Abstract
Interactions of herbicides and natural environmental stressors such as pH and food availability are poorly understood. We tested a chemical formulation of triclopyr (Release®) at environmentally relevant test concentrations (0.25 and 0.50 mg/L) in combination with two levels of pH (pH 5.5 and 7.5), and two levels of food availability (high and low). Population level effects of each stressor alone and in combination with the others were investigated using Simocephalus vetulus, a zooplankton species, and Rana pipiens tadpoles (Gosner stage 25), both common to forest ponds and wetlands. Herbicide treatments resulted in significant decreases in survival of both test species as well as reproduction and development time for Simocephalus vetulus at levels 5–10× below predicted worst case environmental concentrations (2.6 mg/L). This laboratory study demonstrates a probable risk of toxic effects of Release® herbicide which may be significantly increased by low food availability and by low pH at environmentally relevant concentrations.
Keywords: Multiple stressors, pH, Zooplankton, Triclopyr, Amphibian
Introduction
Despite the fact that fluctuations in natural environmental conditions are known to alter effects of chemical stressors, interactive effects of stressors are not well addressed in standard toxicity test protocols (Hanazato and Dodson, 1995; Horne and Dunson, 1995; Folt et al., 1999). Combined chemical and physical stressors have been shown to create effects that are either greater than or less than those of the stressors alone. In cases where natural variation in environmental variables enhances the toxicity of chemical stressors and exacerbating conditions are likely to be common, this knowledge should be incorporated into environmental regulation and risk mitigation methods to ensure sufficient protection of non-target organisms.
This study was conducted to examine the effects of Release® in combination with two important environmental variables, pH and food availability, on wetland zooplankton and amphibians. It is part of a multiple-tiered research program for investigating the effects of herbicides used in forestry management (Thompson, 2004; Edginton et al., 2003; Chen et al., 2004; Edginton et al., 2004; Thompson et al., 2004; Wojtaszek et al., 2004; Wojtaszek, 2004; Wojtaszek et al., 2005). Zooplankton and amphibians are ubiquitous in freshwater environments and known to be particularly sensitive indicators of stress (Schindler, 1987; Murphy et al., 2000). They may also be considered to be particularly vulnerable to multiple stressors as they have limited ability to migrate out of sub-optimal conditions.
Herbicides are routinely used in forestry and agriculture to control the growth of unwanted plant species and increase crop production. However, in the field application of these chemicals, non-target habitats may be exposed to varying degree as the result of accidental overspray, drift and off-target movement (Thompson, 2004). Release® or Garlon 4®, trade names for the butoxy ethyl ester of triclopyr (TBEE), are used for the suppression of broadleafed plants in both Canadian and US forest vegetation management regimes (Thompson and Pitt 2003; Shepard et al. 2004). They also increase yields of spruce/fir stands and pine plantations, and are used to clear utility right-of-ways and eliminate invasive species. In surface waters, TBEE has a half-life of 3–8 days (Kreutzweiser et al., 1995; Wojtaszek et al., 2005) and is rapidly absorbed by biota, sediments and allochthonous materials (Solomon et al., 1988; Barron et al., 1990; Kreutzweiser et al., 1995; Thompson et al., 1995).
Wetlands are important habitat for vertebrates (amphibian larvae, fish), invertebrates (aquatic insects, zooplankton), and aquatic plants (algae, aquatic macrophytes) which exhibit a range of sensitivity depending on the taxon and the endpoint measured (Roshon et al., 1999). Tolerance limits for variables such as pH, food, oxygen, etc, may be narrow or wide, depending on the species. Ambient pH and food availability can vary greatly in lakes, ponds, and wetlands due to seasonal changes in temperature, productivity, and snowmelt particularly in the temperate forest regions of the US and Canada (Semkin and Jeffries, 1986; Saunders et al., 2000). For example, in wetlands and lakes of north-central Ontario, Canada, pH can range from 4.2–7 (Mallory et al., 1998). Both low pH and food deficiency are known to negatively affect zooplankton and amphibians resulting in lower survival, increased development times and smaller body sizes (Tessier and Consolatti, 1991; Horne and Dunson, 1995; Kupferberg, 1997; Folt et al., 1999; Locke and Sprules, 2000). Food levels below 600 μg carbon L-1 can be limiting to zooplankton populations if sustained over the growing season (Lampert, 1978). The combination of sub-optimal pH and food in combination with exposure to anthropogenic chemicals can result in multiple stressor effects that can diminish or enhance individual performance and population growth (Folt et al., 1999; Chen et al., 2004; Edginton et al., 2004).
In this study, we examine the individual and interactive effects of Release®, a commercial formulation of TBEE with food availability and pH. Treatment levels of herbicide, algal food, and pH are representative of conditions in natural systems. The herbicide treatments yielded initial concentrations 5–10× below predicted environmental concentrations (PEC calculated based upon maximum label rates as recommended for forestry applied directly to 15 cm of water). In these tests, exposure concentrations were held relatively constant. However, in natural surface waters the more toxic ester form of triclopyr dissipates rapidly with time to 50% dissipation ranging from < 1 day up to 8 days depending on numerous factors influencing the primary dissipation mechanisms of photolysis, hydrolysis and microbial degradation (Solomon et al., 1988; Kreutzweiser et al., 1995; Wojtaszek et al., 2005).
The primary mode of action for triclopyr in plants is associated with differential cell division and growth (Ahrens, 1994) resulting in impairment of vascular transport and ultimately death of the plant. Aquatic animals, for example salmonid fry, are known to sorb TBEE from water, and then de-esterify the compound to form triclopyr acid (Barron et al., 1990). Where the de-esterification rate is less than the uptake rate, mortality may result from lethal tissue concentrations of triclopyr acid or total residues. While the exact mode of action in aquatic animals is not well understood, it has been considered to act as a general narcotic through disruption of cellular homeostasis (Barron et al., 1990; Edginton et al., 2004). Irrespective of the mode of action, toxicological literature suggests that fish, amphibians, zooplankton, plants and invertebrates are generally equi-sensitive upon exposure to formulated products containing triclopyr as the butoxyethyl ester (TBEE), (Wan et al., 1987; Johansen and Geen, 1990; Berrill et al., 1994; Roshon et al., 1999; Edginton et al., 2003; Wojtaszek, 2004). Existing triclopyr studies warrant further study of potential interactive effects on aquatic organisms.
