min session, the monkeys were removed briefly from the observation arena by a trained handler and evaluated for ataxia, defined as the inability to balance on a stainless steel transport pole held in the horizontal plane. During each ataxia assessment, a score of 0 indicated that the monkey was able to balance normally on the transport pole, a score of 1 indicated inability to balance, and a score of 2 indicated that the monkey could neither balance on nor support its weight on the pole. In addition, a measurement of muscle resistance was taken in order to determine myorelaxant effects (cf. Licata et al. 2009). After rating the ability of the monkey to balance on the pole, the experimenter then grasped one leg and gently extended it to assess the degree of resistance to flexion. A score of 0 indicated that the monkey retracted its leg normally, a score of −1 indicated delayed and/or reduced resistance to leg flexion, and a score of −2 indicated no flexion of the leg.
Scoring of videotapes was conducted by observers trained to use the behavioral scoring system described by Platt et al. (2002). Eight behaviors (Platt et al. 2002) were scored by recording their presence or absence in 15-s intervals during three 5-min observation periods across the session (0–5 min, 12–17 min, 24–29 min). Frequency scores were calculated from these data as the proportion of 15-s intervals in which a particular behavior occurred, and the maximum possible score was 20.
Data analysis and drug preparation
Effects of doses of compounds were evaluated by conducting a priori Bonferroni t-tests (parametric data) or Dunn’s Q statistic (non-parametric data), comparing individual doses to vehicle injection. Potency values (dose engendering 50% maximum effect, ED50) were calculated in individual monkeys by log-linear regression analysis. For all comparisons, the alpha level was set at p ≤ 0.05.
TP003 was provided by Merck, Sharp, & Dohme Research Laboratories (Harlow, UK), and was prepared in a vehicle of 10% benzyl alcohol, 50% propylene glycol, and 40% sterile water. All other drugs were purchased from Tocris-Cookson (Ellisville, MO, USA), and were dissolved in 50% propylene glycol, 50% sterile water.
RESULTS
Conflict
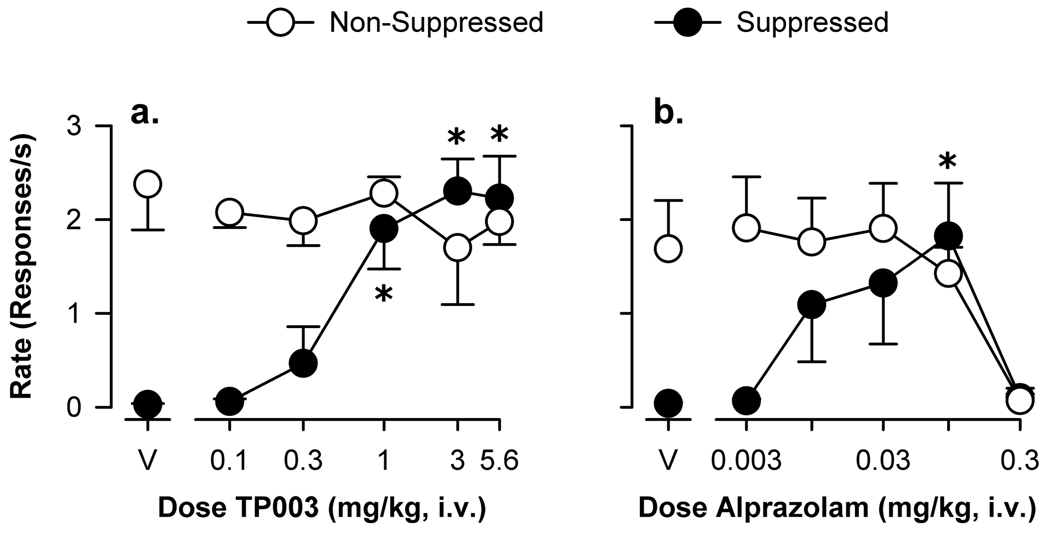
Figure 1a shows the effects of TP003 on the fixed-ratio schedule of food pellet delivery (non-suppressed responding) and the concurrent schedule of food delivery and electric shock presentation (suppressed responding). Following vehicle administration, rates of responding during both components were similar to those observed during training sessions (i.e. between 2.0–3.0 responses/s during the non-suppressed component, and less that 0.1 responses/s during the suppressed component). TP003 increased the mean rates of suppressed responding compared to vehicle at doses of 1.0 to 5.6 mg/kg (Bonferroni t tests, p<0.05) with an ED50 value of 0.53 mg/kg, i.v.. Across the doses tested, TP003 did not affect response rates during the non-suppressed component.
