Abstract
Elevation of cellular cyclic AMP (cAMP) levels inhibits cell cycle reentry in a variety of cell types. While cAMP can prevent the activation of Raf-1 and extracellular signal-regulated kinases 1 and 2 (ERK1/2) by growth factors, we now show that activation of ERK1/2 by ΔRaf-1:ER is insensitive to cAMP. Despite this, ΔRaf-1:ER-stimulated DNA synthesis is still inhibited by cAMP, indicating a cAMP-sensitive step downstream of ERK1/2. Although cyclin D1 expression has been proposed as an alternative target for cAMP, we found that cAMP could inhibit ΔRaf-1:ER-induced cyclin D1 expression only in Rat-1 cells, not in CCl39 or NIH 3T3 cells. ΔRaf-1:ER-stimulated activation of CDK2 was strongly inhibited by cAMP in all three cell lines, but cAMP had no effect on the induction of p21CIP1. cAMP blocked the fetal bovine serum (FBS)-induced degradation of p27KIP1; however, loss of p27KIP1 in response to ΔRaf-1:ER was less sensitive in CCl39 and Rat-1 cells and was completely independent of cAMP in NIH 3T3 cells. The most consistent effect of cAMP was to block both FBS- and ΔRaf-1:ER-induced expression of Cdc25A and cyclin A, two important activators of CDK2. When CDK2 activity was bypassed by activation of the ER-E2F1 fusion protein, cAMP no longer inhibited expression of Cdc25A or cyclin A but still inhibited DNA synthesis. These studies reveal multiple points of cAMP sensitivity during cell cycle reentry. Inhibition of Raf-1 and ERK1/2 activation may operate early in G1, but when this early block is bypassed by ΔRaf-1:ER, cells still fail to enter S phase due to inhibition of CDK2 or targets downstream of E2F1.
The growth factor-stimulated Raf-MEK-extracellular signal-regulated kinase 1 and 2 (ERK1/2) pathway regulates gene expression and cell cycle reentry (38, 41, 47, 51, 59, 80). The importance of this cascade in the regulation of the cell cycle is underlined by a number of observations. For example, inhibition of the ERK1/2 pathway blocks the proliferation of many cell types in G1 (38, 51, 68), while activated forms of Raf and MEK are sufficient to promote cell cycle reentry and to transform cells (17, 38, 51, 61, 71, 80). Activation of Raf requires the Ras GTPases, products of the c-Ras proto-oncogenes, which are activated in response to occupancy of growth factor receptors (41, 47, 59). Once activated, ERK1/2 accumulate in the nucleus, where they phosphorylate and regulate transcription factors, including members of the Ets and AP-1 families (4, 15, 65, 71, 78). Activation of the ERK1/2 pathway promotes the expression of cyclin D1, cyclin E, and cyclin A and the degradation of p27KIP1 (38, 51, 80). These are key events in the activation of the cyclin-dependent kinases CDK4 and CDK2, which promote cell cycle reentry by phosphorylating the retinoblastoma tumor suppressor protein, pRb (38, 51, 56, 62), thereby derepressing and releasing the transcription factor E2F (21).
The ability of cyclic AMP (cAMP) to regulate cell cycle reentry was first reported >25 years ago (57). While cAMP can promote the proliferation of some cells, such as Swiss 3T3 fibroblasts and thyrocytes (3, 5, 45, 60, 73), it inhibits proliferation in most cells (2, 9, 13, 16, 25, 37, 39, 40, 50, 70, 72, 79). The ability of cAMP to inhibit the growth of cells transformed by Ki-Ras (70) and Raf (32) in vitro, and to cause tumor regression in vivo (2), has fueled interest in the use of cAMP-elevating agents in chemotherapy (46). Despite this, the precise mechanism by which cAMP inhibits cell cycle reentry remains undefined (reviewed in references 35 and 69). cAMP prevents cells from entering S phase (50), focusing attention on signaling events during G1. Furthermore, cAMP inhibits proliferation stimulated by either G protein-coupled receptors or receptor tyrosine kinases (50), suggesting that it targets a signaling pathway that is central to cell cycle reentry.
It has been shown that increasing intracellular cAMP levels can block activation of Raf-1 (9, 13, 20, 81) and the subsequent activation of MEK and ERK1/2 (9, 13, 20, 30, 34, 81) without inhibiting signaling from receptors to Ras-GTP (9, 13). This cross talk has attracted much interest (35, 69), not least because these are some of the properties one would ideally require of an “anti-Ras” drug. The mechanism by which cAMP inhibits Raf-1 activation is complex, involving multiple cAMP-dependent phosphorylation events in the N-terminal regulatory domain (19, 20, 54, 66, 81) and perhaps the kinase domain (53). Since the ERK1/2 pathway is required for cell cycle reentry, the ability of cAMP to prevent ERK1/2 activation would seem to be a plausible mechanism for the cAMP-mediated G1 arrest (35, 69). Indeed, in many cases there seems to be good correlation between these events. For example, in Rat-1 and NIH 3T3 fibroblasts, cAMP inhibits ERK1/2 activation and proliferation (9, 13, 20), whereas cAMP fails to inhibit ERK1/2 (24) and can actually cooperate with growth factors to promote cell cycle reentry in Swiss 3T3 cells (45) and thyrocytes (3, 60, 73) and differentiation in PC12 cells (10, 23, 75). In contrast, in some cell types, inhibition of ERK1/2 can be partially dissociated from the antiproliferative effects of cAMP (16, 52). Despite this, no study has unambiguously addressed the role of ERK1/2 inhibition in the antiproliferative effects of cAMP, and other models have been proposed. For example, cAMP can prevent cyclin D1 expression (25, 43, 63, 72, 79) or p27KIP1 degradation (37, 39, 40, 58), both rate-limiting steps in cell cycle reentry (11, 56). However, since both of these events are dependent upon prior activation of ERK1/2 (4, 38, 68, 80), it is difficult to determine if their inhibition by cAMP is a direct effect or, more simply, a consequence of inhibition of ERK1/2. To address this problem most clearly requires a means of activating ERK1/2 in a cAMP-insensitive manner in cells in which ERK1/2 activation would otherwise normally be blocked by cAMP.
Here, we show that cAMP fails to inhibit activation of ERK1/2 by the ΔRaf-1:ER fusion protein in three different fibroblast cell lines. Despite this, ΔRaf-1:ER-stimulated DNA synthesis is still strongly inhibited by cAMP, and this correlates with loss of Cdc25A and cyclin A expression and inhibition of CDK2 activity. Finally, if we activate E2F1 directly (74), to circumvent the G1 CDKs (18), cAMP no longer inhibits expression of Cdc25A or cyclin A but still inhibits E2F1-induced DNA synthesis. Thus, cAMP can inhibit cell proliferation at multiple points, both early and late in the G1/S transition, and inhibition of the ERK1/2 pathway is not a prerequisite for cAMP-induced growth arrest, even in those cells in which it occurs.
MATERIALS AND METHODS
4-Hydroxytamoxifen (4-HT) and β-estradiol (E2) were purchased from Sigma, prepared as 1 mM stock solutions in ethanol, and stored at −70°C. Fetal bovine serum (FBS) and all other cell culture reagents were from Invitrogen. The following antibodies were used: c-Fos (sc-52), Fra-1 (sc-183), Fra-2 (sc-604), JunB (sc-46), CDK2 (sc-163), and Cdc25A (sc-7389) from Santa Cruz Biotechnology and cyclin D1 (CC12) and p27Kip1 (NA35) from Calbiochem; antibodies to cyclin E (RB-012-P) and cyclin A (MS-384-P) were from Neo Markers (MS-384-P); p21CIP1 (556431) was from Pharmingen; total ERK1/2 antibody (MK12) was from Becton Dickinson-Signal Transduction Laboratories; and phospho-ERK1/2 and phospho-Tyr15-cdc2 were from Cell Signaling Technology. Polyclonal antibodies to the hormone-binding domain of the estrogen receptor were from Steven Robbins, University of California—San Francisco. Horseradish peroxidase-conjugated secondary antibodies were from Bio-Rad, and detection was done with the enhanced chemiluminescence (ECL) system (Amersham). Protease inhibitors were from Sigma. All other reagents were of the highest grade commercially available. pCMVNeoHA-Cdc25A was provided by Ingrid Hoffmann (German Cancer Researech Centre-DKFZ), and pBabePuroHA-ER:E2F1 was provided by Kristian Helin (European Institute of Oncology).
Cells and cell culture.
All cells were maintained in phenol red-free Dulbecco's modified Eagle's high-glucosemedium containing penicillin-streptomycin, glutamine, and 10% (vol/vol) FBS. Rat-1 cells expressing ΔRaf-1:ER (RR1 cells) (14, 15) were maintained in 400 μg of G418 ml−1, while NIH 3T3 cells expressing ΔRaf-1:ER* (80) and CCl39 cells expressing ΔRaf-1:ER* (CR1-11 cells) (76) were maintained in 2 μg of puromycin ml−1. CR1-11 cells expressing hemagglutinin (HA)-Cdc25A were derived by transfection, dual selection in puromycin and G418, and ring cloning after limiting dilution. Rat-1 cells expressing HA-ER-E2F1 were derived by retroviral infection, puromycin selection, and ring cloning after limiting dilution. For cell stimulations, Rat-1 and CCl39 cell derivatives were changed to serum-free medium 24 h before stimulation. In the case of NIH 3T3 cells, this was modified to 48 h in Dulbecco's modified Eagle's medium containing 0.5% (vol/vol) FBS with 500 mg of fatty acid-free bovine serum albumin and 10 ml of insulin-transferrin-selenium supplement (ITS-X; Invitrogen) per liter to prevent apoptosis when NIH 3T3 cells were incubated overnight under serum-free conditions.
Cell stimulation and ERK1 immune complex kinase assay.
