Summary
The important roles that carbohydrates play in biological processes and their potential application in diagnosis, therapeutics, and vaccine development have made them attractive synthetic targets. Despite ongoing challenges, tremendous progresses have been made in recent years for the synthesis of carbohydrates. The chemical glycosylation methods have become more sophisticated and the synthesis of oligosaccharides has become more predictable. Simplified one-pot glycosylation strategy and automated synthesis are increasingly used to obtain biologically important glycans. On the other hand, chemoenzymatic synthesis continues to be a powerful alternative for obtaining complex carbohydrates. This review highlights recent progress in chemical and chemoenzymatic synthesis of carbohydrates with a particular focus on the methods developed for the synthesis of oligosaccharides, polysaccharides, glycolipids, and glycosylated natural products.
Keywords: Carbohydrates, chemical synthesis, chemoenzymatic synthesis, oligosaccharides, polysaccharides
Introduction
Carbohydrates play important roles in many biological processes including cell recognition, cell migration, inflammation, and bacterial and viral infections. The development of efficient and general synthetic routes for oligosaccharides remains a major challenge because of the complexity of their structures. Unlike proteins and nucleic acids, there is no general synthetic route for carbohydrates. Because of the polyhydroxyl nature of carbohydrates, a major challenge in carbohydrate synthesis is to modify a specific hydroxyl group in the presence of others. Nevertheless, a number of powerful methods including metal-catalyzed synthesis, one-pot glycosylation, and automated solid-phase synthesis have been developed to address many challenges in carbohydrate chemistry.
As an alternative to chemical synthesis, chemoenzymatic approaches are often employed for the synthesis of oligosaccharides and glycoconjugates. Chemoenzymatic methods combine the flexibility of chemical synthesis and the efficiency and selectivity of enzymatic methods to obtain diverse complex carbohydrates. The enzymes commonly used are glycosyltransferases, glycosidases, lipases, and their mutants with or without sugar nucleotide biosynthetic enzymes.
As the synthesis and application of glycopeptides/glycoproteins, glycosaminoglycans, and bacterial polysaccharides will be discussed in separate articles in this special issue, this review will summarize recent (within last two years) advance in the method development of chemical and chemoenzymatic synthesis of oligosaccharides, polysaccharides, glycolipids, and glycosylated natural products.
Chemical synthesis of oligosaccharides
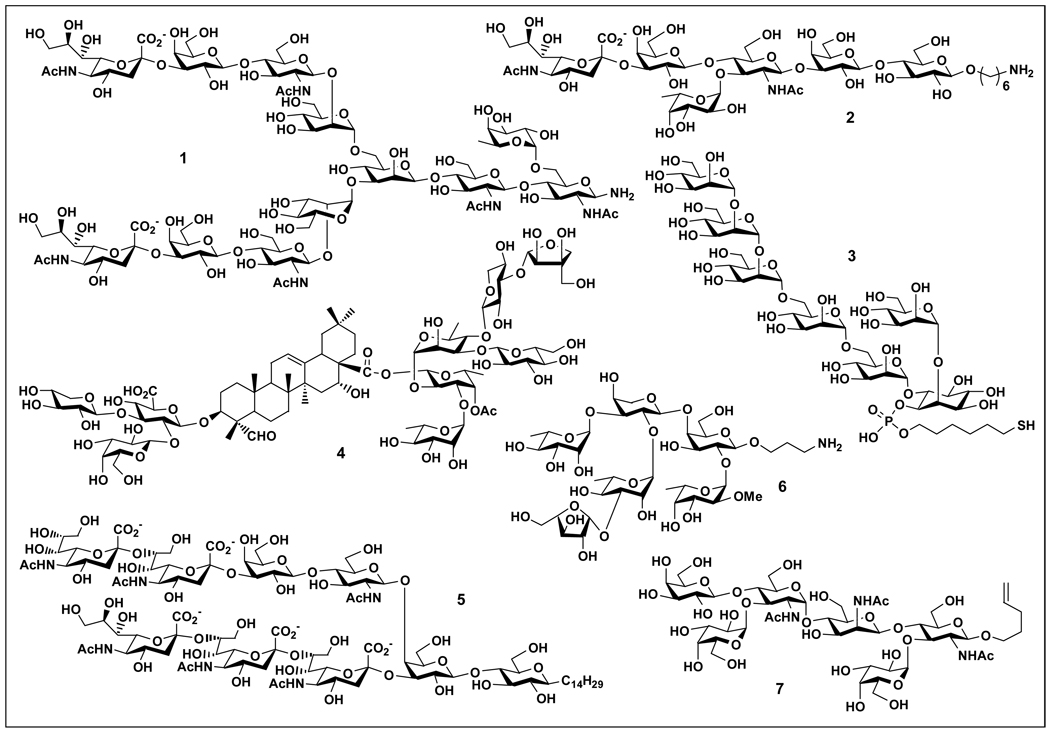
Great progress has been made in the chemical synthesis of complex carbohydrates, as demonstrated by the synthesis of sialic acid rich biantennary N-linked glycan 1 found in human Follicle-stimulating Hormone (hFSH) [1], sialyl Lewisx 2 [2], PIM glycans (such as PIM6 3) from Mycobacterium tuberculosis [3], and the immuno-adjuvant QS-7 Api 4 isolated from the bark of Quillaja saponaria [4] (Figure 1). Due to the complexity of their structures, chemical synthesis of oligosaccharides requires careful planning, including choosing protecting groups, synthetic strategies, and glycosylation methods. Numerous novel methods have been developed and expanded in recent years to meet the demand.
Figure 1.
Structures of complex oligosaccharides obtained by chemical synthesis.
