Abstract
Efflux of glutathione (GSH) from astrocytes has been suggested as a key factor for neuroprotection by astrocytes. Here we evaluated if the Nrf2 activator curcumin affects basal and stimulated (Ca2+ omission) GSH efflux from cultures of astroglial cells. Stimulated efflux of GSH was observed at medium concentration of 0, 0.1 mM Ca2+, but not at 0.2 or 0.3 mM Ca2+. Astroglia treated with 30 μM curcumin increased the cellular content of GSH in parallel with elevated basal and stimulated efflux. Conversely treatment with buthionine sulfoximine lowered efflux of GSH. The efflux stimulated by Ca2+- omission was not affected by the P2X7-receptor antagonist Blue Brilliant G (100 nM) or the pannexin mimetic/blocking peptide 10Panx1 but inhibited by the gap junction blocker carbenoxolone (100 μM) and a hemichannel blocker Gap26 (300 μM). RNAi directed against Nrf2 partly inhibited the effect of curcumin. The results show that elevated cellular GSH by curcumin treatment enhance efflux from astroglial cells, a process which appear to be a prerequisite for astroglial mediated neuroprotection.
Keywords: Astrocyte, Glutathione, Connexin 43, Nrf2, Curcumin
Introduction
Astrocytes protect neurons in several ways. One route is via a glutathione (GSH) “shuttle” that involves synthesis and efflux of glutathione from astrocytes [1]. GSH is then catabolized in the extracellular space by astrocytic γ-glutamyltranspeptidase, a reaction that yields a γ-glutamyl-dipeptide amino acid and cysteinylglycine [1]. This dipeptide is taken up or broken down to cysteine and glycine [2]. Cysteine is a substrate for the EAAC1 transporter and is used intracellularly for neuronal GSH synthesis [3]. Interestingly and importantly from a potential therapeutic standpoint this intricate astrocytic defense system can be stimulated. Thus, it has been demonstrated that tert-butylhydroquinione via the transcription factor NF-E2-related factor 2 (Nrf2) and the antioxidant response element (ARE) induce the expression of a number of genes involved in the GSH defence system described above [4]. This system was recently elegantly shown to be neuroprotective in vivo against experimental amyotropic lateral sclerosis (ALS) by Nrf2 overexpression selectively in astroglial cells [5]. The GSH efflux is thus a key in this defense system. The multidrug resistance protein 1 is one efflux pathway for GSH which is upregulated by tert-butylhydroquinione [4, 6]. Another system for GSH efflux is inhibited by gap junction blockers and extracellular Ca2+. The system most likely consists of half gap junctions, termed connexons or hemichannels [7, 8]. As extracellular Ca2+ is decreased in situations like ischemia [9] efflux of GSH via such Ca2+-regulated astroglial hemichannels could be one route to supply neurons with the rate-limiting GSH precursor cysteine. Here we have further characterized the basal and stimulated efflux of GSH by omission of Ca2+ when its synthesis is increased or decreased. The γ-glutamylcysteine synthetase inhibitor buthionine sulfoximine (BSO) was used to reduce the intracellular GSH concentration and and curcumin to stimulate synthesis of GSH via increased expression of genes like γ-glutamylcysteine synthetase [10]. The use of curcumin was based on earlier studies demonstrating increased levels of GSH and the synthesizing enzymes in cell lines and primary cultures of astrocytes (see [10]).
Experimental Procedures
Materials
Eagle's minimum essential medium with Earle's salts (MEM), penicillin–streptomycin (PEST), amino acids, vitamins, and l-glutamine were purchased from Invitrogen (Merelbeke, Belgium), and fetal calf serum from Merck (Berlin, Germany). The artificial cerebrospinal fluid (ACSF) consisted of (mM): NaCl (128), KCl (3), CaCl2 (2), MgSO4 (1.2), KH2PO4 (0.4), NaHCO3 (25) and d-glucose (10) and Gey's balanced salt solution of (mM): NaCl (119), KCl (5), CaCl2 (2), MgCl2 (1) MgSO4 (0.3), KH2PO4 (0.2), NaHCO3 (27), Na2HPO4 (0.67) and d-glucose (5.5). In experiments were the effects of low extracellular levels of Ca2+ was evaluated CaCl2 was omitted from the medium or CaCl2 was added at the indicated concentrations. Chemical analysis of the Ca2+ concentration in the respective media was not performed. The solutions were equilibrated with gas mixtures (see further below) containing 5% CO2 which resulted in a pH ∼7.4. All salts in ACSF were from Sigma or Merck (Darmstadt, Germany) and methanol was from Rathburn (Rathburn Chemicals Ltd, Walkersburn, UK). Carbenoxolone (CBX), BSO and Brilliant blue G (BBG) were bought from Sigma (St. Louis, MO, USA). The connexin 43 (Cx43) mimetic/blocking peptide (Gap26) (VCYDKSFPISHVR) and the pannexin1 mimetic/blocking peptide (WRQAAFVDSY), 10Panx1, were synthesized by solid-phase chemistry and purified by HPLC to 95% purity (Sigma-Genosys) [11, 12]. Curcumin of 80% purity was also purchased from Sigma. Curcumin and 10Panx1 were dissolved in DMSO and diluted in ACSF and ACSF/0 Ca2+ to a final DMSO concentration of 0.1%.