For ERK assays, experiments were performed on 6-cm-diameter plates of confluent, quiescent cells. Following stimulation with appropriate growth factors, agonists, and drugs, the cells were lysed in ice-cold TG lysis buffer (20 mM Tris- HCl [pH 8], 1% Triton X-100, 10% glycerol, 137 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 μM leupeptin, 10 μg of aprotinin ml−1), and ERK1 activity was assayed by immune complex kinase assay as described previously (4, 27).
Cell stimulation and ΔRaf-1:ER immune complex kinase assay.
Cell monolayers were incubated with 1 μM E2 with or without cAMP agonists for the appropriate times at 37°C before lysis in ice-cold TG lysis buffer as described for ERK assays. One hundred micrograms of cell lysate was subjected to immunoprecipitation for 2 h with 3 μl of anti-hbER antiserum, and immune precipitates were collected on protein A-Sepharose beads. Immune complexes were washed three times in TG lysis buffer and once in Raf kinase buffer (30 mM Tris, pH 7.5, 10 mM MgCl2 1 mM MnCl2, 1 mM dithiothreitol). The immune complexes were then resuspended in a total volume of 30 μl of Raf kinase buffer containing 300 ng of catalytically inactive MEK-1, 10 μM cold ATP, and 10 μCi of [γ-32P]ATP and incubated at 30°C for 30 min. The reactions were terminated by the addition of 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiled at 95°C for 5 min. Samples were resolved on an SDS-10% PAGE gel, subjected to autoradiography, and quantified by phosphorimager.
Assay of CDK2 activity.
Cells were lysed, and CDK2-associated histone H1 kinase activity was assayed as described previously (27).
Western blot analysis.
Whole-cell extracts in TG lysis buffer or samples from immunoprecipitates were boiled in 4× sample buffer, resolved on SDS-PAGE gels (10, 12, or 14% acrylamide), and analyzed by Western blotting using Amersham ECL or ECL+ reagents as described previously (4, 27, 68).
Assay of cell cycle reentry.
DNA synthesis was monitored by the incorporation of [3H]thymidine into trichloroacetic acid-precipitable material as described previously (4, 50, 68).
Reproducibility of results.
Unless otherwise stated, results are from single experiments representative of between three and seven giving similar results. ERK1 immune complex kinase assays were performed on single or duplicate cell samples; we have found this to be a highly sensitive and reproducible assay (4, 13, 14, 27, 68). ΔRaf-1:ER kinase assays were performed on duplicate stimulated cell samples. [3H]thymidine incorporation was assayed from duplicate stimulated cell samples.
RESULTS
Activation of ERK1/2 by ΔRaf-1:ER is not inhibited by cAMP.
The ability of cAMP to inhibit the ERK1/2 pathway varies in different cell lines (10, 16, 24, 69), presumably reflecting cell- or tissue-specific expression of key regulators of the ERK1/2 or cAMP pathway (35). To try to minimize such effects, we focused on three fibroblast cell lines which are growth inhibited by cAMP and exhibit different degrees of inhibition of ERK1/2 by cAMP. cAMP causes a delay in activation of ERK1/2 (52) and inhibits the proliferation of CCl39 cells (50). CR1-11 cells (76) are a clone of CCl39 cells expressing ΔRaf-1:ER* in which the kinase domain of Raf-1 is fused to a modified version of the hormone-binding domain of the murine estrogen receptor, analogous to ER (48), which can be activated by 4-HT but not E2. cAMP inhibits proliferation of NIH 3T3 cells and causes a more substantial inhibition of ERK1/2 (20, 64). NIH 3T3 cells expressing ΔRaf-1:ER* were described previously (80). Finally, Rat-1 cells are very sensitive to the antiproliferative effects of cAMP, and ERK1/2 activation is essentially abolished by cAMP in these cells (9, 13, 34, 81). Rat-1 cells expressing ΔRaf-1:ER (RR1 cells) have been described previously (14, 15). For simplicity, we refer to either ΔRaf-1:ER (wild-type ER) or ΔRaf-1:ER* (mutant ER, refractory to E2) simply as ΔRaf-1:ER in this report, as we have observed no differences in their sensitivities to a variety of agents which increase cAMP levels, including dibutyryl-cAMP (db-cAMP), 3-isobutyl-1-methylxanthine (IBMX), and cholera toxin (K. Balmanno and S. J. Cook, unpublished observations). Significantly, the ΔRaf-1:ER chimeras contain the kinase domain of Raf-1, including the proposed protein kinase A (PKA) phosphorylation site at Ser621 (53), but lack the N-terminal Ras binding domain and PKA phosphorylation sites at Ser43, Ser233, and Ser259 (19, 20, 66, 81).
In the first instance, we used RR1 cells to determine if the ΔRaf-1:ER fusion protein is inhibited by agents that elevate cAMP. The addition of E2 to quiescent RR1 cells led to a pronounced activation of ΔRaf-1:ER (Fig. 1A) and the endogenous ERK1 (Fig. 1B). Activation of ΔRaf-1:ER was unaffected by pretreatment of cells with 100 or 500 μM IBMX (Fig. 1A), a cell-permeant cyclic nucleotide phosphodiesterase inhibitor (13). Similarly, activation of ERK1, assayed from the same lysates, was also unaffected by IBMX (Fig. 1B). This was not an artifact of the RR1 cells, since epidermal growth factor (EGF) stimulation of ERK1 via the endogenous Ras-dependent pathway was still inhibited in a dose-dependent fashion by IBMX (Fig. 2A). In contrast, over the same dose range, E2-stimulated (ΔRaf-1:ER-mediated) ERK1 activation was not inhibited by IBMX (Fig. 2A, left). E2-stimulated ERK1 activation was also refractory to db-cAMP (Fig. 2A, right) or cholera toxin (Balmanno and Cook, unpublished). Thus, in RR1 cells, cAMP can inhibit growth factor-stimulated activation of Raf-1 and ERK1/2 (9, 13, 81) but cannot inhibit ΔRaf-1:ER or its ability to activate the ERK1/2 pathway.
FIG. 1.
cAMP does not inhibit ΔRaf-1:ER in RR1 cells. Quiescent, serum-starved RR1 cells expressing ΔRaf-1:ER were treated with 100 or 500 μM IBMX or vehicle control (solid bars) for 30 min prior to stimulation with E2 for 1 h. (A) Activation of ΔRaf-1:ER, assayed by its ability to phosphorylate catalytically inactive MEK1, was monitored after immunoprecipitation with anti-estrogen receptor antibodies as described in Materials and Methods. (B) The same lysates as in panel A were also used to immunoprecipitate endogenous ERK1, which was assayed by its ability to phosphorylate myelin basic protein. In both cases, the results are means plus ranges from duplicate cell cultures taken from a single experiment. Similar results were obtained in two other experiments. PI, phosphorimager.
FIG. 2.
cAMP does not inhibit ΔRaf-1:ER-mediated ERK1 activation in RR1, CR1-11, or NIH 3T3/ΔRaf-1:ER cells. All experiments were performed on quiescent, serum-starved cells. (A) RR1 cells were pretreated with increasing concentrations of IBMX for 30 min prior to stimulation with EGF or E2(left) or treated with 1 μM E2 in the absence (solid bars) or presence (hatched bars) of 1 mM db-cAMP (right). ERK1 activity was determined by immune complex kinase assay, and the results are from a single representative experiment. (B) CR1-11 cells were treated with control vehicle (4-HT) or 1 mM db-cAMP (4-HT+cAMP) for 5 min before stimulation with increasing concentrations of 4-HT as indicated for 20 h. ERK1 activity was determined by immune complex kinase assay and quantified on a phosphorimager. The results are expressed as phosphorimager (PI) units and are taken from a single experiment representative of three that had similar results. For reference, the same lysates were used to assay CDK2 activity, which was completely inhibited (Balmanno and Cook, unpublished). (C) NIH 3T3/ΔRaf-1:ER cells were treated with 1 mM db-cAMP (cAMP) or vehicle control (Con) for 30 min prior to being stimulated for 2 h with 10 or 100 nM 4-HT. Activation of ERK1 was determined by immune complex kinase assay. The data are means plus ranges of duplicate cell dishes and are taken from a single experiment representative of three.
This effect was not confined to RR1 cells. In CR1-11 cells, 4-HT caused a dose-dependent activation of ERK1, and even a 20-h exposure to db-cAMP failed to inhibit this response (Fig. 2B), though it inhibited CDK2 activation assayed in parallel (Balmanno and Cook, unpublished). Similarly, in NIH 3T3 cells, the ability of ΔRaf-1:ER to activate ERK1 was not blocked by db-cAMP (Fig. 2C). In all cases, immunoblots with phosphospecific antibodies confirmed that activation of ERK2 was also unaffected by cAMP elevation (Balmanno and Cook, unpublished). Thus, in three different fibroblast cell lines, activation of ERK1/2 by ΔRaf-1:ER is resistant to levels of cAMP that inhibit the normal growth factor-stimulated pathway.
Signaling from ΔRaf-1:ER to AP-1 proteins is insensitive to cAMP.