Figure 1.
Anti-conflict effects of TP003 (a) and alprazolam (b) in rhesus monkeys trained under a multiple schedule of food presentation (non-suppressed responding) and food + shock presentation (suppressed responding). Abscissae, cumulative intravenous dose of drug in mg/kg, i.v. Ordinates, response rate as responses per second. Each data point represents the mean (± S.E.M.) from three or four monkeys. Points above “V” represent data after vehicle administration. Asterisks represent significant differences relative to vehicle (Bonferroni t-tests, p<0.05).
We also determined the anti-conflict effects of alprazolam as a comparative standard. Administration of alprazolam engendered a characteristic increase in the rates of suppressed responding at low to intermediate doses and attenuated the rates of non-suppressed responding at higher doses (Figure 1b), and the ED50 values from each component is included in Table 1. As can be seen in the table, alprazolam was ~45-fold more potent than TP003, and attenuated non-suppressed responding at doses ~10-fold higher than those that increased rates of suppressed responding. For comparison purposes, the previously reported potencies of HZ-166 and zolpidem, two subtype-selective drugs, are also shown in Table 1.
Table 1.
Potencies of TP003, alprazolam, HZ-166 and zolpidem to alter suppressed and non-suppressed responding in the conflict procedure
| Drug | Selectivity | Suppressed ED50 (95% CL) | Non-suppressed ED50 (95% CL) | Ratioa |
|---|---|---|---|---|
| TP003 | α3 | 0.53 (0.48–1.1) | > 5.6 | > 11 b |
| Alprazolam | α1,2,3,5 | 0.012 (0.0004–0.055) | 0.12 (0.068–0.26) | 10 |
| HZ-166 c | α2,3 | 0.80 (0.17–2.0) | > 10 | > 13 d |
| Zolpidem e | α1 | – f | 0.34 (0.13–4.2) | – |
Ratio= Non-suppressed ED50/ Suppressed ED50
The highest dose tested (5.6 mg/kg) did not decrease rates of non-suppressed responding to < 50% in any monkey. To calculate the potency ratio, an ED50 of 5.6 was assigned.
derived from Fischer et al. 2010
the highest dose tested (10 mg/kg) did not decrease rates of non-suppressed responding to < 50% in any monkey. To calculate the potency ratio, an ED50 of 10 was assigned.
derived from Rowlett et al. 2006
could not be calculated
Ataxia and myorelaxation
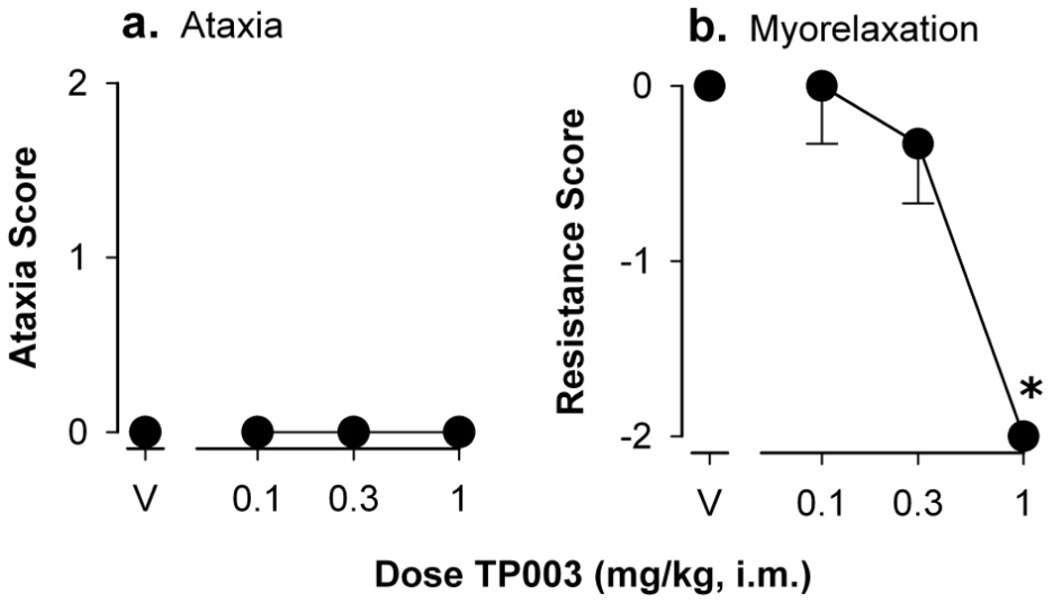