Primary Astrocyte Cultures
Primary cultures of astrocytes were prepared from the hippocampi of newborn (P1-P2) Sprague–Dawley rats as previously described [13]. In brief, the rats were decapitated and the hippocampi were carefully dissected. The tissue was mechanically passed through a nylon mesh (80 μm mesh size) into culture medium consisting of MEM supplemented to the following composition: 20% (v/v) fetal calf serum, 1% penicillin–streptomycin, 1.6 time the concentrations of amino acids and 3.2 times the concentration of vitamins (in comparison to MEM), 1.6 mM l-glutamine, 7.15 mM glucose and 48.5 mM NaHCO3. The cells were grown in 35 mM wells at 37°C in a humidified atmosphere of 95% air and 5% CO2. The medium was changed after 3 days in culture and thereafter three times a week. Cells were used after 14–19 days in culture when a confluent monolayer had been formed. Curcumin and BSO were added to the culture medium 24 h prior to the experiments.
siRNA Mediated Knock-Down of Nrf2
Down regulation of the Nrf2 expression was achieved by siRNA technique as described earlier [14]. The astrocytes were transiently transfected using ON-TARGETplus SMARTpool siRNA against rat Nrf2 (Thermo Scientific Dharmacon, CO, USA). ON-TARGET plus non-targeting pool (Thermo Scientific Dharmacon, CO, USA) was used as negative control. The astrocytes were grown in 12-well plates to confluency (13–14 days in vitro). The transfection was initiated by incubating the cultures with OptiMEM (Invitrogen, CA, USA) for 30 min. Nrf2 ON-TARGETplus SMARTpool siRNA or ON-TARGET plus non-targeting pool (100 nM, final concentration) was mixed with Lipofectamine 2000 (3 μl; Invitrogen, CA, USA) in OptiMEM and incubated for 20 min prior to addition to the astrocytes (final volume 1 ml). After 5 h, the transfection mixture was replaced with OptiMEM containing 20% serum and the astrocytes were incubated for 19 h. The cultures were thereafter further incubated in normal growth medium with or without curcumin (30 μM) for another 24 h. The efficiency of the knockdown was evaluated by western blot (Fig. 6b) using α-Nrf2 antibody (dilution 1:1,000, R&D Technologies). The optical densities of Nrf2 blot was correlated to the densities of tubulin and showed a decrease in the level of Nrf2 by approximately 70% compared to untreated samples.
Fig. 6.
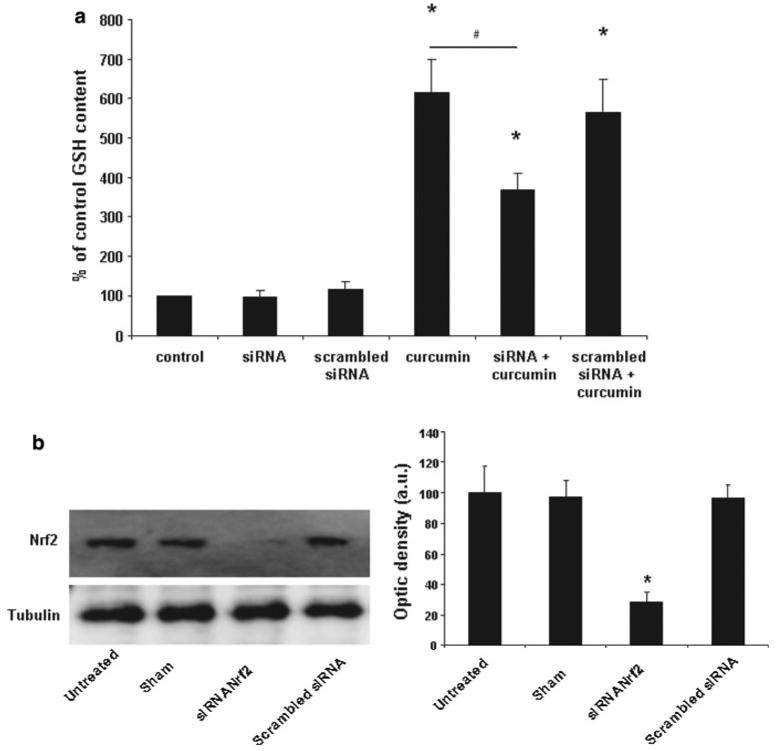
a The effect of curcumin (30 μM) on intracellular GSH concentration is significantly reduced by siRNA mediated knockdown of Nrf2. No effect was observed on basal levels of GSH or when the cells were transfected with scrambled siRNA. Values are given as percent relative to GSH concentration in the control group (n = 8 ± SEM). The level of GSH in cells treated with 30 μM curcumin after treatment with siRNA against Nrf2 was significantly lower (Wilcoxon signed rank test, p < 0.05) compared to the levels induced by curcumin in non-subjected cells and to levels in cells treated with a scrambled siRNA. Stars mark a significant difference (p < 0.05) compared to control and # marks significant difference between curcumin and siRNA treated cultures. b Left: Treatment with siRNA directed against Nrf2 lowered the expression of the Nrf-2 protein by approximately 70%. Right: Densitometric analysis of Nrf2 protein expression in astrocyte cultures treated with siRNA directed against Nrf-2. Data are plotted as ratio of the Nrf2/tubulin obtained in each condition
Efflux Experiments
