Abstract
Transforming growth factor-βs (TGF-β) are multifunctional proteins capable of either stimulating or inhibiting mitosis, depending on the cell type. These diverse cellular responses are caused by stimulating a single receptor complex composed of type I and type II receptors. Using a chimeric receptor model where the granulocyte/monocyte colony-stimulating factor receptor ligand binding domains are fused to the transmembrane and cytoplasmic signaling domains of the TGF-β type I and II receptors, we wished to describe the role(s) of specific amino acid residues in regulating ligand-mediated endocytosis and signaling in fibroblasts and epithelial cells. Specific point mutations were introduced at Y182, T200, and Y249 of the type I receptor and K277 and P525 of the type II receptor. Mutation of either Y182 or Y249, residues within two putative consensus tyrosine-based internalization motifs, had no effect on endocytosis or signaling. This is in contrast to mutation of T200 to valine, which resulted in ablation of signaling in both cell types, while only abolishing receptor down-regulation in fibroblasts. Moreover, in the absence of ligand, both fibroblasts and epithelial cells constitutively internalize and recycle the TGF-β receptor complex back to the plasma membrane. The data indicate fundamental differences between mesenchymal and epithelial cells in endocytic sorting and suggest that ligand binding diverts heteromeric receptors from the default recycling pool to a pathway mediating receptor down-regulation and signaling.
INTRODUCTION
Transforming growth factor-βs (TGF-β) control a variety of cellular processes as diverse as mitotic inhibition or stimulation (Massagué, 1996; Moses and Serra, 1996). It is unclear how the same receptor complex can mediate such different cellular phenotypes. The most commonly accepted receptor model for TGF-β action consists of a heteromeric complex composed of type I and type II receptors (Wrana et al., 1992, 1994). Once associated, the type I receptor becomes phosphorylated primarily within the juxtamembrane GS domain (amino acids 185–192) by the constitutive serine/threonine kinase activity of the type II receptor (Franzén et al., 1995; Wieser et al., 1995). Phosphorylation of the GS domain is proposed to activate the type I receptor, resulting in signal propagation to downstream effector molecules (Massagué, 1998). In addition, specific residues in nearby regions have also been suggested to have both positive and negative regulatory functions (Wieser et al., 1995; Charng et al., 1996; Souchelnytskyi et al., 1996; Doréet al., 1998). For instance, threonine 200 has been shown to have a fundamental role in mediating all aspects of TGF-β signaling (Wieser et al., 1995), whereas replacement of threonine 204 with an acidic residue, such as aspartate, can generate a type I receptor capable of signaling (albeit to a lesser extent) independent of ligand or an associated type II receptor (Wieser et al., 1995; Charng et al., 1996; Luo and Lodish, 1996). What makes these data most intriguing is that none of the aforementioned residues have been shown to be phosphorylated (Heldin et al., 1997). As such, the mechanism(s) through which they might act is still enigmatic. Additionally, there have been no reports as to whether these amino acids might modulate TGF-β receptor endocytic activity.
Once ligand binding occurs, typically a growth factor receptor is removed from the plasma membrane by an endocytic process, resulting in receptor down-regulation. Endocytosis consists of several interconnected pathways, including the initial internalization of receptors, sorting endosomes, recycling of receptors back to the plasma membrane, and/or shunting to proteosome/lysosomes for degradation. Previous reports regarding the TGF-β receptor have been contradictory and ranged from no significant down-regulation (Massagué, 1985; Wakefield et al., 1987) to a 50% decrease in surface binding (Frolik et al., 1984). The discrepancy is likely due to the existence of several different TGF-β receptor complexes on the plasma membrane with distinct endocytic activities (Chen and Derynck, 1994; Henis et al., 1994; Anders et al., 1997; Doréet al., 1998; Gilboa et al., 1998). Using a chimeric receptor model consisting of the ligand binding domain of the granulocyte/monocyte colony-stimulating factor (GM-CSF) α or β receptor fused to the transmembrane and cytoplasmic tail of the type I or type II TGF-β receptor, we have shown (in fibroblasts) that only heteromeric receptors (type I/II interactions) are down-regulated, whereas homomeric receptors (types I/I and II/II interactions) were proposed to recycle back to the plasma membrane following initial internalization (Anders and Leof, 1996; Anders et al., 1997).
Although TGF-β receptors appear to be endocytosed through a clathrin-mediated process (Anders et al., 1997), the mechanism(s) regulating the endocytic event(s) remains unknown. In the present report we wished to test whether specific amino acid residues in TGF-β type I and type II receptors known to modulate signaling, as well as key residues within motifs that regulate endocytosis in other receptor systems, might also control endocytosis of the TGF-β receptor complex. The data from the present study indicate 1) that TGF-β receptor endocytic activity in both fibroblasts and epithelial cells appears to be controlled independently of type I receptor tyrosine-based sorting elements; 2) although type I receptor phosphorylation plays an essential role in regulating TGF-β receptor signaling in fibroblasts and epithelial cells, it is only obligate for receptor endocytosis in fibroblasts, delineating fundamental differences in the intracellular recognition of the receptor complex between the two cell types; 3) TGF-β receptors constitutively recycle in the absence of ligand; and 4) ligand binding diverts heteromeric receptor complexes from the default recycling pathway to one associated with down-regulation and signaling.
