Abstract
Although many Alzheimer’s disease (AD) patients have a family history of the disease, it is rarely inherited in a predictable way. Functional magnetic resonance imaging (fMRI) studies of non-demented adults carrying familial AD mutations provide an opportunity to prospectively identify brain differences associated with early AD-related changes. We compared fMRI activity of 18 non-demented autosomal dominant AD mutation carriers with fMRI activity in 8 of their non-carrier relatives as they performed a novelty encoding task in which they viewed novel and repeated images. Because age of disease onset is relatively consistent within families, we also correlated fMRI activity with subjects’ distance from the median age of diagnosis for their family. Mutation carriers did not show significantly different voxelwise fMRI activity from non-carriers as a group. However, as they approached their family age of disease diagnosis, only mutation carriers showed increased fMRI activity in the fusiform and middle temporal gyri. This suggests that during novelty encoding, increased fMRI activity in the temporal lobe may relate to incipient AD processes.
Keywords: PSEN1, APP, fMRI, familial Alzheimer’s disease
1. Introduction
Previous studies using functional magnetic resonance imaging (fMRI) to evaluate Alzheimer’s disease (AD) risk have demonstrated that during memory tasks, non-demented older adults having increased genetic risk for AD showed differences in brain activity compared with those without such a risk (Bondi et al., 2005; Bookheimer et al., 2000; Filippini et al., 2009; Fleisher et al., 2005; Johnson et al., 2006; Lind et al., 2006; Xu et al., 2009). These studies have focused almost exclusively on the contributions to brain activity of allele ε4 of Apolipoprotein E (APOE4), the genetic influence that has most consistently been shown to confer an increased risk of developing late onset AD across numerous studies (Bertram and Tanzi, 2008). However, although possession of an APOE4 allele increases the risk of developing AD at a younger age, the allele is neither necessary nor sufficient to cause the disease (Corder et al., 1993). Therefore, without later determining which subjects eventually develop AD, it is not possible to determine which APOE genotype-related differences are related to incipient AD in healthy adults and which are intrinsic to APOE4 without regard to AD processes.
Rare, early-onset (typically before age 60), familial forms of AD exist, which are caused by mutations in one of three genes: presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP). For carriers of these mutations, AD onset is governed by a fully-penetrant autosomal dominant inheritance (Bertram and Tanzi, 2008). Additionally, in such familial Alzheimer’s disease (FAD), although age of onset may vary between families, it has been shown to be similar within families (Fox et al., 1997; Murrell et al., 2006). Therefore, for presymptomatic carriers of these mutations, it is possible to predict approximately when disease symptoms will begin. This certainty provides an excellent model for identifying the earliest AD-related changes prospectively. Differences between mutation carriers and non-carriers that are also seen in APOE4+ subjects performing a similar task may be evidence of AD-related processes that transcend gene polymorphism. Correlation of those variations with the number of years until expected age of disease symptoms in FAD mutation carriers would provide further support that the changes were associated with AD processes.
We compared the brain activity of non-demented adults carrying mutations in PSEN1 or APP with the brain activity of their family members who did not carry such a mutation as they performed a non-associative novelty encoding task. Although mutations in PSEN1 and APP may have different effects on the brain, our goal was to identify functional brain patterns that generalized to prodromal AD without being specific to any one polymorphism. Also, the small number of subjects at risk for the individual mutation types makes meaningful comparisons more challenging. We therefore considered mutation carriers as a group regardless of mutation. The novelty encoding task we used engages regions of the hippocampus and surrounding cortex (Zeineh et al., 2000), and various similar tasks have been shown to be sensitive to differences in APOE genotype in cognitively intact adults (Bondi et al., 2005; Filippini et al., 2009; Johnson et al., 2006; Trivedi et al., 2006). Of these studies, two used tasks most similar to ours in that they involved the encoding of novel pictures (Bondi et al., 2005; Filippini et al., 2009) without the element of recognition decisions found in some other studies (Johnson et al., 2006; Trivedi et al., 2006). We hypothesized that our results would be most similar to those in a picture novelty encoding study of older (Bondi et al., 2005) (rather than younger (Filippini et al., 2009)) adults who, like our subjects, were presumably closer to AD onset in those destined to develop the disease. Specifically, we hypothesized that PSEN1 and APP mutation carriers in our study would show increased fMRI activity while viewing novel versus repeated images compared with mutation non-carriers. We further hypothesized that this increased activity would occur among regions that, in older APOE4+ subjects, had shown greater fMRI activity during the task in prior studies. These regions included parts of the temporal lobe (the fusiform and parahippocampal gyri and the hippocampus), the superior parietal lobule, and the medial and middle frontal gyri (Bondi et al., 2005). We anticipated that differences would be most striking in the temporal lobe, which is known to be affected early by Alzheimer’s disease changes (Braak and Braak, 1995).