The ataxic and myorelaxant effects of TP003 as assessed in the quantitative observational procedures are shown in Figure 2. Administration of vehicle did not engender ataxic or myorelaxant effects in any monkey. TP003 also did not increase ataxia scores across the dose range tested. However, dose-dependent decreases in muscle resistance scores were observed after TP003 administration, with an effect significantly different from vehicle administration at 1.0 mg/kg (Dunn’s Q, p<0.05). The median scores from the maximally effective dose of TP003, and for comparison the previously reported scores of alprazolam and zolpidem, are shown in Table 2.
Figure 2.
Ataxia (a) and resistance (myorelaxation, b) scores of TP003 in squirrel monkeys as determined using quantitative observational procedures. Abscissae, dose of TP003 in mg/kg, i.m. Ordinates, assessment score as described in Materials and Methods section. Each data point represents the median and inter-quartile range from four monkeys. Points above “V” represent data after vehicle administration. Asterisks represent significant differences relative to vehicle (Dunn’s Q statistic, p<0.05).
Table 2.
Ataxic- and myorelaxant-like effects of TP003, alprazolam and zolpidem in the observation procedure. Median scores (±interquartile range) are from the maximally effective dose tested (shown in parenthesis).
| Drug | Myorelaxation | Ataxia |
|---|---|---|
| Vehicle | 0 (0 – 0) | 0 (0 – 0) |
| TP003 (1 mg/kg) | −2 (−2 – −2) * | 0 (0 – 0) |
| Alprazolam (1 mg/kg) a | −2 (−1.835 – −2) * | 1.67 (1.67 – 1.835) * |
| Zolpidem (10 – 17.8 mg/kg) a | −1.835 (−1.17 – −2) * | 2 (1.5 – 2) * |
derived from Licata et al. 2009
Dunn’s Q statistic, p<0.05
Sucrose pellet consumption
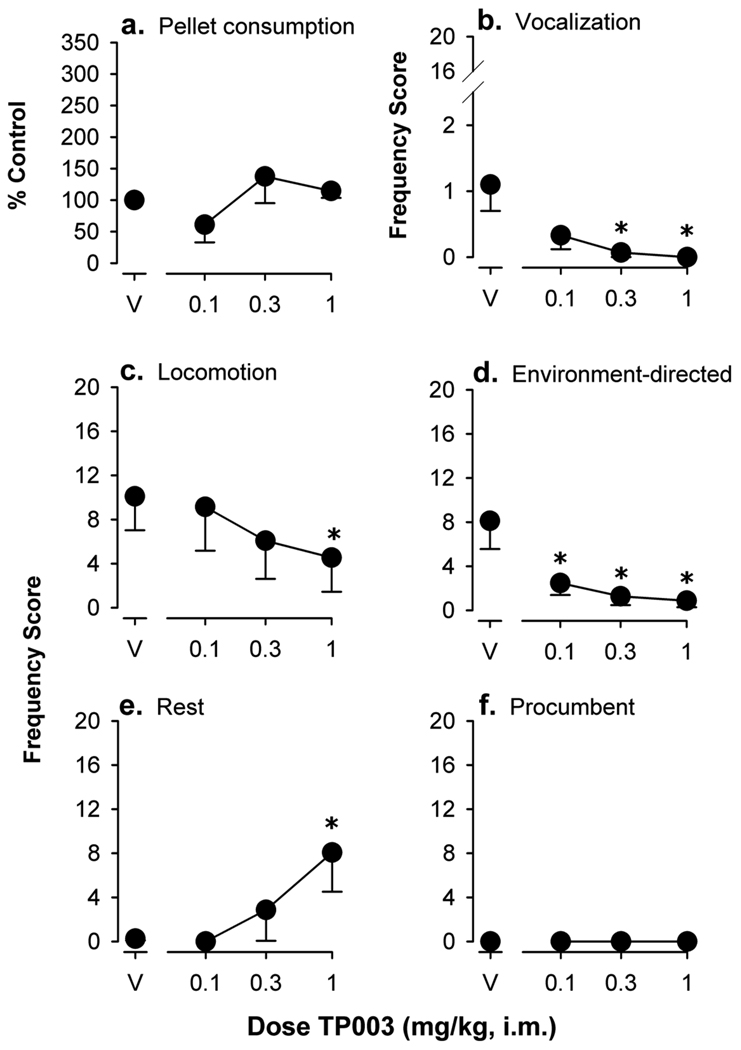
The hyperphagic effects of TP003, as determined in the assay of sucrose-pellet consumption, are shown in Figure 3a. Under baseline conditions, monkeys consumed a mean (± S.E.M.) of 19 ± 4 sucrose pellets during the 10-min access period. Sucrose pellet consumption was not altered following administration of vehicle. Across the doses tested, TP003 also did not significantly affect the consumption of sucrose pellets. The percent increase in sucrose pellet consumption following administration of TP003, and for comparison alprazolam and zolpidem, are shown in Table 3.
Figure 3.