Algal food levels reflect eutrophic to mesotrophic conditions in ponds and pH levels (5.5–7.5) span a range commonly encountered in wetlands. Specifically, we tested three hypotheses: 1) At environmentally relevant levels, Release® has no significant effects on survival, reproduction or development time of Simocephalus vetulus or survival of Rana pipiens; 2) There are no significant interactions between herbicide, low pH, and low food stressors; and 3) The two wetland taxa, S. vetulus and R. pipiens, are equally sensitive to the three stressors.
Methods
Zooplankton and amphibian species that have widespread geographic distributions and are representative of organisms in forest wetland environments were chosen as test organisms. S. vetulus, is a freshwater cladoceran species, common in shallow forest ponds. It is broadly distributed throughout subtropical and temperate regions and has been used in numerous population and toxicity studies (Willis et al., 1995). The amphibian R. pipiens also has a broad distribution in forest ponds and wetlands throughout North America. It is especially common in northeastern North America where Release® is used in forestry management. Gosner stage 25 larvae were used in the experiments because of their demonstrated sensitivity to herbicide stress (Edginton et al., 2003; Chen et al., 2004; Edginton et al., 2004; Thompson et al., 2004; Wojtaszek et al., 2004) and the coincident seasonal timing of this age class with typical forest herbicide application schedules. Both species use algae as their main food resource and we employed Cryptomonas erosa for both culturing and experiments. This algal species has high nutritional value (Chen and Folt, 1993) and is representative of taxonomic groups found in most lakes and ponds.
Cultures of S. vetulus were established from individuals collected in October 1999 from a 4 meter deep, tannin-stained forest pond located in Bomoseen State Game Park in western Vermont (Coordinates: N 43.66619, W 073.22845). Zooplankton cultures were maintained at 20°C in filtered (1.6 μm) water from Storrs Pond, NH to which EDTA (5.9 × 10−6 M Na2EDTA) was added to enhance cryptomonad survivorship (Kirk, 1988). Storrs Pond is a eutrophic pond with pH ranging from 6–7. Zooplankton cultures were maintained at pH~7.0 in a 14:10 hour light:dark cycle and fed 300 μg carbon·L−1 exponentially growing C. erosa daily. Storrs Pond, near Dartmouth College, provided a local year round source of water for all the lab's zooplankton cultures and experiments. The pre-experimental procedures were conducted according to previously described methods to control for maternal and age effects (Folt et al., 1999; Chen et al., 2004). R. pipiens eggs and tadpoles were obtained from Carolina Biological kept in filtered Storrs Pond water. The tadpoles were maintained at 20°C on a 14:10 hour light:dark cycle and fed a mixture of exponentially growing Cryptomonas erosa and Chlamydomonas reinhardi (~300 μg carbon·L−1 total) and Tetramin fish food. When tadpoles reached Gosner stage 25, they were used for herbicide experiments.
S. vetulus and R. pipiens were studied in separate static renewal experiments with each being exposed to either individual or multiple stressors in specified combinations. We chose a static renewal approach for the herbicide, pH, and food treatments even though in the field, the herbicide undergoes rapid degradation and food may be diminished by the herbicide treatment. However, based on a half-life of 3–8 days, the concentrations at the end of 21 days could still be higher than the highest test concentration (i.e. 5× below the EEC).
Experiments were conducted in multiple blocks using a 3-way factorial design. Treatments for S. vetulus experimental blocks included 2 pH levels (pH 5.5 and 7.5), 2 food levels (300 and 800 μg carbon·L−1), and 2 concentrations of Release® (0.25 and 0.5 acid equivalent (a.e.) mg·L−1). R. pipiens experiments consisted of the same pH and herbicide treatments but lower food concentrations which were based on values found in the literature to be limiting to tadpoles (40 and 800 μg carbon·L−1) (Seale, 1982). All pH treatments were adjusted to within 0.2 pH units of the specified treatments by titrating either HCl or NaOH into beakers containing the designated herbicide and food treatments in non-EDTA water. All treatment conditions were renewed every other day (replacement every 48 hours) during the course of experiments. Prior to experiments, pH adjustments and measurements were tested to insure that pH would not change in the 48 hours between renewal of treatment conditions. In addition, the algal food species, C. erosa was tested in herbicide treatments to insure that there were no direct effects of the herbicide on food availability during the 48 hours exposures between renewals in the experiments.
The test material used in this experiment was the commercial formulation Release® which contains the active ingredient triclopyr (3, 5, 6-trichloro-2-pyridl-oxyacetic acid) formulated as a butoxyethyl ester at a concentration of 480 g a.e. L−1 (Lot # ME09161103). This formulation also contains kerosene as a carrier to enhance uptake. Nominal test concentrations of 0.25 and 0.50 mg a.e. L−1 were analytically verified by gas chromatographic techniques. Analytical verification of aqueous test concentrations confirmed averages within 10% of nominal levels. Total triclopyr residues were determined in 1 mL subsamples derived from thawed and mixed whole water samples. Subsample aliquots were acidified by addition of 0.2 mL of concentrated HCl to convert triclopyr residues to triclopyr acid and subsequently derivatized to form triclopyr methyl ester as an analyte amenable to gas chromatographic analysis. Derivatization was performed by diluting acidified samples to 10 mL constant volume in methanol, addition of 1 mL of boron trifluoride reagent and heating for 1 hour at 105–110°C. The derivative was cleaned-up by partitioning from water to hexane. Hexane extracts were dried with sodium sulfate, evaporated and reconstituted in a final volume of 10 mL iso-octane. Following addition of 2,4-D methyl ester as an internal standard, aliquots of 2 uL were analyzed by gas chromatography on an HP5890 series II chromatograph equipped with an autosampler and electron capture detector. Analyte separations involved temperature programmed chromatography on a DB-17 (0.53 mm i.d. × 15 m, 1 uM film thickness) (J&W scientific) with quantitation based on relative peak area response of the triclopyr and 2,4-D methyl esters based on a 5 point calibration curve. Results of concurrent quality control sample analyses (n=17) demonstrated quantitative recovery efficiency and excellent precision of the method (102.1 ± 9.3%). Limits of quantitation were estimated conservatively as 0.0015 mg a.e. L−1 in water. Herbicide test concentrations are expressed in terms of mg a.e. L−1 of triclopyr to facilitate comparison to the majority of the published literature and do not imply that effects are solely the result of exposure to the free acid form of TBEE. Conversion to units of equivalent concentrations for the ester TBEE can be made by multiplying by a factor of 1.35.