2. Methods
2.1 Subjects
Subjects were 26, cognitively intact or mildly cognitively impaired first-degree relatives of PSEN1 or APP mutation-carriers (age 19–46; mean 31.6 years) (Table 1). Cognitively intact subjects were those who did not meet the definition of AD or mild cognitive impairment (MCI) as defined below. Each underwent extensive clinical, cognitive, biochemical, and imaging evaluations. Subjects were cognitively intact except for five subjects who had MCI (Petersen, 2004). MCI was defined as those mutation carriers having intact daily functioning yet having an average z-score at least 1.5 standard deviations below mutation non-carriers on a composite score derived from scores on two tests in at least one of four cognitive domains. These domains included language, visuospatial ability, verbal memory, and executive function. The language domain included tests of Category Fluency for animals and Object Naming from the Spanish English Neuropsychological Assessment Scale, or SENAS (Mungas et al., 2004). The visuospatial domain included Rey-Osterrieth Complex Figure Copy (Wechsler, 1987) and Block Design from the WAIS-R (Loewenstein et al., 1995). Verbal memory included Word-List Learning Delayed Recall and Memory Verbal Prose Delayed Recall (Artiola-i-Fortuny et al., 1999). Finally, the frontal/executive function domain included the Stroop Interference Score (Stroop, 1935) and Color Trails Interference Score. We included the five mildly impaired subjects in order to more fully model the spectrum of earliest AD-related changes in fMRI response to a memory task. However, we controlled for cognition in our fMRI analysis as discussed in the Statistical analyses portion of the Methods section. All subjects included scored a 0 (asymptomatic) or 0.5 (questionable cognitive impairment) on the Clinical Dementia Rating (CDR) (Morris, 1997) Scale. Those who scored above 0 on the CDR and had both a Mini Mental State Exam (MMSE) (Folstein et al., 1975) score less than 24 and a Cognitive Assessment Screening Instrument (CASI) (McCurry et al., 1999) score less than 85 were excluded from the study. Of the 18 familial AD mutation carriers, 5 had CDR scores of 0.5, and the remainder were presymptomatic (CDR = 0). In addition, subjects were normotensive and had no history of head injury requiring hospitalization, of drug or alcohol dependency, or of neurological or psychological conditions that could affect cognition other than possible early declines related to incipient Alzheimer’s disease. None were taking medications designed to improve cognition. Functional MRI scans that were unusable due to excessive motion (> 2 mm) or severe artifacts were not included in the current study.
Table 1.
Demographic characteristics of subjects
| Mutation Carriers | Mutation Non-Carriers | |
|---|---|---|
| Number of subjects | 18 | 8 |
| Age (years) a,b | 30.9 (19 – 43) | 31.1 (19 – 46) |
| Relative age (years) a,b | −14.4 (1 – 35) | −14.8 (2 – 35) |
| Males/Females a | 3/15 | 1/7 |
| Family gene risk (PSEN1/APP)a | 15/3 | 5/3 |
| APOE4+ a | 2 | 1 |
| Education (years) a,b | 11.5 (7.0 – 19.0) | 11.9 (6.0 – 17.0) |
| CDR (# scoring 0.0/0.5) | 13/5 | 7/1 |
| MMSE a | 27.9 (22 – 30) | 28.4 (26 – 29) |
| CASI a | 90.5 (74.7 – 100.0) | 91.8 (81.0 – 96.4) |
| Stroop interference a,c | −2.7 (−21.2 – 10.7) | 3.01 (−7.5 – 10.7) |
| Category fluency (animals) a | 23.0 (8.0–30.0) | 24.3 (20.0 – 30.0) |
| Word list learning, delayed recall a,d | 11.2 (4.0 – 15.0) | 12.3 (9.0 – 15.0) |
A two-tailed Mann-Whitney test did not show a significant difference between mutation carriers and non-carriers.
Listed as mean (range).
Number reflects actual performance relative to a calculated expected performance based on the individual’s Stroop word-naming and color-naming scores.
From the Spanish English Neuropsychological Assessment Scales (SENAS)
Subjects were either Mexicans residing in Mexico or Mexican-American. All spoke Spanish well enough to perform Spanish language neuropsychological testing. A fluently bilingual psychometrician performed all neuropsychological assessments, which included tests that were either non-verbal or available and validated in Spanish. All cognitive assessments were performed with both subjects and researchers blind to subjects' genetic status except in the case of a single subject who had undergone clinical presymptomatic testing.
Seventeen of the subjects included in the current study were previously reported in a study that evaluated their white matter integrity (Ringman et al., 2007). Of the subjects included in that study, three were excluded from the current study for testing lower than our exclusionary criteria here. An additional two were excluded who had not received an fMRI scan, and one was excluded for having a technically inadequate fMRI scan.
Twenty-one of the subjects in the current study were included in a previous fMRI study that examined the effect of APOE genotype and familial mutation in cognitively intact familial AD mutation carriers (Ringman et al., 2010). That study did not investigate variations in brain activity with familial age of disease onset, which is the focus of the current study. Our study includes five mutation carriers having a CDR score of 0.5 who were excluded from the previous study. An additional two subjects were included in the previous study that are excluded from the current one: a subject having a scan with a somewhat limited field of view, and a mutation non-carrier who was several years past the age of disease diagnosis for the family.
Study procedures were approved by the Institutional Review Boards at UCLA and the National Institute of Neurology and Neurosurgery in Mexico City. All subjects signed written, informed consent. Subjects were told that they would be tested for the FAD mutation for which they were at risk but in the context of the research protocol would not learn the result.
2.2 Genetic testing
Genotyping was performed in the laboratory of Daniel Geschwind at UCLA based on blood samples coded with a unique identifier. DNA was extracted and APOE genotyping was performed using standard techniques. The presence of A431E and L235V substitutions in PSEN1 were assessed using RFLP analyses. The presence of the V717I substitution in APP was assessed using direct sequencing.
2.3 Imaging procedures
We acquired 30-slice whole brain functional MRI scans (3 Tesla Siemens Allegra) using an echo-planar imaging (EPI) scan sequence while the subjects performed a novelty encoding task (3.1 mm slices, aligned to the anterior and posterior commissure plane; 3.1 × 3.1 mm in-plane resolution; TR = 3400 ms; TE = 35 ms; flip angle = 90°). Two scans were acquired to aid in coregistration to the standard Montreal Neurological Institute (MNI) template brain. One was a T2-weighted image (TR = 5000 ms; TE = 30 ms) having a bandwidth matched to that of the EPI sequences. The other was a whole brain axial T1-weighted “Magnetization Prepared Rapid Gradient Echo” (MPRAGE) 3-D MRI sequence (1mm slices/0 mm gap; TR = 1900 ms; TE = 4.38 ms; TI = 1100 ms; flip angle 15 degrees; 1×1 mm in-plane resolution).
