Abstract
The gene encoding the prohormone proopiomelanocortin (POMC) is mainly expressed in two regions in vertebrates, namely corticotrophs and melanotrophs in the pituitary and a small population of neurons in the arcuate nucleus of the hypothalamus. In this latter region, POMC-derived peptides participate in the control of energy balance and sensitivity to pain. Neuronal expression of POMC is conferred by two enhancers, nPE1 and nPE2, which are conserved in most mammals, but no transcription factors are yet known to bind to these enhancers. In this work, by means of a one-hybrid screening, we identify that nPE2 possesses an element recognized by transcription factors of the nuclear receptor superfamily. This element, named NRBE, is conserved in all known nPE2 enhancers and is necessary to confer full enhancer strength to nPE2-driven reporter gene expression in transgenic mice assays, indicating that the phylogenetic conservation of the element is indicative of its functional importance. In a search for candidate nuclear receptors that might control POMC we observed that estrogen receptor alpha (ESR1) - a known regulator of energy balance at the hypothalamic level - can bind to the NRBE element in vitro. In addition we observed by immunofluorescence that ESR1 is coexpressed with POMC in around 25–30% of hypothalamic neurons of males and females during late embryonic stages and adulthood. Thus, our results indicate that hypothalamic expression of POMC is controlled by nuclear receptors and establish ESR1 as a candidate regulator of POMC.
Keywords: proopiomelanocortin, melanocortin, transcriptional regulation, estrogen receptor, transcriptional enhancer
1. Introduction
The proopiomelanocortin gene (POMC) is mainly expressed in pituitary corticotrophs and melanotrophs, and in a group of neurons in the arcuate nucleus of the hypothalamus. POMC encodes a prohormone that, upon posttranslational processing, gives rise to several bioactive peptides with important roles in vertebrate physiology. Pituitary POMC hormones play a major role in the stress response while central POMC-derived neuropeptides participate in the regulation of food intake and pain sensitivity (Kieffer et al., 2002; Coll and Loraine Tung, 2009).
Although much is known about the regulatory elements and transcription factors that regulate POMC expression in the pituitary, the cis-trans code that dictates POMC expression in the hypothalamus is largely unknown (Jenks, 2009). In recent years, our group has demonstrated that neuronal expression of POMC is conferred by two distal enhancers, nPE1 and nPE2, located 12 and 10.5 kb upstream of the transcription start site of mouse Pomc, respectively (de Souza et al., 2005). Both enhancers exist only in mammals and have distinct evolutionary origins. An in silico paleogenomics study demonstrated that nPE2 was exapted from a CORE-SINE retrotransposon between 200 to 320 million years ago whereas nPE1 is a more recent acquisition that occurred in the lineage leading to Eutherians (placental mammals) between 85 to 170 million years ago (Santangelo et al., 2007). Despite their different evolutionary origins, nPE1 and nPE2 are able to independently direct reporter gene expression to POMC arcuate neurons of transgenic mice (de Souza et al., 2005). This example of convergent evolution suggests that nPE1 and nPE2 might share common motifs recognized by a common set of transcription factors. In addition, each enhancer harbors unique highly conserved elements that may provide distinct hormonal regulation of Pomc expression. To date, only two transcription factors are known to modulate the expression of POMC in response to hormones that reach the hypothalamus (Jenks, 2009). These factors are STAT3 and FOXO1, that regulate POMC in the brain in response to leptin and insulin, respectively (Kitamura et al., 2006). However, the proposed binding sites for these factors are located within the proximal mouse Pomc promoter, around 400 bp upstream of the transcriptional start site. Thus, no transcription factors are yet known to bind to the neuronal POMC enhancers.
Here, we report the identification of a conserved element (NRBE) in the POMC enhancer nPE2 that can bind to transcription factors of the nuclear receptor superfamily. Members of this family possess a zinc-finger DNA binding domain, and a ligand-binding domain capable to interact with several types of ligands including steroid hormones. Other members of this superfamily have no known ligand and are referred to as orphan receptors (Mangelsdorf et al., 1995; Giguère, 1999). We have found that the estrogen receptor alpha (ESR1) is a candidate nuclear receptor factor to regulate neuronal POMC expression since it is able to bind to the NRBE motif of nPE2 and is expressed in POMC neurons during development and adulthood. Our results represent a first step in identifying transcription factors responsible for POMC expression in the mammalian hypothalamus.