The efflux experiments were carried out by incubating the cells with ACSF or ACSF with no Ca2+ added (400 μl) for 10 min. The fluid was then removed and filtered before immediate HPLC analysis or storage in −20°C (maximally 2 weeks). The incubation procedure was repeated 7 times (70 min in total) with Ca2+ omission occurring during the fourth and fifth incubation period. All inhibitors were present during a 30 min preincubation period and the entire incubation period (60 min in total before Ca2+ removal). All solutions were equilibrated with a gas mixture containing 5% CO2 to reach a pH of ∼7.4. Thereafter, all solutions were put in an incubator in a humidified atmosphere of 95% air and 5% CO2 at 36.5°C for at least 30 min. After the seventh incubation, the cells were scraped into 400 μl of 0.3 M HClO4 and sonicated. After centrifugation at 11,000g the supernatant was removed and filtered (Acrodisc, 0.2 μm, Pall Corporation, Ann Arbor, MI, USA). The supernatant was used to determine the cellular content of glutathione and amino acids. The protein pellet was dissolved in 100 μl 2% SDS and the protein content was measured using the bicinchoninic acid method [15].
HPLC Determination of Amino Acids and GSH
All chromatography was performed using a Varian 5000 or 5500 HPLC pump coupled to a fluorescence detector (Schoeffel FS 970) for detection of the fluorescence intensities of OPA-derivatives of glutathione and amino acid (see below for details). Data were processed with Millennium and Maxima software (Waters Corporation, Milford, MI, USA). All separations were performed at room temperature. Sample injection was made using a Waters 717 autosampler.
GSH and amino acids were determined using OPA derivatization and fluorescence detection essentially as described earlier [16]. A solution of β-mercaptoethanol, Na2-EDTA and NaN3 (final concentration 20, 1, 5 mM, respectively) was added to the samples and standards to keep GSH in its reduced form and prevent bacterial growth. The OPA-solution was prepared weekly and consisted of OPA (40 mg) dissolved in methanol (400 μl), β-mercaptoethanol (40 μl), borate buffer (2.0 ml, 0.8 M, pH 12) and H2O (1.6 ml). Every 2 days β-mercaptoethanol (10 μl) was added to the solution. Amino acids were derivatized (25 μl of sample mixed with 25 μl OPA solution) in the autosampler before injection. The amino acid derivatives were separated on a Nucleosil C18 column (200 × 4.6 mM; Macherey-Nagel, Germany) with a mobile phase consisting of NaH2PO4 (50 mM, pH 5.28) and methanol in a gradient from 25 to 95% methanol. A flow rate of 1 ml/min was used. Detection was carried out by excitation at 333 nm and emission over 418 nm. To test for possible interactions with GSH, standard solutions with or without drugs were analysed. No differences in the peak heights of GSH were found with any of the drugs used.
Statistics
Statistical analysis was performed with SPSS software (SPSS Inc., Chicago, Illinois, USA). One-way ANOVA followed by Tukey's test for multiple comparisons and Wilcoxons signed rank test were used for statistical analysis. A p value of <0.05 was considered statistically significant. Data shown in figures are from at least 3 independent cultures and expressed as means ± SEM.
Results
The efflux profile from the primary astroglial cells by omission of Ca2+ was similar to that reported from organotypic cultures [8], i.e. the efflux rates of GSH, glutamate, taurine (not shown) and phosphoethanolamine were particularly elevated (Fig. 1a, b). The stimulated efflux by omission of Ca2+ was not affected by the P2X7- receptor antagonist Brilliant Blue G (BBG, 100 nM) or the pannexin mimetic/blocking peptide 10Panx1 (300 μM) but inhibited by the gap junction blocker carbenoxolone (100 μM) and the connexin43 mimetic/blocking peptide Gap26 (300 μM, Fig. 2).
Fig. 1.