S. vetulus Adult Experiments
Adult S. vetulus experiments were conducted in three blocks, each of which contained 4 replicates per treatment with a single individual contained in each replicate. The pH of larger batches of media was monitored using a Beckman electrode, and adjusted by adding HCl or NaOH prior to addition of food and herbicide. Adult S. vetulus were placed individually in 145 ml jars containing filtered pond water with the appropriate pH, food (C. erosa), and herbicide levels. The jars were capped and rotated on a plankton wheel on a cycle of 2 minutes of rotation and 15 minutes stationary (1 revolution per 100 seconds) to ensure uniform distribution of algae. Incubators were maintained at 20°C on a 14h:10h light:dark cycle. In order to maintain fairly constant herbicide concentrations, food levels, and pH levels in each treatment, individual animals were transferred into newly prepared experimental treatments every other day at which time neonates were removed and counted. Survival was measured daily and experiments were terminated after 8 days.
S. vetulus Juvenile Experiments
In the juvenile S. vetulus experiments, there were 10 replicates per treatment run in a single experiment with a single individual in each replicate. Neonates (< 24 h old) were placed individually in 40-ml glass vials containing 30-ml of pond water and appropriate pH, food, and herbicide levels. Experimental animals were not rotated on a plankton wheel in juvenile experiments to prevent neonates from being caught in the surface tension. Individuals were transferred every other day into new vials containing the appropriate stressor treatments. Juvenile experiments were terminated when all individuals had either died or produced their first clutch. Age of first reproduction and survival were measured for each individual.
R. pipiens Survival Experiments
Gosner stage 25 tadpoles received from Carolina Biological or collected in the field were acclimated to treatment pH levels 24 hours prior to the start of the experiments, half to pH 5.5 and half to pH 7.5. R. pipiens experiments were conducted with 4 replicates per treatment in each of two blocks with a single tadpole in each replicate. Survival of controls in the second block was poor and the block was terminated. An additional block could not be run due to availability of tadpoles. During the experiment, individual tadpoles were placed in 250 mls of filtered pond water with the specified herbicide, pH, and food (C. erosa) treatments. Every other day, tadpoles were transferred into jars containing new filtered pond water with the respective herbicide, pH, and food treatments. Individual tadpoles were monitored every day for survival and the experiments were terminated after 10 days.
Statistical Analysis
Survival of S. vetulus adults and R. pipiens tadpoles was analyzed using SAS LIFETEST (SAS, statistics version 5). Pairwise comparisons of survival responses in each treatment were made using a Logrank test. Comparisons of development times of neonates were also made using SAS LIFETEST and Logrank tests in pairwise comparisons. Interactions between stressors analyzed using LIFETEST were examined by comparing the significance of the effect of a single stressor (e.g, pH effect alone comparing low vs. high pH with no herbicide and high food) to the significance of the effect of that single stressor in the presence of a second stressor (e.g. pH effect comparing low vs. high pH in the presence of herbicide or low food). Reproduction was measured as cumulative reproduction (total number of neonates produced per female during the experiment) and reproductive rate (eggs per female per day) (Chen and Folt, 1996). Cumulative reproduction reflects both reproduction and survival, because it measures reproduction per individual over the entire survival period. The reproductive responses of S. vetulus to pH, food, and herbicide treatments were compared using a GLM three-way ANOVA (SAS, statistics version 5) and data were analyzed for main effects and interactions. Reproduction data were tested for normal distribution and error variances and residuals were visually inspected to check for homoscedasticity. Block effects were considered in the ANOVA's to determine whether constant conditions were maintained in the cultures throughout the experiments.
Results
S. vetulus Adult Survival
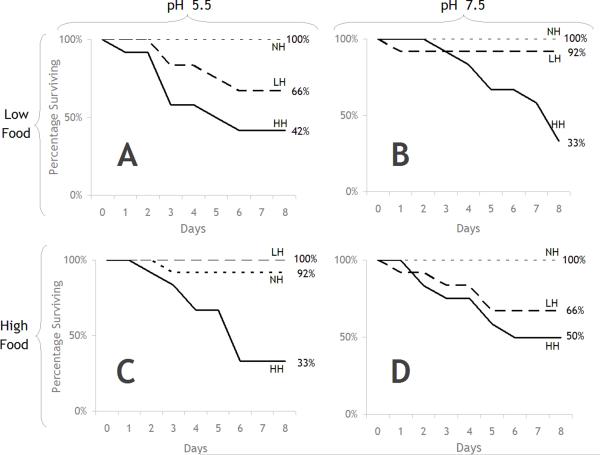
In the experiments measuring adult S. vetulus response to Release®, there were significant increases in mortality due to herbicide concentrations at both pH 5.5 and 7.5 (Table 1, lines e, f, g, and j; Figure 1a, b). In the high herbicide treatment (0.5 mg/L) that is approximately one fifth of the predicted environmental concentration, there was 50–67% mortality at both pH levels by the end of 8 days. Interactions between pH and herbicide and food and herbicide were detected when examining the pair-wise comparisons of survival curves between each treatment (Table 1). Although there were no significant differences between pH treatments in high food and no herbicide (Table 1, line a, p=0.3173, Figure 1a), there were significant effects of pH in the presence of low herbicide (Table 1, line k, pH 7.5 < pH 5.5; p=0.0321, Figure 1b) suggesting an herbicide × pH interaction. In addition, there were no differences in effects between low and high herbicide in high food, pH 7.5 (Table 1, line i, p=0.2719) but there were significant differences between herbicide levels in low food, pH 7.5 (Table 1, line r, p=0.0065). In comparisons of low vs. high food in various herbicide and pH treatment conditions, there were only significant effects of food on S. vetulus survival under conditions of low herbicide and low pH in which low food resulted in a 34% decline in survival (Table 1, line p). Food level also altered the comparison between survival at high vs. low pH in high herbicide concentration, where in high food survival was greater in high pH than in low pH (Table 1, line k vs. line l, p=.0321 vs p=0.1665).
Table 1.