2. Materials and methods
2.1. One-hybrid screening
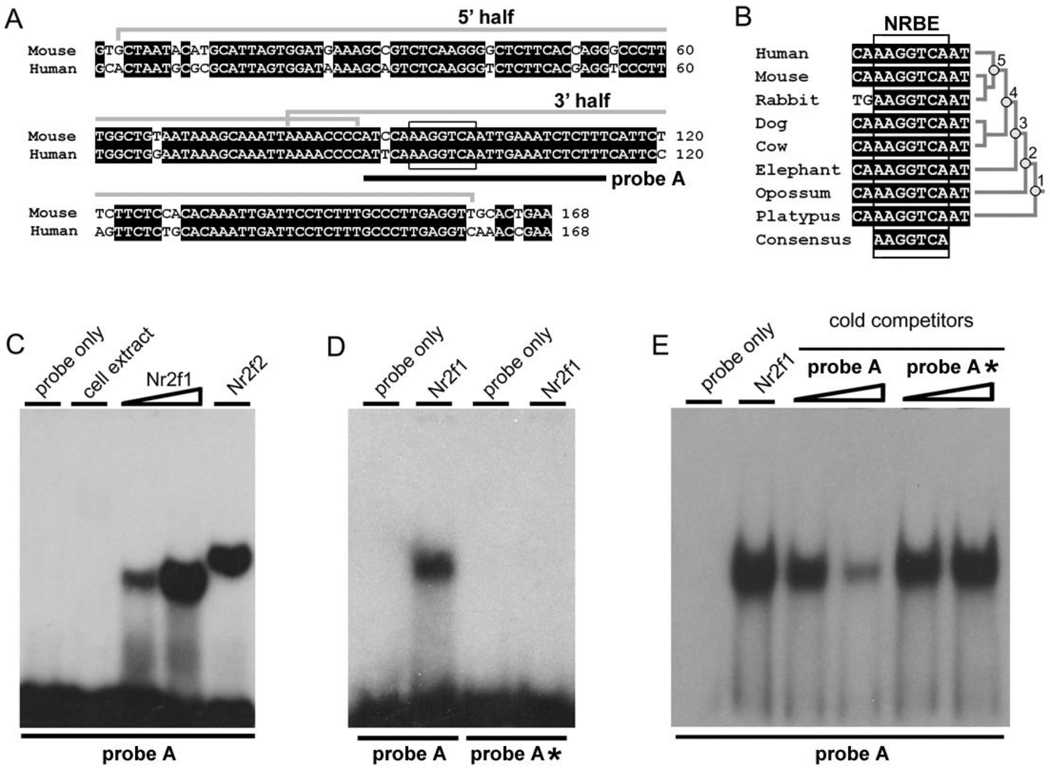
Identification of transcription factors binding to nPE2 was done using the Matchmaker One-Hybrid Kit (Clontech) and following the instructions of the manufacturer. Specific oligonucleotide primers were synthesized to PCR-amplify two regions of mouse nPE2 that were used as baits: the 5' half (77 bp) and 3' half (79 bp) as shown in Fig. 1A. Each nPE2 half was cloned into the EcoRI and SstI sites, upstream of the yeast reporter plasmid pHIS-1 that carries a HIS3 reporter gene (Clontech). Both baits share an 8-bp region (AAAACCCC) of overlapping sequences in the middle of nPE2. Each plasmid was inserted by homologous recombination into the HIS3 locus of yeast strain YM4271 and selected by growing in histidine-deficient medium, whereby leaky HIS3 expression allows for the isolation of recombinants. After selection, both yeast strains were transformed with an adult mouse brain cDNA library cloned into pACT2 (ML4008AH; BD Biosciences) and grown on plates lacking histidine and leucine and supplemented with 10 mM of 3-aminotriazole (Sigma), an inhibitor of the HIS3 gene product added to reduce background growth. Yeast colonies growing on this medium had their plasmids isolated and sent for sequencing. Of several dozen yeast colonies isolated, four clones contained plasmids with partial cDNA segments of transcription factors, all of which encoded nuclear receptors and bound to the 3’ half bait of nPE2.
Fig. 1.
Characterization of a nuclear receptor binding site in the nPE2 neuronal enhancer. (A) Alignment of mouse and human nPE2 with identical nucleotide positions highlighted in black. Grey brackets indicate mouse regions used as baits in one-hybrid screening (5’ and 3’ half probes). The consensus for nuclear receptor binding element NRBE is indicated with a black box. Probe A used in EMSA studies is depicted with a black line. (B) Alignment of nPE2 sequences encompassing NRBE from various mammalian species and their phylogenetic relationship. (C) Gel shift of probe A, which encompasses mouse NRBE, and bacterially-expressed Nr2f1 (increasing amounts) and Nr2f2. Control lanes include a probe A without cell extract (probe only) or with BL21 bacterial extract without recombinant nuclear receptor expression (cell extract). (D) Gel shift with Nr2f1 extracts and probes A and A*, the latter harboring a mutated NRBE site. (E) Gel shift with [32]P-labelled probe A incubated with cell extracts expressing Nr2f1 in the presence of increasing amounts of cold probe A or cold probe A*.
2.2. Electrophoretic mobility shift assays
A mouse COUP-TFII cDNA clone was purchased from the American Type Culture Collection (ATCC®, Cat. No. 10434520) and a human ESR1 cDNA clone was purchased from OriGene Technologies (Cat. No. SC 125287). A full-length mouse COUP-TFI cDNA was obtained from one of the clones isolated in the one hybrid screening. For expression of recombinant proteins each cDNA was cloned into expression vector pET-28a (Novagen), to make His6x-fusion proteins, and transformed into E. coli BL21 strain. Protein production was induced by 1 mM isopropyl β-D-1-thiogalactopyranoside in a bacterial culture at OD600 0.6 and 37 °C. After induction, the culture was incubated for an additional 4 h and harvested by centrifugation. Cells were lysed in extraction buffer (20 mM Tris-HCl pH 8, 100 mM NaCl, 0.5% Triton-X100, 1 mM EDTA and protease inhibitors) and cell debris was pelleted and discarded. The remaining solution was stored at −70 °C until used as protein extracts. The quality of protein extracts quality was determined by SDS-PAGE followed by Coomasie Brilliant Blue staining. A non-transformed E.coli BL21 extract was prepared, to be used as a control for EMSA reactions.
Electrophoretic mobility shift assays were performed using double-stranded oligonucleotides. We designed a probe A encompassing Nuclear Receptor Binding Element (5’-ATCCAAAGGTCAATTGAAATCTCTTT-3’) and a mutated version probe A* harboring nucleotide replacements in NRBE (5’-ATCCAAGCTCGAATTGAAATCTCTTT-3’). The sense strand was end-labelled using [32P]γ-ATP (PerkinElmer) and T4 polynucleotide kinase (Invitrogen) and then annealed to its complementary strand.
Electrophoretic mobility shift assay reactions were performed by incubating 10,000 cpm of labelled DNA (~ 0.5 nM) with 10 µg of recombinant protein in reaction buffer (20 mM HEPES pH8, 10 mM MgCl2, 1 mM EDTA, 10% glycerol, 100 ng of sonicated salmon sperm DNA, 1 mM DTT and protease inhibitors) at room temperature for 30 min. Final reaction volume was 20 µl. Reactions were loaded on 8% polyacrylamide gels containing 5% glycerol in 0.5X TBE. After electrophoresis, gels were dried and exposed to X-ray film (Kodak BioMax MS).
2.3. Transgenic mice
Transgenic mice expressing enhanced green fluorescent protein (EGFP, Clontech) under the control of mouse Pomc regulatory regions have been described previously (Cowley et al., 2001). The transgene includes −13 to +8 kb around the mouse Pomc transcription start site and contains the EGFP coding region inserted in Pomc exon 2, before the translation initiation codon. The construct includes both nPE1 and nPE2 (de Souza et al., 2005).