MATERIALS AND METHODS
Materials
Recombinant human GM-CSF was generously provided by DNAX Research Institute (Palo Alto, CA), whereas recombinant human TGF-β2 was purchased from R&D Systems (Minneapolis, MN). Cell culture media, horse serum, and geneticin (G418 sulfate) were purchased from Life Technologies (Gaithersburg, MD). Fetal bovine serum (FBS) was obtained from Summit (Fort Collins, CO) and hygromycin B was purchased from Boehringer-Mannheim (Indianapolis, IN). Monensin was purchased from Sigma Chemical Co. (St. Louis, MO), whereas antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell Culture
Parental AKR-2B and NIH-3T3 fibroblasts were maintained in DMEM supplemented with 5% (vol/vol) FBS, whereas parental Mv1Lu and R1B (Mv1Lu clone lacking type I TGF-β receptors; Laiho et al., 1990) mink lung epithelium, Madin-Darby canine kidney (MDCK), and NRK-49F cultures were maintained in DMEM supplemented with 10% (vol/vol) FBS. Chimeric receptor expressing clones were constructed in a two-step process with the cDNA constructs described previously (Anders and Leof, 1996). Initially, a wild-type chimeric βI or βII receptor was transfected into each cell line. The designation βI or βΙΙ refers to the ligand binding domain of the GM-CSF β receptor coupled to the transmembrane and cytoplasmic domains of the TGF-β type I or type II receptor, respectively. Cells were plated at 105 cells/well in six-well dishes 24 h before transfection. Cells were washed with serum-free DMEM and incubated in 2 ml of DMEM for 10 min before transfection with a mixture of 2 μg of plasmid and 4 μl of TransIt-LT2 (Pan Vera, Madison, WI) in a final volume of 100 μl of Opti-MEM for 8 h at 37°C. Medium was replaced with 10% FBS/DMEM for 16 h and then changed to selection medium (5% FBS/DMEM supplemented with 300 μg/ml hygromycin B for AKR-2B and NIH-3T3 or 10% FBS/DMEM 300 μg/ml hygromycin for Mv1Lu, MDCK and NRK-49F) for 24 h before trypsinization and replating at 1:40 dilution. Fourteen days posttransfection isolated colonies were expanded. Clones were screened by fluorescent activated cell sorting (FACS) for plasma membrane expression of the chimeric receptor as previously described (Anders and Leof, 1996). One representative clone was chosen for the second transfection based upon high membrane expression of the chimeric receptor and normal induction of plasminogen activator inhibitor protein-1 (PAI-1, fibroblasts) or normal inhibition of thymidine incorporation in response to TGF-β2 (epithelial cells).
The second transfection used mutant αI or αII chimeric receptors (ligand binding domain from GM-CSF α receptor fused to the transmembrane and cytoplasmic region of the TGF-βΙ or II receptor) to produce the chimeric high-affinity ligand-binding α/β complex. Chimeric β receptor-expressing clones were plated at 7.5 × 104 cells in six-well plates and transfected with 2 μg of a mutant α receptor plasmid in TransIt-LT2 and Opti-MEM. After recovery, cells were placed in selection medium (5% FBS/DMEM or 10%FBS/DMEM supplemented with 600 μg/ml geneticin and 350 μg/ml hygromycin B) for 24 h before 1:40 dilution.
Site-directed Mutagenesis of Chimeric cDNA
Chimeric αΙ receptor cDNA was mutated at amino acid threonine 200 to valine by using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA) and mutagenic primers CAGGTTTACCATTGCTTGTTCAGAGAgtATTGCGAGAACTATTGTG and CACAATAGTTCTCGCAATTacTCTCTGAACAAGCAATGGTAAACCTG, where the lowercase letters indicate the base changes necessary to introduce the appropriate amino acid change. The chimeric αI receptor containing the tyrosine to serine mutation at position 182 (Y182S) was generated with mutagenic primers CGTTGAAAGACTTAATTTcTGATATGACAACGTCAGG and CCTGACGTTGTCA-TATCAgAAATTAAGTCTTTCAACG, whereas the Y249S construct used the primers GGCAGAGATTTcTCAAACTGTAATG and CATTACAGTTTGAgAAATCTCTGCC. Introduction of K277R and P525L mutations, which inhibit type II receptor kinase activity, into the chimeric αII constructs was previously described (Anders et al., 1998). These constructs were transfected into MB-102 parental Mv1Lu cells and clones selected. Mutant constructs were verified by automated DNA sequencing and ligated into the eukaryotic expression vectors pNa at the SalI and HindIII sites for αI and αII constructs and into pHa at the SalI or XbaI and BamHI sites for the βI or βII constructs, respectively.
Receptor Function and Expression
Receptor binding assays were used to determine expression of chimeric receptors on the plasma membrane, as described previously (Anders and Leof, 1996; Doréet al., 1998). Functional analysis of the chimeric receptors in AKR-2B clones involved both the induction of endogenous PAI-1 protein and transiently transfected PAI-1 luciferase (3TP-Lux) reporter gene activity by GM-CSF, through the chimeric receptors, and TGF-β2, through the endogenous TGF-β receptor (Anders and Leof, 1996). Representative cell lines from each transfection group chosen for further analysis were Y182S clone 1, T200V clones 10 and 12, and Y249S clone 7. Functional analysis of Mv1Lu clones involved inhibition of [3H]thymidine incorporation in the absence or presence of 10 ng/ml GM-CSF or TGF-β2. Representative clones chosen from each transfection group were wild-type receptor clones 9 and 18; Y182S clones 1, 4, and 9; T200V clones 2, 7, and 13; Y249S clones 8, 16, and 22; K277R clones 102-1, -3, and -18, 117-10 and -15, 126-1 and -12; and P525L clones 102-5, -10, and -13, 117-7 and -9, 126-2 and -5.
Endocytosis
The endocytic response to ligand binding was performed as previously described (Anders et al., 1997; Doréet al., 1998). Briefly, to determine receptor down-regulation, cells were incubated at 37°C with 10 ng/ml (500 pM) cold GM-CSF for the times indicated. Wells were then washed twice at 4°C with phosphate-buffered saline, pH 3.0, and the remaining surface binding determined by incubating for 2 h at 4°C with 100 pM 125I-GM-CSF alone or in the presence of 25-fold molar excess of cold GM-CSF before cell lysis. A modification of the down-regulation assay was used to assess the effect of monensin on receptor trafficking. Cells were pretreated with 100 μM monensin in 5% FBS/DMEM for 30 min at 37°C. Medium was then replaced with fresh 5% FBS/DMEM/monensin containing 10 ng/ml cold GM-CSF for the indicated times and processed as described above. To describe receptor recycling, 37°C 5% FBS/DMEM/monensin-containing medium was added directly to cells for the indicated times.