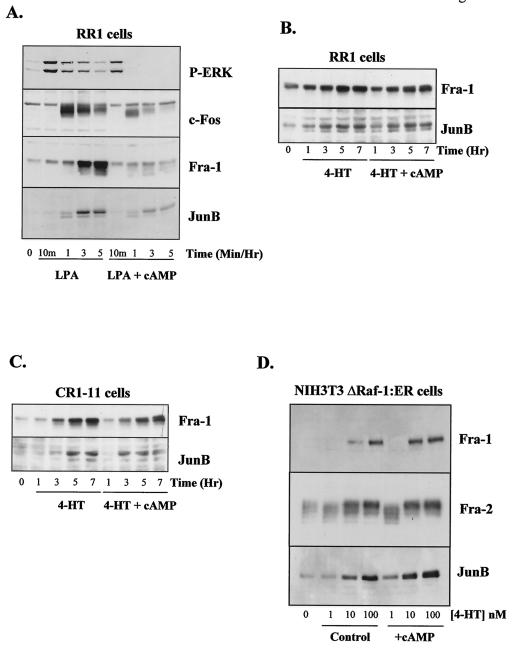
Since cAMP could not inhibit ΔRaf-1:ER-stimuated ERK1/2 activation, we examined targets downstream of the ERK1/2 pathway. The ERK1/2 cascade regulates the expression of members of the AP-1 family, including c-Fos, Fra-1, and JunB (4, 15, 65, 71, 78). In RR1 cells, lysophosphatidic acid caused a biphasic, sustained activation of ERK1/2, as assessed with phosphospecific antibodies (Fig. 3A), and this was accompanied by a rapid expression of c-Fos and a more delayed expression of Fra-1 and JunB. db-cAMP caused a partial inhibition of the early peak of ERK1/2 activation at 10 min and a complete loss of the sustained phase of the response. This resulted in a small reduction of the early peak of c-Fos expression after 1 h, while the sustained phase of the response at 3 and 5 h was reduced to 41% ± 21% of control levels. The slower expression of Fra-1 and JunB, which is absolutely dependent upon ERK1/2 activation (15), was also strongly inhibited by cAMP treatment (Fig. 3A); for example, Fra-1 expression was reduced to 26% ± 11% of controls levels, while JunB was reduced to 40% ± 5% of control values. In contrast to the growth factor-regulated expression of AP-1 proteins, cAMP had only a modest effect on expression of Fra-1 or JunB in response to ΔRaf-1:ER in RR1 cells (Fig. 3B). For example, ΔRaf-1:ER-stimulated expression of Fra-1 was reduced only to 86% ± 5% of control values, while JunB levels were 108% ± 14% of control levels.
FIG. 3.
cAMP fails to inhibit the expression of AP-1 proteins by ΔRaf-1:ER. All experiments were performed on quiescent serum-starved cells. (A) RR1 cells were stimulated with 100 μM lysophosphatidic acid (LPA) in the absence or presence of 1 mM db-cAMP for the indicated times. Phosphorylation of ERK1 and ERK2 (P-ERK) and expression of c-Fos, Fra-1, and JunB were assayed by performing Western immunoblotting on whole-cell lysates using the appropriate antibodies. (B and C) RR1 cells (B) and CR1-11 cells (C) were stimulated with 100 nM 4-HT in the absence or presence of 1 mM db-cAMP for the indicated times. Expression of Fra-1 and JunB was assayed by performing Western immunoblotting on whole-cell lysates using the appropriate antibodies. (D) NIH 3T3/ΔRaf-1:ER cells were stimulated with 1, 10, or 100 nM 4-HT in the absence or presence of 1 mM db-cAMP for 5 h. Expression of Fra-1, Fra-2, and JunB was assayed by performing Western immunoblotting on whole-cell lysates using the appropriate antibodies. In all cases, the results are from single experiments representative of between three and seven others that gave similar results.
ΔRaf-1:ER-stimulated expression of Fra-1 and JunB was also largely insensitive to cAMP in CR1-11 cells (Fig. 3C). For example, cAMP reduced Fra-1 and JunB levels only to 93% ± 4% and 80% ± 7% of control levels. Similar results were observed in NIH 3T3 cells, where activation of ΔRaf-1:ER caused an increase in expression of Fra-1, Fra-2, and JunB which was completely insensitive to db-cAMP (Fig. 3D). In this analysis, we could not examine the effect of cAMP on c-Fos, since ΔRaf-1:ER causes little if any increase in c-Fos expression in RR1 cells (15) or CR1-11 cells (Balmanno and Cook, unpublished). Thus, when ΔRaf-1:ER is used to bypass the cAMP block in ERK1/2 activation, the expression of key AP-1 proteins is either no longer blocked by cAMP or exhibits greatly reduced sensitivity.
ΔRaf-1:ER-stimulated DNA synthesis is still inhibited by cAMP.
Given the importance of the ERK1/2 pathway in promoting cell cycle reentry, the ability to inhibit ERK1/2 activation seems a plausible explanation for the antiproliferative effects of cAMP. The ability of ΔRaf-1:ER to activate ERK1/2, and its downstream AP-1 targets, in a cAMP-insensitive manner allowed us to address this question by simply determining whether cAMP could still inhibit ΔRaf-1:ER-stimulated DNA synthesis.
In NIH 3T3 cells, activation of ΔRaf-1:ER with 4-HT caused a dose-dependent stimulation of DNA synthesis which exhibited a bell-shaped dose-response curve. The downturn at high doses of 4-HT may reflect the induction of p21CIP1 and inhibition of CDK2 (80). Significantly, db-cAMP (Fig. 4A) or IBMX (Balmanno and Cook, unpublished) completely inhibited DNA synthesis whether stimulated by 4-HT or FBS. This effect was also observed in CR1-11 cells expressing ΔRaf-1:ER* (Fig. 4B). For example, IBMX inhibited both thrombin-stimulated DNA synthesis and the smaller ΔRaf-1:ER-stimulated DNA synthesis in a dose-dependent manner with similar 50% inhibitory concentrations (Fig. 4B). Finally, we also observed that db-cAMP completely inhibited both serum-stimulated and the more modest ΔRaf-1:ER-stimulated DNA synthesis in RR1 cells (Fig. 4C). These results, in three different fibroblast cell lines, clearly demonstrate that although ΔRaf-1:ER can circumvent the cAMP block of ERK1/2 activation and AP-1 gene expression, it cannot bypass the block in cell cycle reentry, providing unambiguous evidence for some other cAMP-sensitive event(s) downstream of ERK1/2 which prevents entry into S phase.
FIG. 4.
cAMP can still inhibit ΔRaf-1:ER-stimulated DNA synthesis. (A) NIH 3T3/ΔRaf-1:ER cells were stimulated with increasing concentrations of 4-HT in the absence (4-HT) or presence (4-HT+cAMP) of 1 mM db-cAMP, and cell cycle reentry was assayed by [3H]thymidine incorporation ([3H]Thy Inc) (left), or the cells were stimulated with 10 nM 4-HT or 10% (vol/vol) FBS in the absence (Con) or presence (cAMP) of 1 mM db-cAMP, and cell cycle reentry was assayed by [3H]thymidine incorporation (right). The results are means ± ranges of duplicate cell cultures. (B) CR1-11 cells were pretreated for 30 min with increasing concentrations of IBMX and then stimulated for 24 h with either 100 nM thrombin (Thr) or 100 nM 4-HT. Cell cycle reentry was assayed by [3H]thymidine incorporation, and the results are the means ± ranges of duplicate cell cultures. (C) RR1 cells were pretreated with 1 mM db-cAMP or vehicle control and then stimulated for 20 h with either 10% (vol/vol) FBS (left) or 100 nM 4-HT (right). Cell cycle reentry was assayed by [3H]thymidine incorporation, and the results are the means ± ranges of duplicate cell cultures. SF, serum free; cA, cAMP. In all cases, the results are from a single experiment representative of three that had the same result.
Activation of CDK2 is an alternative target for cAMP downstream of the ERK1/2 pathway.
Cell cycle reentry requires the expression of the D-type cyclins, which activate CDK4 and initiate phosphorylation of pRb (51, 56) to promote cyclin E expression. Expression of cyclin E and cyclin A and the assembly of cyclin E-CDK2 and cyclin A-CDK2 complexes provide further rounds of pRb phosphorylation. The CDK inhibitor p21CIP1 is induced by some antiproliferative stimuli and acts to inhibit CDK2-containing complexes, although it may also promote the assembly of cyclin D-CDK4 (42). p27KIP1, an inhibitor of CDK2 complexes, accumulates to high levels in quiescent cells and must be degraded for cells to exit G1 and enter S phase (11). Both cyclin D1 and p27KIP1 have been proposed to be alternative targets for the antiproliferative effects of cAMP. However, since these events are dependent upon prior activation of ERK1/2, it is difficult to determine if their inhibition by cAMP is a direct effect or, more simply, due to inhibition of ERK1/2 upstream. The ability of ΔRaf-1:ER to activate the ERK1/2 pathway independently of cAMP allowed us to resolve this issue. These studies were again performed in CR1-11, RR1, and NIH 3T3/ΔRaf-1:ER cells in the hope of gaining some consensus on the possible targets of cAMP downstream of the ERK1/2 pathway.
(i) Effect of cAMP on activation of CDK2 in CR1-11 cells.
db-cAMP strongly inhibited the FBS-stimulated activation of CDK2 in CR1-11 cells (Fig. 5A) without reducing CDK2 expression (Fig. 5B). Treatment with db-cAMP had no effect on p21CIP1 expression but did strongly inhibit FBS-stimulated cyclin D1 expression, as reported previously (43). We were unable to reproducibly assay CDK4 activity due to technical difficulties. However, we used the phosphorylation of Ser795 of Rb as a recognized surrogate marker for CDK4 activity, since it is known that this site is phosphorylated by CDK4, but very poorly by CDK2, and phosphorylation of Rb-Ser795 is required to inactivate the Rb cell cycle arrest function (12). We found that the FBS-stimulated phosphorylation of Rb-Ser795 was abolished by cAMP treatment, and this was consistent with the inhibition of cyclin D1 expression (Fig. 5B). cAMP also caused a strong inhibition of Cdc25A and cyclin A expression and prevented the degradation of p27KIP1. These results would be consistent with cAMP blocking cyclin D1 expression (43), since cyclin A and Cdc25A are induced downstream of cyclin D1. However, different results were obtained when we activated ΔRaf-1:ER. Although db-cAMP abolished CDK2 activation induced by ΔRaf-1:ER (Fig. 5C), it failed to inhibit the expression of cyclin D1 or the phosphorylation of the CDK4 site P-Rb-Ser795, assayed in parallel (Fig. 5D). Thus, when the cAMP block of ERK1/2 activation is bypassed by ΔRaf-1:ER, cAMP can no longer inhibit cyclin D1 expression or CDK4 activity in CCl39 cells.