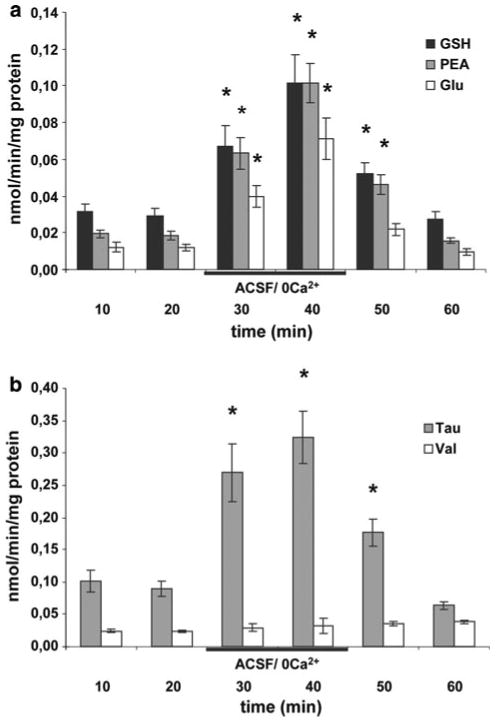
a Time course of stimulated efflux of glutathione (GSH), phosphoethanolamine (PEA) and glutamate (Glu) caused by omission of Ca2+. The efflux rates reached their maxima 10 min after the introduction of ACSF/0 Ca2+. b Time course of efflux for taurine (Tau) and valine (Val) following omission of Ca2+. No change in efflux was observed for Val. Data are presented as mean efflux rate (n = 6 ± SEM). Stars in a and b indicate a significant difference between efflux in ACSF and ACSF/ 0 Ca2+ (p < 0.05)
Fig. 2.
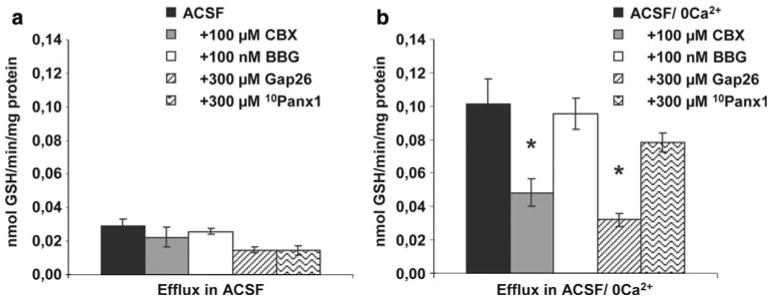
a The basal efflux of GSH in ACSF was not changed by the gap junction inhibitor carbenoxolone (CBX), the P2X7-receptor antagonist Brilliant Blue G (BBG), the the connexin hemichannel mimetic/blocking peptide Gap26 or the pannexin hemichannel mimetic/blocking peptide 10Panx1. b The gap junction blocker carbenoxolone (CBX) and the connexin hemichannel blocking peptide Gap26 significantly reduced the efflux of GSH caused by omission of Ca2+ while the P2X7-receptor antagonist Brilliant Blue G (BBG) and the pannexin hemichannel mimetic/blocking peptide 10Panx1 did not cause significant effects. Data are presented as mean efflux rate (n = 6 ± SEM). Stars mark a significant difference (p < 0.05) in efflux with inhibitors compared to efflux in ACSF/0 Ca2+ without inhibitors (n = 6 ± SEM)
Stimulated efflux of GSH was observed at 0.1 mM Ca2+, but not at 0.2 or 0.3 mM Ca2+ (Fig. 3). Blocking the synthesis of GSH by adding BSO (1 mM for 24 h) to the culture medium decreased the cellular content of GSH by ∼70% (Fig. 5). This treatment also decreased the basal efflux (∼70% lower compared to culturing without BSO) and efflux of GSH stimulated by omission of extracellular Ca2+ (∼85% lower compared to cells cultured without BSO) (Fig. 4a, b). The effect of BSO on basal efflux was selective for GSH, i.e. the efflux of other amino acids was unaffected. Treatment with BSO caused no change in efflux stimulated by omission of Ca2+ for phosphoethanolamine or taurine (not shown) but reduced that of glutamate (Fig. 4b). The cellular content of phosphoethanolamine was unchanged but that of glutamate increased by BSO treatment (Fig. 5).
Fig. 3.
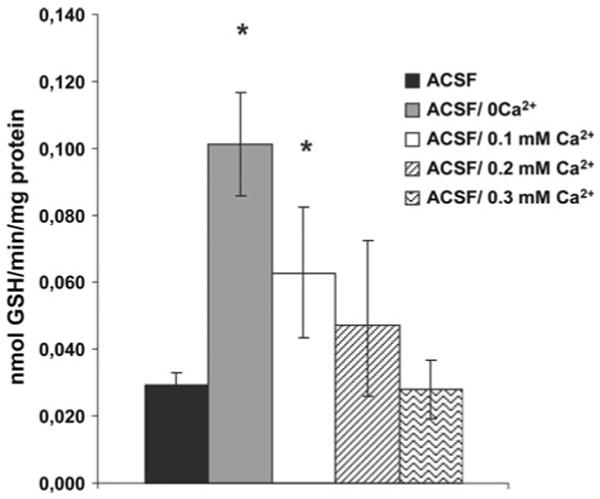
The efflux of GSH was stimulated by 0.1 mM Ca2+ but not by 0.2 mM Ca2+ or 0.3 mM Ca2+. Data are presented as mean efflux rate (n = 6 ± SEM). Stars mark a significant different efflux (p < 0.05) compared to efflux in ACSF
Fig. 5.