Advances in chemical glycosylation
For decades, chemical sialylation methods suffer from low yields and poor stereoselectivity. However, great progress has been made recently to address these problems. Takahashi and co-workers reported an efficient chemical sialylation method using 5N,4O-carbonyl protected thiosialosides as donors for stereoselective synthesis of GPIc ganglioside oligosaccharide 5 (Figure 1) containing multiple sialic acid residues with both α2,3- and α2,8-sialyl linakges [5]. While an isopropylidene protection group at C7 and C8 hydroxyl groups in the 5N,4O-carbonyl protected thiosialoside donor was an excellent donor for the formation of Siaα2,3Gal linkage, di-O-chloroacetyl protection group at C7 and C8 hydroxyl groups in the donor was preferred for the formation of Siaα2,8Sia linkages [5]. On the other hand, fully protected thiosialoside donor with a 5N,4O-carbonyl group and 5N,4O-carbonyl protected sialoside acceptors with free hydroxyl groups at C8 and C9 were good pairs for the synthesis of Siaα2,9Sia linkages for the formation of α2,9-linked oligosialic acids [6].
A highly convergent and stereoselective method was developed for the synthesis of tetrasaccharide and hexasaccharide (6, Figure 1) epitopes of rhamnogalacturonan II found in the cell wall of some plants [7•]. The synthesis was achieved by tuning the reactivity of glycosyl donor and acceptor building blocks. Introduction of β-linked arabino-l-furanoside by a conformationally constrained arabinosyl donor is of particular interest.
Recent progress in obtaining β-linked mannopyranosides was demonstrated in the synthesis of β-N-acetylmannosamine-containing hexasaccharide repeating unit 7 (Figure 1) found in the vegetative cell wall of Bacillus anthracis by the formation of trans-glucosidic linkage followed by inverting the C2 stereocenter of glucose [8], and the development of a novel direct β-mannosylation method by activating anomeric hydroxy using phthalic anhydride and trifluoromethanesulfonic anhydride (Tf2O) as promoters [9]. On the other hand, an intramolecular aglycon delivery (IAD) strategy developed by Ito and co-workers was proven efficient in direct stereoselective synthesis of β-l-rhamnopyranosides using 2-O-naphthylmethyl-3-O-TMS-α-L-rhamnopyranoside as the donor [10••].
Other recent carbohydrate synthetic methods include the use of 4-(pyridine-2-yl)thiazol-2-yl thioglycosides as bidentate ligands for the synthesis of pneumococcal oligosaccharides [11] and the use of N’-glycosyltoluenesulfonohydrazides (GSHs) as glycosyl donors without protecting groups [12].
Transition metals such as gold and nickel catalysts have been used in novel glycosylation reactions. For example, AuCl3 or AuBr3 in acetonitrile has been used to activate stable benzyl protected propargyl and methyl glycosides as glycosyl donors for the glycosylation of simple alcohols, cholesterol, and carbohydrates [13,14]. Gold(I) catalyst PPh3AuOTf has been used with glycosyl ortho-hexynylbenzoate donor in efficient synthesis of a tetrasaccharide N,N,N-trimethyl-d-glucosamine-chitotriomycin as an inhibitor for insect and fungal β-N-acetylglucosaminidases [15]. It has also been shown to be a superior catalyst for 1,2-anhydrosugar donors in glycosylation reactions even with hindered sugar alcohol acceptors [16]. On the other hand, nickel catalysts have been used with N-para-methoxybenzylidene protected α-2-deoxy-2-amino glycoside donor in stereoselective synthesis of hexosamine-containing oligosaccharides [17•].
One-pot glycosylation of oligosaccharides
One-pot glycosylation methods allow multiple glycosylation reactions taking place successively in the same reaction flask without the need for intermediate isolation. There are two common approaches for the one-pot glycosylation of oligosaccharides. One relies on the reactivity differences of glycosyl donors and acceptors and the other uses pre-activation of the glycosyl donor followed by addition of the acceptor. In addition, a regioselective and combinatorial one-pot protection of monosaccharides has also been reported [18••].
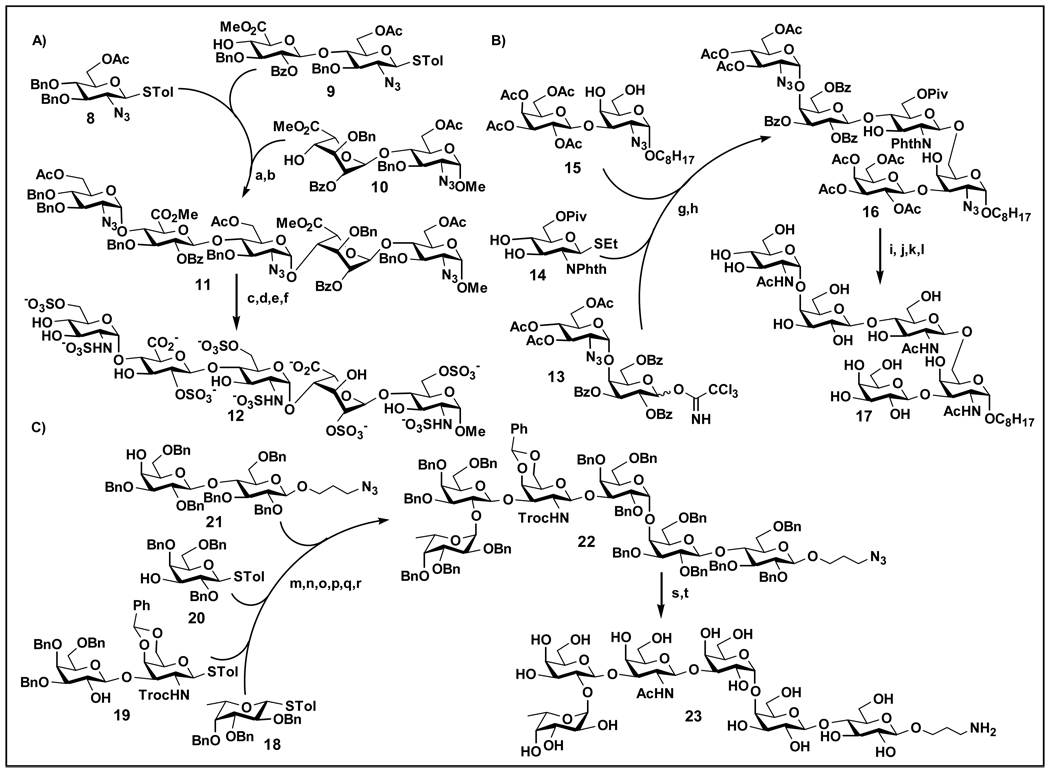
Reactivity-based one-pot glycosylation of oligosaccharides was demonstrated in the synthesis of heparin-like oligosaccharides using thioglycosides as building blocks [19•]. Activation of the most reactive building block followed by successive addition of building blocks of lower reactivity and activation led to the desired oligosaccharide 12 (Figure 2A). A similar strategy was employed in the synthesis of pentasaccharide 17 which showed antibiotic activity against Helicobacter pylori using different donors and promoters in a one-pot two-step system (Figure 2B) [20]. A one-pot strategy that combines reductive opening of benzylidene acetal and glycosylation was also used in the synthesis of Lewisx and sialyl Lewisx oligosaccharides [21, 22].
