Abstract
Individuals with chronic asthma show a progressive decline in lung function that is thought to be due to structural remodeling of the airways characterized by subepithelial fibrosis and smooth muscle hyperplasia. Here we show that the tumor necrosis factor (TNF) family member LIGHT is expressed on lung inflammatory cells after allergen exposure. Pharmacological inhibition of LIGHT using a fusion protein between the IgG Fc domain and lymphotoxin β receptor (LTβR) reduces lung fibrosis, smooth muscle hyperplasia and airway hyperresponsiveness in mouse models of chronic asthma, despite having little effect on airway eosinophilia. LIGHT-deficient mice also show a similar impairment in fibrosis and smooth muscle accumulation. Blockade of LIGHT suppresses expression of lung transforming growth factor-β (TGF-β) and interleukin-13 (IL-13), cytokines implicated in remodeling in humans, whereas exogenous administration of LIGHT to the airways induces fibrosis and smooth muscle hyperplasia, Thus, LIGHT may be targeted to prevent asthma-related airway remodeling.
Individuals with chronic asthma show evidence of structural remodeling of the airways, including accumulation of extracellular matrix proteins such as collagen and thickening of smooth muscle. Current therapies for asthma are beneficial in controlling symptoms and airway inflammation but have little effect on lung remodeling. For example, in bronchial biopsies from individuals with asthma similar levels of subepithelial fibrosis are seen after anti-inflammatory therapy with corticosteroids1,2, suggesting that the mechanisms that regulate remodeling may be distinct from those that induce eosinophilia or other aspects of lung inflammation. The severity of asthma and level of lung function impairment are also associated with increased mass of peribronchial smooth muscle3. It has been suggested that airway remodeling is the result of a complex interplay between immune cells and these structural cells, driven by a network of cytokines and growth factors, notably TGF-β and IL-13 (refs. 4,5). Many of these soluble mediators are involved in immune responses as well as tissue repair. Thus, new targets for airway remodeling are needed for the development of therapeutics for diseases of the lung, including asthma.
The TNF superfamily consists of many membrane-bound and soluble proteins with proinflammatory effects on innate and adaptive immune responses. The TNF family ligand LIGHT (TNFSF14; homologous to lymphotoxins, shows inducible expression, competes with HSV glycoprotein D for HVEM, a receptor expressed by T lymphocytes), is a homotrimer expressed on the surface of several immune cells. LIGHT binds the herpesvirus entry mediator (HVEM; TNFRSF14) and also is a shared ligand with membrane lymphotoxin (LTαβ) for LTβR6,7. As other TNF superfamily members are being recognized as key mediators in asthmatic inflammation, including OX40 ligand (TNFSF4)8,9, as well as TNF itself10, we hypothesized that LIGHT might be involved in driving aspects of lung inflammation or have a role in airway remodeling. In line with this, a recent report found that sputum LIGHT levels in people with asthma correlated with decreased lung function11. Using two mouse models of chronic asthma and a therapeutic blocking strategy, we now show a role for LIGHT in controlling the extent of airway remodeling with resultant regulation of the proremodeling cytokines IL-13 and TGF-β.
RESULTS
Blockade of LIGHT or LTab reduces airway remodeling
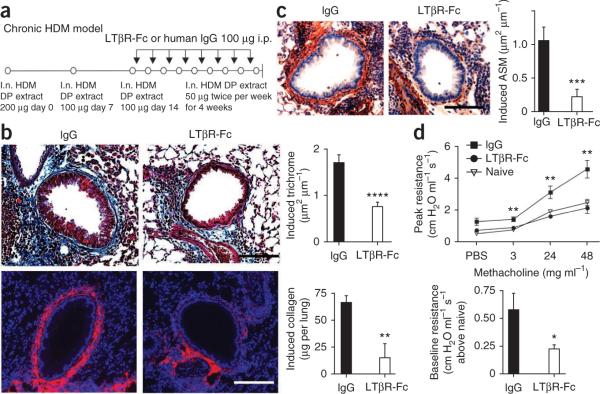
We used a model of house dust mite (HDM)-induced chronic asthma to test the effects of blocking the interactions of LIGHT or LTαβ with HVEM and LTβR. Wild-type (WT) mice develop acute airway inflammation after three challenges with HDM extract12 and then undergo a fibrotic response in the lung, together with other structural changes reminiscent of those found in human asthma, when challenges are extended to twice per week for several weeks. We used a fusion protein of Fc with the extracellular portion of the lymphotoxin β receptor (LTβR-Fc) that can prevent LIGHT-LTβR, LIGHT-HVEM and LTαβ-LTβR interactions13. We administered LTβR-Fc after development of acute airway inflammation, 24 h before each additional intranasal HDM challenge (Fig. 1a). Both increased peribronchial smooth muscle area and lung fibrosis were induced in control mice after chronic HDM exposure (Fig. 1b,c), but mice receiving LTβR-Fc showed much less fibrosis, as measured by peribronchial trichrome staining14, airway collagen-1 expression and assays for total lung collagen (Fig. 1b). α-smooth muscle actin expression was also significantly lower in the LTβR-Fc–treated mice (Fig. 1c and Supplementary Fig. 1).
Figure 1.
Blockade of LIGHT or LTαβ inhibits airway remodeling and AHR induced by HDM. (a) Protocol for HDM-induced remodeling. WT mice were given three intranasal (i.n.) challenges with HDM extract, once per week. LTβR-Fc or IgG was given 24 h before each additional intranasal HDM challenge over the next 4 weeks. i.p., intraperitoneal. (b) Lung sections were stained for Masson's trichrome (top left and middle) and collagen-1 (bottom left and middle) and scored for the extent of fibrosis (top right, n = 54–75 airways per group). Induced total lung collagen was measured (bottom right, pooled from four mice per group, two experiments shown). (c) Lung sections stained for α-smooth muscle actin (left) and scored for extent of induced peribronchial smooth muscle (right, n = 49–70 airways per group). Induced reflects levels above those detected in mice receiving three intranasal challenges before LTβR-Fc treatment. (d) Peak airway resistance with increasing doses of methacholine and baseline resistance without methacholine exposure (six or seven mice per group). *P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001, means ± s.e.m., Mann-Whitney test. Data are from two or three independent experiments. Scale bars, 100 μm.