Results of pair-wise comparisons of survival curves of individual treatments using a log-rank test comparison. Values in bold indicate significant differences at the p<0.05 level.
| combinations (e.g. NH/HH “no herbide vs. high herbicide” indicated comparision | ||||||
|---|---|---|---|---|---|---|
| Line |
Release* |
Food** |
pH |
S. vetulus survival |
S. vetutus development |
R. pipiens survival |
| a | NH | High | 5.5/7.5 | 0.3173 | 0.0556 | No mortality |
| b | NH | Low | 5.5/7.5 | No mortality | 0.3843 | No mortality |
| c | NH | High/Low | 7.5 | No mortality | 0.9360 | No mortality |
| d | NH | High/Low | 5.5 | 0.3173 | 0.0017 | No mortality |
| e | NH/HH | High | 7.5 | 0.0020 | 0.0029 | 0.0062 |
| f | NH/HH | High | 5.5 | 0.0059 | 0.0001 | 0.0069 |
| g | NH/LH | High | 7.5 | 0.0321 | 0.0022 | 0.0067 |
| h | NH/LH | High | 5.5 | 0.3173 | 0.0001 | 0.0067 |
| i | LH/HH | High | 7.5 | 0.2719 | 0.0137 | 0.0714 |
| j | LH/HH | High | 5.5 | 0.0007 | 0.0939 | 0.0180 |
| k | LH | High | 5.5/7.5 | 0.0321 | 0.0751 | 0.7037 |
| l | LH | Low | 5.5/7.5 | 0.1665 | 0.5099 | 0.2662 |
| m | HH | High | 5.5/7.5 | 0.9411 | 0.0947 | 0.0300 |
| n | HH | Low | 5.5/7.5 | 0.1351 | 0.2283 | 0.0100 |
| o | LH | High/Low | 7.5 | 0.1581 | 0.0961 | 0.7930 |
| p | LH | High/Low | 5.5 | 0.0370 | 0.0074 | 0.4270 |
| q | HH | High/Low | 7.5 | 0.9075 | 0.0284 | 0.7399 |
| r | LH/HH | Low | 7.5 | 0.0065 | 0.5389 | 0.6700 |
Release: NH= 0.0 mg/L; LH=0.25 mg/L ; HH=0.50 mg/L
Food: Low=750 cells/L; High= 2000 cells/L
Figure 1.
Adult S. vetulus survival in response to treatments of Release® (0.25 and 0.50 mg a.e. L−1), pH, and food. A) in low food and low pH treatments; B) in low food treatments and high pH; C) in high food and low pH; and D) in high food and high pH. NH = no herbicide, LH = low herbicide, HH = high herbicide.
Adult Reproduction
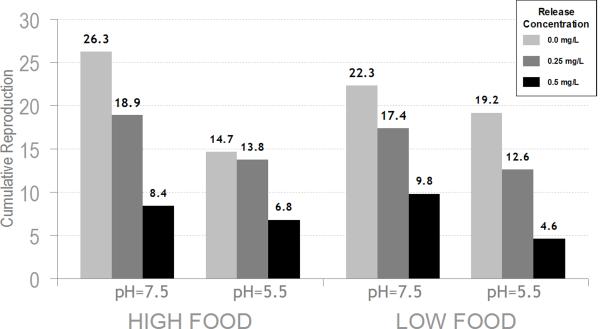
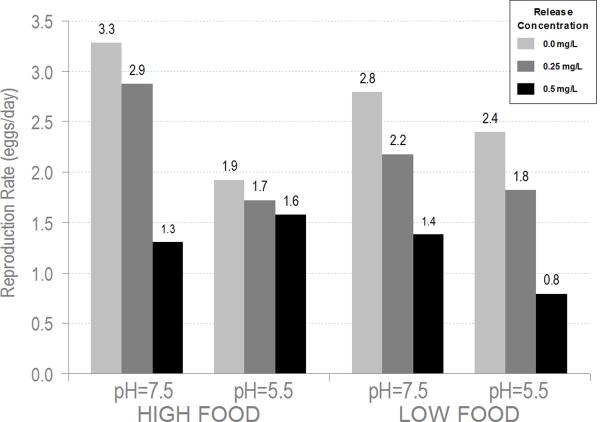
The response of adult S. vetulus reproduction to Release® was strongly affected by both pH and herbicide concentration irrespective of food availability. In both pH levels and both food levels, there was a decline in cumulative reproduction (CR) and reproductive rate (RR) with increasing herbicide concentration (Figure 2a and 2b). However, reproduction at pH 5.5 was consistently lower than at pH 7.5 for both herbicide treatments and controls. In a 3-way ANOVA (Table 2), there was a significant pH effect (p<0.0001 CR, p<0.0002 RR) and a significant herbicide effect (p<0.0001 CR, p<0.0001 RR) for both cumulative reproduction and reproductive rate, respectively. Although there was also a significant block effect, the herbicide effect was consistent across the three blocks and the pH effect was significant in the first and third blocks. There was a significant 3-way interaction with pH, food, and herbicide for reproductive rate (p=0.0360) and a similar trend, although not significant for cumulative reproduction (p=0.0910). However, this may have been driven by the significant block effect in which only the second block showed a significant 3-way interaction.
Figure 2.
Adult S. vetulus reproduction in response to treatments of Release® (0.25 and 0.50 mg a.e. L−1), pH and food. A) Cumulative reproduction; B) Reproductive rate. Histogram bar colors represent herbicide concentrations.
Table 2.
Results for 3-way ANOVA for effects of food, pH, herbicide, and block on cumulative reproduction and reproductive rate of S. vetulus. Values in bold indicate significant differences at the p<0.05 level.
| Treatments |
Cumulative Reproduction P-vafue |
Reproductive Rate P-vaiue |
|---|---|---|
| Food | 0.6660 | 0.1665 |
| pH | <0.0001 | <0.0002 |
| Herbicide | <0.0001 | <0.0001 |
| Block | <0.0001 | <0.0001 |
| Food * pH | 0.4376 | 0.3412 |
| Food * Herbicide | 0.8395 | 0.6291 |
| pH * Herbicide | 0.3537 | 0.1407 |
| pH * Food * Herbicide | 0 0910 | 0.0360 |
Juvenile Survival and Development
Release® did not have significant effects on survival of juvenile S. vetulus since in most treatments 90% of the individuals survived. In fact, the juveniles had higher proportional survival than the adults which, at first, was surprising. This may have been due to the fact that the Release® stratified in the unmixed vials in which the juvenile experiments were conducted (Thompson, unpublished data). In order to maintain consistency with juvenile experiments for Vision® and to prevent juveniles from being caught in the surface tension of the media (Chen et al., 2004), the protocol for the juvenile Release® experiments was not changed. However, additional Release® treatments were run in rotated jars and the juvenile mortality was found to be higher than in stationary jars (Chen, unpublished data). In high herbicide concentrations, neonates exposed in jars on rotating wheels exhibited significantly shorter survival (4.8 days) than those in unrotated jars (7.4 days, p=0.0357). Therefore, the results from our unrotated experiments likely underestimated the toxicity effects on survival of juveniles in a well-mixed system.