2.4 Memory activation task
During fMRI scanning, subjects performed a novelty encoding task shown previously to be sensitive to genetic risk for Alzheimer’s disease (Bondi et al., 2005). In this task, subjects viewed 10 blocks of novel and repeated pictures of scenery while indicating with a button press whether the scene was indoor or outdoor (Zeineh et al., 2000). Each block contained 16 pictures, lasting 2.55 seconds each, and novel and repeated blocks were alternated. A rest block lasting 30.6 seconds was presented at the beginning and at the end of the session. Before the scanning session, subjects were shown the repeated stimuli and told how to perform the task. While in the scanner, subjects were again provided verbal and written instructions. Stimuli were presented using MacStim presentation software (WhiteAnt Occasional Publishing).
2.5 Functional MRI data analysis
FSL 4.1.4 tools (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) were used to analyze the fMRI data. Skulls were first stripped from the coplanar T2-weighted scan using FSL “Brain Extraction Tool” (BET) (Smith, 2002). We performed individual preprocessing and individual and group analyses using FSL “FMRI Expert Analysis Tool” (FEAT). Preprocessing included both brain extraction using BET and motion correction with FSL’s “Motion Correction using FMRIB's Linear Image Registration Tool” (MCFLIRT) (Jenkinson et al., 2002). We coregistered each functional scan to its coplanar T2-weighted image and MPRAGE scans using 6 parameter rigid body transformations. The MPRAGE scans were normalized to the MNI standard brain using 12 parameter affine transformations. A high-pass temporal filter of 81.6 seconds was applied to the functional scans, and images were spatially smoothed using a Gaussian smoothing kernel of 5 mm full width half maximum. Motion parameters were added to the model to reduce the effect of motion on the data.
2.6 Statistical analyses
Statistical analyses were performed on each scan within FEAT using “FMRIB’s Improved Linear Model” (FILM) (Woolrich et al., 2001). For each individual, we contrasted fMRI activity that occurred while the subject viewed novel pictures with activity while they viewed repeated images. As a control, we also examined fMRI activity that was greater during novel or repeated blocks versus rest. We next compared the fMRI activity for these contrasts between mutation carriers and non-carriers using FSL “FMRIB’s Local Analysis of Mixed Effects” (FLAME) (Beckmann et al., 2003) to perform a two-sample unpaired t test that generated Z statistic images (Z > 2.3, adjusted to p < 0.05 using cluster thresholding to correct for multiple comparisons). In these analyses, we also evaluated the positive correlation between relative age and fMRI activity while controlling for cognition by including it as a covariate of no interest. As a control, we also examined the negative relationship between relative age and fMRI activity. Relative age was calculated as actual age minus the median age of dementia diagnosis in the individual’s family. In our subjects, median age of dementia diagnosis spanned only 15 years, ranging from 39 to 54 years old. Because of this, in both mutation carriers and non-carriers, relative age was strongly correlated with chronological age (r = 0.81; p = 0.00000012), and voxel associations with relative age therefore reflect chronological age as well. However, in mutation carriers, relative age additionally reflects approximately how close the subject is to symptomatic AD, with a less negative (higher) number indicating a nearer estimated onset. Cognition was controlled for using a number that was the mean of the Z scores for MMSE and CASI for each subject. Because the MMSE and CASI tests are similar, using an average of these test scores for each subject helps to reduce the variability that may occur if only one test were used.
In order to ensure that our significant results were not due to APOE genotype, we performed a Spearman partial correlation test that evaluated the effect of relative age in mutation carriers on average fMRI activity in significant voxels, while controlling for possession of an APOE4 allele.
With the exception of the voxelwise analyses, which were performed using the statistical parametric mapping within the FSL software, all statistical comparisons were performed using non-parametric tests (with an alpha criterion of p < 0.05) unless otherwise noted. Non-parametric tests do not require data to be normally distributed and minimize the effects of any existing data outliers. This is appropriate for small sample sizes, such as ours, whose data are rarely normally distributed. However, non-parametric tests are also conservative and may diminish the ability to detect a relationship if one exists. The relationship between relative age and fMRI activity identified in our voxelwise parametric test was later verified using an ROI analyses with a non-parametric test. We used normal approximations of the two-tailed Mann-Whitney tests to compare values between groups, and Spearman rank correlations to measure the relationships between continuous variables within each group. When the relationships between more than two variables were being evaluated, we used Spearman partial correlation tests (Schemper, 1991), which are analogous to the parametric multiple regression tests.
2.7 Region of interest (ROI) analysis
Because of our small sample size, we wished to verify our fMRI results using non-parametric statistical tests to gain mean activity in the region showing a significant effect of relative age in mutation carriers. Although the regions that showed activity significantly correlated with relative age in the mutation carriers spanned more than one functional brain region, these regions were contiguous and were limited to the temporal lobe. We therefore considered this region of significant activity in aggregate. For each subject, we obtained mean fMRI percent signal change across the ROI during the novel versus repeat contrast using FSL’s featquery tool. We then performed statistical tests on the resulting mean numbers, both including and excluding subjects designated as having MCI.
The right hippocampus previously showed increased fMRI activity in younger and older cognitively intact APOE4+ carriers performing a task similar to ours (Bondi et al., 2005; Filippini et al., 2009). Additionally, the hippocampus and posteromedial cortex (defined here as the precuneus and posterior cingulate cortex) are affected early by AD processes (Braak and Braak, 1995; Frisoni et al., 2009; Jagust et al., 2006; Langbaum et al., 2009; Pihlajamaki et al., 2010). We therefore sought to investigate how mutation status was related to fMRI activity in these areas. Subtle differences in these regions might not be detected in a voxelwise analysis due to the strict multiple comparisons corrections required. We therefore created ROIs for these regions by isolating the regions across the brain that were more active while subjects viewed novel scenes versus repeated scenes, and limiting the resulting binary masks to regions within the left hippocampus, right hippocampus and posteromedial cortex. Boundaries to those regions were based on the Harvard-Oxford ROIs (Desikan et al., 2006) available within FSL. We used the FSL "featquery" function to determine the mean fMRI activity within those ROIs while subjects viewed novel versus repeated images. We then compared values between mutation carriers and non-carriers using a Mann-Whitney two-tailed test. For the mutation carriers, we also evaluated the relationship between fMRI activity and relative age while adjusting for cognition using Spearman partial correlations.