We next performed invasive pulmonary function testing. After exposure to nebulized methacholine, mice treated with LTβR-Fc had reduced airway hyperresponsiveness (AHR) as compared with IgG-treated mice (Fig. 1d). We also found a decrease in baseline airway resistance (Fig. 1d), which is probably related to the reduction in peribronchial smooth muscle and airway fibrosis15. Bronchoalveolar lavage fluid (BALF) eosinophil numbers, and the overall extent of peribronchial cellular infiltrates scored by lung histology, were similar in both LTβR-Fc and control IgG groups (Supplementary Fig. 2a). BALF neutrophil, B cell and CD4+ and CD8+ T lymphocyte numbers in the lung, and the number of bronchial epithelial cells staining for mucus (periodic acid–Schiff), were not significantly different after LTβR-Fc treatment (Supplementary Fig. 2). Thus, we found the reduction in remodeling features and AHR after LTβR-Fc treatment were independent of effects on airway eosinophilia and histological infiltration detected after chronic allergen challenge.
LIGHT is required for airway remodeling
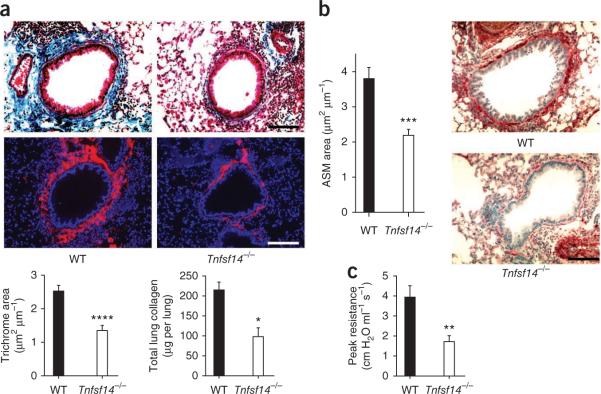
LIGHT expression was induced on lung CD45+ cells after acute HDM challenge (Supplementary Fig. 3). We found LIGHT on B and T cells but not macrophages or granulocytes. To directly investigate the role of LIGHT, we subjected LIGHT-deficient (Tnfsf14−/−) mice16 to the chronic HDM protocol. Similarly to mice treated with LTβR-Fc, Tnfsf14−/− mice had reduced peribronchial fibrosis, collagen-1 and α-smooth muscle actin expression, total lung collagen abundance (Fig. 2a,b), and showed reduced AHR as compared with WT mice (Fig. 2c). LIGHT-deficient mice had only a modest reduction in lung inflammatory infiltrates, as determined by histologic scoring, and no statistical difference in airway eosinophilia (Supplementary Fig. 4).
Figure 2.
LIGHT-deficient mice are resistant to airway remodeling induced by HDM. WT and Tnfsf14−/− mice received HDM intranasally once per week for 3 weeks, then twice per week for 4 weeks. Mice were killed 1 day after the last challenge. (a) Lung sections stained with Masson's trichrome (top) and collagen-1 (middle) and scoring for fibrosis (bottom left, n = 35–36 airways per group, means ± s.e.m., Mann-Whitney test). Total lung collagen was also measured (bottom right, eight mice per group, means ± s.e.m., Mann-Whitney test). (b) Peribronchial smooth muscle area (left, n = 34–35 airways per group, means ± s.e.m., Mann-Whitney test) and lung sections stained for α-smooth muscle actin (right). Levels reflect those above lung measurements from naive mice (a and b). (c) Invasive lung function test and resistance after challenge with 48 mg ml−1 methacholine (means ± s.e.m., Mann-Whitney test from six or seven mice per group). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Scale bars, 100 μm.
Because there is no reagent that can specifically block the interaction of LIGHT with HVEM and LTβR without affecting LTαβ-LTβR interactions, we treated Tnfsf14−/− mice with LTβR-Fc to determine whether LTαβ is active in the absence of LIGHT. LTβR-Fc– and control IgG–treated Tnfsf14−/− mice had similar fibrosis and smooth muscle hyperplasia (Supplementary Fig. 5a,b) and no difference in AHR (Supplementary Fig. 5c). Although LTαβ and LIGHT could act sequentially, the data from the LIGHT-deficient mice suggests that LIGHT is the primary mediator of the remodeling process.
To confirm our findings in another model, we used ovalbumin (OVA) as the antigen for sensitization and chronic repetitive airway challenges. Mice treated with LTβR-Fc after acute inflammation was established had a strong reduction of induced fibrosis and smooth muscle actin expression (Supplementary Fig. 6). Scoring revealed only a modest reduction of inflammatory lung infiltrates, and airway eosinophilia was not different in LTβR-Fc–treated or Tnfsf14−/− mice compared to controls (Supplementary Fig. 7). Thus, LIGHT is crucial for the induction of airway remodeling in both HDM- and OVA-induced chronic asthma models.
LIGHT regulates lung TGF-β
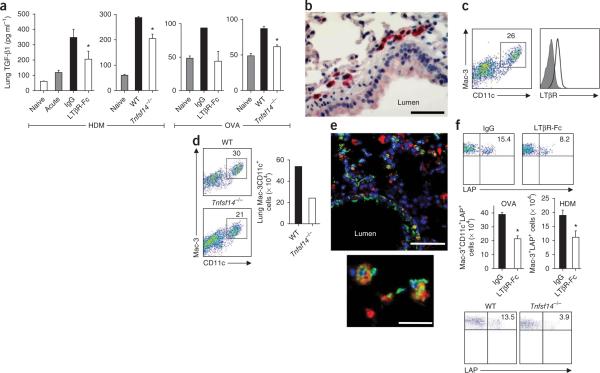
Studies of human and mouse cells have suggested that TGF-β is a principle mediator of airway remodeling, and its expression is elevated in lung samples from individuals with asthma5. TGF-β can induce epithelial-mesenchymal transition (EMT), differentiation of myofibroblasts, production of extracellular matrix proteins, including collagen-1, and smooth muscle cell proliferation5,17. We detected a significantly increased concentration of lung TGF-β1 after chronic HDM or OVA challenges (Fig. 3a). However, significantly less free TGF-β1 was expressed in lung homogenates from WT C57BL/6 mice treated with LTβR-Fc, or from Tnfsf14−/− mice (Fig. 3a).
Figure 3.