FIG. 5.
Effects of cAMP on activation of CDK2 and expression of cyclin D1, cyclin A, Cdc25A, and CDKIs in CR1-11 cells. Quiescent CR1-11 cells were stimulated for the indicated times with 10% (vol/vol) FBS (A and B) or 100 nM 4-HT (C and D) in the absence (solid symbols) or presence (open symbols) of 1 mM db-cAMP. Whole-cell lysates were used to assay CDK2 activity, quantified as phosphorimager (PI) units (A and C), or were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with antibodies specific for cyclin (cyc) D1, phospho (P)-Rb-Ser795, p27KIP1, p21CIP1, cyclin A, Cdc25A, phospho-CDK2-Tyr15, and CDK2 (B and D). Phospho-CDK2-Tyr15 phosphorylation was assessed by immunoblotting following immunoprecipitation of CDK2. In all cases, the results are from a single experiment representative of three that had similar results.
To examine other possible causes for the inhibition of ΔRaf-1:ER-induced CDK2 activation by cAMP, we examined the expression of other CDK2 regulators (Fig. 5D). ΔRaf-1:ER-induced p21CIP1 expression was unaffected by cAMP. The ΔRaf-1:ER-induced loss of p27KIP1 was partially reversed by cAMP, but only at later times (18 to 24 h), whereas inhibition of CDK2 activity was also observed at 14 h, when cAMP had no effect on p27KIP1 levels. By far the most striking effect of cAMP was to inhibit ΔRaf-1:ER-induced expression of cyclin A and Cdc25A, both of which are required for CDK2 activation. Loss of cyclin A expression would be predicted to prevent the assembly of CDK2 complexes late in G1 and at the start of S phase. To address this directly, we immunoprecipitated CDK2 and blotted it with the anti-phospho-Tyr15 CDK1/cdc2 antibody (which also recognizes Tyr15-phosphorylated CDK2), since it is known that both CDK1/cdc2 (67) and CDK2 (31) are only phosphorylated at Tyr15 after the CDK binds to its cyclin. Consistent with the loss of cyclin A, cAMP reduced the phosphorylation of Tyr15 of CDK2 in response to FBS or ΔRaf-1:ER (Fig. 5B and D).
These results indicate that in CR1-11 cells, signaling from ΔRaf-1:ER via ERK1/2 to cyclin D1 and phosphorylation of P-Rb-Ser795 is insensitive to doses of cAMP that inhibit the serum response, suggesting that reduced expression of cyclin D1 is unlikely to be the mechanism for the cAMP-dependent block of proliferation downstream of ERK1/2. Inhibition of CDK2 activation, which correlates most clearly with inhibition of Cdc25A and cyclin A expression, is a more likely mechanism.
(ii) Effect of cAMP on activation of CDK2 in RR1 cells.
In RR1 cells, cAMP was able to inhibit both FBS- and ΔRaf-1:ER-induced CDK2 activation (Fig. 6A and C) without reducing CDK2 expression levels (Fig. 6B and D). We were unable to detect p21CIP1 expression in Rat-1 cells; this was probably due to methylation of the promoter, since p21CIP1 is expressed if these cells are treated with methyltransferase inhibitors (1, 27).
FIG. 6.
Effects of cAMP on activation of CDK2 and expression of cyclin D1, cyclin A, Cdc25A, and CDKIs in RR1 cells. Quiescent RR1 cells were stimulated for the indicated times with 10% (vol/vol) FBS (A and B) or 100 nM 4-HT (C and D) in the absence (▪) or presence (□) of 1 mM db-cAMP. Whole-cell lysates were used to assay CDK2 activity, quantified as phosphorimager (PI) units (A and C), or were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with antibodies specific for cyclin (cyc) D1, p27KIP1, cyclin A, Cdc25A, phospho (P)-CDK2-Tyr15, and CDK2 (B and D). Phospho-CDK2-Tyr15 phosphorylation was assessed by immunoblotting following immunoprecipitation of CDK2. The results are from a single experiment representative of three that had similar results.
Pretreatment of quiescent RR1 cells with db-cAMP inhibited FBS-stimulated cyclin D1 expression. However, in contrast to the situation in CR1-11 cells, db-cAMP also inhibited the expression of cyclin D1 induced by ΔRaf-1:ER in RR1 cells. This suggests that even when cAMP fails to inhibit ERK1/2 activation by ΔRaf-1:ER, it can still exert an ERK1/2-independent inhibitory effect on cyclin D1 expression in these cells. In common with CR1-11 cells, db-cAMP was able to completely reverse the FBS-stimulated decrease in 27KIP1 levels at all time points tested. In contrast, the ΔRaf-1:ER-induced degradation of p27KIP1 was only partially reversed by db-cAMP treatment, indicating that signaling from ΔRaf-1:ER to p27KIP1 is significantly less sensitive to cAMP. Finally, db-cAMP inhibited both serum- and ΔRaf-1:ER-induced expression of Cdc25A and cyclin A and prevented the assembly of cyclin CDK2 complexes, as judged by inhibition of CDK2 Tyr15 phosphorylation (Fig. 6B and D).
Effect of cAMP on activation of CDK2 in NIH 3T3- Raf-1:ER cells.
Finally, in NIH 3T3 cells, db-cAMP had no effect on CDK2 expression levels (Fig. 7B) but completely inhibited CDK2 activation induced by ΔRaf-1:ER (Fig. 7A). Despite this, db-cAMP did not inhibit the expression of cyclin D1 and p21CIP1 or the degradation of p27KIP1 in response to ΔRaf-1:ER (Fig. 7B). The ability of ΔRaf-1:ER to induce expression of cyclin E was also unaffected by db-cAMP (Fig. 7C), and since cyclin E expression requires prior activation of cyclin D-CDK4 (29, 82), this again implies that cAMP does not prevent the activation of CDK4 by ΔRaf-1:ER. In common with the studies of CR1-11 and RR1 cells, cAMP inhibited the expression of cyclin A (Fig. 7C). The induction of Cdc25A in response to activation of ΔRaf-1:ER in NIH 3T3 cells was modest compared to that seen in CR1-11 and RR1 cells but was still inhibited by cAMP; for example, at 1 nM 4-HT, cAMP reduced Cdc25A expression by 57% (Fig. 7C).
FIG. 7.
Effects of cAMP on activation of CDK2 and expression of cyclin D1, cyclin A, Cdc25A, and CDKIs in NIH 3T3/ΔRaf-1:ER cells. Quiescent NIH 3T3/ΔRaf-1:ER cells were stimulated with increasing concentrations of 4-HT in the absence (4-HT) or presence (4-HT+cAMP) of 1 mM db-cAMP. Whole-cell lysates were used to assay CDK2 activity, quantified as phosphorimager (PI) units (A), or were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with antibodies specific for cyclin (cyc) D1, p21CIP1, or p27KIP1 (B) or Cdc25A, cyclin E, cyclin A, and CDK2 (C). The results are taken from a single experiment representative of three that had similar results.
In summary, cAMP was able to prevent ΔRaf-1:ER-induced CDK2 activation and proliferation in all three cell lines, and this correlated most clearly with the inhibition of Cdc25A and cyclin A expression.
Stable expression of Cdc25A fails to rescue CR1-11 cells from cAMP-induced growth arrest.
The ability of cAMP to block ΔRaf-1:ER-stimulated expression of Cdc25A suggested that it was a potential target, which might account for the antiproliferative effects of cAMP downstream of ERK1/2. To test this, we sought to determine if stable ectopic expression of Cdc25A could collaborate with ΔRaf-1:ER to overcome the cAMP-induced growth arrest. To this end, we derived doubly resistant clones of CR1-11 cells expressing both ΔRaf-1:ER and HA-tagged Cdc25A from a cytomegalovirus promoter. Despite clear expression of Cdc25A (Fig. 8A), we found that the ΔRaf-1:ER-mediated stimulation of CDK2 activity (Fig. 8B) and DNA synthesis (Fig. 8C) was still inhibited by cAMP. These results suggested that ectopic expression of Cdc25A fails to overcome the cAMP growth arrest and that the relevant cAMP target was either the decrease in cyclin A expression or some other, as yet unidentified, target.
FIG. 8.
Ectopic expression of Cdc25A fails to overcome cAMP growth arrest. (A) CR1-11 cells were transfected with pCMVNeo-HA-Cdc25A, and positive clones were identified by Western immunoblotting with anti-HA antibody (WB:HA). The expression of HA-Cdc25A is shown in one of the positive clones (lane 2) but not in the control CR1-11/Neo cells (lane 1). The asterisk indicates the position of proteolytic breakdown products of the full-length HA-CDc25A. (B and C) Duplicate dishes of CR1-11/Neo or CR1-11/Cdc25A cells were serum starved and then restimulated with 4-HT in the presence or absence of 0.5 mM db-cAMP for 24 h. The cells were assayed for CDK2 activity (B) or [3H]thymidine incorporation ([3H]Thy Inc) (C), and the data are shown as means plus ranges of duplicate dishes from a single experiment representative of three. PI, phosphorimager; cA, cAMP.
Activation of E2F1 overcomes the cAMP block of Cdc25A and cyclin A expression but not the inhibition of DNA synthesis.
Attempts to stably express cyclin A in any of the cell lines we have used have failed, and this is consistent with the reports that constitutive, deregulated expression of cyclin A can cause apoptosis (8, 33). Accordingly, we used another approach to examine the importance of cyclin A as a target of cAMP. One of the major functions of the G1 CDKs (CDK4 and CDK2) is to phosphorylate and inactivate Rb, thereby derepressing and releasing the E2F transcription factor (21). Members of the E2F family control the expression of a number of genes that are believed to be important for entry into and progression through S phase, including those for cyclin A, Cdc25A, dihydrofolate reductase, and thymidine kinase. Indeed, overexpression (18) or activation (74) of E2F1 can induce S-phase entry and overcome G1 arrest imposed by p16INK4a, p21CIP1, p27KIP1, or dominant-negative CDK2 without activating CDK4 or CDK2 (18). Consequently, we sought to determine if E2F1 could overcome the antiproliferative effects of cAMP.