Finally, we reconciled our results with those of a previous study having an overlapping subject sample (Ringman et al., 2010). To do this, we created a mask of the voxels previously showing more activity in mutation non-carriers compared with carriers as subjects viewed novel versus repeated items. We then evaluated the mean activity within that ROI during the same contrast for the subjects in the current study using a normal approximation of a Mann-Whitney two-tailed test.
3. Results
Using a the normal approximation of a two tailed Mann-Whitney test with a threshold of p<0.05, mutation carrier and non-carriers were not significantly different in MMSE, CASI, or CDR score, sex, familial gene mutated, age, relative age or possession of APOE4 (Table 1).
When all subjects were considered together, fMRI activity during novel pictures exceeded fMRI activity during previously viewed pictures in broad regions of the brain including areas important to episodic memory (such as the hippocampus, parahippocampal and fusiform gyri, precuneus and posterior cingulate gyrus) and dorsolateral prefrontal cortex, which is recruited during working memory (Table 2).
Table 2.
Regions of greater fMRI response: novel vs. repeated images – all subjects. Sample MNI coordinates reflect activation peaks for a region.
| Laterality | Brodmann area | Sample MNI coordinates | Max. Z score | |
|---|---|---|---|---|
| Frontal lobe | ||||
| Precentral g. | L | 4 | −38, −10, 58 | 3.82 |
| L | 6 | −38, −14, 62 | 3.80 | |
| Medial frontal g. | R/L | 6 | −2, −6, 52 | 3.75 |
| Dorsolateral PFC | L | 46 | −46, 32, 14 | 3.82 |
| Parietal lobe | ||||
| Postcentral g. | L | 2 | −36, −36, 62 | 2.76 |
| L | 7 | −6, −54, 70 | 3.70 | |
| Precuneus | R/L | 7 | 18, −68, 52 | 4.91 |
| R/L | 31 | −26, −70, 22 | 4.96 | |
| Posterior cingulate g. | R/L | 29 | 20, −50, 14 | 4.95 |
| R/L | 30 | 16, −48, 6 | 7.05 | |
| Temporal lobe | ||||
| Parahippocampal g. | R/L | 36 | 20, −32, −16 | 6.98 |
| Fusiform g. | R/L | 37 | −32, −44, −16 | 7.06 |
| Middle temporal g. | R/L | 39 | 42, −74, 14 | 7.06 |
| Hippocampus | R/L | 20, −8, −28 | 4.84 | |
| Occipital lobe | ||||
| Cuneus | R/L | 7 | −24, −72, 34 | 5.97 |
| R/L | 18 | 12, −80, 14 | 5.99 | |
| R/L | 19 | 14, −88, 30 | 5.98 | |
| R/L | 30 | −10, −58, 6 | 7.01 | |
| Middle occipital g. | R/L | 19 | 38, −76, 6 | 7.08 |
| Cerebellum | R/L | 4, −58, −18 | 3.87 | |
| Thalamus | R/L | −16, −12, 2 | 2.83 |
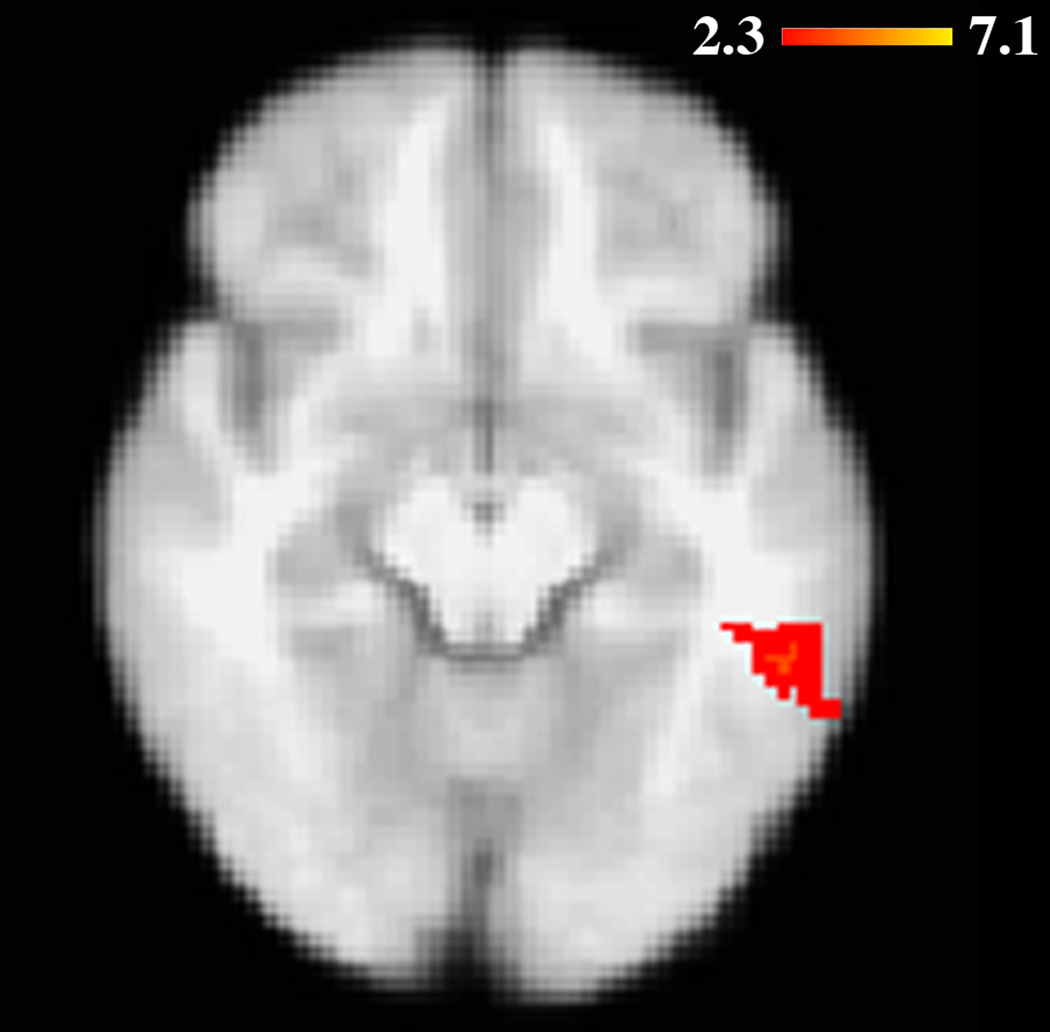
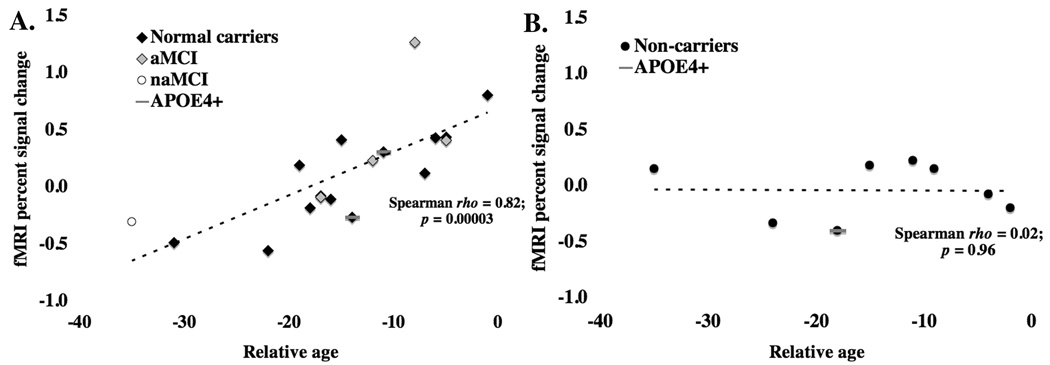
When the fMRI activity during novel versus repeated images was compared between mutation carriers and non-carriers, there was no significant difference in fMRI activity. However, when fMRI activity was covaried with relative age for this contrast, while adjusting for cognition (see Methods), mutation carriers (but not non-carriers) showed increased activity in the middle temporal and fusiform gyri as age to disease symptoms grew nearer (Table 3, Figure 1). This relationship remained significant in carriers even when cognition was not adjusted for (Spearman rho = 0.82; p = 0.00003) (Figure 2) and when the MCI subjects were excluded (Spearman rho = 0.70; p = 0.007). The relationship in all mutation carriers likewise remained significant after adjusting for APOE genotype using a Spearman partial correlation test (Schemper, 1991), a non-parametric test analogous to parametric multiple regression (R2 [full model] = 0.73; p [full model] = 0.00005; R2 [partial contribution of relative age] = 0.60; p [partial contribution of relative age] = 0.00003). No voxels were significantly correlated with relative age in the mutation non-carriers.