LIGHT controls lung TGF-β1 production and accumulation of LAP+ macrophages. Mice were chronically challenged with HDM or OVA. (a) Free TGF-β1 concentrations assessed in lung homogenates from WT mice treated as in Figure 1 (data from six to eight mice per group; acute signifies levels before immunoglobulin treatment; levels of two naive mice also shown, means ± s.e.m., Mann-Whitney, *P < 0.03); WT and Tnfsf14−/− mice treated as in Figure 2 (data from three or four mice per group, means ± s.e.m., t test, *P < 0.02); WT mice treated as in Supplementary Figure 6 (data from four pooled mice per group run in duplicate, mean ± s.e.m., except single IgG group); WT and Tnfsf14−/− mice treated as in Supplementary Figure 6 (data from four pooled mice per group run in quadruplicate, means ± s.e.m., t test, *P < 0.01). (b) Lung sections from WT mice in Supplementary Figure 6 stained for LTβR expression. Scale bar, 50 μm. (c) Lung cells from WT mice in Supplementary Figure 6 analyzed for Mac-3 and CD11c (left), and the gated population evaluated for LTβR expression (right). Filled histogram indicates isotype staining. (d) Lung cells from WT and Tnfsf14−/− mice in Supplementary Figure 6 analyzed for Mac-3 and CD11c (top and bottom) and absolute numbers of Mac-3+CD11c+ cells (right, pooled lung cells from four mice per group). (e) Immunofluorescent staining of lung sections from a representative WT mouse from Figure 1 stained for Mac-3 (red), LAP (green) and DAPI (blue). Scale bar, 50 μm. Image zoom also depicted (bottom). Scale bar, 25 μm. (f) Gated Mac-3+CD11c+ cells from lungs of mice in Figure 1 and Supplementary Figure 6 analyzed for LAP expression (top), enumeration of total LAP+ macrophages per lung (middle, n = 4 mice per group, means ± s.e.m., two experiments shown for OVA and one for HDM, *P < 0.05, t test) and flow analysis for LAP expression gating on Siglec-F+CD11c+ macrophages (bottom).
Several cell types might produce TGF-β1 in the lung and be targets of LIGHT. Immunohistochemistry revealed strong expression of LTβR in cells close to the subepithelial region, where smooth muscle hyperplasia and fibrosis occur during airway remodeling (Fig. 3b). This was consistent with the normal location of lung macrophages. Flow cytometry analysis revealed a population of cells expressing CD11c and Mac-3 that also strongly expressed LTβR (Fig. 3c). Further characterization confirmed that these were macrophages coexpressing F4/80 and Siglec-F (Supplementary Fig. 8)18,19. Macrophages have been implicated in many lung remodeling diseases, including asthma, chronic obstructive pulmonary disease and hypersensitivity pneumonitis20–22, and it has been suggested that they promote remodeling through production of TGF-β23.
We found that LIGHT-deficient mice had reduced percentages and absolute numbers of lung macrophages after chronic challenge with either HDM or OVA (Fig. 3d). TGF-β is initially complexed with latency-associated peptide (LAP), and LAP expression correlates with TGF-β production in many cell types24–26, including macrophages27. Immunofluorescent staining of lung sections from chronically challenged mice revealed that LAP was expressed predominantly on Mac-3+ cells, in addition to airway epithelium, further suggesting that macrophages are a major source of LAP and TGF-β in the remodeled lung (Fig. 3e). Notably, treatment of chronically challenged WT mice with LTβR-Fc led to a strong reduction in the percentages and total numbers of LAP+ macrophages (Fig. 3f), and LIGHT-deficient mice also had a lower proportion of these cells after challenge (Fig. 3f). This implies that LIGHT regulates macrophage accumulation in the lung and directly or indirectly regulates the number of TGF-β–producing cells.
LTβR signaling induces fibrosis and macrophage TGF-β
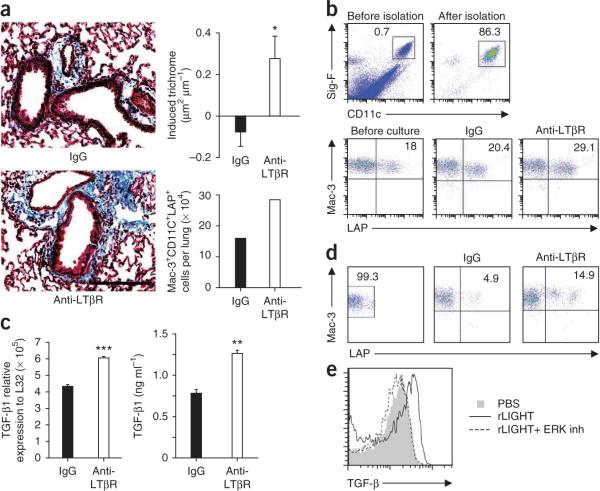
As LTβR was expressed on lung macrophages, we tested whether LTβR signaling could induce remodeling. We sensitized and challenged WT mice with OVA, followed by injection of agonist antibody to LTβR13 given two times per week for 2 weeks, but without further antigen challenge. Mice receiving the agonist antibody showed a marked induction of fibrosis above the level found in acutely challenged mice (Fig. 4a). Furthermore, there was a doubling in the number of LAP+ lung macrophages in the agonist group (Fig. 4a).
Figure 4.
LTβR stimulation promotes fibrosis and TGF-β production by lung macrophages. (a) Lung sections stained with Masson's trichrome (left) and extent of induced peribronchial fibrosis (top right; 44–68 airways per group IgG and anti-LTβR, means ± s.e.m., Mann-Whitney test, *P < 0.05). Scale bar, 100 μm. Mac-3+ CD11c+LAP+ cells per lung were enumerated (bottom right, pooled from six mice per group). WT mice were immunized and acutely challenged with OVA over 28 d and then injected with LTβR agonist antibody (anti-LTβR) or rat IgG every 3–4 d for 2 weeks. (b,c) Analysis of Siglec-F+CD11c+ lung macrophages (b, top) from naive mice after stimulation with rat IgG or anti-LTβR and analyzed for surface LAP expression after 2 d (b, bottom), TGF-β1 mRNA (c, left, ***P < 0.0005) or TGF-β1 protein after HDM was added in the last 8 h of culture (c, right, **P < 0.005). Results are triplicates from each group. (d) Flow cytometry analysis of LAP−Siglec-F+CD11c+Mac-3+ lung macrophages sorted (left) and stimulated with rat IgG or anti-LTβR and analyzed for LAP expression (right). (e) Flow cytometry analysis of intracellular TGF-β in purified lung macrophages stimulated with recombinant LIGHT, in the presence or absence of ERK inhibitor. Data are representative of at least two experiments.