RESULTS
Chimeric Receptor Signaling
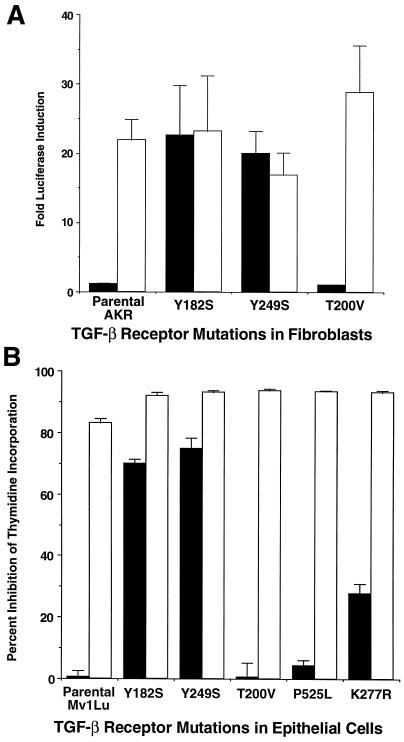
To determine whether TGF-β receptor endocytosis was controlled by specific amino acids in the cytoplasmic tails of the type I or type II receptor, we initially focused our studies on elements within the type I and II receptors previously reported to modulate receptor activity (Wieser et al., 1995; Saitoh et al., 1996; Souchelnytskyi et al., 1996). Point mutations were made in the chimeric αI and αII receptor and clones isolated expressing the generated mutant receptor in the context of a wild-type βII or βI chimera, respectively. Following selection of clones (e.g., similar cell surface high-affinity ligand binding), initial studies determined the effect of specific type I receptor mutations on ligand-dependent induction of endogenous PAI-1 protein (our unpublished data), luciferase activity from a TGF-β–responsive reporter in fibroblasts (3TP-Lux; Figure 1A), and incorporation of [3H]thymidine in epithelial cells (Figure 1B). Although chimeric type I receptors containing the Y182S or Y249S mutation (residues within two putative tyrosine-based internalization consensus sites) induced either luciferase activity or growth inhibition with GM-CSF in fibroblasts and epithelial cells, respectively, clones expressing the T200V chimeric type I receptor were unable to stimulate similar activity despite having a functional signaling pathway as documented by TGF-β activation of endogenous receptors (Figure 1). Consistent with our previously published data in fibroblasts (Anders et al., 1998), chimeric type II receptor kinase mutations (K277R and P525L) were unable to signal in epithelial cells (Figure 1B). The results show that 1) type I receptor tyrosine-based sorting elements are not required for chimeric TGF-β receptor signaling in fibroblasts or epithelial cells; and 2) T200 in the chimeric type I receptor is critical for transcriptional responses in fibroblasts and growth inhibition in epithelial cells.
Figure 1.
Chimeric receptor regulation of TGF-β signaling. Parental cell lines (AKR-2B and Mv1Lu) and clones expressing wild-type chimeric type II TGF-β receptors and the indicated chimeric type I receptor mutation were assayed for the ability to transduce a known TGF-β response when stimulated with either 10 ng/ml GM-CSF (▪), through activation of the mutant chimeric TGF-β receptor, or 10 ng/ml TGF-β2 (□), through the endogenous TGF-β receptors. (A) AKR-2B cells and clones expressing the indicated mutant type I receptor were transiently cotransfected with 3TP-Lux and β-galactosidase control plasmids and treated with GM-CSF or TGF-β2. Data represent the mean fold induction (±SE) of luciferase by TGF-β2 or GM-CSF from two experiments on clones Y182S-1, Y249S-7, and T200V-10 and -12. Fold induction is corrected for β-galactosidase expression and then calculated relative to unstimulated basal expression (5% FBS/DMEM) by the same clone during each experiment. (B) Inhibition of thymidine incorporation was determined in parental Mv1Lu cells and clones expressing mutant chimeric receptors following 20 h of treatment with GM-CSF or TGF-β. The results represent the mean (±SE) of triplicate experiments from parental Mv1Lu cells and three Y182S, T200V, Y249S, K277R, and P525L mutant receptor expressing clones as described in MATERIALS AND METHODS. Data are presented as percentage of inhibition of thymidine incorporation compared with untreated cycling cells of the same clone.
Tyrosine-based Endocytosis of Type I Receptor
Because TGF-β receptor down-regulation in fibroblasts requires both formation of a type I/II receptor complex and type II receptor kinase activity (Anders et al., 1997, 1998), this suggests that a role of the type I receptor is to provide specific residues/motifs that facilitate receptor endocytosis. One such motif shown to enhance the endocytosis of several receptors is tyrosine-based and can be represented by the sequence Y-polar-polar-hydrophobic (Ohno et al., 1995; Marks et al., 1996; Rapoport et al., 1997). Although identical tyrosine-based motifs seen in other proteins were not found in the type I receptor cytoplasmic region, tyrosines at 182 and 249 were selected based on location to known regulatory regions within the receptor and similarity to previously identified adaptor protein-2 recognition sites (Rapoport et al., 1997). Residue Y182 (sequence YDMT) was chosen due to its proximity to the regulatory GS domain (amino acids 185–192), whereas Y249 (sequence YQTV) for its proximity to the putative ATP binding site (K232) and similarity to the lamp-1 internalization sequence YQTI (Honing et al., 1996). It would not be unexpected that either of these residues would be exposed and interact with the endocytic machinery following GS domain phosphorylation and activation of the type I receptor. To address this possibility, the endocytic activity of chimeric TGF-β receptors containing the Y182S or Y249S mutations in the type I receptor was determined in both fibroblasts and epithelium. As shown in Table 1, although differences were observed in the endocytic rate of fibroblasts and epithelial cells (Doréet al., 1998), mutation of Y182 or Y249 to serine had no adverse effect on ligand-induced down-regulation in either cell type compared with the wild-type heteromeric chimera control (A105 or MB-18). For instance, by 4 h there was an ∼80% decrease in receptor binding in all tested lines (Table 1). Thus, in contrast to that observed for other plasma membrane receptors, endocytosis of chimeric TGF-β receptors is controlled independent of tyrosine-based sorting elements in the type I receptor.
Table 1.
Down-regulation of chimeric receptors is not affected by type I TGF-β receptor tyrosine mutations
| Cell line | Slope | Percent receptor binding at 4 h | n |
|---|---|---|---|
| A105 | −0.661 ± 0.082 | 32.2 ± 2.7 | 3 |
| A-Y182S | −0.664 ± 0.057 | 26.6 ± 5.7 | 3 |
| A-Y249S | −0.962 ± 0.090 | 25.7 ± 4.0 | 3 |
| M-Heteromer | −1.301 ± 0.067 | 17.1 ± 2.3 | 2 |
| M-Y182S | −0.981 ± 0.082 | 18.4 ± 0.8 | 2 |
| M-Y249S | −0.906 ± 0.059 | 26.2 ± 2.1 | 2 |
Decreased surface binding of 125I-GM-CSF was determined for AKR-2B and Mv1Lu clones expressing a wild-type chimeric type II TGF-β receptor and a type I TGF-β receptor with tyrosine to serine mutations at either Y182 or Y249, when treated with 10 ng/ml GM-CSF at 37°C. Down-regulation of the normal mesenchymal (A105) and epithelial (M-Heteromer) heteromer containing wild-type type I and type II chimeric receptors is shown for comparison. The rate of down-regulation was determined from the slope of the line calculated from four data points over the first 60 min. Data for the mesenchymal cells represent 1 AKR-2B clone for each receptor type (A105, Y182S-1, and Y249S-7), whereas the epithelial cell data represent the mean of 2 M-heteromers (MB-9 and -18) and three separate M-Y182S (Y182S-1, -4, and -9) and Y249S (Y249S-8, -16, and -22) mutant clones. Assays from the indicated (n) number of experiments were each performed in duplicate.