Figure 2.
One-pot chemical synthesis of A) protected heparan sulphate-type pentasaccharide 12 [19•]; B) pentasaccharide 17 [20]; and C) Globo H hexasaccharide 23 [23]. (a) 9, NIS/TfOH, CH2Cl2, −45 °C to rt; (b) 10, NIS/TfOH, CH2Cl2, −45 °C to rt; (c) LiOOH, THF; (d) Et3N·SO3, DMF; (e) H2, Pd/C; (f) Pyr·SO3, H2O; (g) 14, TMSOTf, CH2Cl2, −70 °C, 1 h; (h) 15, NIS/TfOH, CH2Cl2, −50 to −10 °C, 2 h; (i) PPh3, THF/H2O, rt; (j) NH2CH2CH2NH2, CH3CN-EtOH-toluene, 80 °C, 18 h; (k) Pyr., Ac2O, rt; (l) 1 M NaOMe/MeOH, 2 d; (m) p-TolSCl, AgOTf, TMSOTf, −78 °C; (n) 19, TTBP, −78 to −20 °C; (o) p-TolSCl, AgOTf, −78 °C; (p) 20, TTBP, −78 to −20 °C; (q) p-TolSCl, AgOTf, −78 °C; (r) 21, TTBP, −78 to −20 °C; (s) NaOH, THF; Ac2O, Pyr., DMAP; (t) trimethylphosphine (PMe3), THF, NaOH; H2, Pd(OH). Abbreviation: p-TolSCl, p-tolylsulfenyl chloride.
Iterative one-pot glycosylation based on pre-activation of glycosyl donors was demonstrated in the synthesis of Globo-H hexasaccharide 23 (Figure 2C) [23]. In this strategy, p-tolylthioglycosyl donor was pre-activated by p-toluenesulfenyl triflate (p-TolSOTf) promoter. After reaction with substoichiometric amount (0.9 equiv.) of p-tolylthiolated acceptor, the reaction temperature was warmed up to room temperature to decompose the slightly excess activated donor. The obtained p-tolylthiolated oligosaccharide unit can be activated again for another glycosylation process in one-pot. The procedure can be repeated to add additional building blocks. This method was also applied for the synthesis of complex α2,3-sialylated and fucosylated biantennary N-glycan dodecasaccharide which contains both hard-to-obtain α-sialyl linkages and acid labile α-fucosyl linkages using a sialyl disaccharide building block [24]. The reactivity-independent pre-activation approach was also confirmed to be efficient for the synthesis of oligosaccharides containing N-glycolylneuraminic acid [25] and α2,9-linked sialyl trisaccharides with polymer-assisted deprotection [26]. In addition, it was combined with the reactivity-based one-pot approach in the synthesis of branched oligosaccharides Lewisx pentasaccharide and dimeric Lewisx octasaccharide [27].
Automated oligosaccharide synthesis
Unlike peptides and oligonucleotides, automated oligosaccharide synthesis is much more challenging for various reasons including the presence of multiple hydroxyl groups and branch structures in glycans and the requirement for regio- and stereoselectivity in glycosylation.
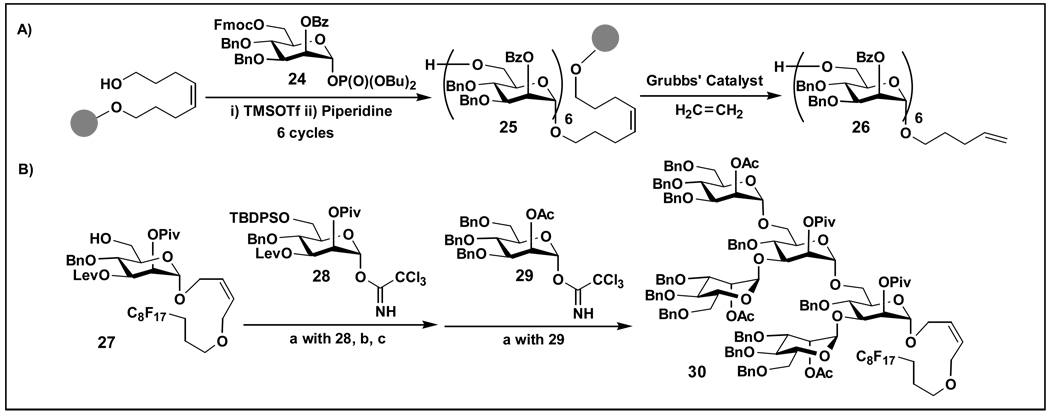
Solid-phase automated carbohydrate synthesis requires a number of steps including the attachment of the acceptor (nucleophile) to the solid support, successive coupling of the monosaccharide building blocks, releasing oligosaccharides from the solid support, and purification and deprotection of the final product [28]. Although a large excess of sugar donors are still required, great advances have been achieved recently as demonstrated in the synthesis of lipomannan backbone α1,6-hexamannoside 26 (Figure 3A) [29], tumor-associated carbohydrate antigens Gb-3 and Globo-H [30], and oligoglucosamines [31].