We then purified naive lung macrophages and cultured them with agonist antibody to LTβR. Macrophage LAP expression and TGF-β1 mRNA levels increased after stimulation of the LTβR (Fig. 4b,c). The concentration of free TGF-β1 in the culture supernatant did not increase (data not shown), but when HDM was added, LTβR stimulation significantly increased release of TGF-β1 (Fig. 4c). This is probably related to a requirement for an inflammatory signal for proteolytic release of active TGF-β1 from LAP. Additionally, stimulation of LTβR on sorted LAP− macrophages resulted in direct upregulation of surface LAP (Fig. 4d). We also cultured purified lung macrophages with soluble recombinant LIGHT (rLIGHT) (Fig. 4e) in the presence or absence of an inhibitor of extracellular signal–regulated kinase (ERK; U0126), a kinase previously shown to regulate TGF-β production from macrophages27. LIGHT stimulation increased expression of intracellular TGF-β, and ERK inhibition completely abrogated TGF-β induction (Fig. 4e). Together, these results suggest that one mechanism by which LIGHT can promote fibrosis is through direct stimulation of LTβR on lung macrophages, resulting in both increased accumulation of these cells in the lung and increased TGF-β expression.
LIGHT directly induces airway remodeling dependent on TGF-β
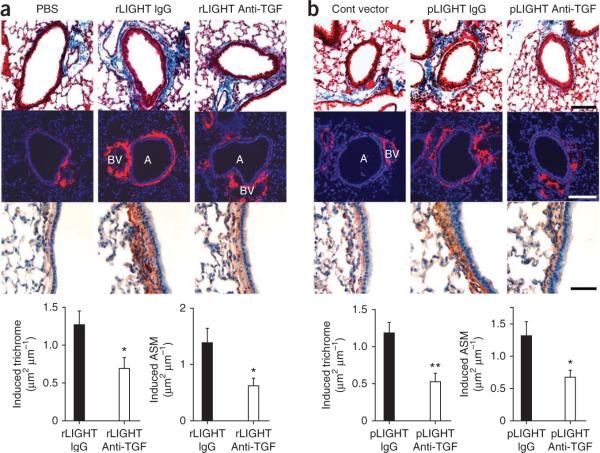
To test whether LIGHT can directly induce airway fibrosis and smooth muscle hyperplasia in vivo by a TGF-β–dependent mechanism, we established acute lung inflammation in WT mice with intranasal HDM given once per week for 3 weeks. This was then followed by intranasal administration of recombinant LIGHT given eight times over the next 2 weeks (in the absence of antigen). Mice that received rLIGHT developed airway fibrosis and smooth muscle hyperplasia compared with those that received PBS, which was reduced by TGF-β blockade (Fig. 5a). To confirm this, we challenged mice with the same acute HDM protocol followed by intranasal administration of a plasmid vector encoding mouse LIGHT. Similarly, these mice showed marked fibrosis and smooth muscle hyperplasia, which we did not observe in mice receiving an empty vector (Fig. 5b). These features were also suppressed by blocking TGF-β. Thus, LIGHT can induce remodeling in the airway, and this process is partly dependent on TGF-β.
Figure 5.
LIGHT-induced airway remodeling is in part dependent on TGF-β. (a,b) Lung sections were stained for trichrome (top row, scale bar, 100 μm), collagen-1 (second row, scale bar, 100 μm), α-smooth muscle actin (third row, scale bar, 50 μm) and scored for fibrosis and smooth muscle area (bottom row, 40 airways per group, means ± s.e.m., Mann-Whitney, *P < 0.05, **P < 0.005). WT mice primed with HDM over 3 weeks were treated with intranasal rLIGHT or PBS given eight times over 2 weeks (a) or pCDNA3 mouse LIGHT plasmid or control plasmid given four times over 2 weeks (b). Antibody to TGF-β (anti-TGF) or isotype control IgG was also injected as indicated. Induced reflects levels above those detected in lungs of mice receiving PBS (a) or control plasmid (b). A, airway; BV, blood vessel.
LIGHT regulates lung IL-13
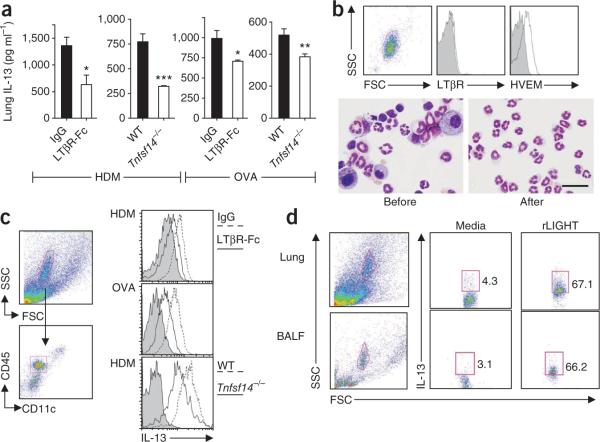
IL-13 is also a pleiotropic regulator of lung remodeling, inducing some activities similarly to TGF-β4. IL-13 is elevated in lung samples with remodeling from individuals with asthma28,29 and can induce epithelial cell proliferation30, myofibroblast proliferation and smooth muscle actin expression31. Furthermore, TGF-β and IL-13 can act synergistically to induce features of asthmatic remodeling32,33. The concentration of IL-13 was decreased by 50% in lung homogenates from HDM-challenged, LIGHT-deficient mice, as well as from those treated therapeutically with LTβR-Fc (Fig. 6a). We found a smaller, but significant, decrease in OVA-challenged mice that lacked LIGHT or were treated with LTβR-Fc (Fig. 6a).
Figure 6.