Transgenes were constructed using standard molecular cloning techniques and sequence quality was assessed by automated PCR sequencing. The control transgene carrying the intact NRBE of nPE2 was constructed by modifying transgene number 11 from de Souza et al. (2005). This transgene lacks nPE1 and the nPE2 sequences were replaced by a truncated version Δ2-nPE2 as in Fig. 4 of Santangelo et al. (2007). The NRBE* transgene is identical except that NRBE sequences were mutated as in probe A* (see Section 2.2). Transgenic mice were generated by pronuclear microinjection of B6CBF2 zygotes as described previously (de Souza et al., 2005; Santangelo et al., 2007) in the Transgenic Core Facility of the Centro de Estudios Científicos in Valdivia, Chile. Microinjected zygotes were transferred to the oviduct of B6CB pseudopregnant females. Newborn pups were decapitated at postnatal day-1 and skin-free heads were fixed with 4% paraformaldehyde in KPBS (0.9% NaCl, 16 mM K2HPO4, 3.6 mM KH2PO4), with 30% sucrose at 4 °C overnight. The following day, heads were embedded in Tissue-Tek and frozen at −20 °C. Serial coronal sections (20 µm) were cut using a cryostat microtome (IEC Microtome, Germany) and sections analyzed by fluorescence microscopy. All animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, United States Public Health Service (USA).
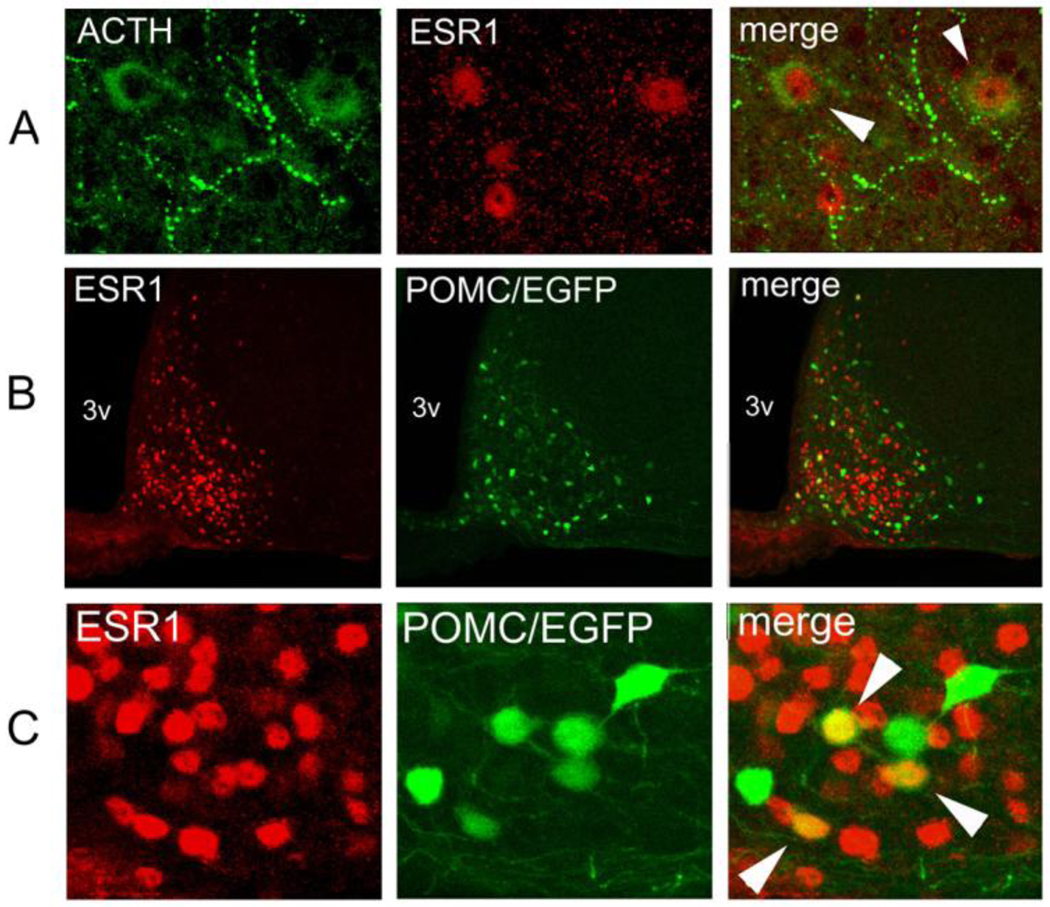
Fig. 4.