For these studies, we used the conditional ER-E2F1 fusion protein, which is also activated by 4-HT and is sufficient to induce expression of Cdc25A and cyclin A and cell cycle reentry in otherwise serum-starved cells (74). Since E2F1 can promote G1 arrest in some cells by promoting the expression of p21CIP1 (28, 49) we limited these studies to Rat-1 cells in which the p21CIP1 promoter is methylated and silenced (1). We expressed HA-ER-E2F1 in Rat-1 cells (R1-ER-E2F1 cells) by retroviral infection; two positive clones were analyzed and gave the same results, so representative experiments from one are shown. Expression of the HA-ER-E2F1 construct was readily detectable by subjecting whole-cell lysates to anti-HA immunoblotting (Fig. 9A). Stimulation of serum-starved R1-ER-E2F1 cells with 4-HT resulted in the rapid expression of Cdc25A, followed by the slower expression of cyclin A, demonstrating the functionality of the ER-E2F1 fusion (Fig. 9B). Significantly, db-cAMP failed to inhibit the expression of either Cdc25A or cyclin A induced by activation of ER-E2F1 (Fig. 9B). Despite this, cAMP still inhibited DNA synthesis in these cells. For example, R1-ER-E2F1 cells exhibited a 20-fold increase in DNA synthesis following stimulation with FBS, and this was strongly inhibited by cAMP (Fig. 9C). Activation of ER:E2F1 with 4-HT caused a more modest threefold increase in DNA synthesis, and this probably reflects the fact that we were activating just a single member of the E2F family whereas FBS stimulation will activate multiple E2Fs (21). In any case, the ER-E2F1-induced DNA synthesis was still completely inhibited by cAMP treatment (Fig. 9D). Thus, despite the fact that ER-E2F1 can completely overcome the cAMP block of Cdc25A and cyclin A expression, ER-E2F1-stimulated DNA synthesis remains fully inhibited by cAMP.
FIG. 9.
Activation of ER-E2F1 overcomes the cAMP block of Cdc25A and cyclin A expression but not inhibition of DNA synthesis. (A) Rat-1 cells were infected with pBabePuro HA-ER-E2F1 retrovirus, and positive clones were detected by Western immunoblotting with anti-HA antibodies (WB:HA). The blot shows the expression of HA-ER-E2F1 in one representative positive clone (lane 2) compared to Rat-1 Puro control cells (lane 1). (B) R1-ER-E2F1 cells were treated with 100 nM 4-HT for the indicated times in the absence or presence of 0.5 mM db-cAMP, and whole-cell lysates were assayed for expression of Cdc25A and cyclin A (cyc A) by Western immunoblotting. The asterisk indicates the position of a nonspecific reactive band which served as a useful loading control. (C and D) R1-ER-E2F1 cells were serum starved and then stimulated with 10% FBS (C) or 100 nM 4-HT (D) for 24 h in the absence (solid bars) or presence (hatched bars) of 0.5 mM db-cAMP. The cells were assayed for [3H]thymidine incorporation ([3H]Thy Inc), and the data are means plus ranges of duplicate dishes from a single representative experiment. cA, cAMP.
DISCUSSION
The ability of cAMP to inhibit Raf-1 activation and so prevent ERK1/2 activation has attracted much interest as a possible mechanism for cAMP-mediated G1 arrest (35, 69). Here, we show that ΔRaf-1:ER can circumvent the cAMP block of ERK1/2 activation even in cells in which the endogenous growth factor-regulated pathway to ERK1/2 is still inhibited. Despite this, ΔRaf-1:ER-mediated CDK2 activation and cell cycle reentry are still blocked by cAMP, demonstrating unambiguously that cAMP inhibition of ERK1/2 is not the only, and perhaps not the major, mechanism for cAMP-mediated growth inhibition
Activation of the ERK pathway by ΔRaf-1:ER is not inhibited by cAMP.
cAMP prevents Raf-1 activation without preventing activation of Ras (9, 13, 81), and two models have been proposed to account for the inhibition of Raf-1. First, it was demonstrated that cAMP-dependent protein kinase A could phosphorylate Raf-1 at Ser43, thereby reducing binding of Ras-GTP to Raf-1 (81). The recent demonstration that cAMP can block the Ras-Raf-1 interaction in whole cells (20) suggests that this may be a physiologically relevant mechanism of Raf-1 inhibition. An alternative model proposed that PKA phosphorylated Ser621 in the C-terminal kinase domain, thereby inhibiting activity (53). We reasoned that ΔRaf-1:ER could help to resolve which mechanism was more likely to operate in whole cells, since it lacks the entire N terminus (61). Thus, if phosphorylation of the N-terminal regulatory domain was the relevant mechanism, then activation of ERK1/2 by ΔRaf-1:ER should be insensitive to cAMP. The results of our analysis, in three different cell lines, are clear and consistent. Assay of the ΔRaf-1:ER fusion protein in Rat-1 (RR1) cells revealed that it was not inhibited by increases in cAMP (Fig. 1A and 2A), unlike endogenous Raf-1 (9, 13, 81). In addition, activation of ERK1/2 by ΔRaf-1:ER was not inhibited by high doses of IBMX, which still abolished EGF stimulation of ERK1/2 through the endogenous Ras-dependent pathway, the most stringent internal control. Finally, cAMP also failed to block ERK1/2 activation by ΔRaf-1:ER in NIH 3T3 and CCl39 fibroblasts, indicating that this is a highly reproducible effect.
While we have not addressed the mechanism for inhibition of Raf-1 directly, our results suggest that cAMP sensitivity resides within the N terminus of Raf-1, which is missing in ΔRaf-1:ER, and argues against a role for phosphorylation of Ser621. Phosphorylation of Ser621 has been observed only upon overexpression of Raf-1 with the PKA catalytic subunit in Cos and Sf9 cells (53). Furthermore, Ser621 is constitutively phosphorylated in vivo (54), suggesting that PKA-catalyzed phosphorylation at this site may be an artifact of overexpression or in vitro assay conditions. In contrast, cAMP can promote phosphorylation of Raf-1 at Ser259, and this can inhibit Raf-1 activation (19, 20). Finally, the identification of a third cAMP-inducible phosphorylation site at Ser233, which is also inhibitory, reveals a remarkable complexity in the regulation of Raf-1 by cAMP (20). It seems that phosphorylation of Ser43, Ser233, and Ser259 is required for complete inhibition of Raf-1 by cAMP, since only the mutation of all three residues to alanine is sufficient to make Raf-1 insensitive to cAMP (20). The fact that the cAMP-dependent inhibitory phosphorylation sites are all within the N terminus of Raf-1 is consistent with our demonstration that ΔRaf-1:ER is refractory to cAMP and with reports that the isolated kinase domain of Raf is refractory to cAMP (20, 66, 77). cAMP may also act independently of PKA to activate the small GTPase Rap1, which can antagonize Ras-dependent activation of Raf-1 (64). However, recent studies with novel cAMP analogues argue that Rap1 activation is not needed for cAMP-induced inhibition of ERK1/2 (22).
cAMP blocks ΔRaf-1:ER-induced CDK2 activation and DNA synthesis, revealing additional cAMP-sensitive steps downstream of ERK1/2.
Despite signaling from ΔRaf-1:ER via ERK1/2 to AP-1 being largely resistant to cAMP, DNA synthesis remained fully sensitive to cAMP inhibition in all three cell lines. These results unequivocally demonstrate that cells must have at least one other cAMP-sensitive step, acting downstream of ERK1/2, to prevent cell cycle reentry. Consequently, we examined the expression of several key cell cycle regulators acting in G1 in the three different cell lines, hoping to find a consensus mechanism.
(i) Cyclin D1 and cyclin E.
Expression of cyclin D1 is a rate-limiting step in the progression through G1, and cAMP inhibits cyclin D1 expression in response to serum and growth factors (43, 63, 72). In CR1-11 cells, cAMP inhibited cyclin D1 expression in response to FBS but not in response ΔRaf-1:ER; similarly, in NIH 3T3 cells, cAMP failed to inhibit cyclin D1 expression in response to ΔRaf-1:ER. While we have not been successful in assaying CDK4 activity directly, two observations suggest that cAMP fails to inhibit activation of CDK4 by ΔRaf-1:ER. First, in CR1-11 cells, cAMP inhibited the phosphorylation of Rb at Ser795 in response to FBS but not in response to ΔRaf-1:ER. Since it is known that Ser795 is phosphorylated very effectively by CDK4, but not by CDK2 (12), this surrogate assay confirms the results with cyclin D1 expression and implies that CDK4 activation by ΔRaf-1:ER is not inhibited by cAMP in CR1-11 cells. Second, in NIH 3T3 cells, we observed that cAMP did not inhibit ΔRaf-1:ER-stimulated expression of cyclin D1 or cyclin E. It is known that “knock in” of cyclin E at the cyclin D locus can rescue the phenotype of cyclin D1−/− mice, suggesting that cyclin E expression is the major rate-limiting target for cyclin D1 (29). Furthermore, CDK4-catalyzed phosphorylation is required to disrupt the interaction of histone deacetylase with Rb-hSWI/SNF and thereby relieve the repression of cyclin E (82). Thus, the fact that cAMP fails to inhibit cyclin E expression in response to ΔRaf-1:ER in NIH 3T3 cells suggests that signaling from cyclin D1 via CDK4 to cyclin E is intact in these cells. Clearly, future studies should aim to analyze CDK4 activity in detail, but together these observations suggest that cyclin D1 expression is not an alternative target for cAMP in CR1-11 and NIH 3T3 cells. Rather, it seems that the effect of cAMP on cyclin D1 correlates broadly with the ability of cAMP to inhibit ERK1/2 activation; when the cAMP block of ERK1/2 activation is bypassed by ΔRaf-1:ER, the expression of cyclin D1 also becomes insensitive to cAMP. RR1 cells are a notable exception here, as cAMP was able to inhibit expression of cyclin D1 in response to both FBS and ΔRaf-1:ER. This suggests that in Rat-1 cells, cAMP can also exert an inhibitory effect downstream of ERK1/2, perhaps inhibiting cyclin D1 transcription, impairing mRNA stability, or promoting cyclin D1 turnover. In summary, in two out of three cell lines, cAMP failed to block the expression of cyclin D1 or the activation of CDK4, as judged by two independent surrogate assays, in response to ΔRaf-1:ER.