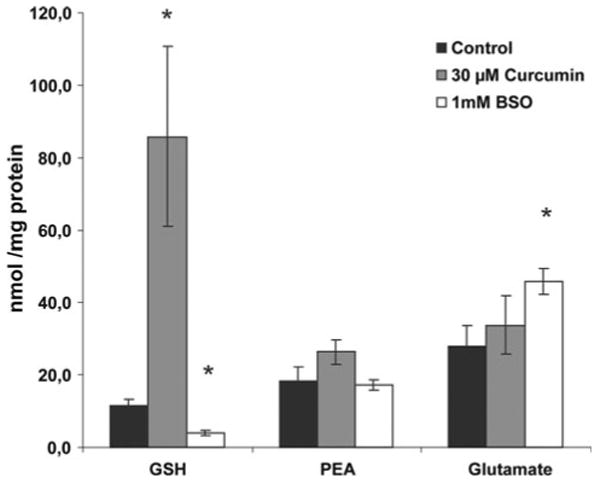
Cellular concentration of GSH, phosphoethanolamine (PEA) and glutamate (Glu) in astrocyte cultures treated for 24 h with 30 μM curcumin or 1 mM buthionine sulfoximine (BSO). The cellular concentration of GSH was increased by treatment for 24 h with 30 μM curcumin and decreased by treatment with 1 mM BSO. Treatment with BSO also increased the cellular concentration of Glu. Stars mark a significant different efflux (p < 0.05) compared to control value
Fig. 4.
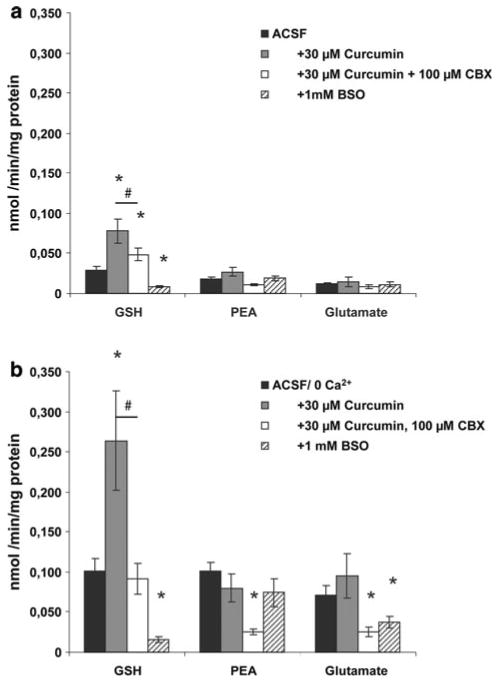
a Effects on efflux of GSH, phosphoethanolamine (PEA) and glutamate (Glu) in ACSF after treatment of the astrocyte cultures for 24 h with 30 μM curcumin or 1 mM buthionine sulfoximine (BSO). The efflux of GSH in ACSF was decreased by treatment for 24 h with BSO and increased by treatment with curcumin. The increased basal efflux of GSH in ACSF was decreased by the gap junction inhibitor carbenoxolone (CBX). No significant effects were observed for efflux of PEA or Glu. Stars mark significant different efflux (p < 0.05) by treatment for 24 h compared to no treatment. # marks significant difference between curcumin treated samples with or without carbenoxolone. b Effects on efflux of GSH, phosphoethanolamine (PEA) and glutamate (Glu) in ACSF/ 0 Ca2+ after treatment of the astrocyte cultures for 24 h with 30 μM curcumin or 1 mM buthionine sulfoximine (BSO). The stimulated efflux of GSH was decreased by treatment for 24 h with BSO and increased by treatment with curcumin. The gap junction blocker carbenoxolone (CBX) reduced the enhanced stimulated efflux of GSH in curcumin treated cultures. No significant effects by treatment with curcumin were observed on efflux of PEA or Glu in ACSF/0 Ca2+. The stimulated efflux of PEA and Glu were reduced by CBX (only evaluated in curcumin treated slices). The stimulated efflux of Glu was reduced after treatment of the cultures with BSO for 24 h. Data are presented as mean efflux rate (n = 6 ± SEM). Stars mark a significant difference (p < 0.05) in efflux with inhibitors or treatment compared to efflux in ACSF/0 Ca2+ without inhibitors. # marks significant difference between curcumin treated samples with or without carbenoxolone
Astrocytes cultured for 24 h with 30 μM curcumin increased the efflux rates of GSH in basal medium and in medium with omitted Ca2+ by 90 and 160%, respectively (Fig. 4a–b). The basal and stimulated efflux of GSH by Ca2+ omission in curcumin treated cells was dramatically reduced by carbenoxolone but notably not down to levels reached in cultures not treated with curcumin (Fig. 4a–b). The basal efflux and the efflux stimulated by Ca2+ omission of phosphoethanolamine and glutamate were not changed by treatment with curcumin. Addition of curcumin to the culture medium increased the cellular content of GSH approximately 6 times whereas the levels of phosphoethanolamine and glutamate were unaffected (Fig. 5). The effects of curcumin on GSH cellular content were reduced considerably by prior treatment of the cells with siRNA against Nrf2 (Fig. 6a) whereas no effect of siRNA was observed on GSH levels in non-curcumin treated cultures.