Figure 3.
Automated synthesis of A) hexamannoside 26 [29] and B) branched pentasaccharide 30 [32••]. (a) TMSOTf, CH2Cl2, 5 °C, 30 min; (b) TBAF, THF, rt, 5h; (c) 1 M NH2NH2, Pyr., AcOH, rt, 30 min.
As a potential alternative for automated synthesis of carbohydrates, a fluorous tag (-C8F17)-assisted solution-phase synthesis has been developed and used in the synthesis of branched and linear mannose oligosaccharides (Figure 3B) [32••]. The fluorous tag linked to the glycosyl acceptors allows all glycosylation and deprotecting reactions to be carried out in solution-phase while facilitates easy purification by fluorous solid phase extraction (FSPE). It greatly enhances the efficiency and the yields compared to solid-phase synthesis. On the other hand, an ionic liquid (IL) supported glycosylation method that uses imidazolium cation tagged donors has also been employed in the synthesis of mannose oligosaccharides [33,34].
Chemoenzymatic synthesis of carbohydrates
Chemoenzymatic approaches combine the flexibility of chemical synthesis and high regio- and stereoselectivity of enzyme-catalyzed reactions to achieve highly efficient synthesis of complex carbohydrates. In general, there are two types of chemoenzymatic methods based on the sequence of the reaction events. One is to apply chemical synthesis first to generate substrate analogs for enzymatic synthesis. The other is to apply enzymatic synthesis first before chemical diversification taking place [35].
Since most enzymatic reactions are optimal or maintain their activities under standard physiological condition, multiple-enzyme reactions can be carried out in one-pot to produce the desire product. Multiple-enzyme processes are especially important for glycosyltransferase-catalyzed reactions as they require sugar nucleotide donors which are either expensive or are not commercially available but can be generated from simpler starting materials using sugar nucleotide biosynthetic enzymes. In addition, longer and more complex oligosaccharides require the combination of several glycosyltransferases for efficient production of desired products [36,37].
Current chemoenzymatic synthetic efforts have been mainly focused on obtaining challenging targets including structurally diverse homogeneous glycopeptides and glycoproteins, glycolipids, glycosylated natural products, sialyloligosaccharides (sialosides), bacterial polysaccharides, heparan sulfates or heparin, and other complex oligosaccharides or polysaccharides. Recent advances in the chemoenzymatic synthesis of sialosides and related polysaccharides, complex oligosaccharides, glycolipids, as well as glycosylated natural products are highlighted here.
Chemoenzymatic synthesis of sialyl oligosaccharides, polysaccharides, and macrocycles
Sialic acids are a family of acidic monosaccharides which have been found predominantly as the terminal units on glycans and glycoconjugates in nature. Unlike common monosaccharides, chemical sialylation is much more difficult and less efficient due to the presence of an electron-withdrawing carboxyl group at the anomeric carbon, a sterically hindered quaternary anomeric carbon, and a deoxy carbon next to the anomeric center. In addition, chemical approaches will be impractical in generating sialosides with most of the naturally occurring sialic acid forms as many sialic acid modifications are labile to final deprotection steps in chemical synthesis. Therefore, chemoenzymatic approaches are excellent choices for synthesizing sialic acid-containing molecules.
Taking the advantages of high expression level in E. coli expression systems, high activity, and substrate promiscuity of bacterial sialoside biosynthetic enzymes, Chen and co-worker have established and applied a highly efficient one-pot multiple-enzyme system for the chemoenzymatic synthesis of naturally occurring and non-natural sialosides. In this system, mannose, ManNAc, or their derivatives are chemically or enzymatically synthesized as sialic acid precursors. These compounds are converted by a sialic acid aldolase catalyzed reaction to form sialic acids and their derivatives, which are activated by a cytidine 5’-monophosphate (CMP)-sialic acid synthetase, and transfer to proper acceptors by a suitable sialyltransferase to form the targeted sialosides. The promiscuity of these enzymes and the power of the one-pot multiple-enzyme chemoenzymatic approach has been showcased in combinatorial chemoenzymatic synthesis of a library of 72 biotinylated α2,6-linked sialosides. The produced sialosides can be directly used in NeutrAvidin-coated microtiter plates for high-throughput screening to identify the preferred ligands for sialic acid-binding protein without the tedious purification processes [38••]. Using CstII, a multifunctional sialyltransferase from Campylobacter jejuni, in the one-pot multiple-enzyme system, GD3 and GT3 ganglioside oligosaccharides have been obtained [39].
The substrate flexibility of bacterial sialoside biosynthetic enzymes were highlighted in the enzymatic synthesis of fluorinated sialosides and fluorinated CMP-sialic acids [40], which are important substrate analogs for X-ray crystal structural studies of sialyltransferases [41]. More interestingly, a recombinant E. coli sialic acid aldolase was shown to accept disaccharides containing a reducing mannose or ManNAc derivative as substrates to produce more complex disaccharides containing a sialic acid at the reducing end [42].
Oligosaccharides and polysaccharides containing internal sialic acids are particularly difficult to obtain from natural sources or by chemical synthesis. The Chen group reported the first successful example of controlled chemoenzymatic synthesis of size-defined polysaccharides with sialic acid-containing repeating units by sialyltransferase-catalyzed block transfer of oligosaccharides (Figure 4) [43••]. CMP-activated disaccharide and tetrasaccharide analogs 33 and 37, respectively, were excellent donor substrates for a recombinant Photobacterium damsela α2,6-sialyltransferase (Pd2,6ST). This method was also extended to obtain structurally-defined macrocyclic oligosaccharides of varied sizes [44]. The substrate flexibility of sialyltransferases opens the door for the efficient synthesis of biologically important oligosaccharides and polysaccharides containing sialic acid.