Quantitative behavioral effects of TP003 in squirrel monkeys as determined in the observation procedure. Abscissae, dose of TP003 in mg/kg, i.m. Ordinates, pellet consumption as the mean number of sucrose pellets consumed expressed as percentage of baseline control (a) or frequency score as described in Materials and Methods section (b–f). Each data point represents the mean (± S.E.M.) from five monkeys. Points above “V” represent data after vehicle administration. Asterisks represent significant differences relative to vehicle (Bonferroni t-tests, p<0.05).
Table 3.
Effects of TP003, alprazolam and zolpidem on observational measures related to sedation. Frequency scores (±S.E.M.) are from the maximally effective dose tested (shown in parenthesis).
| Drug | Pellet Consumption (% Control) | Locomotion | Environment-directed | Rest | Procumbent |
|---|---|---|---|---|---|
| Vehicle | 100 | 10.1 ± 3.1 | 8.1 ± 2.6 | 0.3 ± 0.2 | 0 ± 0 |
| TP003 (0.3 – 1) | 137 ± 42 | 4.5 ± 3.1 * | 0.9 ± 0.6 * | 8.1 ± 3.6 * | 0 ± 0 |
| Alprazolam (0.3 – 1) a | 255 ± 21 * | 0.8 ± 0.3 * | 0 ± 0 * | 6.7 ± 3.6 | 16.1 ± 2.5 * |
| Zolpidem (10 – 17.8) a,b | 292 ± 55* | 1.2 ± 0.9 * | 1 ± 0.8 * | 3.4 ± 2.8 | 9.4 ± 4.0 * |
Sedative-motor effects
Figure 3 also shows observable behavioral effects of TP003 in squirrel monkeys. Dose-dependent decreases in vocalization (Figure 3b, minimum effective dose = 0.3 mg/kg), locomotion (Figure 3c, minimum effective dose = 1.0 mg/kg) and environment-directed behavior (Figure 3d, minimum effective dose = 0.1 mg/kg) were observed following administration of TP003 (Bonferroni t tests, p<0.05). The frequency measures of rest and procumbent posture induced by TP003 are also shown in Figure 3. TP003 dose-dependently increased the frequency of rest posture (Figure 3e, minimum effective dose = 1.0 mg/kg; Bonferroni t tests, p<0.05). In contrast, increases in procumbent posture were not observed across the doses tested (Figure 3f). The mean frequency scores for passive visual and self-directed behaviors (a combination of scores for grooming and scratching) were not altered by TP003 across the dose range tested (0.1–1.0 mg/kg, data not shown). The behavioral frequency scores that were engendered by the maximally effective dose of TP003, and for comparison the previously reported scores of alprazolam and zolpidem, are also shown in Table 3.