LIGHT promotes IL-13 production by lung eosinophils. (a) IL-13 content in mice primed and challenged with HDM or OVA. Lung homogenates from mice treated as in Figures 1 and 2 and Supplementary Figure 6 were analyzed (five to seven mice per group from Figure 1, three or four mice per group from Figure 2 and triplicates of pooled samples from four to seven mice per group from Supplementary Figure 6, means ± s.e.m., t test, *P < 0.05, **P < 0.01, ***P < 0.005). (b) Flow cytometry analysis of sorted granulocytes (>95% eosinophils by cytospin, bottom; scale bar, 50 μm) from WT mice after acute intranasal OVA challenge for LTβR (top middle) and HVEM expression (top right). Isotype control in gray. (c) Flow cytometry analysis of CD45+CD11c− granulocyte-gated lung eosinophils (top and bottom left) from mice in Supplementary Figure 6 for intracellular IL-13 directly ex vivo (middle right). Siglec-F+CD11c− eosinophils from mice in Figure 1 (top right) and Figure 2 (bottom right) were stained for IL-13 expression. (d) Flow cytometry analysis of BALF (bottom left) and lung cells (top left) and intracellular IL-13 (middle and right) was analyzed in cells gated on forward and side scatter (left, >95% eosinophils). Cells from WT mice immunized and challenged with OVA over 8 days were cultured for 48 h with rLIGHT or medium added during the last 24 h. Data are representative of two independent experiments.
T helper type 2 (TH2) cells might be a source of IL-13 (ref. 4) and a potential target of LIGHT activity. T cells express HVEM, but not LTβR, and HVEM signals can increase proliferation and cytokine secretion from T cells to augment their response to antigen7. However, we did not detect differences in the numbers of TH2 cells in the airway or lung of Tnfsf14−/− mice or after LTβR-Fc treatment in WT mice (Supplementary Fig. 9a). Additionally, CD4+ T cell production of IL-13 and IL-5 was unaltered in comparison with controls (Supplementary Fig. 9b,c), indicating that LIGHT does not regulate TH2 cells during chronic repetitive allergen challenges.
Other cell types that might be a source of IL-13 include eosinophils, mast cells and structural cells4. Because binding of LIGHT to HVEM increases cytokine production in granulocytes in vitro34, we reasoned that HVEM might be expressed on eosinophils. Indeed, sorted BALF eosinophils (>95% of sorted granulocytes) from acute OVA- or HDM-challenged mice expressed HVEM but not LTβR (Fig. 6b and data not shown). We then performed intracellular cytokine staining for IL-13 in eosinophils from WT mice after sensitization and chronic challenge with HDM or OVA (Fig. 6c). Lung CD45+CD11c− granulocytes were predominantly eosinophils and constitutively expressed intracellular IL-13, which suggests these were highly differentiated cells at the end of the chronic antigen challenge (Fig. 6c). In contrast, those obtained from mice receiving LTβR-Fc or LIGHT-deficient mice had reduced IL-13 expression (Fig. 6c). To directly test the effect of LIGHT, we cultured less differentiated eosinophils isolated from the lung and BALF of antigen-sensitized WT mice that had received only two intranasal antigen challenges. In this case, the eosinophils did not spontaneously produce IL-13, but IL-13 expression was strongly promoted by stimulation with rLIGHT (Fig. 6d). In contrast, the gated lymphocyte population in these cultures showed minimal induction of IL-13 after LIGHT stimulation (Supplementary Fig. 10). Collectively, these data indicate that LIGHT is required in models of chronic asthma for maximal expression of two of the primary cytokines thought to promote airway remodeling, and that LIGHT-LTβR or LIGHT-HVEM interactions promote proremodeling activities of lung macrophages and lung eosinophils, two of the cell types associated with chronic asthma and remodeling in humans.
DISCUSSION
We show that LIGHT is crucial for airway remodeling and airway hyperresponsiveness in two mouse models of chronic asthma. Notably, our data present a new role for this molecule in lung remodeling and fibrotic disease and suggest LIGHT may be a relevant target for suppressing fibrosis and smooth muscle hyperplasia. Several published reports confirm an association of LIGHT with elements of tissue remodeling. In individuals with scleroderma, a disease that can lead to pulmonary fibrosis, BALF concentrations of LIGHT were higher in those with lung involvement compared to those with only nonpulmonary manifestations35. Moreover, a first report evaluating LIGHT expression in individuals with asthma was recently published11. The authors measured soluble LIGHT in the sputum of a heterogeneous group of 242 people with asthma, including 48 with severe asthma. After analysis of approximately 40 soluble mediators, only LIGHT was found to inversely correlate with lung function, as assessed by baseline forced expiratory volume in one second (that is, higher LIGHT levels were associated with lower baseline forced expiratory volumes, or lung function).
As LIGHT has two receptors, targeting its interaction with LTβR and HVEM may prevent multiple proremodeling activities during the development of chronic asthma. We identified TGF-β as being regulated by LIGHT and found that lung macrophages expressing LTβR are responsive to LIGHT. Of note, rLIGHT has also been found to increase expression of matrix metalloproteinase-9 (MMP-9) in macrophages36. MMP-9 is found in the BALF of people with asthma37, and MMP-9–deficient mice show reduced peribronchial fibrosis after chronic allergen challenge38. Together with our results, these studies suggest that LIGHT-LTβR interactions on lung macrophages might regulate the expression of both TGF-β and MMP-9 during chronic asthma. Although TGF-β is almost universally considered to be involved in lung remodeling5, one report in a mouse model of chronic HDM administration found it was dispensable for driving structural changes in the lung39. This may be due to differences in the frequency and periodicity of HDM exposure in that study compared to our and others' studies. Other models with OVA have shown a clear role for TGF-β in airway remodeling40,41, and we found TGF-β expression was reduced by targeting LIGHT in both our HDM and OVA models. This is concordant with the demonstration that direct administration of LIGHT via the airways induced remodeling in a TGFβ-dependent manner.
IL-13 is similarly considered to have a major role in remodeling4 and is required for structural changes in the lungs in OVA-, fungal- and HDM-induced animal models42–44. We found LIGHT promotes IL-13 expression in eosinophils and markedly impaired eosinophil IL-13 production was observed after treatment with LTβR-Fc. The importance of eosinophils in human asthmatic remodeling has been shown after treatment with IL-5-specific antibodies, which reduces eosinophils in the lung and leads to a reduction in airway deposition of extracellular matrix proteins45. Eosinophils have also been shown to contribute to airway remodeling in OVA-induced animal models of chronic asthma14,46. One report found that eosinophils were not required for HDM-driven remodeling47, but with the caveat that this experimental protocol differed in dose, duration and frequency of challenges compared to that used here. These differences in terms of the molecules required for remodeling may simply reflect variations that might occur in humans and, rather than being contradictory, may provide key insight into subpopulations of people with asthma. Regardless of the ultimate target, or targets, of LIGHT activity, our data show that in various antigen models LIGHT is a primary orchestrator of the remodeling processes.