Role of Type I Receptor T200 in Down-regulation and Signaling
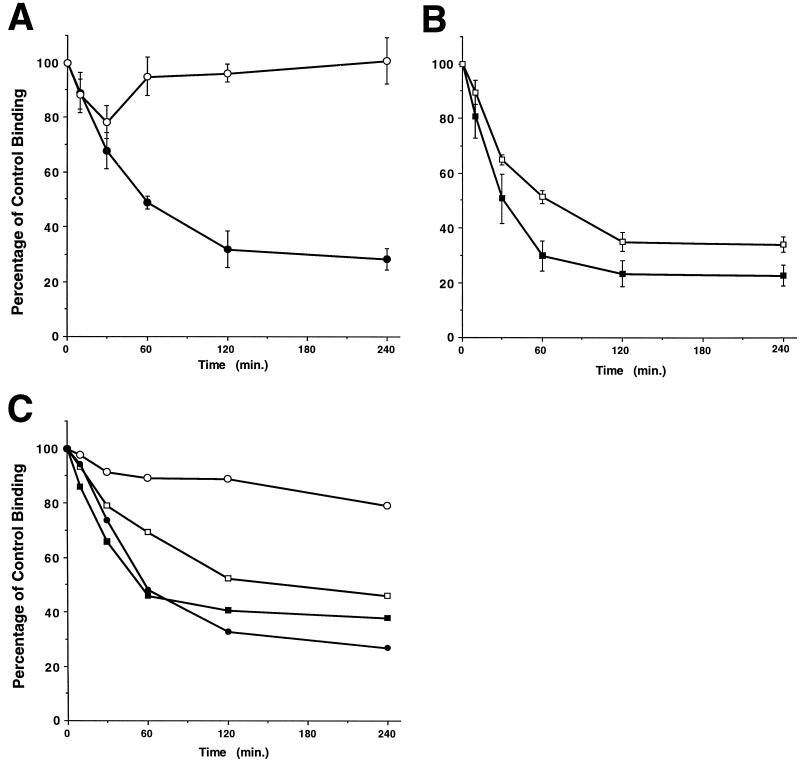
The preceding data indicate that type I receptor tyrosine-based endocytic motifs do not significantly modulate the initial endocytic activity of TGF-β receptors in either fibroblasts or epithelial cells. Because we had previously shown a delineation of endocytosis and signaling in fibroblasts (Anders et al., 1998), we next determined whether other mutations reported to effect receptor signaling (Franzén et al., 1995; Wieser et al., 1995) would modulate receptor endocytosis. Similar to that shown previously in Mv1Lu epithelial cells (Doréet al., 1998), mutation of type I receptor residues S172 or T176 in fibroblasts had no significant effect on either the rate or extent of receptor down-regulation (our unpublished data). In contrast, when T200V clones were assayed for ligand-induced down-regulation of the chimeric receptors, a differential response was observed dependent upon the cell type (Figure 2). Although an initial decrease in receptor binding was observed following 30-min ligand treatment in fibroblasts (Figure 2A), by 60 min binding had returned to 94% of control levels and remained constant over the next 3 h. However, whereas mutation of T200 resulted in a type I TGF-β receptor with impaired endocytic activity in fibroblasts, epithelial clones expressing the same receptor mutation down-regulate similar to wild-type receptors (Figure 2B).
Figure 2.
Down-regulation of chimeric mutant T200V type I receptors. Cell surface binding in response to 10 ng/ml GM-CSF was performed as described in MATERIALS AND METHODS. Each point represents the mean (± SE) for all clones of the series assayed in duplicate from two independent experiments. (A) AKR-2B fibroblast clones T200V-10 and -12 (○) are compared with A105 wild-type control (●). (B) Mv1Lu epithelial clones T200V-2 and -7 (□) are compared with the mean of MB-9 and -18 as wild-type control (▪). (C) Mean percentage of down-regulation of two different epithelial (▪, □) and three fibroblast (●, ○) cell lines. Stable clones expressing the T200V mutation (open symbols) in Mv1Lu (clones 2 and 7) and MDCK (clones 13, 17, 32, and 38) epithelial lines and 3T3-NIH (clones 6, 7, and 9), AKR-2B (clones 10 and 12), and NRK-49F (clones 1 and 3) fibroblast lines were used. Control wild-type expressing clones (closed symbols) used for comparison were Mv1Lu-9 and -18, MDCK-1 and -5, NIH-3T3-2 and -10, AKR-2B clone A105, and NRK-49F-7.
To determine whether this cell type-specific response to the T200V mutation was a common theme in epithelial and mesenchymal cells, we transfected the α1 T200V constructs in conjunction with wild-type β2 constructs into MDCK epithelial cells, as well as NIH-3T3 and NRK-49F fibroblasts (Figure 2C). As noted previously in Mv1Lu and AKR-2B cells, the T200V mutation expressed in other cell lines demonstrated a similar response. The combined down-regulation response of the T200V mutant-expressing clones, in epithelial cells, was similar to that of clones expressing wild-type receptors (54% decrease in surface binding for T200V compared with 62% for wild-type, at 4 h). This is in contrast to fibroblasts in which the T200V mutation significantly inhibited chimeric receptor down-regulation over 4 h. (21% for T200V compared with 74% for wild-type). Thus, the T200V mutation represents the first type I receptor site capable of modulating both signaling and endocytic activity of the TGF-β receptor complex and documents differential endocytic sorting between fibroblasts and epithelial cells.