Herbicide effects and interactions with pH and food were clearly observed in the measurement of neonate development rates (Figure 3a and b, Table 1). In treatments with no herbicide, individuals matured between 6–7 days. In treatments with low (0.25 mg/L) and high (0.5 mg/L) herbicide, time to maturity was >9 days. Moreover, although not significant, there was a consistently longer time to maturity in the low vs. high pH (line a, p=0.0556, Table 1). There was also pronounced effects of food concentrations resulting in a longer and more variable time to maturity in low food concentrations than high food concentrations across all treatments including controls. For example, mean age of first reproduction varied from 8.8–13.4 days in high food but 10–16.7 days in low food (Table 3). Furthermore, pair-wise comparisons between treatments of development curves (Fig 3a and 3b) showed trends of slower development times in low food than high food in no herbicide and low pH (Table 1, line d, p = 0.0017) and slower development and lower percent matured in high herbicide and high pH (Table 1, line q, p = 0.0284). This finding shows that food deficiencies can significantly increase development times and that the effect is enhanced by concomitant exposure to even low levels of Release® herbicide.
Figure 3.
Juvenile S. vetulus development time in response to treatments of Release® (0.25 and 0.50 mg a.e. L−1), pH, and food. A) in low food and low pH treatments; B) in low food treatments and high pH; C) in high food and low pH; and D) in high food and high pH. NH = no herbicide, LH = low herbicide, HH = high herbicide.
Table 3.
Development time (mean and standard deviations) and percentage of individuals of S. vetulus that survived to maturity.
| Release* | Food** | pH | Development Time | % Survival | |
|---|---|---|---|---|---|
| means | stand dev. | ||||
| NH | Low | 5.5 | 11.2 | 1.8 | 100 |
| NH | Low | 7.5 | 10.0 | 2.5 | 100 |
| NH | High | 5.5 | 8.8 | 1.0 | 100 |
| NH | High | 7.5 | 9.9 | 1.9 | 100 |
| LH | Low | 5.5 | 15.6 | 3.4 | 100 |
| LH | Low | 7.5 | 15.6 | 2.5 | 60 |
| LH | High | 5.5 | 12.2 | 1.1 | 90 |
| LH | High | 7.5 | 13.4 | 1.5 | 70 |
| HH | Low | 5.5 | 16.7 | 3.6 | 60 |
| HH | Low | 7.5 | 15.6 | 3.4 | 90 |
| HH | High | 5.5 | 11.6 | 0.8 | 100 |
| HH | High | 7.5 | 12.1 | 0.3 | 90 |
Release: NH= 0.0 mg/L; LH=0.25 mg/L ; HH=0.50 mg/L
Food: Low=750cells/L; High= 2000 cells/L
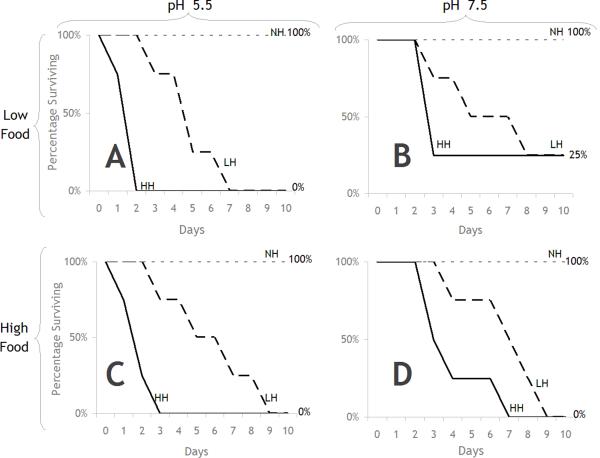
R. pipiens Survival
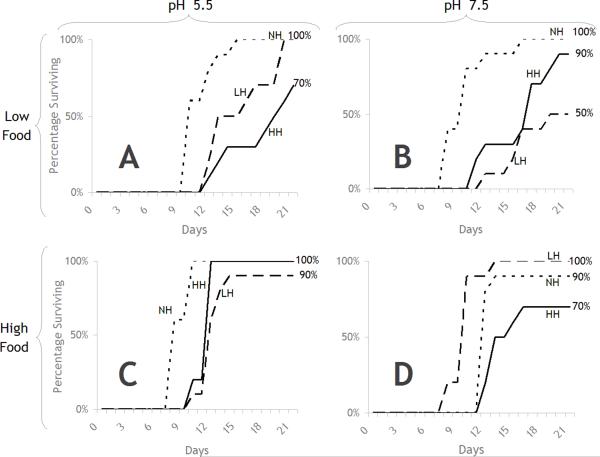
Overall survival of R. pipiens tadpoles was poor in the second block of the two experiments conducted. Therefore, data from only the first block could be used. Figure 4a and 4b show the survival of R. pipiens in the first block to various treatments. There was no significant effect of pH or of food on survival in the absence of herbicide. However, survival was significantly reduced following exposure to either herbicide test concentration (Table 1, line e, f, g, h, p = 0.0062, 0.0069, 0.0067, 0.0067). In high herbicide levels, the toxicity of the herbicide was significantly greater in pH 5.5 than pH 7.5 (Table 1, lines m, n, p = 0.0300, 0.0100) but at low herbicide levels there was no significant pH effect (Figures 4a and b). When combined with low food availability, rapid and essentially complete mortality was observed, with the response being more immediate and dramatic at the higher herbicide test concentration. Results demonstrated a clear interaction between herbicide, food, and pH. Pair-wise comparisons of survival curves for each treatment resulted in patterns of herbicide effects similar to those as described for adult S. vetulus (Table 1). However, the interactive effects of herbicide with food and pH differed between the two species. In particular, R. pipiens exposed to high herbicide levels exhibited more severe mortality in pH 5.5 than pH 7.5 at both low and high food concentrations (Table 1, lines m, n, Figures 4a and b).
Figure 4.
Gosner stage 25 R. pipiens survival in response to treatments of Release® (0.25 and 0.50 mg a.e. L−1), pH, and food. A) in low food and low pH treatments; B) in low food treatments and high pH; C) in high food and low pH; and D) in high food and high pH. NH = no herbicide, LH = low herbicide, HH = high herbicide.
Discussion
Our results demonstrate that Release® has significant negative effects on the survival, reproduction, and development of S. vetulus at concentrations well below predicted environmental concentration levels. Moreover, the toxic effects of the herbicide are in some cases exacerbated by low pH and in other cases by food concentration. There were parallel responses across species with the effect of each of the stressors on the target species being dependent upon the response variable measured. For both species, Release® significantly affected survival and also reproduction in S. vetulus at low and high concentrations. Moreover, pH and food effects interacted significantly with the herbicide to diminish survival of both species and also development of S. vetulus juveniles.