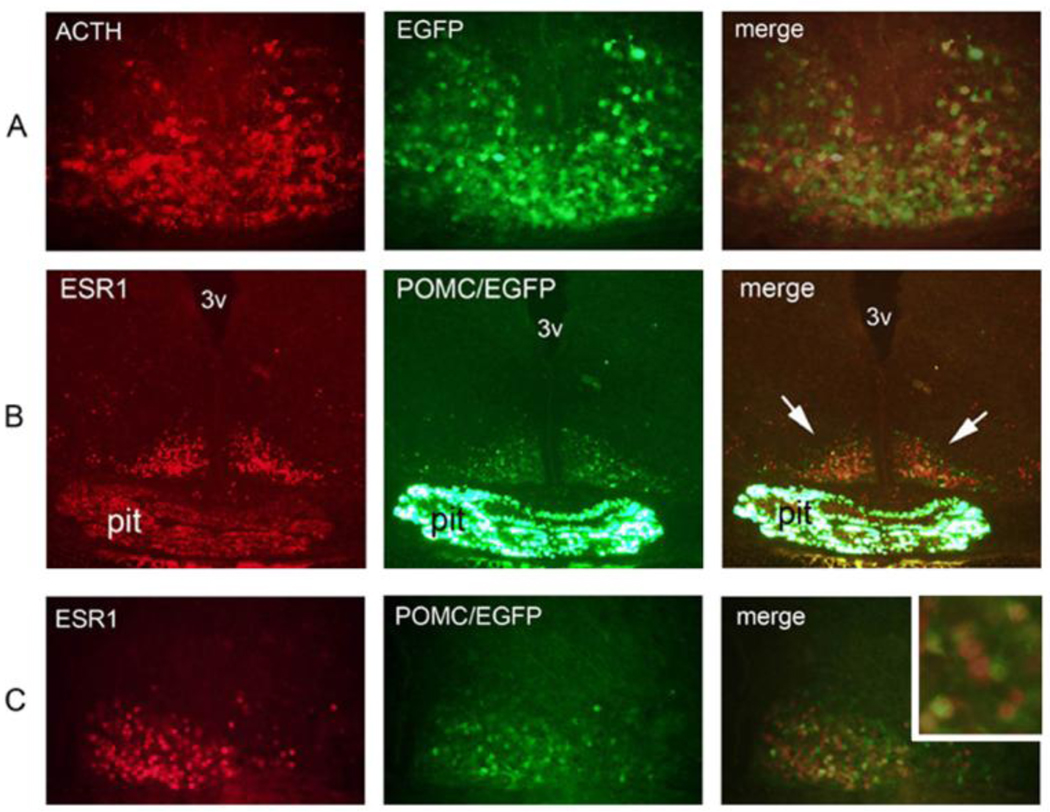
Colocalization of estrogen receptor α (ESR1) and POMC in the adult hypothalamus. (A) Immunofluorescence of the rat arcuate nucleus against POMC-derived ACTH (green), ESR1 (red) and the superimposition of both images (merge). Colocalization, denoted by arrowheads, is seen between nuclear ESR1 and cytosolic ACTH. (B) Immunofluorescence against ESR1 (red) on a coronal hypothalamic section of a Pomc-EGFP transgenic mouse (green). 3v: third ventricle. (C) Higher magnification of the same experiment as on B. Colocalization of ESR1 and EGFP is indicated in several neurons with white arrowheads.
2.4. Sequence analysis
nPE2 sequences from various mammalian species were obtained by searching the genomes in the Ensembl website (http://www.ensembl.org). Alignments were performed using Clustal W (http://www.ebi.ac.uk/clustalw). Searches for nuclear receptor binding sites were performed by visual inspection and the MatInspector program (Genomatix, Germany).
2.5. Double immunohistochemistry
Double immunohistochemistry was performed as described (de Souza et al., 2005). Mice were perfused with 4% paraformaldehyde in KPBS and brains were excised, postfixed in 4% paraformaldehyde/KPBS overnight at 4°C, and sectioned (50 µm) with a Vibratome 1000 (Ted Pella, Redding, CA). Brain slices were treated with 1% H2O2 in KPBS for 20 min, washed twice with KPBS, and incubated overnight at 4°C with the primary antibodies diluted in KPBS, 0.3% Triton X-100 and 2% normal goat serum. The primary antibodies were monoclonal anti-COUP-TFI (1:100 dilution, H8132, Perseus Proteomics, Japan) or anti-COUP-TFII (1:100 dilution, H7147, Perseus Proteomics, Japan). The next day, slices were washed in KPBS and incubated with biotinylated anti-mouse IgG antibody (Vector) diluted 1:200 in KPBS, 0.3% Triton X-100 for 2 h at room temperature (RT). After washing in KPBS, slices were incubated with avidin/biotin-horseradish peroxidase complex (Vectastain Elite ABC kit; Vector) for 1 h at room temperature, washed in KPBS, and developed with 25 mg of diaminobenzidine (DAB; Sigma)/ml and 0.05% H2O2 in TBS (150 mM NaCl, 50 mM Tris-HCl, pH 7.5). Second immunohistochemistry was done with rabbit polyclonal anti-ACTH-IC-1 (1:1,000; National Hormone and Pituitary Program) and detected with biotinylated anti-rabbit IgG antibody and the Vectastain ABC kit (Vector). Peroxidase activity was finally developed with benzidine hydrochloride (Sigma) as described in Gelman et al. (2003). Stained slices were mounted onto SuperFrost Plus slides (Fisher).
2.6. Immunofluorescence
Immunofluorescence was done in Vibratome slices of adult Pomc-EGFP transgenic mice similarly as described above. Primary antibodies were two different rabbit polyclonal anti-ESR1 antibodies used at 1:1000 (Upstate) or 1:200 (Santa Cruz) dilutions, and a mouse monoclonal anti-ACTH antibody (1:200; Santa Cruz). The primary antisera was detected with AlexaFluor555-coupled anti-rabbit IgG and/or anti-mouse IgG (1:1000; Molecular Probes, Invitrogen). After washing in KPBS, brain slices were mounted on slides and covered with Vectashield medium (Vector Labs). Confocal images were taken with a Nikon confocal microscope and the percentage of green and red colocalized fluorescence in arcuate neurons was counted.
To obtain Pomc-EGFP mouse embryos, timed pregnancies were established and embryos collected at embryonic days (E) E11.5, E13.5, E15.5 and E17.5, fixed in 4% paraformaldehyde for 2–3 hours, washed, dehydrated, included in embedding medium (Tissue-Tek) and sectioned on a cryostat (Microm HM 505N; Micron, Germany) as described previously (Santangelo et al., 2007). Immunofluorescence on embryo slices mounted on SuperFrost Plus slides (Fisher) was performed as described above, except that a rabbit polyclonal anti-ACTH primary antibody (1:1,000, National Hormone & Pituitary Program) was also used.
3. Results
3.1. Nuclear receptor transcription factors bind to nPE2
In order to identify transcription factors controlling POMC expression via nPE2, we divided this enhancer into two halves of approximately 70 bp each and used each half as bait on a yeast one-hybrid screening of a cDNA library of adult mouse brain (Fig. 1A). After two independent screenings using the 5’ half fragment of nPE2 no transcription factor was isolated. In contrast, the 3’ half of nPE2 led to the isolation of four clones that could grow on histidine-deficient medium. Interestingly, the four clones are transcription factors that belong to the nuclear receptor family: Nr2f1 (COUP-TFI), Nr2f2 (COUP-TFII), Nr2f6 (EAR2) and Nr3b1 (estrogen-related receptor α, ESRRA).