As well as regulating TGFβ and IL-13, LIGHT may control additional cellular functions that contribute to remodeling. rLIGHT has been shown to enhance proliferation of vascular smooth muscle cells48 as well as promote expression of α-smooth muscle actin49, actions mediated by HVEM and LTβR, respectively. Bronchial epithelial cells may also be a target. LTβR was found on epithelial cells in human lungs50, and we found weak expression of LTβR in lungs of chronically challenged mice, albeit less than on macrophages. Bronchial epithelial cells can undergo EMT driven by TGF-β5,17, and this might be one source of the increased smooth muscle mass characteristic of airway remodeling. However, we saw induction of fibrosis as well as increased numbers of lung macrophages after injection of an agonist antibody to LTβR without a corresponding increase in peribronchial smooth muscle hyperplasia (data not shown). Notably, we did detect smooth muscle hyperplasia in mice receiving intranasal rLIGHT and LIGHT plasmid, suggesting that HVEM and not LTβR has a role in driving the increase in smooth muscle mass. Future studies are needed to determine whether smooth muscle cells or bronchial epithelial cells are direct targets of LIGHT activity in vivo.
Neutralizing LIGHT reduced airway hyperresponsiveness despite no robust effects on the peribronchial immune infiltrates. This is consistent with previous reports that suggest that airway hyperresponsiveness in chronically challenged mice correlates more with remodeling changes than the degree of cellular infiltrates in the lung15,51,52. The precise relationship between airway inflammation, hyperresponsiveness and remodeling in human asthmatics is unclear, but there is evidence that baseline lung function and severity of asthma is related to the smooth muscle mass3. The dichotomy between eosinophil accumulation and fibrosis that we detected is also consistent with previous data that have suggested that airway remodeling may be a distinct process from eosinophilic lung inflammation1,2,53,54. Some studies have additionally suggested that IL-13 might directly contribute to the degree of hyperresponsiveness55, and therefore the combination of less fibrosis, smooth muscle hyperplasia and IL-13 after LTβR-Fc treatment or in Tnfsf14−/− mice most likely explains the relatively normal lung function we observed.
In summary, we report that the TNF family member LIGHT is essential for airway remodeling in several mouse models of chronic asthma. Blockade of LIGHT, after acute inflammation was established in the lung, substantially reduced airway fibrosis and smooth muscle hyperplasia, two factors associated with a decline in lung function in human asthmatics. This previously unknown role for LIGHT in airway remodeling may lead to strategies targeting this molecule in asthmatic airway remodeling or other diseases involving fibrosis and remodeling.
ONLINE METHODS
Mouse models of airway remodeling and interventions
Eight- to twelve-week-old C57BL/6 mice (Jackson Laboratories) or Tnfsf14−/− mice16 (bred in house on the BL/6 background) were used. For HDM experiments, mice were given intranasal administrations of Dermatophagoides pteronyssinus extracts (Greer Laboratories, 8.42 endotoxin units per mg HDM): 200 μg on day 0 and 100 μg on days 7 and 14 to induce an acute response similar to that previously described12. To induce a chronic response, 50 μg of HDM was then given twice per week for 4 weeks.
In some experiments, mice challenged acutely with HDM were given 10 μg of intranasal recombinant LIGHT (R&D Systems) or PBS on days 16–18 and on alternating days for five doses. For mouse LIGHT in vivo gene transfer experiments, 50 μg of pCDNA3 mouse LIGHT or empty vector was given on days 16, 20, 23 and 27 after HDM challenge. This dose and frequency was based on ranges used in previous reports of successful intranasal plasmid gene transfer in the lung56,57. Full-length mouse Tnfsf14 was cloned into pCDNA3.1(+) at HindIII and NotI, and endotoxin-free plasmid DNA was purified using the EndoFree Plasmid Mega Kit (Qiagen) following the manufacturer's directions. Antibody to TGFβ (clone 1D11.16.8, Bio X Cell) or isotype control was given in 300-μg doses i.p. every 3–4 d for 2 weeks beginning 1 day after the last HDM challenge.
LTβR-Fc and agonist antibody to LTβR were generated in house as previously described13. LTβR-Fc and control human IgG (Bethyl) (100 μg) were given i.p. starting on day 29 for OVA, and day 16 for HDM protocols, and given 24 h before each subsequent intranasal challenge for 4 weeks. LTβR agonist antibody was given on day 30 and every 3–4 d for 2 weeks.For experiments to assess undifferentiated eosinophils, WT mice were injected i.p. with 50 μg of OVA (Sigma) adsorbed to 0.5 mg of alum (Pierce) on day 0, challenged with intranasal OVA on days 7 and 8 and killed on day 9. BALF and lung cells were then cultured with recombinant LIGHT (100 ng ml−1, R&D Systems) for 24 h before intracellular cytokine staining. All experiments were in compliance with the regulations of the La Jolla Institute for Allergy and Immunology Animal Care Committee in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care.
Airway hyperresponsiveness
In some experiments, invasive pulmonary function testing was performed using the Flexivent system (Scireq), and airway resistance was analyzed by Scireq Flexivent 5.1 software as previously described58.
Airway Inflammation Analysis
We performed bronchoaleveolar lavage and processed lung for histology as previously described54. Lung eosinophils were detected as the Siglec-F+CD11c− population of isolated lung cells19. BALF neutrophils were detected as the GR-1 granulocyte population. All BALF cell counts were confirmed by manual differential counting of cytospins. Total cell counts were performed using a hemacytometer (Hausser-Scientific).
Lung cytokines
Right lungs were individually placed in 500 μl of RPMI (Gibco) with 0.5% Triton-X and homogenized, followed by centrifugation. In some experiments, lungs were snap frozen in liquid nitrogen before homogenizing as previously described59 and analyzed for IL-13 (R&D) and TGFβ (Promega and R&D) 1 by ELISA.