(ii) CDK2 activation.
Elevation of cAMP was able to inhibit CDK2 activation in all cell lines tested, whether induced by FBS or ΔRaf-1:ER. cAMP had no effect on CDK2 expression, suggesting that it influences signaling from ΔRaf-1:ER to one or the other of the regulators of CDK2.
(iii) p21CIP1.
Depending on expression levels, p21CIP1 can facilitate CDK4 assembly (42) or inhibit the activity of CDK2 and cause cell cycle arrest (80). Although cAMP has been shown to increase p21CIP1 levels in at least two studies (44, 58), we did not observe any effect of cAMP on p21CIP1 expression in CCl39 or NIH 3T3 cells. Furthermore, cAMP was able to inhibit CDK2 and cell proliferation in Rat-1 cells, which fail to express p21CIP1 (1). Thus, there is no absolute requirement for p21CIP1 in cAMP-mediated growth arrest in fibroblasts, and the effect of cAMP on p21CIP1 may be cell type specific.
(iv) p27KIP1.
cAMP has been shown to inhibit the mitogen-dependent down regulation of p27KIP1 in a variety of cell types (37, 39, 40, 43), and we could certainly demonstrate that cAMP prevented the FBS-stimulated degradation of p27KIP1 in CR1-11 and RR1 cells. In contrast, cAMP was less effective at inhibiting p27KIP1 degradation in response to ΔRaf-1:ER in CR1-11 and RR1 cells. Finally, in NIH 3T3 cells, the ΔRaf-1:ER-induced loss of p27KIP1 was clearly not reversed by cAMP, suggesting that p27KIP1 is not likely to be an alternative target for cAMP in these cells.
(v) Cdc25A.
cAMP inhibited the expression of Cdc25A in response to FBS, but also in response to activation of ΔRaf-1:ER, in all cell lines tested, though the induction of Cdc25A was quite modest in NIH 3T3 cells. Cdc25A is a phosphatase that serves to dephosphorylate inhibitory sites in the ATP-binding pocket of CDK2 and is required for entry into S phase (7, 26, 36, 55). Clearly, inhibition of Cdc25A expression would therefore prevent the activation of CDK2 even in the presence of an appropriate cyclin. However, if we stably expressed HA-Cdc25A from a cytomegalovirus promoter, we found that it failed to overcome the cAMP growth arrest even in collaboration with activated ΔRaf-1:ER, suggesting that cAMP must target some other event to inhibit CDK2 and cell cycle reentry.
(vi) Cyclin A.
cAMP inhibited the expression of cyclin A by ΔRaf-1:ER in all cell lines tested, perhaps via an inhibitory cAMP response element in the cyclin A promoter (6). Blockade of cyclin A expression would certainly be expected to prevent the activation of CDK2 late in G1 and throughout S phase, and the reduced level of CDK2 Tyr15 phosphorylation certainly confirmed that cyclin-CDK2 complex assembly was inhibited by cAMP. Since the cyclin A gene is an E2F target gene (21, 74) and overexpression or activation of E2F1 can promote S-phase entry (74) and circumvent the G1 CDKs (18), we expressed the conditional construct ER-E2F1 in Rat-1 cells. We found that cAMP failed to inhibit the expression of Cdc25A and cyclin A but still inhibited ER-E2F1-induced DNA synthesis. The simplest explanation for this observation is that cAMP does not inhibit E2F1 per se but rather blocks the expression of specific E2F1 target genes, other than the Cdc25A or cyclin A gene, which are required for DNA synthesis. The identities of the relevant genes remain to be defined.
Multiple sites of cAMP-induced arrest during the G1→S transition.
A major theme that emerges from this study is that cells possess multiple sites of cAMP sensitivity during the G1→S transition. The first, inhibition of Raf-1, blocks all ERK1/2-dependent events early in G1 (Fig. 10A), while a second, blockade of Cdc25A and cyclin A, and perhaps reversal of p27KIP1 degradation (Fig. 10B), acts close to the G1→S-phase transition to prevent CDK2 activation. Even when CDK2 is activated and E2F has been released from Rb-mediated inhibition, our results with ER-E2F1 indicate that cAMP can still target specific events downstream of E2F1 to prevent DNA synthesis (Fig. 10C). The extent to which the early Raf-1 block is used may vary in different cell lines, depending on the natures of the Raf genes expressed (35, 69). For example, several studies have shown that B-Raf is not inhibited and may actually be stimulated by cAMP (10, 23, 75). Consequently, cAMP may fail to inhibit ERK1/2 activity in cells expressing B-Raf, or in tumors harboring mutationally activated B-Raf (17), but still inhibit cell cycle reentry through effects on CDK2 activity or targets downstream of E2F1. So the inhibition of CDK2 may be the more important mechanism for cAMP-induced cell cycle arrest in CCl39 cells (6, 52) and vascular smooth muscle cells(14). In contrast, the inhibition of ERK1/2 may well be an important antiproliferative mechanism in Rat-1 cells, since the overexpression of B-Raf in these cells reduces their sensitivity to growth inhibition by cAMP-elevating agents (23). The corollary to this model is that activation of ERK1/2 and CDK2 should be completely independent of cAMP in those cells in which cAMP fails to inhibit proliferation, and this certainly appears to be the case in Swiss 3T3 cells and thyrocytes (3, 5, 45, 60, 73).
FIG. 10.
Model depicting sites of cAMP sensitivity during cell cycle reentry. (A) cAMP can promote phosphorylation of Raf-1 at S43, S233, and S259 (indicated by PPP), thereby blocking activation of Raf-1 by growth factors and preventing ERK1/2 activation. As a result, most ERK1/2-dependent events downstream of Raf-1 will be blocked by cAMP (indicated by shading). (B) ΔRaf-1:ER is insensitive to cAMP. As a result, many ERK1/2-dependent events downstream, such as expression of AP-1 proteins and cyclin (cyc) D1, are no longer blocked by cAMP. However, cAMP can still prevent the activation of CDK2 by inhibiting the expression of cyclin A, Cdc25A, and perhaps p27KIP1 (indicated by shading). The extent to which the early cAMP block at Raf-1 (A) operates will depend on the expression of cAMP-insensitive Raf isoforms, such as B-Raf. In such cases, where cAMP fails to inhibit ERK1/2 activation, the later checkpoint at CDK2 may be more important for cAMP-mediated growth arrest. (C) Once CDK2 has been activated, cAMP can still inhibit E2F1-stimulated DNA synthesis, presumably by inhibiting the expression of select E2F1 target genes (indicated by shading), but not by inhibiting E2F1 per se, since Cdc25A and cyclin A expression proceed normally. For details, see Discussion.
In summary, although the cAMP block in ERK1/2 activation is bypassed by ΔRaf-1:ER, cAMP can still inhibit ΔRaf-1:ER-stimulated DNA synthesis. It is particularly striking how far the proliferative signal proceeds through G1 when the cAMP block in ERK1/2 activation is bypassed. Serum and growth factor-stimulated increases in AP-1 proteins and cyclin D1, and loss of p27KIP1, are all blocked by cAMP, whereas the same responses to ΔRaf-1:ER are either refractory to cAMP or exhibit greatly reduced sensitivity. The notable exception to this is cyclin D1 in RR1 cells, and future studies should aim to examine this system in more detail. It is only events late in G1, such as CDK2 activity, which retain sensitivity to cAMP when activated by ΔRaf-1:ER. Even when CDK2 is bypassed, cAMP can still inhibit DNA synthesis induced by the direct activation of E2F1. These multiple sites may allow the rapid cessation of DNA synthesis following an increase in cAMP levels.
Acknowledgments
We thank Kristian Helin and Ingrid Hoffmann for provision of cDNA constructs, Steve Robbins for provision of anti-ER antibodies, and Susan MacDonald and Tharin Wendell for provision of purified catalytically inactive MEK1. We are grateful to Richard Marais for useful discussions and for providing us with a preprint of his study in advance of publication.
This work was funded by grants from Cancer Research United Kingdom, formerly the Cancer Research Campaign (SP2458/0201 and SP2382/0403), and a competitive strategic grant from the BBSRC. S.J.C. is a Senior Cancer Research Fellow of Cancer Research UK.