Analysis of the nucleotide sequence of nPE2 revealed the presence of an AAGGTCA motif with close similarity to a half-site recognized by nuclear receptors (Fig. 1A). This motif is 100% conserved in the nPE2 of all available mammalian species, including the marsupials, opossum and wallaby and the monotreme, platypus (Fig. 1B). To test whether this element binds to the identified factors, we performed electromobility-shift assays (EMSA) with a [32P]-end labelled double stranded DNA probe encompassing the AAGGTCA element (probe A, Fig. 1A) and bacterially-expressed Nr2f1 and Nr2f2. As shown in Fig. 1C–D, these factors bind to probe A but not to a mutated probe A* in which the AAGGTCA motif was substituted with AGCTCGA. Similarly, an excess of cold probe A inhibited binding of Nr2f1 to the radiolabelled probe A but not an excess of the mutated cold probe A* (Fig. 1E). Thus, the element present in nPE2 is a Nuclear Receptor Binding Element, that we named NRBE. No consensus nuclear receptor binding elements are present in other parts of nPE2 or in nPE1.
3.2. Functional analysis of NRBE in transgenic mice
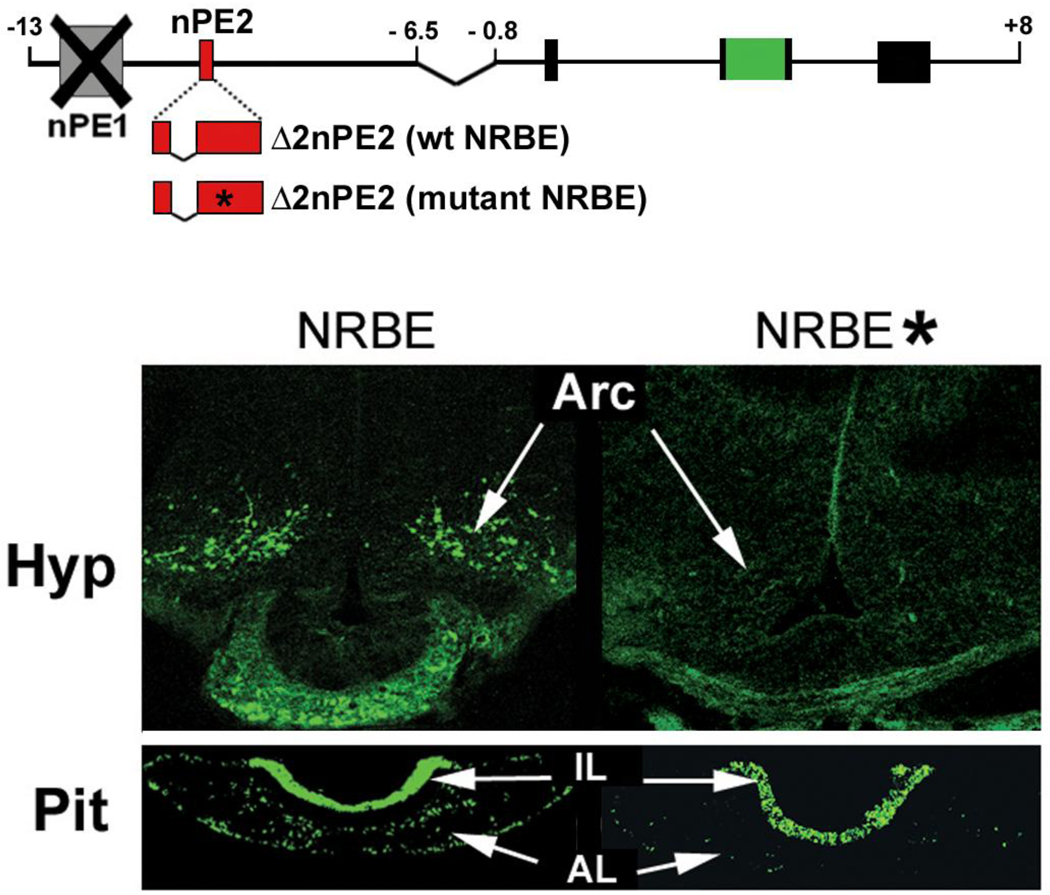
Next we sought to examine whether NRBE was important for nPE2 function in transgenic mouse expression assays. In a previous work we observed that the deletion of a region of nPE2 that encompasses NRBE (region 3 in Santangelo et al., 2007) abolishes nPE2 enhancer activity in transgenic mice. However, shorter deletions that remove each half of this fragment independently did not impair transgenic expression of the reporter gene, indicating that each half portion was sufficient for nPE2 activity (constructs Δ2 and Δ4 in Santangelo et al., 2007). Therefore, we decided to only included the 3’ half of nPE2 region 3 in the designed transgenes (as in Fig. 4 of Santangelo et al., 2007) to test the importance of NRBE sequences in comparison with a similar transgene carrying nucleotide substitutions in NRBE as those present in probe A* (Fig. 2). Both transgenes included the proximal pituitary promoter as a positive control to identify mouse pedigrees that lack EGFP expression all tissues due to insertion into transcriptionally inactive heterochromatin (Fig. 2).
Fig. 2.
Analysis of the importance of NRBE sequences in nPE2 enhancer activity in POMC hypothalamic neurons of transgenic mice. The two transgenes used are depicted above. Hypothalamic (Hyp) and pituitary (Pit) sections of representative postnatal day 1 founder transgenic mice carrying a transgene with wild-type NRBE or mutated NRBE sequences (*) show green fluorescence from EGFP expression. Arc: arcuate nucleus, AL: anterior lobe, IL: intermediate lobe.
A transgenic mouse founder analysis performed at postnatal day 1 showed EGFP expression in hypothalamic arcuate neurons in three out of four mice carrying intact NRBE (Fig. 2). In contrast, from five independent founder transgenic mice carrying the mutant NRBE version only one showed EGFP expression in arcuate neurons, despite the fact that all of them expressed EGFP in pituitary melanotrophs and corticotrophs (Fig. 2). These results suggest that NRBE plays an important role on reporter gene expression in POMC hypothalamic neurons and that a nuclear receptor transcription factor binds to this site to regulate Pomc expression in this brain area.