Discussion
Stimulated GSH synthesis in astroglia is neuroprotective via a shuttle that involves GSH efflux as described in the introduction [1]. Here we characterized and showed that the efflux of GSH can be enhanced by curcumin and by low extracellular Ca2+.
The stimulating effect on efflux by omission of extracellular Ca2+ is in line with several reports. Stimulated efflux of GSH [7, 8], glutamate [17], taurine [8, 17], NAD+ [18] and ATP [19] has been demonstrated earlier from astroglial cells in low or nominal Ca2+-free media. Most studies concerning efflux by Ca2+-omission have been performed in cultures. However, similar effects on glutamate efflux have been observed in vitro from acutely prepared optic nerves of mice, indicating that this efflux pathway also exist in vivo [17]. The most likely efflux route when extracellular Ca2+ is reduced in the incubation medium is via connexin hemichannels. This is based on the findings that the efflux was blocked by general gap junction blockers and a more specific connexin mimetic peptide, Gap26 [20]. Further, no effect was observed using the P2X7-receptor blocker BBG and the pannexin mimetic/blocking peptide 10Panx1. The lack of effects of P2X7-receptor blockers are in accordance with several studies [7, 8]. In addition and as far as we know none of the putative transport proteins, pannexin hemichannels, P2X7-receptors, volume sensitive anion channels, voltage dependent anion channels, volume sensitive outwardly rectifying channels, cystic fibrosis transmembrane conductance regulator and vesicular release pathways are blocked by extracellular Ca2+. The threshold concentration for effects on efflux of GSH was 0.1 mM Ca2+ whereas incubation in 0.2 or 0.3 mM Ca2+ did not stimulate efflux. This agrees well with an earlier study on stimulated efflux of glutamate from primary cultures of astroglia by low extracellular Ca2+ [17]. Low extracellular Ca2+-concentrations are reached during anoxia and spreading depression [9]. It is interesting to note that the newly developed techniques for measurements of extracellular Ca2+ reveal that the earlier used techniques greatly underestimate the drops in Ca2+ [21]. It is thus possible but remains to be demonstrated that more physiological nervous activity can induce dramatic decreases in extracellular Ca2+ that are compatible with connexin hemichannel opening.
The treatment with curcumin was selective and strongly activated both the basal and stimulated efflux of GSH but did not increase efflux of other components measured. In curcumin treated cells both the basal efflux of GSH and the efflux stimulated by omission of Ca2+ were decreased by carbenoxolone. However, carbenoxolone did not reduce GSH efflux down to basal levels in curcumin treated cells. This indicates that other GSH efflux pathways that are not sensitive to carbenoxolone is/are elevated by curcumin. Interestingly multidrug resistance protein 1 is upregulated by Nrf2 [22]. The effect of curcumin on GSH levels was in our study reduced by Nrf2 directed siRNA. It therefore appears likely that the elevated basal efflux of GSH is in part due to efflux via elevated levels of multidrug resistance protein 1 [23].
Both basal and stimulated GSH efflux rates were enhanced by curcumin and reduced by lowering the intracellular GSH by buthionine sulfoximine. GSH efflux is thus closely linked to its intracellular levels. The finding agrees well with early studies by Sagara and coworkers who showed that astrocytes release GSH depending on their concentration in a wide range [24]. The discrepancy in our case between the increase in cellular level (about 6 × control) and the less prominent elevated efflux (about 2.5 × control) of GSH is mostly likely due to saturation of the efflux. This was suggested already by Sagara and coworkers who showed that the efflux of GSH was linear at low cellular levels of GSH but saturated >40 nmol/mg protein [24]. In our case treatment with curcumin increased the cellular level to about 85 nmol/mg protein. This large elevation of cellular GSH in comparison to basal cellular levels is likely due to that curcumin to a higher degree increase the expression of the modulatory subunit of γ-glutamylcysteine ligase in comparison to the catalytic subunit [10]. The high proportion of modulatory subunit will increase the Ki of glutamylcysteine ligase for GSH and lower the Km of γ-glutamylcysteine ligase for glutamate [25]. As this enzyme is rate-limiting for GSH synthesis the increase in the modulatory subunit will elevate synthesis to reach high levels of GSH [25].