Role of Type II Receptor Kinase in Endocytosis
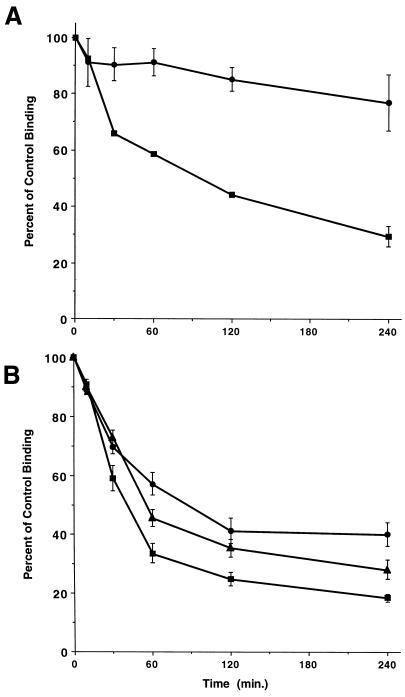
We have previously shown that type II receptor kinase activity is critical for both receptor down-regulation and signaling in fibroblasts (Anders et al., 1998). For instance, cells expressing a wild-type chimeric type I receptor and a kinase-impaired chimeric type II receptor have diminished endocytic activity, whereas cultures expressing a wild-type chimeric type II receptor and a kinase-deficient type I receptor internalize ligand and down-regulate like wild-type receptors (Anders et al., 1998). Because the T200V mutation blocks type I receptor phosphorylation (Wieser et al., 1995), and epithelial cells expressing this receptor mutation down-regulate similar to wild-type receptors (Figure 2), this suggests that the kinase activity of the type II receptor might not be obligate for chimeric TGF-β receptor down-regulation in epithelial cells. As such, we wished to directly assess the role of the type II receptor kinase in regulating endocytosis in Mv1Lu epithelial cells. Consistent with previously published data (Anders et al., 1998), a mutation (P525L) in the chimeric type II receptor that alters the ability of the receptor to transphosphorylate dramatically reduces receptor down-regulation in fibroblasts (Figure 3A). However, when the same mutation or the K277R mutation in the ATP binding site was expressed in epithelial cells, a distinct endocytic response was observed. For instance, although there was a slight effect on the extent of down-regulation relative to wild-type, the mutant receptor complex still decreased binding 60–70% following addition of ligand (Figure 3B). Thus, the data indicate a fundamental difference for type I receptor phosphorylation in the recognition and processing of the TGF-β receptor complex in fibroblasts and epithelial cells.
Figure 3.
Down-regulation of type II kinase mutant receptors depends upon cell type. Specific surface binding of 125I-GM-CSF was determined in clones expressing a wild-type chimeric type I TGF-β receptor and point mutations in the type II TGF-β receptor at either K277 (▴) or P525 (●), when treated with 10 ng/ml GM-CSF at 37°C for the indicated times. Down-regulation of the normal heteromer (▪) containing wild-type type I and type II chimeric receptors is shown for comparison. (A) Data represent the mean (±SE) for each AKR-2B clone (wild-type A105 and P525L-13 and -22) assayed in duplicate from three separate experiments. (B) Data represent the mean (±SE) for each Mv1Lu clone (wild-type MB-9 and -18; K277R 102-1, -3, and -18, 117-7 and 9, 126-2 and -5; and P525L 102-5, -10, and -13, 117-10 and –15, and 126-1 and -12) assayed in duplicate from two separate experiments.
Receptor Recycling
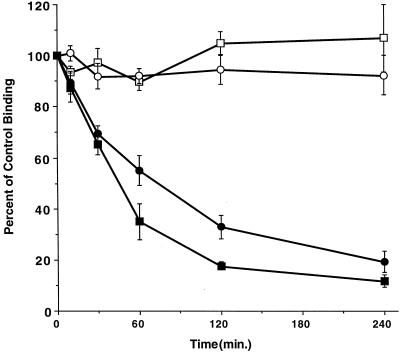
The T200V mutant receptor expressing AKR-2B clones fail to decrease plasma membrane receptor number in the presence of ligand, despite an initial decrease similar to wild-type receptors (Figure 2). This biphasic response suggests the receptors initially internalize, and then are recycled back to the cell surface in fibroblasts. To determine whether the T200V mutant receptor complex is in fact recycled, ligand-induced down-regulation was performed in the presence of monensin to inhibit vesicular trafficking to the plasma membrane. As shown in Figure 4, addition of monensin resulted in an approximate 85% decrease in the surface binding of both the T200V mutant and control wild-type fibroblasts. This is contrasted by the <10% decrease in T200V receptor binding observed in the absence of monensin. These results (Figures 2A and 4) indicate that in the presence of ligand, the T200V mutant receptors initially engage the endocytic machinery, and then are recycled to the plasma membrane.
Figure 4.
Monensin induced down-regulation of T200V chimeric receptor. Down-regulation assays were performed on AKR-2B clones containing wild-type type I and II chimeric receptors (□) or a wild-type type II and T200V type I mutant (▵) in the presence of 100 μM monensin and 10 ng/ml GM-CSF. The data represent the mean (± SE) of three separate experiments done in duplicate on two independent mutant clones (T200V-10 and -12) and wild-type (A105) control cells. The ligand-induced down-regulation of the T200V clones in the absence of monensin reported in Figure 2 is shown for comparison (▴). Preliminary experiments demonstrated that monensin had no effect on ligand binding and decreased wild-type cell surface receptor binding in a dose-dependant manner, with 100 μM being maximally effective (our unpublished data).
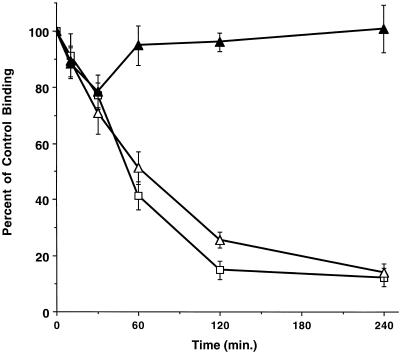
Because the T200V mutation 1) defined a site capable of altering the endocytic response to ligand-activated chimeric TGF-β receptors (Figure 2A); and 2) provided evidence that mutant chimeric TGF-β receptors used a recycling pathway in fibroblasts (Figure 4), it would not be unexpected if wild-type chimeric TGF-β receptors were similarly regulated. To address this question, specific binding in the absence of previous ligand treatment was determined in the presence or absence of monensin in fibroblasts and epithelial cells. Although binding remained constant in the absence of monensin, the addition of monensin to either cell type resulted in a time-dependent loss in cell surface binding (Figure 5). To determine whether the decreased receptor binding resulted from a preferential loss of either the type I or type II receptors, FACS analysis was performed following 1-h monensin treatment and indicated that both type I and II receptors decreased to a similar extent (our unpublished data). Thus, the wild-type chimeric TGF-β receptor complex constitutively recycles in the absence of ligand. These results are consistent with a model whereby ligand binding diverts the receptor complex from this constitutive recycling pathway to one resulting in receptor down-regulation.