Figure 4.
Synthesis of size-defined polysaccharides [43••] and macrocyclic carbohydrates [44]. (a) pyruvate, CTP, MgCl2, E. coli K12 sialic acid aldolase, NmCSS, Tris-HCl buffer (100 mM, pH 8.5), 37 °C; (b) CuI, DIPEA, CH3CN/H2O, rt; (c) Pd2,6ST, Tris-HCl buffer (100 mM, pH 7.5), 37 °C; (d) NaOMe/MeOH, rt; (e) pyruvate, CTP, MgCl2, E. coli K12 sialic acid aldolase, NmCSS, Pd2,6ST, Tris-HCl buffer (100 mM, pH 8.5), 37 °C. Abbreviation: NmCSS, N. meningitidis CMP-sialic acid synthetase; Pd2,6ST, Photobacterium damsela α2,6-sialyltransferase.
In addition, uncommon activities have been discovered for some glycosyltransferases and these properties have been used in the synthesis of uncommon or non-natural oligosaccharides. The Withers group found that a β1,4-galactosyltransferase from Helicobacter pylori can accept sulfur-containing acceptor to synthesize sulfur-linked disaccharide Galβ-S-1,4-GlcNAcβ-pNP, it can also use a mannoside as the acceptor to produce uncommon disaccharide Galβ1,4Manβ-pNP [45].
Chemoenzymatic synthesis of oligosaccharides and glycolipids by glycosidases and trans-glycosidases
In nature, glycosidases generally cleave glycosidic bonds, but they have been often employed in the synthesis of glycosides due to their broader subject specificity and lower cost [46]. Recent examples for the use of glycosidases in the synthesis of oligosaccharides include the enzymatic synthesis of gentiooligosaccharides [47], and the chemoenzymatic synthesis of oligosaccharide containing 2-acetamido-2-deoxy-β-d-galactopyranosyluronic acid residue by β-N-aceylhexoaminidase from Talaromyces flavus [48]. It was recently reported that a large amount of endo-β-N-acetylglucosaminidase (Endo-A) enhances the transglycosylation activity of the disaccharide oxazoline, and polymerizes the disaccharide oxazoline to form oligosaccharides and polysaccharides in the absence of an external acceptor [49].
Glycosidases are converted to glycosynthases using site-directed mutagenesis of the catalytic nucleophile [50]. These enzymes can carry out transglycosylation reaction without hydrolyzing the product, therefore increasing the yield of desired product. A recent example illustrated the use of glycosynthase mutants derived from rice BGlu1 β-glucosidase for the synthesis of long-chain oligosaccharides [51]. In addition, enzymatic transglycosylation was employed for the synthesis α-acarviosinyl-(1,9)-3-α-glycopyranosylpropen [52]. Withers and co-worker recently reported the use of direct evolution for the conversion of a glycosphingolipid-synthesizing enzyme with poor activity for certain sphingolipids into an enzyme that is comparable to the parent enzyme with the native lipid substrate [53].
Chemoenzymatic synthesis of glycosylated natural products
Glycosylated natural products are attractive targets for drug development. The carbohydrate moiety of many natural products has important effects on the specificity and pharmacology of the compounds. Current discovery of promiscuous glycosyltransferases and biosynthetic enzymes for producing sugar nucleotides of uncommon sugars provides a great opportunity for enzyme-catalyzed glycosylation of diverse natural products. A recent example was provided by the Liu group who discovered that a macrolide glycosyltransferase can tolerate different substrates including variety of cyclic and linear substrates [54]. When multiple hydroxyl groups were present, good regiospecificity was observed with cyclic substrates, but none was observed for the linear substrates. In addition, directed evolution has been applied in the Thorson group as an effective method to produce glycosyltransferase mutants that have extended promiscuity for the synthesis of a large library of natural products [55,56]. The success of this relies heavily on the development of high-throughput screen methods.
Conclusions and future perspectives
In conclusion, a wide range of chemical synthetic methods have been developed and employed for the synthesis of carbohydrates including one-pot glycosylation, automated solid-phase approach, and solution-phase approaches. Enzymes such as glycosyltransferases, glycosidases, and their mutants including glycosynthases continue to be powerful tools for the synthesis of oligosaccharides and glycoconjugates. Current advances in functional genomics studies and modern technologies on proteins X-ray crystal structural studies, site-directed mutagenesis, and directed evolution will provide a continuously expanding rich reservoir of efficient enzyme catalysts. The availability and easy access to complex carbohydrates will realize the medicinal applications of carbohydrates.
Acknowledgements
This contribution was supported in part by National Institutes of Health Grant GM076360, National Science Foundation CAREER Award 0548235, and the Arnold and Mable Beckman Foundation. X.C. is a Beckman Young Investigator, an Alfred P. Sloan Research Fellow, and a Camille Dreyfus Teacher-Scholar.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Nagorny P, Fasching B, Li X, Chen G, Aussedat B, Danishefsky SJ. Toward fully synthetic homogeneous beta-human follicle-stimulating hormone (beta-hFSH) with a biantennary N-linked dodecasaccharide. Synthesis of beta-hFSH with chitobiose units at the natural linkage sites. J Am Chem Soc. 2009;131(16):5792–5799. doi: 10.1021/ja809554x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanashima S, Castagner B, Esposito D, Nokami T, Seeberger PH. Synthesis of a sialic acid alpha(2–3) galactose building block and its use in a linear synthesis of sialyl Lewis X. Org Lett. 2007;9(9):1777–1779. doi: 10.1021/ol0704946. [DOI] [PubMed] [Google Scholar]
- 3.Boonyarattanakalin S, Liu X, Michieletti M, Lepenies B, Seeberger PH. Chemical synthesis of all phosphatidylinositol mannoside (PIM) glycans from Mycobacterium tuberculosis. J Am Chem Soc. 2008;130(49):16791–16799. doi: 10.1021/ja806283e. [DOI] [PubMed] [Google Scholar]
- 4.Deng K, Adams MM, Gin DY. Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants. J Am Chem Soc. 2008;130(18):5860–5861. doi: 10.1021/ja801008m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka H, Nishiura Y, Takahashi T. An efficient convergent synthesis of GP1c ganglioside epitope. J Am Chem Soc. 2008;130(51):17244–17245. doi: 10.1021/ja807482t. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Nishiura Y, Takahashi T. Stereoselective synthesis of alpha(2,9) di- to tetrasialic acids, using a 5,4-N,O-carbonyl protected thiosialoside. J Org Chem. 2009;74(11):4383–4386. doi: 10.1021/jo900176e. [DOI] [PubMed] [Google Scholar]
- 7. Rao Y, Boons GJ. A highly convergent chemical synthesis of conformational epitopes of rhamnogalacturonan II. Angew Chem Int Ed Engl. 2007;46(32):6148–6151. doi: 10.1002/anie.200701750.. The authors demonstrated the synthesis of a hexasaccharide epitope of rhamnogalacturonan II found in the cell wall of some plants. Such a synthesis is challenging due to the crowdedness around the central arabinopyranoside and the presence of 1,2-cis glycoside residues.