Airway remodeling analysis
The area of peribronchial fibrosis on trichrome-stained sections was evaluated with an image analysis system (Image-Pro Plus; Media Cybernetics)14. Smooth muscle area was evaluated in the same manner. Quantification of total lung collagen was performed using the Sircoll assay as previously described40.
Flow cytometry
Lungs were digested in 2 mg ml−1 collagenase and 1 mg ml−1 DNase (Roche) for 30 min, and lung cells were purified with a 70-μm cell strainer (BD Falcon). Cells were incubated with monoclonal antibody to CD16/CD32 (24G.2) for 10 min to block Fc receptors and then stained for 30 min with various combinations of antibodies to surface markers described in the Supplementary Methods. For staining of mouse LIGHT, we first blocked the expression of LTβ with antibody BBF6 to LTβ (kindly provided by J. Browning) followed by LTβR-Fc and phycoerythrin-conjugated antibody to human immunoglobulin (eBioscience) as previously described60.
Macrophage isolation and culture conditions
To isolate lung resident macrophages, BALF cells were removed by extensive washing with PBS following digestion of lung tissues. After incubation with mouse Fc-blocking antibody (2.4G2), recovered cells were stained with allophycocyanin-conjugated antibody to CD11c and phycoerythryin-conjugated antibody to Siglec-F followed by anti-PE MicroBeads (Miltenyi Biotec). Siglec-F+ cells were enriched on an AutoMACS Pro cell separator (Miltenyi Biotec) following further isolation by a FACSAria II cell sorter (BD Biosciences) to purify Siglec-F+CD11c+ autofluorescent lung resident macrophages. Cells were cultured with LTβR-specific agonist antibody (4H8) or rLIGHT as described in the Supplementary Methods.
Immunostaining
Immunohistochemical detection of LTβR in paraffin-embedded lung sections was performed as previously described58. Immunofluorescent staining for Mac-3, LAP and collagen-1 is described in the Supplementary Methods.
Statistical analyses
Statistical analysis was performed using GraphPad Prism software. A nonparametric t test or Mann-Whitney test was used where indicated. A P value < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Meilan, Y. Adams, X. Tang and M. Macauley for technical assistance and the UCSD histology core for lung section processing and staining. Antibody to LTβ BBF6 was kindly provided by J. Browning, Biogen Idec. This work was supported by US National Institutes of Health (NIH) grant AI070535 to M.C., a fellowship from the American Academy of Asthma, Allergy and Immunology, UCSD Clinical and Translational Research Institute and NIH grant 1K08AI080938-01A1 award to T.A.D. and NIH grants R37AI068033 and AI067890 to C.F.W. This is manuscript number 1285 of the La Jolla Institute for Allergy and Immunology.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Boulet LP, et al. Airway hyperresponsiveness, inflammation and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroids. Am. J. Respir. Crit. Care Med. 2000;162:1308–1313. doi: 10.1164/ajrccm.162.4.9910051. [DOI] [PubMed] [Google Scholar]
- 2.Chakir J, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17 and type I and type III collagen expression. J. Allergy Clin. Immunol. 2003;111:1293–1298. doi: 10.1067/mai.2003.1557. [DOI] [PubMed] [Google Scholar]
- 3.Pepe C, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J. Allergy Clin. Immunol. 2005;116:544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol. Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 5.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 6.Mauri DN, et al. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 7.Ware CF. Network communications: lymphotoxins, LIGHT and TNF. Annu. Rev. Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 8.Salek-Ardakani S, et al. 0×40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 2003;198:315–324. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seshasayee D, et al. In vivo blockade of 0×40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 2007;117:3868–3878. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry MA, et al. Evidence of a role of tumor necrosis factor α in refractory asthma. N. Engl. J. Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 11.Hastie AT, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J. Allergy Clin. Immunol. 2010;125:1028–1036. e13. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammad H, et al. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Trez C, et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J. Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho JY, et al. Inhibition of airway remodeling in IL-5–deficient mice. J. Clin. Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Southam DS, Ellis R, Wattie J, Inman MD. Components of airway hyperresponsiveness and their associations with inflammation and remodeling in mice. J. Allergy Clin. Immunol. 2007;119:848–854. doi: 10.1016/j.jaci.2006.12.623. [DOI] [PubMed] [Google Scholar]
- 16.Scheu S, et al. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin β in mesenteric lymph node genesis. J. Exp. Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doerner AM, Zuraw BL. TGF-β1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1β but not abrogated by corticosteroids. Respir. Res. 2009;10:100. doi: 10.1186/1465-9921-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zasłona Z, et al. Transcriptome profiling of primary murine monocytes, lung macrophages and lung dendritic cells reveals a distinct expression of genes involved in cell trafficking. Respir. Res. 2009;10:2. doi: 10.1186/1465-9921-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J. Immunol. Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhavsar P, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 21.Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121:156S–159S. doi: 10.1378/chest.121.5_suppl.156s. [DOI] [PubMed] [Google Scholar]
- 22.Bogaert P, Tournoy KG, Naessens T, Grooten J. Where asthma and hypersensitivity pneumonitis meet and differ: noneosinophilic severe asthma. Am. J. Pathol. 2009;174:3–13. doi: 10.2353/ajpath.2009.071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalil N, Whitman C, Zuo L, Danielpour D, Greenberg A. Regulation of alveolar macrophage transforming growth factor-β secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J. Clin. Invest. 1993;92:1812–1818. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochi H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25− LAP+ T cells. Nat. Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 25.Berger P, et al. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. FASEB J. 2003;17:2139–2141. doi: 10.1096/fj.03-0041fje. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi R, Anderson DE, Weiner HL. Cutting Edge: Immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-β–dependent manner. J. Immunol. 2007;178:4017–4021. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 27.Burton OT, et al. Roles for TGF-β and programmed cell death 1 ligand 1 in regulatory T cell expansion and diabetes suppression by zymosan in nonobese diabetic mice. J. Immunol. 2010;185:2754–2762. doi: 10.4049/jimmunol.1001365. [DOI] [PubMed] [Google Scholar]