Figure 5.
Constitutive internalization of chimeric TGF-β receptors. Ligand-independent decrease of GM-CSF surface binding in the presence (closed symbols) or absence (open symbols) of 100 μM in wild-type chimeric A105 fibroblasts (□, ▪) and MB-18 epithelial cells (●, ○). Each point represents the mean (± SE) of three separate experiments done in duplicate.
DISCUSSION
The present data provide several new findings for understanding the endocytic and signaling activities of TGF-β receptor complexes. First, we provide evidence that potential type I receptor tyrosine-based sorting elements are dispensable for endocytic activity and signaling. Second, a type I receptor point mutation is described (T200V), which defines a fundamental difference by which TGF-β receptors are processed in fibroblasts and epithelial cells. Third, contrary to that observed in fibroblasts, type II receptor kinase activity is not obligatory for TGF-β receptor down-regulation in Mv1Lu mink lung epithelial cells. Fourth, evidence is provided that in the absence of ligand, the default pathway for TGF-β receptors is to internalize and recycle back to the cell surface regardless of cell type. Fifth, it is proposed that rather than promoting receptor internalization, the binding of ligand diverts TGF-β receptors from a constitutive recycling pathway to one resulting in down-regulation and signaling. Together these observations indicate that mesenchymal and epithelial cell types have similar, as well as distinct endocytic sorting mechanisms for the same receptor complex, possibly accounting for some of the diverse cellular responses to TGF-β.
Tyrosine-based Motifs Are Not Involved in Chimeric TGF-β Receptor Endocytosis or Signaling
The endocytic process is regulated through the coordinate interplay of a number of plasma membrane and cytosolic proteins (Mellman, 1996; Mukherjee et al., 1997; Schmid, 1997; McNiven, 1998). Receptor endocytosis is initiated by the recognition of distinct sequence elements within the cytoplasmic (primarily) domain of various membrane receptors that control the internalization process, as well as association with coated pit proteins (Pearse and Robinson, 1990; Trowbridge et al., 1993; Robinson et al., 1996). Once internalized, receptors are shuttled to various intracellular compartments where they are sorted for recycling back to the cell surface or down-regulated through the degradative pathways. Whereas no canonical sequence or receptor domain has been identified, a common tyrosine-based motif found to regulate growth factor receptor internalization consists of Tyr-polar-polar-hydrophobic residues (Chang et al., 1993; Carpentier and McClain, 1995; Lamaze and Schmid, 1995). Two similar sites near regions known to regulate type I receptor activity are Y182 (YDMT) and Y249 (YQTV). When these tyrosines were individually mutated to serine, rather than being inhibitory, chimeric TGF-β receptors were down-regulated as effectively as wild-type receptors in either fibroblasts or epithelial cells (Table 1). Although we cannot conclusively eliminate a potential site at Y284 (YASW) within the type II receptor, the results indicate that type I receptor tyrosine-based motifs do not have as fundamental a role in regulating the endocytic activity of chimeric TGF-β receptors as they do for other growth factor receptors (Chang et al., 1993; Carpentier and McClain, 1995; Lamaze and Schmid, 1995).
T200 Defines a Type I Receptor Site Differentially Regulating Endocytosis in Fibroblasts and Epithelial Cells
Because tyrosine-based internalization signals are not likely involved in TGF-β receptor endocytosis, we next determined whether other type I receptor residues with known regulatory activity would affect the endocytic response. The two questions we wished to address were 1) whether a site known to regulate receptor signaling would also modulate receptor endocytosis; and 2) whether there would be distinct responses dependent upon cell type. To that end, both mesenchymal and epithelial cell lines were isolated expressing a wild-type chimeric type II TGF-β receptor and a chimeric type I receptor with S172A, T176V, or T200V mutations. As was previously shown in Mv1Lu epithelial cells (Doréet al., 1998), S172A and T176V mutations had no effect on either endocytosis or signaling in fibroblasts (our unpublished data). This is contrasted by the T200V mutation that defined a fundamental difference in endocytic control between the cell types. For instance, although T200 had no affect on epithelial cell receptor down-regulation, fibroblasts expressing this mutation were unable to down-regulate following addition of ligand (Figure 2). However, because the down-regulation assay detects cell surface binding and not receptors per se, an alternative possibility was that the differential response observed in fibroblasts and epithelial cells did not reflect a fundamental cell type difference in how the sorting machinery recognizes T200, but a sequestering or inactivating of receptors in epithelial cells such that they were unable to bind ligand. To directly address this question, hemagglutinin-tagged chimeric type I receptors were generated and histological analysis performed. Preliminary results indicated differential plasma membrane localization of the T200V mutant receptor in fibroblasts and epithelial cells following ligand addition in direct support of the down-regulation data (our unpublished data).
The divergent response of fibroblasts and epithelial cells to ligand-induced down-regulation of the T200V heteromeric receptor complex indicates significant differences in early endocytic events between the two cell types. Although epithelial cells process the T200V receptor complex similar to a wild-type heteromer, the down-regulation response of the T200V mutation in fibroblasts suggests that initially internalized receptors are either recycled back to the cell surface or replaced from intracellular stores. To address this question, the sodium ionophore monensin was used to block the recycling of internalized receptors back to the plasma membrane (Gladhaug and Christoffersen, 1988; Smith and Hunt, 1990; Lenferink et al., 1998; Shitara et al., 1998). In the presence of monensin, both wild-type and T200V mutant receptor complexes showed a similar decrease in surface binding over time (Figure 4). Although monensin has been shown to interfere with specific receptor transport to the plasma membrane from the trans-Golgi network (Sanderson et al., 1993; Sato et al., 1993), it is unlikely that the continuous decrease in binding noted with monensin treatment results from a defect in trafficking of newly synthesized receptors because we previously showed that 6–8 h was required to obtain control binding levels following receptor down-regulation (Anders et al., 1997). Thus, this replacement rate would not be sufficient to maintain the stable level of surface binding shown in Figure 2A.