Tolerance to herbicide exposure differs greatly among taxa and the endpoints measured. The effects of tricolopyr both as Release® (Garlon 4, TBEE) and in the triethylamine form (TEA, Garlon 3A) have been examined in a variety of aquatic organisms (Peterson et al., 1994; Abdelghani et al., 1997; Gardner et al., 1997; Roshon et al., 1999) with the former being far more toxic to non-target organisms than the latter (Wan et al., 1987; Janz et al., 1991). For some taxa, published lethal and sublethal endpoints for Release® are lower than the predicted environmental concentration of 2.7 mg/L suggesting that these non-target species are exposed to levels that may be sublethal or lethal (Roshon et al., 1999; Edginton et al., 2003; Wojtaszek, 2004). 96-h LC50 values for amphibian embryos (8.3–23.3 mg AE/L) are higher than for larvae (0.79–18.2 mg AE/L) and decrease with decreasing pH (Perkins et al., 2000; Edginton et al., 2003). Rainbow trout were more sensitive to TBEE than aquatic insects in lake enclosure experiments (Kreutzweiser et al., 1995). However, rainbow trout survival and behavioral endpoints appeared less sensitive to TBEE than S. vetulus and R. pipiens in this study (Morgan et al., 1991; Kreutzweiser et al., 1995). For all exposures in this study, population level effects measured as survival, reproduction, or development were detected at both concentrations tested. Mortality of both S. vetulus and R. pipiens occurred at a concentration of 0.25 mg a.e./L suggesting that populations of both of these species may be directly affected by acute exposure concentrations less than one fifth the predicted environmental concentration. Other related studies confirm that larval amphibians such as R. pipiens are highly sensitive to toxic effects of Release® herbicide (Berrill et al., 1994; Edginton et al., 2003; Wojtaszek et al., 2005).
The strong effects of the herbicide observed in this (and many other lab experiments) are a direct function of the exposure magnitude and duration employed in the test. Following standard static renewal testing protocols, this experiment artificially maintained a constant level of exposure through the duration of the test. Under natural exposure regimes the principal toxicant (TBEE) is rapidly degraded by a combination of photolysis, hydrolysis and microbial processes or dissipated from the water column by sorption to sediments and other organic matrices (Bush, 1988; Thompson et al., 1991; Kreutzweiser et al., 1995). In fact, compared to earlier generations of chemical herbicides, most commercially available herbicides have relatively short persistence times (half-lives of days rather than months or years). Thus, the exposure of organisms to a single application of TBEE in the field would diminish rapidly over time. Nonetheless, based on predicted environmental levels and taking into account variability in chemical degradation rates the test concentrations and exposure durations used in this study are considered to be environmentally relevant. Thus, environmental stressors such as pH and food availability appear likely to have measurable effects on population parameters alone or in combination with one another or common anthropogenic stressors (Chandini, 1988; Hanazato and Dodson, 1992; Horne and Dunson, 1995; Belden and Lydy, 2000). Due to the toxicity of Release® to certain algal species, indirect effects of the chemical on herbivores seem possible due to the decline in algal food (Peterson et al., 1994). Although there were no declines in C. erosa during the experiments due to treatment levels of Release®, there were measurable effects of food treatments. Indirect food effects measured as differences between treatment combinations at low vs. high food were most detectable in measures of S. vetulus survival and development (age to maturity) and also in R. pipiens survival. Similar interactive stressor effects were also observed for Vision® in an earlier study using S. vetulus and R. pipiens as test species (Chen et al., 2004). While differences in the interactions occur with these two different herbicide formulations, the fact that interactive effects were observed for both herbicides on both test species suggests that such multiple stress interactions are likely for other chemical stressors and aquatic organisms. This postulate is further supported by the increasing number of studies demonstrating various multiple stressor interactions (Hanazato and Dodson, 1995; Abdelghani et al., 1997; Folt et al., 1999; Forget et al., 1999).
Conclusions
These results have a number of implications for populations of non-target aquatic species in the field. Concentrations much lower (5–10×) than the predicted environmental concentration for Release® or Garlon 4® applications in forestry are significantly toxic to a ubiquitous wetland zooplankton species and a common amphibian species. These results also demonstrate that the effects of Release® and other herbicides depend on the pH of the system and differences of two pH units (pH 5.5–7.5) can be critical to their toxicity (Chen et al., 2004; Edginton et al., 2004). Due to its enhanced toxicity in pH 5.5, Release® creates more of an environmental risk when applied to more acidic wetland habitats. Moreover, reductions in food resources due to seasonal fluctuations or direct chemical effects could greatly affect growth rates of herbivore populations through delays in development of juveniles or reductions in reproduction. Our results suggest that zooplankton and tadpole populations could be at risk in low pH, low productivity wetlands sprayed directly or indirectly with Release®. These habitats are common features of the northeast regions of North America where applications of triclopyr ester herbicides are made to achieve vegetation management objectives in forestry or industrial rights-of–way.
A limited amount of monitoring data for triclopyr residue concentrations in surface waters measuring concentrations of 0.05–0.11 mg L−1 (Wan et al., 1987; McCall et al., 1988; Thompson et al., 1991; Washington State, 2004) suggest that real-world exposure levels may be commonly below test levels employed in this experiment. However, given the lack of monitoring data for triclopyr concentrations in wetlands, the potential for concomitant exposure to other natural stressors such as low pH and low food, and the demonstrable sensitivity of both zooplankton and larval amphibians, we would conclude that broadcast applications of triclopyr herbicide in industrial and forestry use sectors may pose a risk of potential effects to zooplankton and amphibian larvae in wetland ecosystems. A more refined ecological risk assessment directly pertinent to these use patterns would require detailed chemical and biological monitoring in potentially exposed natural wetlands to determine whether actual conditions in operational herbicide applications present risks to zooplankton and amphibian populations.
Acknowledgments
We are grateful to M. Calvi, L. Aucoin, J. Stamp, C. Kemp, T. Dempsey, and B. Mayes for assistance with experiments. We also thank J. Dykes for statistical assistance; A. Edginton for advice on working with tadpoles; B. Staznik for her assistance in the analytical confirmation of test concentrations; and R. Stemberger for providing us with the Simocephalus vetulus culture. This research was supported by the Toxic Substances Research Initiative, Grant 121 from Health Canada and NIH Grant P42 ESO7373-7 to C.L. Folt and C.Y. Chen from the National Institute of Environmental Health Sciences.