REFERENCES
- 1.Allan, L. A., T. Duhig, M. Read, and M. Fried. 2000. The p21(WAF1/CIP1) promoter is methylated in Rat-1 cells: stable restoration of p53-dependent p21(WAF1/CIP1) expression after transfection of a genomic clone containing the p21(WAF1/CIP1) gene. Mol. Cell. Biol. 20:1291-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ally, S., T. Clair, D. Katsaros, G. Tortora, H. Yokozaki, R. A. Finch, T. L. Avery, and Y. S. Cho-Chung. 1989. Inhibition of growth and modulation of gene expression in human lung carcinoma in athymic mice by site-selective 8-Cl-cyclic adenosine monophosphate. Cancer Res. 49:5650-5655. [PubMed] [Google Scholar]
- 3.Ariga, M., T. Nedachi, M. Akahori, H. Sakamoto, Y. Ito, F. Hakuno, and S. Takahashi. 2000. Signalling pathways of insulin-like growth factor-I that are augmented by cAMP in FRTL-5 cells. Biochem. J. 348:409-416. [PMC free article] [PubMed] [Google Scholar]
- 4.Balmanno, K., and S. J. Cook. 1999. Sustained MAP kinase activation is required for the expression of cyclin D1, p21CIP1 and a subset of AP-1 proteins in CCL39 cells. Oncogene 18:3085-3097. [DOI] [PubMed] [Google Scholar]
- 5.Baptist, M., F. Lamy, J. Gannon, T. Hunt, J. E. Dumont, and P. P. Roger. 1996. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J. Cell Physiol. 166:256-273. [DOI] [PubMed] [Google Scholar]
- 6.Barlat, I., B. Henglein, A. Plet, N. Lamb, A. Fernandez, F. R. McKenzie, J. Pouyssegur, A. Vie, and J. M. Blanchard. 1995. TGF-β1 and cAMP attenuate cyclin A gene transcription via a cAMP responsive element through independent pathways. Oncogene 11:1309-1318. [PubMed] [Google Scholar]
- 7.Blomberg, I., and I. Hoffmann. 1999. Ectopic expression of Cdc25A accelerates the G1/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol. Cell. Biol. 19:6183-6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortner, D. M., and M. P. Rosenberg. 1995. Overexpression of cyclin A in the mammary glands of transgenic mice results in the induction of nuclear abnormalities and increased apoptosis. Cell Growth Differ. 6:1579-1589. [PubMed] [Google Scholar]
- 9.Burgering, B. M., G. J. Pronk, P. C. van Weeren, P. Chardin, and J. L. Bos. 1993. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 12:4211-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calleja, V., P. R. Enriquez, C. Filloux, P. Peraldi, V. Baron, and E. Van Obberghen. 1997. The effect of cyclic adenosine monophosphate on the mitogen-activated protein kinase pathway depends on both the cell type and the type of tyrosine kinase-receptor. Endocrinology 138:1111-1120. [DOI] [PubMed] [Google Scholar]
- 11.Coats, S., W. M. Flanagan, J. Nourse, and J. M. Roberts. 1996. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science 272:877-880. [DOI] [PubMed] [Google Scholar]
- 12.Connell-Crowley, L., J. W. Harper, and D. W. Goodrich. 1997. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell 8:287-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook, S. J., and F. McCormick. 1993. Inhibition by cAMP of Ras-dependent activation of Raf. Science 262:1069-1072. [DOI] [PubMed] [Google Scholar]
- 14.Cook, S. J., J. Beltman, K. A. Cadwallader, M. McMahon, and F. McCormick. 1997. Regulation of mitogen-activated protein kinase phosphatase-1 expression by extracellular signal-related kinase-dependent and Ca2+-dependent signal pathways in Rat-1 cells. J. Biol. Chem. 272:13309-13319. [DOI] [PubMed] [Google Scholar]
- 15.Cook, S. J., N. Aziz, and M. McMahon. 1999. The repertoire of fos and jun proteins expressed during the G1 phase of the cell cycle is determined by the duration of mitogen-activated protein kinase activation. Mol. Cell. Biol. 19:330-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cospedal, R., M. Lobo, and I. Zachary. 1999. Differential regulation of extracellular signal-regulated protein kinases (ERKs) 1 and 2 by cAMP and dissociation of ERK inhibition from anti-mitogenic effects in rabbit vascular smooth muscle cells. Biochem. J. 342:407-414. [PMC free article] [PubMed] [Google Scholar]
- 17.Davies, H., G. R. Bignell, C. Cox, P. Stephens, S. Edkins, S. Clegg, J. Teague, H. Woffendin, M. J. Garnett, W. Bottomley, N. Davis, E. Dicks, R. Ewing, Y. Floyd, K. Gray, S. Hall, R. Hawes, J. Hughes, V. Kosmidou, A. Menzies, C. Mould, A. Parker, C. Stevens, S. Watt, S. Hooper, R. Wilson, H. Jayatilake, B. A. Gusterson, C. Cooper, J. Shipley, D. Hargrave, K. Pritchard-Jones, N. Maitland, G. Chenevix-Trench, G. J. Riggins, D. D. Bigner, G. Palmieri, A. Cossu, A. Flanagan, A. Nicholson, J. W. Ho, S. Y. Leung, S. T. Yuen, B. L. Weber, H. F. Seigler, T. L. Darrow, H. Paterson, R. Marais, C. J. Marshall, R. Wooster, M. R. Stratton, and P. A. Futreal. 2002. Mutations of the BRAF gene in human cancer. Nature 417:949-954. [DOI] [PubMed] [Google Scholar]
- 18.DeGregori, J., G. Leone, K. Ohtani, A. Miron, and J. R. Nevins. 1995. E2F-1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev. 9:2873-2887. [DOI] [PubMed] [Google Scholar]
- 19.Dhillon, A. S., C. Pollock, H. Steen, P. E. Shaw, H. Mischak, and W. Kolch. 2002. Cyclic AMP-dependent kinase regulates Raf-1 kinase mainly by phosphorylation of serine 259. Mol. Cell. Biol. 22:3237-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumaz, N., Y. Light, and R. Marais. 2002. Cyclic AMP blocks cell growth through Raf-1-dependent and Raf-1-independent mechanisms. Mol. Cell. Biol. 22:3717-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 22.Enserink, J. M., A. E. Christensen, J. de Rooij, M. van Triest, F. Schwede, H. G. Genieser, S. O. Doskeland, J. L. Blank, and J. L. Bos. 2002. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4:901-906. [DOI] [PubMed] [Google Scholar]
- 23.Erhardt, P., J. Troppmair, U. R. Rapp, and G. M. Cooper. 1995. Differential regulation of Raf-1 and B-Raf and Ras-dependent activation of mitogen-activated protein kinase by cyclic AMP in PC12 cells. Mol. Cell. Biol. 15:5524-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faure, M., and H. R. Bourne. 1995. Differential effects on cAMP on the MAP kinase cascade: evidence for a cAMP-insensitive step that can bypass Raf-1. Mol. Biol. Cell 6:1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gagelin, C., D. Toru-Delbauffe, J. M. Gavaret, and M. Pierre. 1999. Effects of cyclic AMP on components of the cell cycle machinery regulating DNA synthesis in cultured astrocytes. J. Neurochem. 73:1799-1805. [DOI] [PubMed] [Google Scholar]
- 26.Galaktionov, K., X. Chen, and D. Beach. 1996. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature 382:511-517. [DOI] [PubMed] [Google Scholar]
- 27.Garner, A. P., C. R. Weston, D. E. Todd, K. Balmanno, and S. J. Cook. 2002. ΔMEKK3:ER* activation induces a p38 α/β2-dependent cell cycle arrest at the G2 checkpoint. Oncogene 21:8089-8104. [DOI] [PubMed] [Google Scholar]
- 28.Gartel, A. L., E. Goufman, S. G. Tevosian, H. Shih, A. S. Yee, and A. L. Tyner. 1998. Activation and repression of p21(WAF1/CIP1) transcription by RB binding proteins. Oncogene 17:3463-3469. [DOI] [PubMed] [Google Scholar]
- 29.Geng, Y., W. Whoriskey, M. Y. Park, R. T. Bronson, R. H. Medema, T. Li, R. A. Weinberg, and P. Sicinski. 1999. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell 97:767-777. [DOI] [PubMed] [Google Scholar]
- 30.Graves, L. M., K. E. Bornfeldt, E. W. Raines, B. C. Potts, S. G. Macdonald, R. Ross, and E. G. Krebs. 1993. Protein kinase A antagonizes platelet-derived growth factor-induced signalling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc. Natl. Acad. Sci. USA 90:10300-10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu, Y., J. Rosenblatt, and D. O. Morgan. 1992. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 11:3995-4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafner, S., H. S. Adler, H. Mischak, P. Janosch, G. Heidecker, A. Wolfman, S. Pippig, M. Lohse, M. Ueffing, and W. Kolch. 1994. Mechanism of inhibition of Raf-1 by protein kinase A. Mol. Cell. Biol. 14:6696-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoang, A. T., K. J. Cohen, J. F. Barrett, D. A. Bergstrom, and C. V. Dang. 1994. Participation of cyclin A in Myc-induced apoptosis. Proc. Natl. Acad. Sci. USA 91:6875-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hordijk, P. L., I. Verlaan, K. Jalink, E. J. van Corven, and W. H. Moolenaar. 1994. cAMP abrogates the p21ras-mitogen-activated protein kinase pathway in fibroblasts. J. Biol. Chem. 269:3534-3538. [PubMed] [Google Scholar]
- 35.Houslay, M. D., and W. Kolch. 2000. Cell-type specific integration of cross-talk between extracellular signal-regulated kinase and cAMP signaling. Mol. Pharmacol. 58:659-668. [PubMed] [Google Scholar]
- 36.Jinno, S., K. Suto, A. Nagata, M. Igarashi, Y. Kanaoka, H. Nojima, and H. Okayama. 1994. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 13:1549-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato, J. Y., M. Matsuoka, K. Polyak, J. Massague, and C. J. Sherr. 1994. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79:487-496. [DOI] [PubMed] [Google Scholar]
- 38.Kerkhoff, E., and U. R. Rapp. 1998. Cell cycle targets of Ras/Raf signalling. Oncogene 17:1457-1462. [DOI] [PubMed] [Google Scholar]