Chimeric TGF-β Receptors Constitutively Recycle
The finding that fibroblasts expressing TGF-β receptors with the T200V mutation underwent recycling following initial internalization indicated 1) a mechanism existed by which receptors could be returned to the cell surface; and 2) TGF-β receptor down-regulation depends upon an internalization motif(s) as well as an intracellular targeting motif(s) that diverts receptors from the recycling pathway. Although previous reports had suggested that TGF-β receptors undergo recycling (Massagué and Kelly, 1986; Sathre et al., 1991), these studies were unable to differentiate the multiple ligand binding species on the cell surface (type II, type I/II, and type III receptors). Because unoccupied EGF receptors are known to constitutively recycle (Gladhaug and Christoffersen, 1988; French et al., 1994), we hypothesized that TGF-β receptors might use a similar endocytic strategy. To address that question, cells expressing chimeric receptors were treated with monensin in the absence of ligand and the effect on subsequent cell surface binding determined. As shown in Figure 5, monensin induced a time-dependant decrease in chimeric TGF-β receptor binding. Because GM-CSF binding to the chimeric receptors requires the formation of an α/β complex (Anders and Leof, 1996), the decreased binding could reflect a loss in only one of the two receptor types. To address that question, FACS analysis was performed and showed that both type I and II receptors were internalized to a similar extent (our unpublished data). This result indicates that neither receptor is preferentially recognized by the internalization machinery. Moreover, no significant difference in the rate of down-regulation was observed in monensin-treated cells, with or without ligand (compare the monensin-treated cultures in Figures 4 and 5 to wild-type control in Figures 2 and 3). Thus, in the absence of ligand wild-type chimeric TGF-β receptors are constitutively internalized and recycled in AKR-2B fibroblasts and Mv1Lu epithelial cells.
Proposed Model for Chimeric TGF-β Receptor Endocytosis in Fibroblasts and Epithelial Cells
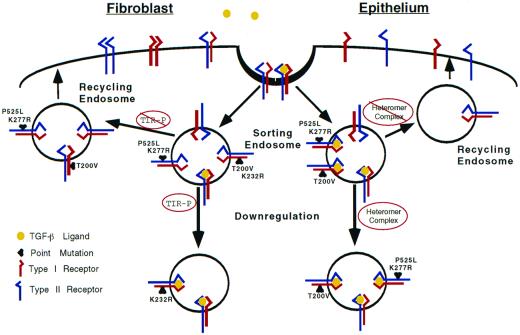
To help define the differences in endocytic control between the epithelial and mesenchymal cell types studied, we propose the following model (Figure 6). TGF-β receptors are constitutively internalized regardless of ligand occupancy. The functional significance of ligand appears to be related to the formation of specific heteromeric complexes recognizable by the endocytic machinery. In fibroblasts, type I receptor phosphorylation is necessary to divert heteromeric receptors from the default recycling pathway. This would account for the lack of down-regulation observed in the type I T200V and type II kinase mutants (Figures 2 and 3). This is in contrast to epithelial cells where the requirement of type I receptor phosphorylation is not as stringent; it is the ligand-occupied heteromeric complex that is recognized. Whether there are other receptor elements or activities regulating receptor trafficking in epithelial cells is presently unknown. In this model we have described common and distinct endocytic mechanisms that are consistent within multiple cell lines. It is unlikely to be a universal theme for all mesenchymal and epithelial cells, because few concepts in biology are absolute. However, a key component of this model is that it lends itself to readily testable questions, including 1) determining whether the results reflect fundamental differences in other mesenchymal and epithelial cells; 2) defining and characterizing endocytic motifs in the type I and/or type II receptor; 3) determining the role(s) of cytosolic proteins such as adaptor protein family members and rab proteins in trafficking of the receptor complex; and 4) determining the relationship between TGF-β receptor signaling and endocytosis and whether this is differentially regulated in fibroblasts and epithelial cells.
Figure 6.
Schematic diagram illustrating potential endocytic routes for heteromeric TGF-β receptor complexes in fibroblasts and epithelial cells. We propose this working model for the trafficking of TGF-β receptor complexes for the two cell types studied. Although ligand is included, it is presently unknown whether it remains receptor bound or dissociates from the receptor complex. Initially, receptors are internalized into a vesicular compartment (sorting endosome), which separates heteromeric and homomeric receptor complexes regardless of cell type (for simplicity only heteromeric complexes are shown). In the absence of ligand, heteromeric receptors are recycled back to the cell surface. A key feature for mesenchymal cells is the phosphorylation status of the type I receptor. Type I receptor phosphorylation is decreased by the P525L or K277R mutations in the type II receptor or the type I receptor T200V mutation; any of these receptor mutations will keep the receptor complex within the recycling pathway. However, if the type I receptor is phosphorylated in fibroblasts, the complex is diverted from the default recycling pathway to one associated with receptor down-regulation. This is in contrast to epithelial cells, which shuttle ligand-bound heteromeric receptor complexes to the down-regulation pathway(s) regardless of phosphorylation status.
ACKNOWLEDGMENTS
We thank Dr. Richard Pagano for helpful discussions and comments. This work was supported by Grants GM-54200 and GM-55816 from the National Institutes of Health.