DISCUSSION
Conventional benzodiazepines bind non-selectively at GABAA receptors containing α1, α2, α3 and α5 subunits; however the role of different GABAA receptor subtypes in the behavioral effects of these drugs has not been characterized fully. We now demonstrate that TP003, a novel ligand with functional selectivity at α3GABAA receptors, is effective in behavioral measures of the anxiolytic and myorelaxant effects of benzodiazepines in monkeys. TP003 also engendered rest posture, reduced locomotion, and environment-directed behaviors; a profile of effects that raise the possibility of a role of α3GABAA receptors in relatively mild sedative effects induced by benzodiazepines. In contrast, TP003 was ineffective in behavioral assays of the ataxic, hyperphagic and more pronounced sedative effects (i.e., procumbent posture) of benzodiazepine-type drugs. Together, these findings provide evidence that sets apart the profile of behavioral effects induced by selective α3GABAA receptor stimulation vs. the profile of behavioral effects engendered by conventional and other subtype-selective benzodiazepines.
Procedures that assess the effects of drugs on experimentally-induced conflict are used often to assess the potential anxiolytic effects of these drugs as in humans (Geller and Seifter 1962; Spealman 1979; Kleven and Koek 1999; Rowlett et al. 2006). Previous studies from our laboratory have used a conflict procedure developed for rhesus monkeys to assess the anxiolytic effects of conventional benzodiazepines and other positive GABAA receptor modulators with either selective affinity or selective efficacy for GABAA receptor subtypes (Rowlett et al. 2005; Rowlett et al. 2006; Fischer et al. 2010). Results from these studies provide evidence for a differential role of GABAA receptors in the anxiolytic effects of benzodiazepines. As an example, L-838,417, a compound with functional selectivity at α2GABAA, α3 GABAA, and α5 GABAA receptors, produced an anti-conflict effect similar to conventional non-selective benzodiazepines (Rowlett et al. 2005). A similar result was observed when XHe-II-053 and HZ-166, drugs with high intrinsic efficacy at α2GABAA and α3GABAA receptors, were assessed in the conflict procedure (Fischer et al. 2010). Together with data suggesting that drugs selective for α1GABAA receptors (e.g. zolpidem) are only marginally effective in this procedure (Rowlett et al. 2005, 2006), these experiments support a key role for α2GABAA and α3GABAA receptors, but not α1GABAA receptors, in benzodiazepine-induced anxiolysis. Subsequent demonstrations of anxiolytic-like effects after administration of the partial α2GABAA and α3GABAA receptor agonist TPA023 in other rodent and primate models of anxiety also support this hypothesis (Atack et al. 2006).
In the present study, administration of TP003 produced an anti-conflict effect to the same degree as observed with the conventional benzodiazepine alprazolam, (see also Rowlett et al. 2006). These data provide clear evidence that a compound with selective efficacy at α3GABAA receptors can produce anxiolytic-like effects in primates, and support a role for this receptor subtype in the anxiolytic effects of benzodiazepines. It is noteworthy that TP003 produced an anti-conflict effect without altering rates of non-suppressed responding over the dose range tested. Similar results were observed when L-838,417 was assessed in the conflict procedure (Rowlett et al. 2005). Interestingly, both TP003 and L-838,417 are without appreciable efficacy at α1GABAA receptors, raising the possibility that α1GABAA receptors may be involved in the response rate-reducing effects of benzodiazepines.