This research was supported by the Toxic Substances Research Initiative, Grant 121 from Health Canada and NIH Grant P42 ESO7373-7 to C.L. Folt and C.Y. Chen from the National Institute of Environmental Health Sciences. The use of vertebrate experimental animals was conducted in the accordance with national and institutional guidelines specified in the Animal Subjects Review Forms approved by the Institutional Animal Care and Use Committee (IACUC) at Dartmouth College.
REFERENCES
- Abdelghani AA, Tchounwou PB, Anderson AC, Sujono H, Heyer LR, Monkiedje A. Toxicity evaluation of single and chemical mixtures of Roundup, Garlon-3a, 2,4-d, and syndets surfactant to channel catfish (Ictalurus punctatus), bluegill sunfish (Lepomis microchirus), and crawfish (Procambarus spp.) Environmental Toxicology and Water Quality. 1997;12:237–243. [Google Scholar]
- Ahrens WH. Herbicide handbook. Seventh Edition 1994. [Google Scholar]
- Barron MG, Mayes MA, Murphy PG, Nolan RJ. Pharmacokinetics and metabolism of triclopyr butoxyethyl ester in coho salmon. Aquatic Toxicology. 1990;16:19–32. [Google Scholar]
- Belden JB, Lydy MJ. Impact of atrazine on organophosphate insecticide toxicity. Environmental Toxicology and Chemistry. 2000;19:2266–2274. [Google Scholar]
- Berrill M, Bertram S, McGillivray L, Kolohon M, Pauli B. Effects of low concentrations of forest-use pesticides on frog embryos and tadpoles. Environmental Toxicology and Chemistry. 1994;13:657–664. [Google Scholar]
- Bush PB, Neary DG, Taylor JW. Effect of triclopyramine and ester formulations on groundwater and surface runoff water quality in the coastal plain. Proc. South. Weed Sci. Soc. 1988;39:262–270. [Google Scholar]
- Chandini T. Changes in food [chlorella] levels and the acute toxicity of cadmium to Daphnia carinata (Daphnidae) and Echinisca triserialis (Macrothricidae) [Crustacea, Cladocera] Bulletin of Environmental Contamination and Toxicology. 1988;41:398–403. doi: 10.1007/BF01688885. [DOI] [PubMed] [Google Scholar]
- Chen CY, Folt CL. Measures of food quality as demographic predictors in freshwater copepods. Journal of Plankton Research. 1993;15:1247–1261. [Google Scholar]
- Chen CY, Folt CL. Consequences of fall warming for zooplankton overwintering success. Limnology and Oceanography. 1996;41:1077–1086. [Google Scholar]
- Chen CY, Hathaway KM, Folt CL. Multiple stress effects of Vision® herbicide, pH, and food on zooplankton and larval amphibian species from forest wetlands. Environmental Toxicology and Chemistry. 2004;23:823–831. doi: 10.1897/03-108. [DOI] [PubMed] [Google Scholar]
- Edginton AN, Sheridan PM, Stephenson GR, Thompson DG, Boermans HJ. Comparative effects of pH and Vision® herbicide on two life stages of four anuran amphibian species. Environmental Toxicology and Chemistry. 2004;23:815–822. doi: 10.1897/03-115. [DOI] [PubMed] [Google Scholar]
- Edginton AN, Stephenson GR, Sheridan PM, Thompson DG, Boermans HJ. Effect of pH and Release® on two life stages of four anuran amphibians. Environmental Toxicology and Chemistry. 2003;22:2673–2678. doi: 10.1897/02-484. [DOI] [PubMed] [Google Scholar]
- Folt CL, Chen CY, Moore MV, Burnaford J. Synergism and antagonism among multiple stressors. Limnology and Oceanography. 1999;44:864–877. [Google Scholar]
- Forget J, Pavillon JF, Beliaeff B, Bocquene G. Joint action of pollutant combinations (pesticides and metals) on survival (LC50 values) and acetylcholinesterase activity of Tigriopus brevicornis (Copepoda, Harpacticoida) Environmental Toxicology and Chemistry. 1999;18:912–918. [Google Scholar]
- Gardner SC, Grue CE, Grassley JM, Lenz LA, Lindenauer JM, Seeley ME. Single species algal (Ankistrodesmus) toxicity tests with Rodeo® and Garlon 3a®. Bulletin of Environmental Contamination and Toxicology. 1997;59:492–499. doi: 10.1007/s001289900505. [DOI] [PubMed] [Google Scholar]
- Hanazato T, Dodson SI. Complex effects of a kairomone of Chaoborus and an insecticide on Daphnia pulex. Journal of Plankton Research. 1992;14:1743–1755. [Google Scholar]
- Hanazato T, Dodson SI. Synergistic, effects of low oxygen concentration, predator kairomone, and a pesticide on the cladoceran Daphnia pulex. Limnology and Oceanography. 1995;40:700–709. [Google Scholar]
- Horne MT, Dunson WA. Effects of low pH, metals, and water hardness on larval amphibians. Archives of Environmental Contamination and Toxicology. 1995;29:500–505. [Google Scholar]
- Janz DM, Farrell AP, Morgan JD, Vigers GA. Acute physiological stress responses of juvenile coho salmon (Oncorhynchus kisutch) to sublethal concentrations of Garlon 4, Garlon 3a and Vision herbicides. Environmental Toxicology and Chemistry. 1991;10:81–90. [Google Scholar]
- Johansen JA, Geen GH. Sublethal and acute toxicity of the ethylene-glycol butyl ether ester formulation of triclopyr to juvenile coho salmon (Oncorhynchus kisutch) Archives of Environmental Contamination and Toxicology. 1990;19:610–616. doi: 10.1007/BF01059083. [DOI] [PubMed] [Google Scholar]
- Kirk KL. Dissertation. Dartmouth College; Hanover NH USA: 1988. The effect of suspended sediments on planktonic rotifers and cladocerans. [Google Scholar]
- Kreutzweiser DP, Thompson DG, Capell SS, Thomas DR, Staznik B. Field evaluation of triclopyr ester toxicity to fish. Archives of Environmental Contamination and Toxicology. 1995;28:18–26. [Google Scholar]
- Kupferberg SJ. The role of larval diet in anuran metamorphosis. American Zoologist. 1997;37:146–159. [Google Scholar]
- Lampert W. Field-study on dependence of fecundity of daphnia spec on food concentration. Oecologia. 1978;36:363–369. doi: 10.1007/BF00348062. [DOI] [PubMed] [Google Scholar]