3.3. Identification of estrogen receptor α (ESR1) as a potential transcription regulator of POMC expression
The nuclear receptors identified by one-hybrid screening are candidates to be direct POMC regulators. However, a necessary condition is that they must be expressed in POMC arcuate neurons. Thus, we discarded Nr2f6 as a possible POMC regulator since knockin mice expressing lacZ from the Nr2f6 locus revealed no expression in the arcuate nucleus (Warnecke et al., 2005). Similarly, an extensive in situ hybridization screening failed to detect Nr3b1 in the adult arcuate nucleus (Gofflot et al., 2007). In turn, COUP factors Nr2f1 and Nr2f2 are reported to be expressed in the arcuate nucleus (Gofflot et al., 2007) but our double immunohistochemistry studies indicate that only Nr2f2 was expressed in a few arcuate cells that, however, did not express POMC (data not shown). Thus, all four factors identified by one-hybrid screening had to be discarded as regulators of POMC. Another nuclear receptor transcription factor that is expressed in the arcuate nucleus of the hypothalamus is estrogen receptor α (ESR1, Nr3a1), as demonstrated by immunohistochemistry studies in mice, rats and sheep (Yaghmaie et al., 2010; Davis et al., 2004; Chakraborty et al., 2003; Gay et al., 2000; Skinner and Herbison, 1997; Lehman and Karsch, 1993). Interestingly, ESR1, as POMC, is known to be involved in the regulation of energy balance at the hypothalamic level, and administration of estrogen reduces food intake (Gao et al., 2007).
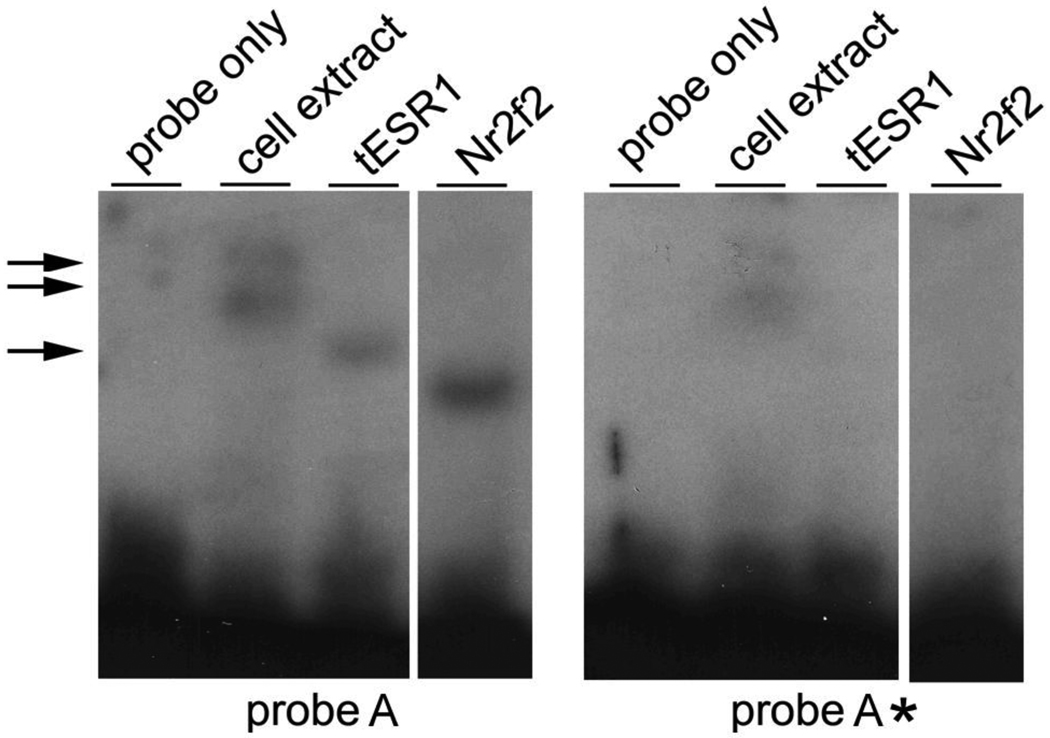
To check whether ESR1 is a suitable candidate to regulate POMC expression, we tested the ability of the DNA-binding domain of human ESR1 (tESR1) expressed in E. coli to bind the NBRE site of nPE2 by EMSA. As expected, ESR1 binds to a DNA fragment containing NRBE but not to a similar probe carrying a mutated NRBE (Fig. 3).
Fig. 3.
Interaction of the DNA-binding domain of the estrogen receptor α (tESR1) to nPE2 sequences encompassing NRBE. Electromobility shift assays were performed with [32P]-labelled probe A containing NRBE or probe A* that carries a mutated NRBE site (right). Lane labels as on Fig. 1. Bacterially-expressed Nr2f2 was used as a positive control. A double arrow indicates unspecific bands seen with the BL21 cell extract under a longer exposure. The single arrow indicates the shifted band that binds tESR1.
3.4. Expression analysis of ESR1 and POMC during mouse development and adulthood
To investigate whether POMC and ESR1 were coexpressed in the adult mouse hypothalamus, we performed immunofluorescence studies using a rabbit polyclonal anti-ESR1 antibody and a monoclonal antibody against POMC-derived adrenocorticotropic hormone (ACTH). Colocalization of both signals was observed in arcuate neurons of the rat hypothalamus (Fig 4A), but the high background around the median eminence - presumably caused by the use of a monoclonal antibody on rodent tissue - prevented the unambiguous detection of ACTH immunoreactivity along the basomedial portion of the arcuate nucleus. To circumvent this difficulty, we made use of transgenic mice expressing EGFP driven by POMC regulatory sequences, in which EGFP is coexpressed with POMC peptides in over 90 % of arcuate neurons (Fig. 4A and Cowley et al., 2001). ESR1 immunoreactivity was detected using a secondary antibody linked to the red fluorochrome AlexaFluor555 and colocalization with green EGFP fluorescence was determined by confocal microscopy (Fig. 4B). A quantitative analysis showed that ESR1 and POMC/EGFP colocalize in 29.7% (range 24–34%) and 26.5% (range 18–36%) of arcuate neurons of male and female mice, respectively (Fig. 4B–C). No obvious difference in colocalization was seen along the antero-posterior levels of the arcuate nucleus. ESR1 was also detected in non-POMC neurons of the ventromedial nucleus, where ESR1 is also known to be expressed at high levels, as well as in a few cells in more dorsal levels of the hypothalamus (not shown).