The stimulated efflux pathway of phosphoethanolamine by low extracellular Ca2+, appear to be the same route as for GSH [8]. Phosphoethanolamine efflux was similar in treated cultures (BSO and curcumin) compared with control cultures. This indicates that the efflux pathway that is controlled by extracellular Ca2+, putatively connexin hemichannels, is unchanged by the treatments and that the altered efflux of GSH is related to changed cellular content rather than to a enhanced efficiency of the hemichannel efflux. However, and as noted above, it appears that the efflux via multidrug resistance protein 1 is upregulated by curcumin. Strangely BSO treatment increased the cellular content of glutamate but decreased efflux of glutamate stimulated by Ca2+ omission (but not the basal efflux). The increase in cellular glutamate is probably due to less incorporation of glutamate in γ-glutamylcysteine as the enzymatic step blocked by BSO is the ligation of glutamate and cysteine [25]. The factors behind the reduced glutamate efflux in medium with no added Ca2+ in BSO treated cultures is at present unknown. However, the efflux is a combination of release and uptake. One major difference between glutamate and GSH/phosphoethanolamine is the avid uptake of glutamate in contrast to GSH/phosphoethanolamine. In an earlier study no effects on the excitatory amino acid transporters were found in BSO treated primary astrocyte cultures [26] which makes elevated Na+-dependent uptake a less likely explanation. Alternatively other pathways for glutamate efflux could be reduced by intracellular GSH or BSO treatment. Indeed it has been shown that BSO decrease cystine uptake [27] which would lead to decreased glutamate efflux via the Xc− system.
The increase in cellular content of GSH in the present experiments is well in line with the findings that curcumin also in vivo can increase GSH levels in normal and ischemic conditions [28, 29]. The results indicate that the elevated basal GSH efflux by curcumin may be one mechanism for the neuroprotection afforded by curcumin [30]. Interestingly, the curcumin-induced but not the basal cellular content of GSH was decreased by siRNA against Nrf2. The lack of decreased basal GSH after siRNA agrees well with that Nrf2 knock-out and wild-type mice have similar GSH content in brain [31]. These findings indicate that, at least in young animals, the constitutive synthesis of GSH is independent of the Nrf2 system [31].
Overall the results shows that the intracellular level of GSH grossly determine the basal and stimulated efflux rates by Ca2+-omission. From a neuroprotective standpoint this implies that increasing the astroglial content of GSH with curcumin will “feed” the neurons with the rate-limiting GSH precursor cysteine. This may be particularly important in conditions such as Parkinson's disease where a dramatic tissue drop of GSH in the substantia nigra is observed and in normal ageing which is accompanied by a decrease in brain GSH levels [32].
Acknowledgments
The work was supported by the Swedish Research Council/Medicine to MS, Parkinsonfonden and Åhlénstiftelsen. MS and SGW are supported by the National Institutes of Health (GM 44842). FB is supported by Fredrik and Ingrid Thurings-, Edit Jacobsons- and Magnus Bergvalls foundations. MN is supported by the Swedish Research Council, LUA/ALF, the region of West Sweden (RUN) and Edit Jacobssons Foundation.
Contributor Information
Malin H. Stridh, Email: malin.stridh@physiol.gu.se, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg, Sweden; Abteilung für Allgemeine Zoologie, Fachbereich Biologie, Technische Universität Kaiserslautern, Erwin-Schrödinger-Straße 13, 67663 Kaiserslautern, Germany.
Fernando Correa, Institute of Biomedicine, University of Gothenburg, Gothenburg, Sweden.
Christina Nodin, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg, Sweden.
Stephen G. Weber, Department of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA
Fredrik Blomstrand, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg, Sweden.
Michael Nilsson, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg, Sweden.
Mats Sandberg, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg, Sweden; Institute of Biomedicine, University of Gothenburg, Gothenburg, Sweden.