Table 3.
Regions in which fMRI activity is higher in mutation carriers with a greater relative age. Sample MNI coordinates reflect activation peaks for a region.
| Laterality | Brodmann area | Sample MNI coordinates | Max. Z score | |
|---|---|---|---|---|
| Middle temporal gyrus | L | 21 | −54, −40, −8 | 3.87 |
| Fusiform gyrus | L | 37 | −52, −42, −12 | 3.88 |
| 20 | −48, −26, −20 | 3.86 |
Figure 1.
As they neared the expected age of AD onset, carriers of PSEN1 and APP mutations showed increased fMRI activity in the left fusiform and middle temporal gyri during novel versus repeated items. Image is shown in radiological orientation.
Figure 2.
Within the region of differential fMRI activity shown in Figure 1, PSEN1 and APP mutation carriers (A), but not non-carriers (B) showed increased fMRI activity as they approached the expected age of disease onset. Results were not driven by those mutation carriers deemed to be suffering from mild cognitive impairment. Amnestic (aMCI) and non-amnestic (naMCI) patients are indicated among the mutation carriers.
To evaluate whether our significant relationship between relative age and fMRI activity in the mutation carriers arose from an increase in fMRI activity while viewing novel images or a decrease while viewing repeated images we performed an ROI analysis comparing the fMRI percent signal change during novel and repeated images (each versus rest). Our ROI included voxels in the fusiform and middle temporal gyri that were significantly correlated with relative age while mutation carriers viewed novel versus repeated images. We found that insignificant increases in fMRI activity while viewing novel items (Spearman rho = 0.22; p = 0.38) and decreases while viewing repeated items (Spearman rho = −0.26; p = 0.29) occurred as subjects neared their expected age of disease symptoms, resulting in an overall significant relationship between novel versus repeated images and relative age.
We also examined the reverse comparisons: the correlation of increased relative age with fMRI activity during repeated images versus novel images, and the correlation of decreased relative age with fMRI activity during the novel versus repeated images. No voxels were significant in either analysis.
Mean fMRI activity was not significantly different between mutation carriers and non-carriers in the right (Z = 0.44; p = 0.66) or left (Z = 0.92; p = 0.36) hippocampus or posteromedial cortex (Z = 0.89; p = 0.37). Likewise, mean fMRI activity in the right hippocampus (R2 = 0.04; p = 0.76), left hippocampus (R2 = 0.05; p = 0.69), and posteromedial cortex (R2 = 0.10; p = 0.46) was not significantly correlated with relative age for mutation carriers after controlling for cognition.