- 39.Kikukawa, M., Y. Okamoto, H. Fukui, and H. Nakano. 1997. Effect of dibutyryl cyclic AMP on the cyclin-dependent kinase inhibitor p27Kip1 in the human hepatoma cells PLC/PRF/5. Anticancer Res. 17:3287-3291. [PubMed] [Google Scholar]
- 40.Kim, T. Y., W. I. Kim, R. E. Smith, and E. D. Kay. 2001. Role of p27(Kip1) in cAMP- and TGF-beta2-mediated antiproliferation in rabbit corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 42:3142-3149. [PubMed] [Google Scholar]
- 41.Kolch, W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289-305. [PMC free article] [PubMed] [Google Scholar]
- 42.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 43.L'Allemain, G., J. N. Lavoie, N. Rivard, V. Baldin, and J. Pouyssegur. 1997. Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle re-entry in fibroblasts Oncogene 14:1981-1990. [DOI] [PubMed] [Google Scholar]
- 44.Lee, T. H., L. Y. Chuang, and W. C. Hung. 2000. Induction of p21WAF1 expression via Sp1-binding sites by tamoxifen in estrogen receptor-negative lung cancer cells. Oncogene 19:3766-3773. [DOI] [PubMed] [Google Scholar]
- 45.Lee, Y. H., J. S. Park, C. H. Park, and S. K. Lee. 1998. Synergistic effect of cyclic AMP and insulin on the expression of cyclin A gene in Swiss 3T3 cells. Biochem. Biophys. Res. Commun. 244:843-848. [DOI] [PubMed] [Google Scholar]
- 46.Lerner, A., D. H. Kim, and R. Lee. 2000. The cAMP signaling pathway as a therapeutic target in lymphoid malignancies. Leuk. Lymphoma 37:39-51. [DOI] [PubMed] [Google Scholar]
- 47.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 48.Littlewood, T. D., D. C. Hancock, P. S. Danielian, M. G. Parker, and G. I. Evan. 1995. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 23:1686-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lomazzi, M., M. C. Moroni, M. R. Jensen, E. Frittoli, and K. Helin. 2002. Suppression of the p53- or pRB-mediated G1 checkpoint is required for E2F-induced S-phase entry. Nat. Genet. 31:190-194. [DOI] [PubMed] [Google Scholar]
- 50.Magnaldo, I., J. Pouyssegur, and S. Paris. 1989. Cyclic AMP inhibits mitogen-induced DNA synthesis in hamster fibroblasts, regardless of the signalling pathway involved. FEBS Lett. 245:65-69. [DOI] [PubMed] [Google Scholar]
- 51.Marshall, C. J. 1999. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr. Opin. Cell Biol. 11:732-736. [DOI] [PubMed] [Google Scholar]
- 52.McKenzie, F. R., and J. Pouyssegur. 1996. cAMP-mediated growth inhibition in fibroblasts is not mediated via mitogen-activated protein (MAP) kinase (ERK) inhibition. cAMP-dependent protein kinase induces a temporal shift in growth factor-stimulated MAP kinases. J. Biol. Chem. 271:13476-13483. [DOI] [PubMed] [Google Scholar]
- 53.Mischak, H., T. Seitz, P. Janosch, M. Eulitz, H. Steen, M. Schellerer, A. Philipp, and W. Kolch. 1996. Negative regulation of Raf-1 by phosphorylation of serine 621. Mol. Cell. Biol. 16:5409-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison, D. K., G. Heidecker, U. R. Rapp, and T. D. Copeland. 1993. Identification of the major phosphorylation sites of the Raf-1 kinase. J. Biol. Chem. 268:17309-17316. [PubMed] [Google Scholar]
- 55.Nilsson, I., and I. Hoffmann. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4:107-114. [DOI] [PubMed] [Google Scholar]
- 56.Palmero, I., and G. Peters. 1996. Perturbation of cell cycle regulators in human cancer. Cancer Surv. 27:351-367. [PubMed] [Google Scholar]
- 57.Pastan, I. H., G. S. Johnson, and W. B. Anderson. 1975. Role of cyclic nucleotides in growth control. Annu. Rev. Biochem. 44:491-522. [DOI] [PubMed] [Google Scholar]
- 58.Rao, S., J. Gray-Bablin, T. W. Herliczek, and K. Keyomarsi. 1999. The biphasic induction of p21 and p27 in breast cancer cells by modulators of cAMP is posttranscriptionally regulated and independent of the PKA pathway. Exp. Cell Res. 252:211-223. [DOI] [PubMed] [Google Scholar]
- 59.Robinson, M. J., and M. H. Cobb. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180-186. [DOI] [PubMed] [Google Scholar]
- 60.Roger, P. P., D. Christophe, J. E. Dumont, and I. Pirson. 1997. The dog thyroid primary culture system: a model of the regulation of function, growth and differentiation expression by cAMP and other well-defined signaling cascades. Eur. J. Endocrinol. 137:579-598. [DOI] [PubMed] [Google Scholar]
- 61.Samuels, M. L., M. J. Weber, J. M. Bishop, and M. McMahon. 1993. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol. Cell. Biol. 13:6241-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santoni-Rugiu, E., J. Falck, N. Mailand, J. Bartek, and J. Lukas. 2000. Involvement of Myc activity in a G(1)/S-promoting mechanism parallel to the pRb/E2F pathway. Mol. Cell. Biol. 20:3497-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sewing, A., C. Burger, S. Brusselbach, C. Schalk, F. C. Lucibello, and R. Muller. 1993. Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J. Cell Sci. 104:545-555. [DOI] [PubMed] [Google Scholar]
- 64.Schmitt, J. M., and P. J. S. Stork. 2001. Cyclic AMP-mediated inhibition of cell growth requires the small G protein Rap1. Mol. Cell. Biol. 21:3671-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaulian, E., and M. Karin. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131-E136. [DOI] [PubMed] [Google Scholar]
- 66.Sidovar, M. F., P. Kozlowski, J. W. Lee, M. A. Collins, Y. He, and L. M. Graves. 2000. Phosphorylation of serine 43 is not required for inhibition of c-Raf kinase by the cAMP-dependent protein kinase. J. Biol. Chem. 275:28688-28694. [DOI] [PubMed] [Google Scholar]
- 67.Solomon, M. J., M. Glotzer, T. H. Lee, M. Philippe, and M. W. Kirschner. 1990. Cyclin activation of p34cdc2. Cell 63:1013-1024. [DOI] [PubMed] [Google Scholar]
- 68.Squires, M. S., P. M. Nixon, and S. J. Cook. 2002. Cell cycle arrest by PD184352 requires inhibition of the extracellular signal-regulated kinases (ERK) 1/2 but not ERK5/BMK1. Biochem. J. 366:673-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stork, P. J. S., and J. M. Schmitt. 2002. Crosstalk between cAMP and MAP kinase signalling in the regulation of cell proliferation. Trends Cell Biol. 12:258-266. [DOI] [PubMed] [Google Scholar]
- 70.Tortora, G., F. Ciardiello, S. Ally, T. Clair, D. S. Salomon, and Y. S. Cho-Chung. 1989. Site-selective 8-chloroadenosine 3′,5′-cyclic monophosphate inhibits transformation and transforming growth factor alpha production in Ki-ras-transformed rat fibroblasts. FEBS Lett. 242:363-367. [DOI] [PubMed] [Google Scholar]
- 71.Treinies, I., H. F. Paterson, S. Hooper, R. Wilson, and C. J. Marshall. 1999. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal to stimulate DNA synthesis. Mol. Cell. Biol. 19:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vadiveloo, P. K., E. L. Filonzi, H. R. Stanton, and J. A. Hamilton. 1997. G1 phase arrest of human smooth muscle cells by heparin, IL-4 and cAMP is linked to repression of cyclin D1 and cdk2. Atherosclerosis 133:61-69. [DOI] [PubMed] [Google Scholar]
- 73.Van Keymeulen, A., J. Bartek, J. E. Dumont, and P. P. Roger. 1999. Cyclin D3 accumulation and activity integrate and rank the comitogenic pathways of thyrotropin and insulin in thyrocytes in primary culture. Oncogene 18:7351-7359. [DOI] [PubMed] [Google Scholar]
- 74.Vigo, E., H. Muller, E. Prosperini, G. Hateboer, P. Cartwright, M. C. Moroni, and K. Helin. 1999. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol. Cell. Biol. 19:6379-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vossler, M. R., H. Yao, R. D. York, M. G. Pan, C. S. Rim, and P. J. Stork. 1997. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89:73-82. [DOI] [PubMed] [Google Scholar]
- 76.Weston, C. R., K. Balmanno, C. Chalmers, K. Hadfield, S. A Molton, R. Ley, E. F. Wagner, and S. J. Cook. 2003. Activation of ERK1/2 by ΔRaf-1:ER* represses Bim expression independently of the JNK or PI3K pathways. Oncogene 22:1281-1293. [DOI] [PubMed] [Google Scholar]
- 77.Whitehurst, C. E., O. Hakime, J. T. Bruder, U. R. Rapp, and T. D. Geppert. 1995. The MEK kinase activity of the catalytic domain of RAF-1 is regulated independently of Ras binding in T cells. J. Biol. Chem. 270:5594-5599. [DOI] [PubMed] [Google Scholar]
- 78.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 79.Williamson, E. A., G. S. Burgess, P. Eder, S. Litz-Jackson, and H. S. Boswell. 1997. Cyclic AMP negatively controls c-myc transcription and G1 cell cycle progression in p210 BCR-ABL transformed cells: inhibitory activity exerted through cyclin D1 and cdk4. Leukemia 11:73-85. [DOI] [PubMed] [Google Scholar]
- 80.Woods, D., D. Parry, H. Cherwinski, E. Bosch, E. Lees, and M. McMahon. 1997. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17:5598-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu, J., P. Dent, T. Jelinek, A. Wolfman, M. J. Weber, and T. W. Sturgill. 1993. Inhibition of the EGF-stimulated MAP kinase signalling pathway by adenosine 3′,5′-monophosphate. Science 262:1065-1069. [DOI] [PubMed] [Google Scholar]
- 82.Zhang, H. S., M. Gavin, A. Dahiya, A. A. Postigo, D. Ma, R. X. Luo, J. W. Harbour, and D. C. Dean. 2000. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell 101:79-89. [DOI] [PubMed] [Google Scholar]