Using previously described techniques (Licata et al. 2009), the present study characterized the motor-altering effects of TP003 across measures related to ataxia and myorelaxation. Studies with mutant mice implicate α1GABAA receptors in the ataxic effects of benzodiazepines, and further suggest that α2GABAA and α3GABAA receptors are involved in benzodiazepine-induced myorelaxation (Rudolph et al. 1999; McKernan et al. 2000; Crestani et al. 2001). Subsequent pharmacological studies in monkeys with subtype-selective GABAA receptor agonists agree with these earlier findings. (Platt et al. 2002; Licata et al. 2005; Rowlett et al. 2005; Licata et al. 2009). For example, whereas both SL651498 and L-838,417 induce myorelaxant effects, neither drug was effective in producing ataxic effects in the same animals (Licata et al. 2005; Rowlett et al. 2005). Moreover, the ataxic, but not myorelaxant effects of benzodiazepine-type compounds were blocked by the α1GABAA-preferring antagonist, βCCT (Licata et al. 2009). The lack of ataxic effects observed with TP003 in the present study is consistent with the idea that α3GABAA receptors do not play a crucial role in benzodiazepine-induced ataxia. Moreover, the results presented here are consistent with the idea that stimulation of α3GABAA receptors alone may be sufficient to produce benzodiazepine-associated myorelaxant effects.
The hyperphagic effects of benzodiazepines have been well documented (e.g. Randall et al. 1960; Cooper and Estall 1985), with cases of night-time bingeing being of particular concern. Studies in animal models have demonstrated increases in food consumption under controlled laboratory conditions, and suggest that different GABAA receptor subtypes may mediate benzodiazepine-induced hyperphagia. Further, these studies suggest that the GABAA receptor subtype mediating this effect may be species dependent. For example, α2GABAA and α3GABAA receptors are important mediators of benzodiazepine-induced hyperphagia in rodents, while α1GABAA and α5GABAA receptors do not play a role (Yerbury and Cooper 1989, Cooper and Ridley 2005, Stephens et al. 2005, Morris et al. 2009). In contrast, an important role for α1GABAA receptors, but not α5GABAA receptors as mediators of benzodiazepine-induced hyperphagia has been documented in primates (Wettstein and Spealman 1986, Kumar et al. 1999, Duke et al. 2006). To our knowledge, little information exists on the role of α3GABAA receptors in this effect in primates. In the present study, TP003 failed to increase the consumption of sucrose pellets at doses that engendered other behavioral effects (e.g., myorelaxant-like effects) and under conditions in which both conventional and α1GABAA-preferring agonists produce approximately 300% increases relative to baseline consumption (Duke et al. 2006). These observations suggest that α3GABAA receptors do not play a key role in the increases in palatable food consumption observed following conventional benzodiazepine administration, and provide further evidence that the contribution of GABAA receptor subtypes in this effect may be species dependent.
Quantitative observational techniques (cf. Platt et al. 2002) were used to assess the effects of TP003 on behaviors typically associated with motor impairment and sedation, as well as vocalizations. Previous findings from our laboratory have demonstrated that conventional benzodiazepines typically have no effects on vocalizations but decrease the frequency of locomotor and environment-directed behaviors (e.g. Platt et al. 2002; Licata et al. 2005). Non-selective benzodiazepines also characteristically induce both rest and procumbent posture (i.e., loose-limbed, sprawled, unable to maintain an upright position), the latter suggestive of a more pronounced, deep sedative effect (Platt et al. 2002; Licata et al. 2005; Rowlett et al. 2005; Duke et al. 2006). In contrast to the characteristic behavioral profile associated with non-selective benzodiazepines, TP003 decreased vocalizations and rest posture with no associated increases in procumbent posture over the dose range tested. Interestingly, the behavioral profile of TP003 also differed from the previously-evaluated functionally selective compounds L-838,417 and SL651498, which did not decrease vocalization, locomotion, or environment-directed behavior, and did not engender rest and procumbent posture. Of particular interest is the lack of rest posture associated with SL651498, which is a full agonist at both α2GABAA and α3GABAA receptor subtypes (Licata et al. 2005). Although clearly speculative, one intriguing possibility is that action at α2GABAA receptors (or partial agonist action at α1GABAA and/or α5GABAA receptors) attenuates rest posture induced by selective α3GABAA stimulation. These findings collectively raise the possibility that mild, but not more pronounced, sedative effects may be induced by α3GABAA–selective agonists. Indirect support for this possibility comes from the observation that a novel α3GABAA–selective agonist (NG2-73) recently was in clinical trials for treatment of insomnia (Wafford and Ebert 2008).