Within the ROI of voxels previously showing more activity in mutation non-carriers compared with carriers as subjects viewed novel versus repeated items (Ringman et al., 2010), mutation non-carriers showed more activity than carriers (Z = 2.14; p = 0.03). This continued to be true when only cognitively intact subjects were included (Z = 2.35; p = 0.02). Activity for mildly impaired mutation carriers was, on average, intermediate to that of cognitively intact mutation carriers and non-carriers. Activity was significantly higher for mutation carriers having MCI compared with those who were cognitively intact (Z = 2.91; p = 0.004). Activity in this region was not significantly correlated with relative age in mutation carriers as shown using a Spearman rank correlation (Spearman rho = 0.33; p = 0.17)
4. Discussion
We found that when non-demented adults carrying fully-penetrant autosomal dominant Alzheimer’s disease mutations approached the familial age of disease diagnosis, they showed greater fMRI activity in the fusiform and middle temporal gyri compared with mutation carriers who were further from the familial age of disease diagnosis. The fusiform gyrus is important in object processing (Tyler et al., 2004) and has been shown previously to be engaged during novelty encoding (Yamaguchi et al., 2004), including during the current task (Zeineh et al., 2000). Likewise, the medial temporal gyrus has been shown in at least one study to respond to novel objects (Pihlajamaki et al., 2005). Additionally, past work has demonstrated that the left fusiform and middle temporal gyri may share patterns of fMRI activity (Emmorey et al., 2010; Gazzaley et al., 2004), suggesting that they may be part of the same functional network. As expected, we did not see a significant relationship between relative age and fMRI activity in non-carriers from the same families.
Our results are consistent with those of a previous study having a similar novel versus repeated items contrast. In that study, older adults at genetic risk for AD showed increased fMRI activity in the fusiform gyrus compared to those without that increased risk (Bondi et al., 2005). However, we did not see a significant effect of genotype for FAD genes in the fusiform gyrus when mutation carriers and non-carriers were compared. Significant results emerged in the fusiform gyrus in the current study only when relative age was covaried with fMRI activity in carriers alone. Additionally, our significant results occurred in a region that was also preferentially active in those with higher AD risk in past studies of older (Bondi et al., 2005) but not younger (Filippini et al., 2009) adults, whose brain activity would presumably continue to change as they neared a typical age of late AD onset. Together this suggests that differential fMRI activity in this region during a novelty encoding task is not specific to FAD, but may also apply to brain changes in incipient late onset AD.
We did not see a significant effect of relative age on fMRI signal in any region other than the fusiform and middle temporal gyri, while the previous authors found differential fMRI activity in the fusiform and parahippocampal gyri, right hippocampus, middle frontal and medial frontal gyri as well as the cerebellum and superior parietal lobule (Bondi et al., 2005). It is possible that in some of these regions, the relationship between risk genotype and fMRI activity during a novel versus repeated items contrast was either specific to late onset AD or to differences inherent to possession of an APOE4 allele but possibly not related directly to AD processes. Such differences could occur developmentally and may or may not create vulnerabilities to later developing the disease as AD-related pathology accumulates. One study that examined fMRI activity in young adults (aged 20–35) during a similar task also found that greater fMRI activity was associated with APOE4 in the right hippocampus and cerebellum in young adults (Filippini et al., 2009). Since AD associated with APOE4 risk would not be expected for several decades after, those results suggest that in the previous studies, either differential fMRI activity in these regions was associated with developmental processes specific to APOE4 or that it related to very slowly developing AD-related changes not found in FAD. It is likely that as these young APOE4+ adults grew closer to the age of AD onset, they would gain additional differences in fMRI activity compared with APOE4− adults, possibly similar to the fusiform gyrus increases seen in our study and in older APOE4+ adults (Bondi et al., 2005). It is also possible that the inclusion of MCI patients in our study created non-linear effects that were not identified by the general linear model in some brain regions.
Our findings of increased fusiform fMRI activity during novelty encoding in those with incipient AD are in contrast with results from other previous studies that examined the relationship between AD genetic risk and fMRI activity during single item encoding of novel images. Two previous studies found that compared with middle-aged adults having lower genetic risk for late onset AD, those having more genetic risk showed less fMRI activity in the medial temporal lobe (including the fusiform gyrus) while viewing novel versus previously viewed items (Johnson et al., 2006; Trivedi et al., 2006). The differences between our results and theirs may be due to dissimilarities in the tasks used. In our task and those used in some previous studies (Bondi et al., 2005; Filippini et al., 2009), novelty encoding occurred while subjects either intentionally learned both novel and repeated items (Bondi et al., 2005) or performed a button press in response to some characteristic of the pictures such that encoding was incidental (Filippini et al., 2009). In contrast, in the two studies that found increased fMRI activity in medial temporal lobe in response to novelty encoding, repeated items were learned before the scanning session, and subjects performed an intentional recognition task during the fMRI task (Johnson et al., 2006; Trivedi et al., 2006). Although the contrast of interest was novel versus repeated items for all studies, the addition of a recognition component to the task (Johnson et al., 2006; Trivedi et al., 2006) would have changed the underlying nature of the task and presumably would have made the task more difficult. In fact, in one of the studies, lower risk subjects responded more quickly to novel items than higher risk subjects did, suggesting that the lower risk subjects performed the task more easily (Johnson et al., 2006). Differences in such details of novelty encoding tasks have previously been shown to influence how brain activity changes with the development of mild cognitive impairment even creating opposing results within similar brain regions (Trivedi et al., 2008).
The fusiform and middle temporal gyri both have demonstrated decreases in cortical thickness in progressive mild cognitive impairment (Chetelat et al., 2005; Convit et al., 2000; Julkunen et al., 2009) and in older adults having memory complaints (Chao et al., 2010) compared with normal older adults. Additionally, cerebral hypoperfusion has been found in the left fusiform gyrus in patients with amnestic mild cognitive impairment (aMCI) who later convert to AD (Caroli et al., 2007). The left fusiform gyrus is likewise among the brain regions shown to have decreased resting state fMRI activity in aMCI patients compared with controls (Qi et al., 2010). Together, these findings suggest that changes to the fusiform and middle temporal gyrus occur in incipient AD, and that our findings may relate to these changes. Since our significant findings resulted from both increases in response to novelty and decreases in response to repeated items, changes to the resting state activity in this region are unlikely to explain our results in full.