- 28.Kaminska M, et al. Airway remodeling in subjects with severe asthma with or without chronic persistent airflow obstruction. J. Allergy Clin. Immunol. 2009;124:45–51. e4. doi: 10.1016/j.jaci.2009.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Woodruff PG, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Booth BW, Sandifer T, Martin EL, Martin LD. IL-13–induced proliferation of airway epithelial cells: mediation by intracellular growth factor mobilization and ADAM17. Respir. Res. 2007;8:51. doi: 10.1186/1465-9921-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito A, Okazaki H, Sugawara I, Yamamoto K, Takizawa H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int. Arch. Allergy Immunol. 2003;132:168–176. doi: 10.1159/000073718. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, et al. Interleukin-13 augments transforming growth factor-β1–induced tissue inhibitor of metalloproteinase-1 expression in primary human airway fibroblasts. Am. J. Physiol. Cell Physiol. 2005;288:C435–C442. doi: 10.1152/ajpcell.00035.2004. [DOI] [PubMed] [Google Scholar]
- 33.Espinosa K, Bosse Y, Stankova J, Rola-Pleszczynski M. CysLT1 receptor upregulation by TGF-β and IL-13 is associated with bronchial smooth muscle cell proliferation in response to LTD4. J. Allergy Clin. Immunol. 2003;111:1032–1040. doi: 10.1067/mai.2003.1451. [DOI] [PubMed] [Google Scholar]
- 34.Heo SK, et al. LIGHT enhances the bactericidal activity of human monocytes and neutrophils via HVEM. J. Leukoc. Biol. 2006;79:330–338. doi: 10.1189/jlb.1104694. [DOI] [PubMed] [Google Scholar]
- 35.Luzina IG, et al. Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum. 2003;48:2262–2274. doi: 10.1002/art.11080. [DOI] [PubMed] [Google Scholar]
- 36.Lee WH, et al. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler. Thromb. Vasc. Biol. 2001;21:2004–2010. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 37.Kelly EA, Jarjour NN. Role of matrix metalloproteinases in asthma. Curr. Opin. Pulm. Med. 2003;9:28–33. doi: 10.1097/00063198-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Lim DH, et al. Reduced peribronchial fibrosis in allergen-challenged MMP-9–deficient mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L265–L271. doi: 10.1152/ajplung.00305.2005. [DOI] [PubMed] [Google Scholar]
- 39.Fattouh R, et al. Transforming growth factor-β regulates house dust mite–induced allergic airway inflammation but not airway remodeling. Am. J. Respir. Crit. Care Med. 2008;177:593–603. doi: 10.1164/rccm.200706-958OC. [DOI] [PubMed] [Google Scholar]
- 40.Le AV, et al. Inhibition of allergen-induced airway remodeling in Smad 3–deficient mice. J. Immunol. 2007;178:7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 41.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti–TGF-β antibody: effect on the Smad signaling pathway. J. Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 42.Tomlinson KL, Davies GC, Sutton DJ, Palframan RT. Neutralisation of interleukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite. PLoS One. 2010;5:e13136. doi: 10.1371/journal.pone.0013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blease K, et al. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 2001;166:5219–5224. doi: 10.4049/jimmunol.166.8.5219. [DOI] [PubMed] [Google Scholar]
- 44.Yang G, et al. Anti–IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28:224–232. doi: 10.1016/j.cyto.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Flood-Page P, et al. Anti–IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 47.Fattouh R, et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite–induced airway disease. Am. J. Respir. Crit. Care Med. 2011;283:179–188. doi: 10.1164/rccm.200905-0736OC. [DOI] [PubMed] [Google Scholar]
- 48.Wei CY, Chou YH, Ho FM, Hsieh SL, Lin WW. Signaling pathways of LIGHT induced macrophage migration and vascular smooth muscle cell proliferation. J. Cell. Physiol. 2006;209:735–743. doi: 10.1002/jcp.20742. [DOI] [PubMed] [Google Scholar]
- 49.Hikichi Y, et al. LIGHT, a member of the TNF superfamily, induces morphological changes and delays proliferation in the human rhabdomyosarcoma cell line RD. Biochem. Biophys. Res. Commun. 2001;289:670–677. doi: 10.1006/bbrc.2001.6039. [DOI] [PubMed] [Google Scholar]
- 50.Boussaud V, Soler P, Moreau J, Goodwin RG, Hance AJ. Expression of three members of the TNF-R family of receptors (4–1BB, lymphotoxin-β receptor and Fas) in human lung. Eur. Respir. J. 1998;12:926–931. doi: 10.1183/09031936.98.12040926. [DOI] [PubMed] [Google Scholar]
- 51.DiGiovanni FA, et al. Concurrent dual allergen exposure and its effects on airway hyperresponsiveness, inflammation and remodeling in mice. Dis. Model Mech. 2009;2:275–282. doi: 10.1242/dmm.001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson JR, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am. J. Respir. Crit. Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 53.Jeffery PK, et al. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am. Rev. Respir. Dis. 1992;145:890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. [DOI] [PubMed] [Google Scholar]
- 54.Doherty TA, Soroosh P, Broide DH, Croft M. CD4+ cells are required for chronic eosinophilic lung inflammation but not airway remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;296:L229–L235. doi: 10.1152/ajplung.90543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang M, et al. Inhibition of arginase I activity by RNA interference attenuates IL-13–induced airways hyperresponsiveness. J. Immunol. 2006;177:5595–5603. doi: 10.4049/jimmunol.177.8.5595. [DOI] [PubMed] [Google Scholar]
- 56.Kitani A, et al. Treatment of experimental (trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-β1 plasmid: TGF-β1–mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor β2 chain downregulation. J. Exp. Med. 2000;192:41–52. doi: 10.1084/jem.192.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.López E, et al. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J. Immunol. 2006;176:1943–1950. doi: 10.4049/jimmunol.176.3.1943. [DOI] [PubMed] [Google Scholar]
- 58.Cho JY, et al. Immunostimulatory DNA inhibits transforming growth factor-β expression and airway remodeling. Am. J. Respir. Cell Mol. Biol. 2004;30:651–661. doi: 10.1165/rcmb.2003-0066OC. [DOI] [PubMed] [Google Scholar]
- 59.Haeberle HA, et al. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J. Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Summers-DeLuca LE, et al. Expression of lymphotoxin-αβ on antigen-specific T cells is required for DC function. J. Exp. Med. 2007;204:1071–1081. doi: 10.1084/jem.20061968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.