- 8.Oberli MA, Bindschadler P, Werz DB, Seeberger PH. Synthesis of a hexasaccharide repeating unit from Bacillus anthracis vegetative cell walls. Org Lett. 2008;10(5):905–908. doi: 10.1021/ol7030262. [DOI] [PubMed] [Google Scholar]
- 9.Kim KS, Fulse DB, Baek JY, Lee BY, Jeon HB. Stereoselective direct glycosylation with anomeric hydroxy sugars by activation with phthalic anhydride and trifluoromethanesulfonic anhydride involving glycosyl phthalate intermediates. J Am Chem Soc. 2008;130(26):8537–8547. doi: 10.1021/ja710935z. [DOI] [PubMed] [Google Scholar]
- 10. Lee YJ, Ishiwata A, Ito Y. Stereoselective synthesis of β-l-rhamnopyranosides. J Am Chem Soc. 2008;130(20):6330–6331. doi: 10.1021/ja801574q.. The authors described a novel and stereoselective synthetic approach for the synthesis of β-l-rhamnopyranosides through intramolecular aglycon delivery. Compared to mannopyranoside, the synthesis of β-l-rhamnopyranosides is much more difficult due to the lack of hydroxyl group at C-6 to form the 4,6-O-benzylidene protection, which is vital for the formation of the β-d-mannopyranosides.
- 11.Pornsuriyasak P, Rath NP, Demchenko AV. 4-(pyridin-2-yl)thiazol-2-yl thioglycosides as bidentate ligands for oligosaccharide synthesis via temporary deactivation. Chem Commun (Camb) 2008;(43):5633–5635. doi: 10.1039/b810569c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudmundsdottir AV, Nitz M. Protecting group free glycosidations using p-toluenesulfonohydrazide donors. Org Lett. 2008;10(16):3461–3463. doi: 10.1021/ol801232f. [DOI] [PubMed] [Google Scholar]
- 13.Hotha S, Kashyap S. Propargyl glycosides as stable glycosyl donors: anomeric activation and glycoside syntheses. J Am Chem Soc. 2006;128(30):9620–9621. doi: 10.1021/ja062425c. [DOI] [PubMed] [Google Scholar]
- 14.Vidadala SR, Hotha S. Methyl glycosides are identified as glycosyl donors for the synthesis of glycosides, disaccharides and oligosaccharides. Chem Commun (Camb) 2009;(18):2505–2507. doi: 10.1039/b822526e. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Li Y, Yu B. Total synthesis and structural revision of TMG-chitotriomycin, a specific inhibitor of insect and fungal beta-N-acetylglucosaminidases. J Am Chem Soc. 2009;131(34):12076–12077. doi: 10.1021/ja9055245. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Tang P, Chen Y, Yu B. Gold(I)-catalyzed glycosidation of 1,2-anhydrosugars. J Org Chem. 2008;73(11):4323–4325. doi: 10.1021/jo8003875. [DOI] [PubMed] [Google Scholar]
- 17. Mensah EA, Nguyen HM. Nickel-catalyzed stereoselective formation of α-2-deoxy-2-amino glycosides. J Am Chem Soc. 2009;131(25):8778–8780. doi: 10.1021/ja903123b.. A general method for the stereoselective synthesis of α-2-deoxy-2-amino glycosides that employ a cationic nickel catalyst and para-methoxybenzylidene as the protecting group for the amine group was developed. The advantage of this method is that α-selectivity is mainly depend on the nature of the ligand on the nickel catalyst, and only a catalytic amount of nickel is required for the reaction.
- 18. Wang CC, Lee JC, Luo SY, Kulkarni SS, Huang YW, Lee CC, Chang KL, Hung SC. Regioselective one-pot protection of carbohydrates. Nature. 2007;446(7138):896–899. doi: 10.1038/nature05730.. The authors reported the first one-pot regioselective and combinatorial approach for the protection of monosaccharides by a single catalyst. This approach, in combination with other advance methods such as one-pot glycosylation should help to accelerate the synthesis of complex carbohydrates.
- 19. Polat T, Wong CH. Anomeric reactivity-based one-pot synthesis of heparin-like oligosaccharides. J Am Chem Soc. 2007;129(42):12795–12800. doi: 10.1021/ja073098r.. A one-pot strategy for the synthesis of heparin-like oligosaccharides was reported. This work represents recent advancement in the one-pot glycosylation of oligosaccharides.