Greater regional memory-related fMRI activity in those at increased risk for developing AD and in those who later decline cognitively has been demonstrated previously (Bookheimer et al., 2000; Miller et al., 2008; Wierenga et al., 2010). It has been hypothesized by some to represent compensation related to AD neuropathology and increased cognitive effort required to perform the same task (Bondi et al., 2005; Bookheimer et al., 2000; Dickerson et al., 2005). Alternatively, such increases may relate to inflammation and other aspects of neuronal distress caused by AD-related pathology and resulting in processes such as aberrant sprouting of cholinergic fibers or inefficient synaptic transmission (Sperling et al., 2010). Hyperperfusion of the medial temporal lobe also has been observed in persons with established AD using arterial spin labeling at rest (Alsop et al., 2008). The current data does not allow us to determine the underlying cause of increases in fMRI activity we found in those at more imminent risk for AD.
Unlike a previous study using an overlapping set of subjects, no significant voxelwise differences in fMRI activity were found during a novelty encoding task when those who carried FAD mutations were compared with their non-mutation carrying relatives. In the previous study we found that mutation non-carriers showed increased activity in the anterior cingulate gyrus and left fronto-polar region compared with cognitively intact mutation carriers (Ringman et al., 2010). However, when mean fMRI activity in this region was evaluated instead using an ROI in the current study, we likewise found that non-carriers showed more fMRI activity than carriers while viewing novel versus repeated items. Additionally, mutation carriers with MCI showed significantly more fMRI activity than cognitively intact mutation carriers, but were not significantly different from non-carriers. These results suggest that cognitively intact mutation carriers and non-carriers process novel and repeated items differently, and that processing changes in carriers as they become cognitively impaired. It is likely that a mutation carrier versus non-carrier finding was not evident in a voxelwise analysis in the current study largely because a voxelwise analysis requires stronger statistical evidence than an ROI analysis to overcome corrections for multiple comparisons, and the five mildly impaired subjects who were included currently weakened the relationship found previously. Additionally, in the current study we controlled for relative age within mutation status and cognition, while in the previous study we instead controlled globally for relative age across groups, familial gene mutation, and APOE genotype status.
Some limitations to interpretations of our data exist. It is possible that due to small sample sizes, we lacked the statistical power to detect additional differences between mutation carriers and non-carriers or relationships with relative age in mutation carriers. We therefore cannot exclude the possibility that such differences exist. Additionally, our voxelwise analyses were performed using parametric statistical tests. We then verified results that appeared to be significant by using an ROI approach and non-parametric testing. Although this gives us confidence that our reported results are not due to outliers, it may result in underreporting of other regions where true relationships are obscured by the inclusion of such data points.
We found that as they neared the estimated age of disease onset, adults destined to develop AD showed increases in fMRI activity in the fusiform and middle temporal gyri in response to novel versus repeated picture stimuli. The increases in fMRI activity in the fusiform gyrus during novelty encoding were consistent with those seen previously in older (Bondi et al., 2005), but not younger (Filippini et al., 2009) APOE4+ adults. Taken together, our results suggest that in adults having greater genetic risk for AD, increased fMRI activity in the fusiform gyrus during novelty encoding is a possible indicator of incipient AD, and that this indicator is not specific to one type of genetic risk factor. Future novelty encoding studies that examine fMRI activity differences in carriers of other genetic risk factors for AD such as CLU and PICALM (Harold et al., 2009; Lambert et al., 2009) and studies that examine fMRI activity in concert with amyloid PET imaging may help elucidate the applicability of our results to incipient AD more generally.
Acknowledgements
This study was supported by PHS K08 AG-22228, California DHS #04-35522, UC MEXUS, and the Shirley and Jack Goldberg Trust. Further support for this study came from Alzheimer's Disease Research Center Grant P50 AG-16570 from the National Institute on Aging, General Clinical Research Centers Program M01-RR00865, and an Alzheimer's Disease Research Center of California grant, the Sidell Kagan Foundation, and the Easton Consortium for Alzheimer's Disease Drug Discovery and Biomarkers. MNB was funded in part by the NIH (T32 NS048004:05).
We are very grateful for the training data for FIRST, particularly to David Kennedy at the CMA, and also to: Christian Haselgrove, Centre for Morphometric Analysis, Harvard; Bruce Fischl, Martinos Center for Biomedical Imaging, MGH; Janis Breeze and Jean Frazier, Child and Adolescent Neuropsychiatric Research Program, Cambridge Health Alliance; Larry Seidman and Jill Goldstein, Department of Psychiatry of Harvard Medical School; Barry Kosofsky, Weill Cornell Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There were no actual or potential conflicts of interest that could inappropriately bias the authors work on this project.