Taken together, the data from the present set of experiments provide further evidence that the behavioral effects of benzodiazepines in primates are likely mediated by different GABAA receptors that contain distinct α subunits. Accordingly, our studies highlight several hypotheses regarding benzodiazepine action. First, our findings suggest that α3GABAA receptors play an important role in benzodiazepine-induced anxiolysis. In addition, results from the present set of experiments also implicate α3GABAA receptors in the myorelaxant and, potentially, the mild sedative effects associated with benzodiazepines. Finally, our results are consistent with the idea that the hyperphagic, ataxic and more pronounced sedative effects of benzodiazepines require actions additional to those at α3GABAA receptors. These hypotheses should provide an important framework for studying the role of different GABAA receptor subtypes in the behavioral effects of benzodiazepine-type drugs, which in turn should help guide both the current clinical use of benzodiazepines as well as the development of improved therapeutic agents for treating anxiety and sleep disorders.
ACKNOWLEDGEMENTS
We acknowledge the assistance of Dr. Annemarie Duggan, Kristen Bano, and Shana Langer with these studies. We also thank Dr. Roger Spealman for comments on an earlier version of this manuscript. This work was supported by USPHS grants DA11792, RR00168, and a collaborative research agreement from Merck Research Laboratories with Dr. Rowlett as the Principal Investigator. Drs. Atack, Reynolds, and Dawson were employees of Merck Research Laboratories (Merck, Sharp & Dohme, Ltd.). Drs. Fischer and Platt had no financial relationship with Merck Research Laboratories. The agreement between Harvard Medical School and Merck Research Laboratories was a scientific collaboration, and did not involve studies that by the publication thereof would engender financial gain or loss from public disclosure.
Footnotes
None of the authors have competing personal financial interests that may be influenced by the publication of this report.
Contributor Information
Bradford D. Fischer, Harvard Medical School, New England Primate Research Center, One Pine Hill Drive, P.O. Box 9102, Southborough, Massachusetts 01772-9102, USA.
John R. Atack, Neuroscience Research Centre, Merck, Sharp & Dohme Research Laboratories, Terlings Park, Eastwick Road, Harlow, Essex CM20 2QR, UK
Donna M. Platt, Harvard Medical School, New England Primate Research Center, One Pine Hill Drive, P.O. Box 9102, Southborough, Massachusetts 01772-9102, USA
David S. Reynolds, Neuroscience Research Centre, Merck, Sharp & Dohme Research Laboratories, Terlings Park, Eastwick Road, Harlow, Essex CM20 2QR, UK
Gerard R. Dawson, Neuroscience Research Centre, Merck, Sharp & Dohme Research Laboratories, Terlings Park, Eastwick Road, Harlow, Essex CM20 2QR, UK
James K. Rowlett, Harvard Medical School, New England Primate Research Center, One Pine Hill Drive, P.O. Box 9102, Southborough, Massachusetts 01772-9102, USA
REFERENCES
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-Dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-30ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2 and α3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Estall LB. Behavioural pharmacology of food, water and salt intake in relation to drug actions at benzodiazepine receptors. Neurosci Biobehav Rev. 1985;9:5–19. doi: 10.1016/0149-7634(85)90028-4. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Ridley ET. Abecarnil and palatability: taste reactivity in normal ingestion in male rats. Pharmacol Biochem Behav. 2005;81:517–523. doi: 10.1016/j.pbb.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias R, Sheppard WF, Fradley RL, Garrett EM, Stanley JL, Tye SJ, Goodacre S, Lincoln RJ, Cook SM, Conley R, Hallett D, Humphries AC, Thompson SA, Wafford KA, Street LJ, Castro JL, Whiting PJ, Rosahl TW, Atack JR, McKernan RM, Dawson GR, Reynolds DS. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AN, Platt DM, Cook JM, Huang S, Yin W, Mattingly BA, Rowlett JK. Enhanced sucrose pellet consumption induced by benzodiazepine-type drugs in squirrel monkeys: role of GABAA receptor subtypes. Psychopharmacology (Berl) 2006;187:321–330. doi: 10.1007/s00213-006-0431-2. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang Z-J, Huang S, He X, Yu J, Zhou H, Johnson EM, Cook JM, Furtmüller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller I, Seifter J. The effects of mono-urethans, di-urethans and barbiturates on a punishment discrimination. J Pharmacol Exp Ther. 1962;136:284–288. [PubMed] [Google Scholar]