- 20.Wang P, Lee H, Fukuda M, Seeberger PH. One-pot synthesis of a pentasaccharide with antibiotic activity against Helicobacter pylori. Chem Commun (Camb) 2007;(19):1963–1965. doi: 10.1039/b618662a. [DOI] [PubMed] [Google Scholar]
- 21.Vohra Y, Vasan M, Venot A, Boons GJ. One-pot synthesis of oligosaccharides by combining reductive openings of benzylidene acetals and glycosylations. Org Lett. 2008;10(15):3247–3250. doi: 10.1021/ol801076w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vohra Y, Buskas T, Boons GJ. Rapid assembly of oligosaccharides: A highly convergent strategy for the assembly of a glycosylated amino acid derived from PSGL-1. J Org Chem. 2009;74(16):6064–6071. doi: 10.1021/jo901135k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Zhou L, El-Boubbou K, Ye XS, Huang X. Multi-component one-pot synthesis of the tumor-associated carbohydrate antigen Globo-H based on preactivation of thioglycosyl donors. J Org Chem. 2007;72(17):6409–6420. doi: 10.1021/jo070585g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun B, Srinivasan B, Huang X. Pre-activation-based one-pot synthesis of an alpha-(2,3)-sialylated core-fucosylated complex type bi-antennary N-glycan dodecasaccharide. Chemistry Eur J. 2008;14(23):7072–7081. doi: 10.1002/chem.200800757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crich D, Wu B. Stereoselective iterative one-pot synthesis of N-glycolylneuraminic acid-containing oligosaccharides. Org Lett. 2008;10(18):4033–4035. doi: 10.1021/ol801548k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka H, Tateno Y, Nishiura Y, Takahashi T. Efficient synthesis of an alpha(2,9) trisialic acid by one-pot glycosylation and polymer-assisted deprotection. Org Lett. 2008;10(24):5597–5600. doi: 10.1021/ol802207e. [DOI] [PubMed] [Google Scholar]
- 27.Miermont A, Zeng Y, Jing Y, Ye XS, Huang X. Syntheses of Lewis(x) and dimeric Lewis(x): construction of branched oligosaccharides by a combination of preactivation and reactivity based chemoselective one-pot glycosylations. J Org Chem. 2007;72(23):8958–8961. doi: 10.1021/jo701694k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeberger PH. Automated oligosaccharide synthesis. Chem Soc Rev. 2008;37(1):19–28. doi: 10.1039/b511197h. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, Wada R, Boonyarattanakalin S, Castagner B, Seeberger PH. Automated synthesis of lipomannan backbone alpha(1–6) oligomannoside via glycosyl phosphates: glycosyl tricyclic orthoesters revisited. Chem Commun (Camb) 2008;30:3510–3512. doi: 10.1039/b804069a. [DOI] [PubMed] [Google Scholar]
- 30.Werz DB, Castagner B, Seeberger PH. Automated synthesis of the tumor-associated carbohydrate antigens Gb-3 and Globo-H: incorporation of alpha-galactosidic linkages. J Am Chem Soc. 2007;129(10):2770–2771. doi: 10.1021/ja069218x. [DOI] [PubMed] [Google Scholar]
- 31.Ko KS, Park G, Yu Y, Pohl NL. Protecting-group-based colorimetric monitoring of fluorous-phase and solid-phase synthesis of oligoglucosamines. Org Lett. 2008;10(23):5381–5384. doi: 10.1021/ol802229b. [DOI] [PubMed] [Google Scholar]
- 32. Jaipuri FA, Pohl NL. Toward solution-phase automated iterative synthesis: fluorous-tag assisted solution-phase synthesis of linear and branched mannose oligomers. Org Biomol Chem. 2008;6(15):2686–2691. doi: 10.1039/b803451f.. The first flourous-tag assisted solution-phase synthesis of oligosaccharides was reported. Despite all challenges, the reported approach seems very promising toward an automated fluorous-phase synthesis of oligosaccharides.
- 33.Pathak AK, Yerneni CK, Young Z, Pathak V. Oligomannan synthesis using ionic liquid supported glycosylation. Org Lett. 2008;10(1):145–148. doi: 10.1021/ol702743x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yerneni CK, Pathak V, Pathak AK. Imidazolium cation supported solution-phase assembly of homolinear alpha(1→6)-linked octamannoside: An efficient alternate approach for oligosaccharide synthesis. J Org Chem. 2009;74(16):6307–6310. doi: 10.1021/jo901169u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao H, Huang S, Cheng J, Li Y, Muthana S, Son B, Chen X. Chemical preparation of sialyl Lewis x using an enzymatically synthesized sialoside building block. Carbohydr Res. 2008;343(17):2863–2869. doi: 10.1016/j.carres.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su DM, Eguchi H, Yi W, Li L, Wang PG, Xia C. Enzymatic synthesis of tumor-associated carbohydrate antigen Globo-H hexasaccharide. Org Lett. 2008;10(5):1009–1012. doi: 10.1021/ol703121h. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Gilbert M, Eguchi H, Yu H, Cheng JS, Muthana S, Zhou LY, Wang PG, Chen X, Huang XF. Chemoenzymatic syntheses of tumor-associated carbohydrate antigen Globo-H and stage-specific embryonic antigen 4. Adv Synth Catal. 2008;350(11–12):1717–1728. doi: 10.1002/adsc.200800129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chokhawala HA, Huang S, Lau K, Yu H, Cheng J, Thon V, Hurtado-Ziola N, Guerrero JA, Varki A, Chen X. Combinatorial chemoenzymatic synthesis and high-throughput screening of sialosides. ACS Chem Biol. 2008;3(9):567–576. doi: 10.1021/cb800127n.. A novel combinatorial chemoenzymatic method was developed for the synthesis of biotinylated sialosides. This approach can be combined with high-throughput screening essays for studying the biological functions of sialic acid-binding proteins without tedious product purification processes.