- Locke A, Sprules WG. Effects of acidic ph and phytoplankton on survival and condition of Bosmina longirostris and Daphnia pulex. Hydrobiologia. 2000;437:187–196. [Google Scholar]
- Mallory ML, McNicol DK, Cluis DA, Laberge C. Chemical trends and status of small lakes near Sudbury, Ontario, 1983–1995: Evidence of continued chemical recovery. Canadian Journal of Fisheries and Aquatic Sciences. 1998;55:63–75. [Google Scholar]
- McCall PJ, Laskowski DA, Bidlack HD. Simulation of the aquatic fate of triclopyr butoxyethyl ester and its predicted effects on coho salmon. Environmental Toxicology and Chemistry. 1988;7:517–527. [Google Scholar]
- Morgan JD, Vigers GA, Farrell AP, Janz DM, Manville JF. Acute avoidance reactions and behavioral-responses of juvenile rainbow-trout (Oncorhynchus mykiss) to Garlon 4, Garlon 3a and Vision herbicides. Environmental Toxicology and Chemistry. 1991;10:73–79. [Google Scholar]
- Murphy J, Phillips C, Beasely V. Aspects of amphibian. In: DW S, et al., editors. Ecotoxicology of Amphibians and Reptiles. SETAC; Pensacola, FL: 2000. pp. 545–571. [Google Scholar]
- Perkins PJ, Boermans HJ, Stephenson GR. Toxicity of glyphosate and triclopyr using the frog embryo teratogenesis assay-Xenopus. Environmental Toxicology and Chemistry. 2000;19:940–945. [Google Scholar]
- Peterson HG, Boutin C, Martin PA, Freemark KE, Ruecker NJ, Moody MJ. Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquatic Toxicology. 1994;28:275–292. [Google Scholar]
- Roshon RD, McCann JH, Thompson DG, Stephenson GR. Effects of seven forestry management herbicides on Myriophyllum sibiricum, as compared with other nontarget aquatic organisms. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere. 1999;29:1158–1169. [Google Scholar]
- Saunders PA, Shaw WH, Bukaveckas PA. Differences in nutrient limitation and grazer suppression of phytoplankton in seepage and drainage lakes of the Adirondack region, NY, USA. Freshwater Biology. 2000;43:391–407. [Google Scholar]
- Schindler DW. Detecting ecosystem responses to anthropogenic stress. Canadian Journal of Fisheries and Aquatic Sciences. 1987;44:6–25. [Google Scholar]
- Seale DB. Obligate and facultative suspension feeding in anuran larvae - feeding regulation in Xenopus and Rana. Biological Bulletin. 1982;162:214–231. [Google Scholar]
- Semkin RG, Jeffries DS. Storage and release of major ionic contaminants from the snowpack in the Turkey lakes watershed. Water Air and Soil Pollution. 1986;31:215–221. [Google Scholar]
- Shepard JP, Creighton J, Duzan H. Forestry herbicides in the United States: an overview. Wildlife Society Bulletin. 2004;32:1020–1027. [Google Scholar]
- Solomon KR, Bowhey CS, Liber K, Stephenson GR. Persistence of hexazinone (Velpar), triclopyr (Garlon), and 2,4-d in a northern Ontario aquatic environment. Journal of Agricultural and Food Chemistry. 1988;36:1314–1318. [Google Scholar]
- Tessier AJ, Consolatti NL. Resource quantity and offspring quality in Daphnia. Ecology. 1991;72:468–478. [Google Scholar]
- Thompson DG. Potential effects of herbicides on native amphibians: A hierarchical approach to ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry. 2004;23:813–814. [PubMed] [Google Scholar]
- Thompson DG, Kreutzweiser DP, Capell SS, Thomas DR, Staznik B, Viinikka T. Fate and effects, of triclopyr ester in a first-order forest stream. Environmental Toxicology and Chemistry. 1995;14:1307–1317. [Google Scholar]
- Thompson DG, Staznik B, Fontaine DD, Mackay T, Oliver GR, Troth J. Fate of triclopyr ester (Release) in a boreal forest stream. Environmental Toxicology and Chemistry. 1991;10:619–632. [Google Scholar]
- Thompson DG, Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR. Chemical and biomonitoring to assess potential acute effects of Vision® herbicide on native amphibian larvae in forest wetlands. Environmental Toxicology and Chemistry. 2004;23:843–849. doi: 10.1897/02-280. [DOI] [PubMed] [Google Scholar]
- Thompson DG, Pitt DG. A review of Canadian forest vegetation management research and practice. Annals of Forest Science. 2003;60:559–572. [Google Scholar]
- Wan MT, Moul DJ, Watts RG. Acute toxicity to juvenile Pacific salmonids of Garlon 3a, Garlon 4, triclopyr, triclopyr ester, and their transformation products - 3,5,6-trichloro-2-pyridinol and 2-methoxy-3,5,6-trichloropyridine. Bulletin of Environmental Contamination and Toxicology. 1987;39:721–728. doi: 10.1007/BF01698468. [DOI] [PubMed] [Google Scholar]
- Washington State D. o. A. Surface water monitoring program for pesticides in salmonid-bearing streams. Washington State Departments of Ecology and Agriculture; Olympia, WA: 2004. [Google Scholar]
- Willis KJ, Ling N, Chapman MA. Effects of temperature and chemical formulation on the acute toxicity of pentachlorophenol to Simocephalus vetulus (schoedler, 1858) (crustacea, cladocera) New Zealand Journal of Marine and Freshwater Research. 1995;29:289–294. [Google Scholar]
- Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR, Thompson DG. Effects of Vision® herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands. Environmental Toxicology and Chemistry. 2004;23:832–842. doi: 10.1897/02-281. [DOI] [PubMed] [Google Scholar]
- Wojtaszek BF. Ph.D. thesis. University of Guelph; Guelph, ON, Canada: 2004. In situ investigation of the effects of Vision® and Release® silvicultural herbicides on plankton and larval amphibians. [Google Scholar]
- Wojtaszek BF, Buscarini TM, Chartrand DT, Stephenson GR, Thompson DG. Effects of Release® herbicide on mortality, avoidance response and growth of amphibian larvae in two forest wetlands. Environmental Toxicology and Chemstry. 2005;24:2533–2544. doi: 10.1897/04-377r.1. [DOI] [PubMed] [Google Scholar]