Expression of POMC is detected in the presumptive hypothalamus already at embryonic day (E) 10.5 (Japón et al., 1994; Santangelo et al., 2007). To examine whether ESR1 is also present in POMC neurons throughout development we performed anti-ESR1 immunofluorescence in POMC-EGFP mouse embryos at E11.5, E13.5, E15.5 and E17.5. In contrast to POMC, ESR1 was not detected in the presumptive hypothalamus at E11.5 and only very few ESR1-positive cells could be seen in the developing arcuate at E13.5 (data not shown). At E15.5 and E17.5, ACTH and EGFP colocalized in most arcuate neurons (Fig. 4A and data not shown). At these later developmental stages, abundant ESR1 expression was detected in the arcuate region, and most EGFP neurons expressed ESR1 (Fig. 4B–C). Thus, ESR1 is a developmental marker of POMC arcuate neurons at E15.5 and E17.5.
4. Discussion
In this study, we report the identification of a conserved motif within the POMC neuronal enhancer nPE2 that is able to bind transcription factors of the nuclear receptor family. This element, that we named, NRBE, is highly conserved in nPE2 of all mammals and is identical to the AGGTCA motif that is one of the two consensus binding half-sites for transcription factors of this type, the other being AGGACA (Giguère, 1999; Deblois and Giguère, 2008). NRBE is located within the 3' half of a 45-bp region of nPE2 that proved to be essential for enhancer function in POMC hypothalamic neurons of transgenic mice (region 3 in Santangelo et al., 2007). Here, we demonstrate that mutating the six nucleotides of NRBE greatly reduces the ability of a truncated nPE2 (Δ2 deletion in Santangelo et al., 2007) to drive reporter gene expression to POMC neurons and suggest that nuclear receptors binding to NRBE participate in the overall nPE2 enhancer activity.
Even though we identified four nuclear receptor transcription factors by one-hybrid assays, the probability of those being the actual factors that bind to NRBE is rather low, since they are not expressed in the adult arcuate nucleus and/or do not colocalize with POMC peptides in this region. An exhaustive in situ hybridization screening of nuclear receptor expression in the murine brain (Gofflot et al., 2007) revealed that ESRRA (Nr3b6) is excluded from the arcuate nucleus, even though it is extensively expressed throughout the brain. In the same screening it was found that COUP-TFI and II (Nr2f1 and Nr2f2) are also excluded from the arcuate nucleus, results that we partially confirmed by immunohistochemistry. We did observe a few scattered immunoreactive cells for COUP-TFII in the arcuate but they did not colocalize with POMC/ACTH. Although EAR2 (Nr2f6) was reported to be expressed at low levels in the arcuate nucleus (Gofflot et al., 2007), knockin mice carrying lacZ within the EAR2 locus expressed the reporter gene only in restricted areas of the central nervous system during development and adulthood that excluded the mediobasal hypothalamus (Warnecke et al., 2005). Because in our study we screened a total mouse brain cDNA expression library, it is expected that we isolated related factors but not the actual transcriptional regulators of POMC expression.
In contrast to these factors, the estrogen receptor α (ESR1) has been reported to be expressed in the arcuate nucleus of several mammalian species (Yaghmaie et al., 2010; Davis et al., 2004; Chakraborty et al., 2003; Gay et al., 2000; Skinner and Herbison, 1997; Lehman and Karsch, 1993). Interestingly, this latter study reported that 15–20% of β-endorphin-immunoreactive arcuate neurons in sheep also contained ESR1 (Lehman and Karsch, 1993). We found that around 26–29% of POMC/EGFP neurons coexpress ESR1 in both male and female adult mice. In addition, we showed that ESR1 colocalizes in the majority of POMC/EGFP cells in the developing mouse arcuate at E15.5 and E17.5, establishing ESR1 as a marker of arcuate development in the mouse. Recombinant ESR1 binds to the NRBE motif of nPE2 in EMSA, in agreement with previous results showing that ESR1 binds to half-sites of the estrogen-responsive element (ERE) both in vitro (Vanacker et al., 1999) and in vivo (Carroll et al., 2005; Lin et al., 2007) and that NRBE is identical to an ERE half-site. That in adulthood not all POMC neurons express ESR1 is compatible with the fact that POMC neurons in the arcuate are known to be heterogeneous in regard to function, as evidenced by expression of different markers. For instance, it has recently been shown that around 40% of POMC neurons are GABAergic (Hentges et al., 2009), 30% coexpress the opioid prohormone dynorphin (Maolood and Meister, 2008), 26 to 36% coexpress the insulin receptor (Williams et al., 2010) and a similar percentage is directly activated by leptin (Münzberg et al., 2003; Williams et al., 2010). Future work should address the extent of overlap between these different subpopulations of hypothalamic POMC neurons.
One of the principal molecules that signals information about the level of peripheral fat stores to the brain is leptin, a hormone produced by adipocytes in proportion to fat content (Coll et al., 2007). Part of the anorectic effect that leptin exerts on the central control of food intake is thought to be mediated by an increase in POMC expression (Schwartz et al., 1997; Thornton et al., 1997) and the activation of POMC neurons in the arcuate nucleus (Cowley et al., 2001). Estrogen is known to have an effect on energy balance that is similar to that of leptin probably because their signaling pathways crosstalk at the hypothalamic level (reviewed in Gao and Horvarth, 2008; Roepke, 2009). Indeed, targeted mutagenesis of the ESR1 gene leads to increased fat mass in both male and female mice (Heine et al., 2000), and estradiol induces a rewiring of neural circuits around POMC neurons that is similar to that of leptin. In addition, estradiol injections partially suppress the obesity phenotype of leptin-deficient mice (Gao et al., 2007) and increase POMC mRNA and its coding peptides in the arcuate nucleus of guinea pigs (Roepke et al., 2008; Thornton et al., 1994) and mice (Pelletier et al., 2007; Gao et al., 2007). Considering previous data and our results showing colocalization of ESR1 and POMC in the developing and adult arcuate, it is tempting to speculate that estrogen exerts its anorectic effect by controlling POMC expression. Altogether, our results establish ESR1 as a candidate for a direct regulator of POMC expression in the mammalian hypothalamus and suggest a possible new level of estrogen action on the control of energy balance.