References
- 1.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of Cys-Gly as precursor for neuronal glutathione. J Neurosci. 1999;19(2):562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, Wallin C, Weber SG, et al. Net efflux of cysteine, glutathione and related metabolites from rat hippocampal slices during oxygen/glucose deprivation: dependence on gamma-glutamyl transpeptidase. Brain Res. 1999;815(1):81–88. doi: 10.1016/s0006-8993(98)01097-x. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama K, Suh SW, Hamby AM, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9(1):119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 4.Shih AY, Johnson DA, Wong G, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vargas MR, Johnson DA, Sirkis DW, et al. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28(50):13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirrlinger J, Schulz JB, Dringen R. Glutathione release from cultured brain cells: multidrug resistance protein 1 mediates the release of GSH from rat astroglial cells. J Neurosci Res. 2002;69(3):318–326. doi: 10.1002/jnr.10308. [DOI] [PubMed] [Google Scholar]
- 7.Rana S, Dringen R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci Lett. 2007;415(1):45–48. doi: 10.1016/j.neulet.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Stridh MH, Tranberg M, Weber SG, et al. Stimulated efflux of amino acids and glutathione from cultured hippocampal slices by omission of extracellular calcium: likely involvement of connexin hemichannels. J Biol Chem. 2008;283(16):10347–10356. doi: 10.1074/jbc.M704153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev. 1985;65(1):101–148. doi: 10.1152/physrev.1985.65.1.101. [DOI] [PubMed] [Google Scholar]
- 10.Lavoie S, Chen Y, Dalton TP, et al. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: importance of the glutamate cysteine ligase modifier subunit. J Neurochem. 2009;108(6):1410–1422. doi: 10.1111/j.1471-4159.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- 11.Gomes P, Srinivas SP, Van Driessche W, et al. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2005;46(4):1208–1218. doi: 10.1167/iovs.04-1181. [DOI] [PubMed] [Google Scholar]
- 12.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nodin C, Nilsson M, Blomstrand F. Gap junction blockage limits intercellular spreading of astrocytic apoptosis induced by metabolic depression. J Neurochem. 2005;94(4):1111–1123. doi: 10.1111/j.1471-4159.2005.03241.x. [DOI] [PubMed] [Google Scholar]
- 14.Kozlova EN, Takenaga K. A procedure for culturing astrocytes from white matter and the application of the siRNA technique for silencing the expression of their specific marker, S100A4. Brain Res Brain Res Protoc. 2005;15(2):59–65. doi: 10.1016/j.brainresprot.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 16.Sandberg M, Butcher SP, Hagberg H. Extracellular overflow of neuroactive amino acids during severe insulin-induced hypoglycemia: in vivo dialysis of the rat hippocampus. J Neurochem. 1986;47(1):178–184. doi: 10.1111/j.1471-4159.1986.tb02847.x. [DOI] [PubMed] [Google Scholar]
- 17.Ye ZC, Wyeth MS, Baltan-Tekkok S, et al. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23(9):3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruzzone S, Guida L, Zocchi E, et al. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD? fluxes in intact cells. Faseb J. 2001;15(1):10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 19.Stout CE, Costantin JL, Naus CC, et al. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277(12):10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 20.Leybaert L, Braet K, Vandamme W, et al. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10(4–6):251–257. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- 21.Fedirko N, Svichar N, Chesler M. Fabrication and use of high-speed, concentric h+- and Ca2+-selective microelectrodes suitable for in vitro extracellular recording. J Neurophysiol. 2006;96(2):919–924. doi: 10.1152/jn.00258.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi A, Suzuki H, Itoh K, et al. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310(3):824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 23.Minich T, Riemer J, Schulz JB, et al. The multidrug resistance protein 1 (Mrp1), but not Mrp5, mediates export of glutathione and glutathione disulfide from brain astrocytes. J Neurochem. 2006;97(2):373–384. doi: 10.1111/j.1471-4159.2006.03737.x. [DOI] [PubMed] [Google Scholar]
- 24.Sagara J, Makino N, Bannai S. Glutathione efflux from cultured astrocytes. J Neurochem. 1996;66(5):1876–1881. doi: 10.1046/j.1471-4159.1996.66051876.x. [DOI] [PubMed] [Google Scholar]
- 25.Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miralles VJ, Martinez-Lopez I, Zaragoza R, et al. Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res. 2001;922(1):21–29. doi: 10.1016/s0006-8993(01)03124-9. [DOI] [PubMed] [Google Scholar]
- 27.Brodie AE, Reed DJ. Buthionine sulfoximine inhibition of cystine uptake and glutathione biosynthesis in human lung carcinoma cells. Toxicol Appl Pharmacol. 1985;77(3):381–387. doi: 10.1016/0041-008x(85)90177-2. [DOI] [PubMed] [Google Scholar]
- 28.Al-Omar FA, Nagi MN, Abdulgadir MM, et al. Immediate and delayed treatments with curcumin prevents forebrain ischemia-induced neuronal damage and oxidative insult in the rat hippocampus. Neurochem Res. 2006;31(5):611–618. doi: 10.1007/s11064-006-9059-1. [DOI] [PubMed] [Google Scholar]
- 29.Jagatha B, Mythri RB, Vali S, et al. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson's disease explained via in silico studies. Free Radic Biol Med. 2008;44(5):907–917. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Sun AY, Simonyi A, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82(1):138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 31.Shih AY, Imbeault S, Barakauskas V, et al. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;280(24):22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 32.Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson's disease: is this the elephant in the room? Biomed Pharmacother. 2008;62(4):236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]