REFERENCES
- Anders RA, Arline SL, Doré JJE, Leof EB. Distinct endocytic responses of heteromeric and homomeric transforming growth factor β receptors. Mol Biol Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Doré JEJ, Arline SA, Garamszegi N, Leof EB. Differential requirements for type I and type II TGFβ receptor kinase activity in ligand-mediated receptor endocytosis. J Biol Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Anders RA, Leof EB. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-β (TGF-β) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-β signaling. J Biol Chem. 1996;271:21758–21766. doi: 10.1074/jbc.271.36.21758. [DOI] [PubMed] [Google Scholar]
- Carpentier JL, McClain D. Insulin receptor kinase activation releases a constraint maintaining the receptor on microvilli. J Biol Chem. 1995;270:5001–5006. doi: 10.1074/jbc.270.10.5001. [DOI] [PubMed] [Google Scholar]
- Chang CP, Lazar CS, Walsh BJ, Komuro M, Collawn JF, Kuhn LA, Tainer JA, Trowbridge IS, Farquhar MG, Rosenfeld MG, Wiley HS, Gill GN. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993;268:19312–19320. [PubMed] [Google Scholar]
- Charng M-J, Kinnunen P, Hawker J, Brand T, Schneider MD. FKBP-12 recognition is dispensable for signal generation by type I transforming growth factor-β receptors. J Biol Chem. 1996;271:22941–22944. doi: 10.1074/jbc.271.38.22941. [DOI] [PubMed] [Google Scholar]
- Chen RH, Derynck R. Homomeric interactions between type II transforming growth factor-beta receptors. J Biol Chem. 1994;269:22868–22874. [PubMed] [Google Scholar]
- Doré JJE, Jr, Edens M, Garamszegi N, Leof EB. Heteromeric and homomeric transforming growth factor-β receptors show distinct signaling and endocytic responses in epithelial cells. J Biol Chem. 1998;273:31770–31777. doi: 10.1074/jbc.273.48.31770. [DOI] [PubMed] [Google Scholar]
- Franzén P, Heldin CH, Miyazono K. The GS domain of the transforming growth factor-beta type I receptor is important in signal transduction. Biochem Biophys Res Commun. 1995;207:682–689. doi: 10.1006/bbrc.1995.1241. [DOI] [PubMed] [Google Scholar]
- French AR, Sudlow GP, Wiley HS, Lauffenburger DA. Postendocytic trafficking of epidermal growth factor-receptor complexes is mediated through saturable and specific endosomal interactions. J Biol Chem. 1994;269:15749–15755. [PubMed] [Google Scholar]
- Frolik CA, Wakefield LM, Smith DM, Sporn MB. Characterization of a membrane receptor for transforming growth factor-β in normal rat kidney fibroblasts. J Biol Chem. 1984;259:10995–11000. [PubMed] [Google Scholar]
- Gilboa L, Wells RG, Lodish HF, Henis YI. Oligomeric structure of type I and type II transforming growth factor beta receptors - homodimers form in the ER and persist at the plasma membrane. J Cell Biol. 1998;140:767–777. doi: 10.1083/jcb.140.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladhaug IP, Christoffersen T. Rapid constitutive internalization and externalization of epidermal growth factor receptors in isolated rat hepatocytes. Monensin inhibits receptor externalization and reduces the capacity for continued endocytosis of epidermal growth factor. J Biol Chem. 1988;263:12199–12203. [PubMed] [Google Scholar]
- Heldin C-H, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Henis YI, Moustakas A, Lin HY, Lodish HF. The types II and III transforming growth factor-beta receptors form homo-oligomers. J Cell Biol. 1994;126:139–154. doi: 10.1083/jcb.126.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Laiho M, Weis FMB, Massagué J. Concomitant loss of transforming growth factor (TGF)-β receptor types I and II in TGF-β-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990;265:18518–18524. [PubMed] [Google Scholar]
- Lamaze C, Schmid SL. Recruitment of epidermal growth factor receptors into coated pits requires their activated tyrosine kinase. J Cell Biol. 1995;129:47–54. doi: 10.1083/jcb.129.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink AEG, Pinkas-Kramarski R, van de Poll MLM, van Vugt MJH, Klapper LN, Tzahar E, van Zoelen EJJ, Yarden Y. Differential endocytic routing of homo-and hetero-dimeric Erb-B tyrosine kinases confers signaling superiority to receptor heterodimers. EMBO J. 1998;17:3385–3397. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Lodish HF. Signaling by chimeric erythropoietin-TGFβ receptors: homodimerization of the cytoplasmic domain of the type I TGFβ receptor and heterodimerization with the type II receptor are both required for intracellular signal transduction. EMBO J. 1996;15:4485–4496. [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. Type-β transforming growth factor receptors in cells chronically exposed to the ligand. Cancer Cells. 1985;3:73–78. [Google Scholar]
- Massagué J. TGFβ signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massagué J, Kelly B. Internalization of transforming growth factor-β and its receptor in Balb/c 3T3 fibroblasts. J Cell Physiol. 1986;128:216–222. doi: 10.1002/jcp.1041280212. [DOI] [PubMed] [Google Scholar]
- McNiven MA. Dynamin: a molecular motor with pinchase action. Cell. 1998;94:151–154. doi: 10.1016/s0092-8674(00)81414-2. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–626. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Moses HL, Serra R. Regulation of differentiation by TGF-β. Curr Opin Genet Dev. 1996;6:581–586. doi: 10.1016/s0959-437x(96)80087-6. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Pearse BMF, Robinson MS. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Amagasa T, Miyazono K, Takagi M, Ichijo H. Identification of important regions in the cytoplasmic juxtamembrane domain of type I receptor that separate signaling pathways of transforming growth factor-β. J Biol Chem. 1996;271:2769–2775. doi: 10.1074/jbc.271.5.2769. [DOI] [PubMed] [Google Scholar]
- Sanderson CM, McQueen NL, Nayak DP. Sendai virus assembly: M protein binds to viral glycoproteins in transit through the secretory pathway. J Virol. 1993;67:651–663. doi: 10.1128/jvi.67.2.651-663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathre KA, Tsang ML-S, Weatherbee JA, Steer CJ. Binding and internalization of transforming growth factor-β1 by human hepatoma cells: evidence for receptor recycling. Hepatology. 1991;14:287–295. [PubMed] [Google Scholar]
- Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-golgi complex. Exp Cell Res. 1993;207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Shitara Y, Kato Y, Sugiyama Y. Effect of brefeldin A and lysosomotropic reagents on intracellular trafficking of epidermal growth factor and transferrin in Madin-Darby canine kidney epithelial cells. J Control Release. 1998;55:35–43. doi: 10.1016/s0168-3659(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Smith A, Hunt RC. Hemopexin joins transferrin as representative members of a distinct class of receptor-mediated endocytic transport systems. Eur J Cell Biol. 1990;53:234–245. [PubMed] [Google Scholar]
- Souchelnytskyi S, ten Dijke P, Miyazono K, Heldin C-H. Phosphorylation of Ser165 in TGF-Symbol“ § 12type I receptor modulates TG.F-Symbol” § 121-induced cellular responses. EMBO J. 1996;15:6231–6240. [PMC free article] [PubMed] [Google Scholar]
- Trowbridge IS, Collawn JF, Hopkins CR. Signal-dependent membrane protein trafficking in the endocytic pathway. [Review] Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB. Distribution and modulation of the cellular receptor for transforming growth factor-β. J Cell Biol. 1987;105:965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate TGF-beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]