- Kleven MS, Koek W. Effects of different classes of partial benzodiazepine agonists on punished and unpunished responding in pigeons. Psychopharmacology (Berl) 1999;144:405–410. doi: 10.1007/s002130051024. [DOI] [PubMed] [Google Scholar]
- Kumar R, Palit G, Singh JR, Dhawan BN. Comparative behavioural effects of benzodiazepine and non-benzodiazepine anxiolytics in rhesus monkeys. Pharmacol Res. 1999;39:437–444. doi: 10.1006/phrs.1998.0455. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Sarma PV, Griebel G, Rowlett JK. Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: studies with the functionally selective ligand SL651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one] J Pharmacol Exp Ther. 2005;313:1118–1125. doi: 10.1124/jpet.104.081612. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Van Linn ML, Rowlett JK. Contribution of alpha1 subunit-containing gamma-aminobutyric acid(A) (GABA (A)) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacology (Berl) 2009;203:539–546. doi: 10.1007/s00213-008-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Morris HV, Dawson GR, Reynolds DS, Atack JR, Stephens DN. Both alpha2 and alpha3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm. Eur J Neurosci. 2006;23:2495–3504. doi: 10.1111/j.1460-9568.2006.04775.x. [DOI] [PubMed] [Google Scholar]
- Morris HV, Nilsson S, Dixon CI, Stephens DN, Clifton PG. Alpha1- and alpha2-containing GABAA receptor modulation is not necessary for benzodiazepine-induced hyperphagia. Appetite. 2009;52:675–683. doi: 10.1016/j.appet.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD, Cook J, Ma C. Selective antagonism of the ataxic effects of zolpidem and triazolam by the GABAA/alpha1-preferring antagonist beta-CCt in squirrel monkeys. Psychopharmacology (Berl) 2002;164:151–159. doi: 10.1007/s00213-002-1189-9. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivam J, editors. Current Protocols in Neuroscience. New York: Wiley; 2005. Unit 9.21. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Lüddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Randall LO, Schallek W, Heise GA, Keith EF, Bagdon RE. The psychosedative properties of methaminodiazepoxide. J Pharmacol Exp Ther. 1960;129:163–171. [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology (Berl) 2006;184:201–211. doi: 10.1007/s00213-005-0228-8. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Savić MM, Clayton T, Furtmüller R, Gavrilović I, Samardzić J, Savić S, Huck S, Sieghart W, Cook JM. PWZ-029, a compound with moderate inverse agonist functional selectivity at GABA(A) receptors containing alpha5 subunits, improves passive, but not active, avoidance learning in rats. Brain Res. 2008;1208:150–159. doi: 10.1016/j.brainres.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD. Comparison of drug effects on responding punished by pressurized air or electric shock delivery in squirrel monkeys: pentobarbital, chlordiazepoxide, d-amphetamine and cocaine. J Pharmacol Exp Ther. 1979;209:309–315. [PubMed] [Google Scholar]
- Stephens DN, Pistovcakova J, Worthing L, Atack JR, Dawson GR. Role of GABAA alpha5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonist. Eur J Pharmacol. 2005;526:240–250. doi: 10.1016/j.ejphar.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Emerging anti-insomnia drugs: tackling sleeplessness and the quality of wake time. Nat Rev Drug Discov. 2008;7:530–540. doi: 10.1038/nrd2464. [DOI] [PubMed] [Google Scholar]
- Wettstein JG, Spealman RD. Behavioral effects of zopiclone, CL 218,872 and diazepam in squirrel monkeys: antagonism by Ro 15-1788 and CGS 8216. J Pharmacol Exp Ther. 1986;238:522–528. [PubMed] [Google Scholar]
- Yerbury RE, Cooper SJ. Novel benzodiazepine receptor ligands: palatable food intake following zolpidem, CGS 17867A, or Ro23-0364, in the rat. Pharmacol Biochem Behav. 1989;33:303–307. doi: 10.1016/0091-3057(89)90504-2. [DOI] [PubMed] [Google Scholar]