Fig. 5.
Colocalization of ACTH or estrogen receptor α (ESR1) and POMC/EGFP in the developing mouse hypothalamus at day E17.5. (A) Immunofluorescence against POMC-derived ACTH (red) on a Pomc-EGFP transgenic mouse embryo (green). Colocalization is seen in most cells (merge). (B) Immunofluorescence against ESR1 (red) on the hypothalamus of a Pomc-EGFP transgenic mouse embryo (green). Arrows in “merge” panel show colocalization on the arcuate region. pit: pituitary; 3v: third ventricle. (C) Higher magnification of the medial basal hypothalamus section shown in B evidences extensive colocalization of ESR1 and POMC/EGFP (merge and higher magnification inset).
Acknowledgments
We thank Vanina Rodríguez, Marta Treimun, Adriana Barriento and Juan Manuel Baamonde for excellent technical assistance and Viviana Bumaschny for help in experiments. This work was supported in part by a National Institutes of Health grant DK068400 (MJL and MR), International Research Scholar Grant of the Howard Hughes Medical Institute (MR), and Agencia Nacional de Promoción Científica y Tecnológica (MR). MR and FSJS are investigators of the National Research Council of Argentina (CONICET). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Role of the funding sources: The funding sources mentioned above played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: FSJdS, MJL and MR have intellectual property and patent interests in the POMC neuronal-specific enhancers and have licensed this intellectual property and related research material to financially-interested companies.
References
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J. Comp. Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Coll AP, Loraine Tung YC. Pro-opiomelanocortin (POMC)-derived peptides and the regulation of energy homeostasis. Mol. Cell. Endocrinol. 2009;300:147–151. doi: 10.1016/j.mce.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor alpha in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145:360–366. doi: 10.1210/en.2003-0996. [DOI] [PubMed] [Google Scholar]
- de Souza FS, Santangelo AM, Bumaschny VF, Avale ME, Smart J, Low MJ, Rubinstein M. Identification of neuronal enhancers of the proopiomelanocortin gene by transgenic mouse analysis and phylogenetic footprinting. Mol. Cell Biol. 2005;25:3076–3086. doi: 10.1128/MCB.25.8.3076-3086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblois G, Giguère V. Nuclear receptor location analyses in mammalian genomes: from gene regulation to regulatory networks. Mol. Endocrinol. 2008;22:1999–2011. doi: 10.1210/me.2007-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am. J. Physiol. Endocrinol. Metab. 2008;294:E817–E826. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- Gay F, Anglade I, Gong Z, Salbert G. The LIM/homeodomain protein islet-1 modulates estrogen receptor functions. Mol. Endocrinol. 2000;14:1627–1648. doi: 10.1210/mend.14.10.0538. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Noaín D, Avale ME, Otero V, Low MJ, Rubinstein M. Transgenic mice engineered to target Cre/loxP-mediated DNA recombination into catecholaminergic neurons. Genesis. 2003;36:196–202. doi: 10.1002/gene.10217. [DOI] [PubMed] [Google Scholar]
- Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, Auwerx J. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–418. doi: 10.1016/j.cell.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Giguère V. Orphan nuclear receptors: from gene to function. Endocr. Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japón MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. doi: 10.1177/42.8.8027530. [DOI] [PubMed] [Google Scholar]
- Jenks BG. Regulation of proopiomelanocortin gene expression: an overview of the signaling cascades, transcription factors, and responsive elements involved. Ann. N.Y. Acad. Sci. 2009;1163:17–30. doi: 10.1111/j.1749-6632.2008.03620.x. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gavériaux-Ruff C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat. Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, Li S, Luu-The V, Labrie F. Estrogenic regulation of proopiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J. Neuroendocrinol. 2007;19:426–431. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- Roepke TA. Estrogen modulates hypothalamic control of energy homeostasis through multiple mechanisms. J. Neuroendocrinol. 2009;21:141–150. doi: 10.1111/j.1365-2826.2008.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo AM, de Souza FS, Franchini LF, Bumaschny VF, Low MJ, Rubinstein M. Ancient exaptation of a CORE-SINE retroposon into a highly conserved mammalian neuronal enhancer of the proopiomelanocortin gene. PLoS Genet. 2007;3:1813–1826. doi: 10.1371/journal.pgen.0030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Herbison AE. Effects of photoperiod on estrogen receptor, tyrosine hydroxylase, neuropeptide Y, and beta-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology. 1997;138:2585–2595. doi: 10.1210/endo.138.6.5208. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Loose MD, Kelly MJ, Rönnekleiv OK. Effects of estrogen on the number of neurons expressing beta-endorphin in the medial basal hypothalamus of the female guinea pig. J. Comp. Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- Vanacker JM, Pettersson K, Gustafsson JA, Laudet V. Transcriptional targets shared by estrogen receptor- related receptors (ERRs) and estrogen receptor (ER) alpha, but not by ERbeta. EMBO J. 1999;18:4270–4279. doi: 10.1093/emboj/18.15.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke M, Oster H, Revelli JP, Alvarez-Bolado G, Eichele G. Abnormal development of the locus coeruleus in Ear2(Nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes. Dev. 2005;19:614–625. doi: 10.1101/gad.317905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghmaie F, Saeed O, Garan SA, Voelker MA, Sternberg H, Timiras PS. Estrogen receptor-alpha immunoreactivity in the arcuate hypothalamus of young and middle-aged female mice. Neuro. Endocrinol. Lett. 2010;31:56–62. [PubMed] [Google Scholar]