References
- Alsop DC, Casement M, de Bazelaire C, Fong T, Press DZ. Hippocampal hyperperfusion in Alzheimer's disease. Neuroimage. 2008;42:1267–1274. doi: 10.1016/j.neuroimage.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artiola-i-Fortuny L, Hermosillo D, Heaton RK, Pardee RE. Manual de Normal y Procedimientos para la Bateria Neuropsicologia en Espanol. Tucson: Neuropsychology Press; 1999. [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer's disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64:501–508. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Caroli A, Testa C, Geroldi C, Nobili F, Barnden LR, Guerra UP, Bonetti M, Frisoni GB. Cerebral perfusion correlates of conversion to Alzheimer's disease in amnestic mild cognitive impairment. J Neurol. 2007;254:1698–1707. doi: 10.1007/s00415-007-0631-7. [DOI] [PubMed] [Google Scholar]
- Chao LL, Mueller SG, Buckley ST, Peek K, Raptentsetseng S, Elman J, Yaffe K, Miller BL, Kramer JH, Madison C, Mungas D, Schuff N, Weiner MW. Evidence of neurodegeneration in brains of older adults who do not yet fulfill MCI criteria. Neurobiol Aging. 2010;31:368–377. doi: 10.1016/j.neurobiolaging.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, Desgranges B, Baron JC. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer's disease. Neurobiol Aging. 2000;21:19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmorey K, Xu J, Gannon P, Goldin-Meadow S, Braun A. CNS activation and regional connectivity during pantomime observation: no engagement of the mirror neuron system for deaf signers. Neuroimage. 2010;49:994–1005. doi: 10.1016/j.neuroimage.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol. 2005;62:1881–1888. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox NC, Kennedy AM, Harvey RJ, Lantos PL, Roques PK, Collinge J, Hardy J, Hutton M, Stevens JM, Warrington EK, Rossor MN. Clinicopathological features of familial Alzheimer's disease associated with the M139V mutation in the presenilin 1 gene. Pedigree but not mutation specific age at onset provides evidence for a further genetic factor. Brain. 1997;120(Pt 3):491–501. doi: 10.1093/brain/120.3.491. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Prestia A, Rasser PE, Bonetti M, Thompson PM. In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer's disease. J Neurol. 2009;256:916–924. doi: 10.1007/s00415-009-5040-7. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, D'Esposito M. Functional connectivity during working memory maintenance. Cogn Affect Behav Neurosci. 2004;4:580–599. doi: 10.3758/cabn.4.4.580. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schurmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frolich L, Hampel H, Hull M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Muhleisen TW, Nothen MM, Moebus S, Jockel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O'Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer's disease in normal aging. Ann Neurol. 2006;59:673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkunen V, Niskanen E, Muehlboeck S, Pihlajamaki M, Kononen M, Hallikainen M, Kivipelto M, Tervo S, Vanninen R, Evans A, Soininen H. Cortical thickness analysis to detect progressive mild cognitive impairment: a reference to Alzheimer's disease. Dement Geriatr Cogn Disord. 2009;28:404–412. doi: 10.1159/000256274. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fievet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossu P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanche H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Langbaum JB, Chen K, Lee W, Reschke C, Bandy D, Fleisher AS, Alexander GE, Foster NL, Weiner MW, Koeppe RA, Jagust WJ, Reiman EM. Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI) Neuroimage. 2009;45:1107–1116. doi: 10.1016/j.neuroimage.2008.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Backman L, Nilsson LG, Petersson KM, Nyberg L. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006;129:1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- Loewenstein DA, Rubert MP, Arguelles T, Duara R. Neuropsychological test performance and prediction of functional capacities among Spanish-speaking and English-speaking patients with dementia. Arch Clin Neuropsychol. 1995;10:75–88. [PubMed] [Google Scholar]
- McCurry SM, Edland SD, Teri L, Kukull WA, Bowen JD, McCormick W, Larson EB. The Cognitive Abilities Screening Instrument (CASI): Data from a cohort of 2524 cognitively intact elderly. Int J Geriatr Psychiatry. 1999;14:882–888. [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9 Suppl 1:173–176. doi: 10.1017/s1041610297004870. discussion 177–178. [DOI] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Crane PK, Haan MN, Gonzalez H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16:347–359. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Murrell J, Ghetti B, Cochran E, Macias-Islas MA, Medina L, Varpetian A, Cummings JL, Mendez MF, Kawas C, Chui H, Ringman JM. The A431E mutation in PSEN1 causing familial Alzheimer's disease originating in Jalisco State, Mexico: an additional fifteen families. Neurogenetics. 2006;7:277–279. doi: 10.1007/s10048-006-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Aronen HJ, Soininen H. Distinct and overlapping fMRI activation networks for processing of novel identities and locations of objects. Eur J Neurosci. 2005;22:2095–2105. doi: 10.1111/j.1460-9568.2005.04380.x. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, K OK, Bertram L, Tanzi RE, Dickerson BC, Blacker D, Albert MS, Sperling RA. Evidence of altered posteromedial cortical FMRI activity in subjects at risk for Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:28–36. doi: 10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Z, Wu X, Wang Z, Zhang N, Dong H, Yao L, Li K. Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage. 2010;50:48–55. doi: 10.1016/j.neuroimage.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Ringman JM, O'Neill J, Geschwind D, Medina L, Apostolova LG, Rodriguez Y, Schaffer B, Varpetian A, Tseng B, Ortiz F, Fitten J, Cummings JL, Bartzokis G. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Medina LD, Braskie M, Rodriguez-Agudelo Y, Geschwind DH, Macias-Islas MA, Cummings JL, Bookheimer S. Effects of Risk Genes on BOLD Activation in Presymptomatic Carriers of Familial Alzheimer's Disease Mutations during a Novelty Encoding Task. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq158. doi:10.1093/cercor/bhq158, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemper M. Non-Parametric Partial Association Revisited. The Statistician. 1991;40:73–76. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC. Reduced hippocampal activation during episodic encoding in middle-aged individuals at genetic risk of Alzheimer's disease: a cross-sectional study. BMC Med. 2006;4 doi: 10.1186/1741-7015-4-1. doi:10.1186/1741-7015-1184-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Hess TM, Fitzgerald ME, Atwood CS, Rowley HA, Asthana S, Sager MA, Johnson SC. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: risk factors for Alzheimer's disease. Neuropsychologia. 2008;46:1667–1678. doi: 10.1016/j.neuropsychologia.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, Moss HE. Processing objects at different levels of specificity. J Cogn Neurosci. 2004;16:351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised: Manual. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- Wierenga CE, Stricker NH, McCauley A, Simmons A, Jak AJ, Chang YL, Delano-Wood L, Bangen KJ, Salmon DP, Bondi MW. Increased functional brain response during word retrieval in cognitively intact older adults at genetic risk for Alzheimer's disease. Neuroimage. 2010;51:1222–1233. doi: 10.1016/j.neuroimage.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Xu G, McLaren DG, Ries ML, Fitzgerald ME, Bendlin BB, Rowley HA, Sager MA, Atwood C, Asthana S, Johnson SC. The influence of parental history of Alzheimer's disease and apolipoprotein E epsilon4 on the BOLD signal during recognition memory. Brain. 2009;132:383–391. doi: 10.1093/brain/awn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. J Neurosci. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11:668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]