- 39.Cheng J, Yu H, Lau K, Huang S, Chokhawala HA, Li Y, Tiwari VK, Chen X. Multifunctionality of Campylobacter jejuni sialyltransferase CstII: characterization of GD3/GT3 oligosaccharide synthase, GD3 oligosaccharide sialidase, and trans-sialidase activities. Glycobiology. 2008;18(9):686–697. doi: 10.1093/glycob/cwn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chokhawala HA, Cao H, Yu H, Chen X. Enzymatic synthesis of fluorinated mechanistic probes for sialidases and sialyltransferases. J Am Chem Soc. 2007;129(35):10630–10631. doi: 10.1021/ja072687u. [DOI] [PubMed] [Google Scholar]
- 41.Ni L, Chokhawala HA, Cao H, Henning R, Ng L, Huang S, Yu H, Chen X, Fisher AJ. Crystal structures of Pasteurella multocida sialyltransferase complexes with acceptor and donor analogues reveal substrate binding sites and catalytic mechanism. Biochemistry. 2007;46(21):6288–6298. doi: 10.1021/bi700346w. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Yu H, Chen X. Disaccharides as sialic acid aldolase substrates: synthesis of disaccharides containing a sialic acid at the reducing end. Angew Chem Int Ed Engl. 2007;46(13):2249–2253. doi: 10.1002/anie.200604799. [DOI] [PubMed] [Google Scholar]
- 43. Muthana S, Yu H, Huang S, Chen X. Chemoenzymatic synthesis of size-defined polysaccharides by sialyltransferase-catalyzed block transfer of oligosaccharides. J Am Chem Soc. 2007;129(39):11918–11919. doi: 10.1021/ja075736b.. Authors reported the first successful example of controlled chemoenzymatic approach for the synthesis of size-defined polysaccharides containing internal sialic acid residues. The results from this study further demonstrate that bacterial sialyltransferases have extremely flexible substrate specificity and can be employed for the synthesis of complex oligosaccharides containing sialic acids.
- 44.Muthana S, Yu H, Cao H, Cheng J, Chen X. Chemoenzymatic synthesis of a new class of macrocyclic oligosaccharides. J Org Chem. 2009;74(8):2928–2936. doi: 10.1021/jo8027856. [DOI] [PubMed] [Google Scholar]
- 45.Namdjou DJ, Chen HM, Vinogradov E, Brochu D, Withers SG, Wakarchuk WW. A beta-1,4-galactosyltransferase from Helicobacter pylori is an efficient and versatile biocatalyst displaying a novel activity for thioglycoside synthesis. Chembiochem. 2008;9(10):1632–1640. doi: 10.1002/cbic.200700775. [DOI] [PubMed] [Google Scholar]
- 46.Kren V, Thiem J. Glycosylation employing bio-systems: from enzymes to whole cells. Chem Soc Rev. 1997;26(6):463–473. [Google Scholar]
- 47.Fujimoto Y, Hattori T, Uno S, Murata T, Usui T. Enzymatic synthesis of gentiooligosaccharides by transglycosylation with [beta]-glycosidases from Penicillium multicolor. Carbohydr Res. 2009;344(8):972–978. doi: 10.1016/j.carres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Bojarová P, Krenek K, Kuzma M, Petrásková L, Bezouska K, Namdjou D-J, Elling L, Kren V. N-Acetylhexosamine triad in one molecule: Chemoenzymatic introduction of 2-acetamido-2-deoxy-β-d-galactopyranosyluronic acid residue into a complex oligosaccharide. J Mol Catal B: Enzym. 2008;50(2–4):69–73. [Google Scholar]
- 49.Ochiai H, Huang W, Wang LX. Endo-beta-N-acetylglucosaminidase-catalyzed polymerization of beta-Glcp-(1→4)-GlcpNAc oxazoline: a revisit to enzymatic transglycosylation. Carbohydr Res. 2009;344(5):592–598. doi: 10.1016/j.carres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaikh FA, Withers SG. Teaching old enzymes new tricks: engineering and evolution of glycosidases and glycosyl transferases for improved glycoside synthesis. Biochem Cell Biol. 2008;86(2):169–177. doi: 10.1139/O07-149. [DOI] [PubMed] [Google Scholar]
- 51.Hommalai G, Withers SG, Chuenchor W, Cairns JR, Svasti J. Enzymatic synthesis of cello-oligosaccharides by rice BGlu1 β-glucosidase glycosynthase mutants. Glycobiology. 2007;17(7):744–753. doi: 10.1093/glycob/cwm039. [DOI] [PubMed] [Google Scholar]
- 52.Lee YS, Lee MH, Lee HS, Lee SJ, Kim YW, Zhang R, Withers SG, Kim KS, Lee SJ, Park KH. Enzymatic synthesis of a selective inhibitor for alpha-glucosidases: alpha-acarviosinyl-(1→9)-3-alpha-d-glucopyranosylpropen. J Agric Food Chem. 2008;56(13):5324–5330. doi: 10.1021/jf703655k. [DOI] [PubMed] [Google Scholar]
- 53.Hancock SM, Rich JR, Caines ME, Strynadka NC, Withers SG. Designer enzymes for glycosphingolipid synthesis by directed evolution. Nat Chem Biol. 2009;5(7):508–514. doi: 10.1038/nchembio.191. [DOI] [PubMed] [Google Scholar]
- 54.Borisova SA, Kim HJ, Pu X, Liu HW. Glycosylation of acyclic and cyclic aglycone substrates by macrolide glycosyltransferase DesVII/DesVIII: analysis and implications. Chembiochem. 2008;9(10):1554–1558. doi: 10.1002/cbic.200800155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams GJ, Goff RD, Zhang C, Thorson JS. Optimizing glycosyltransferase specificity via "hot spot" saturation mutagenesis presents a catalyst for novobiocin glycorandomization. Chem Biol. 2008;15(4):393–401. doi: 10.1016/j.chembiol.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams GJ, Zhang C, Thorson JS. Expanding the promiscuity of a natural-product glycosyltransferase by directed evolution. Nat Chem Biol. 2007;3(10):657–662. doi: 10.1038/nchembio.2007.28. [DOI] [PubMed] [Google Scholar]