Abstract
L-type calcium channels (LTCC) impact the function of nearly all excitable cells. The classical LTCC function is to mediate trans-sarcolemmal Ca2+ flux. This review focuses on the contribution of a mobile segment of the LTCC that regulates ion channel function, and also serves as a regulator of transcription in the nucleus. Specifically we highlight recent work demonstrating an auto-feedback regulatory pathway whereby the LTCC transcription factor regulates the LTCC. Also discussed is acute and long-term regulation of function by the LTCC-transcription regulator.
Introduction
L-type calcium channels (LTCC) couple membrane depolarization to cytosolic calcium entry. In turn, calcium bridges excitation to contraction in cardiac myocytes. Cytosolic Ca2+-entry via LTCC stimulates a larger release of Ca2+ from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyR2). Details of this process are the topic of excellent reviews [1, 2]. A second, less-appreciated contribution of Ca2+ in cardiac myocytes is to couple excitation to transcription. The goal of this review is to introduce a relatively new role for cardiac L-type calcium channels (LTCC) – a direct signal transduction pathway linking LTCC function to transcriptional regulation. The focus of this review is mainly on cardiac LTCC, but there are broader implications given that LTCC are expressed across a wide-range of tissue types [3].
The heart adapts to changing demands by matching output. This occurs on a broad range of time scales from acute beat-to-beat changes to long-term growth related changes. Acute sympathetic nervous stimulation causes a positive inotropism on a relatively rapid time scale. On a considerably longer time scale the growth of the heart during maturation is matched to its functional load[4]. Similarly, exercise or pregnancy stimulates heart growth[5] resulting in a physiological and reversible hypertrophy. Numerous signaling cascades are implicated in the regulation of heart growth including, but not limited to Ca2+-regulated processes[6]. Heart growth is defined here as a change in heart size, principally cardiomyocyte cell size. The detail of size alterations depend on the specific stimuli[7]. Pathological stimuli also promote heart growth, though the signaling pathways are distinct from growth stimulated by physiological cues[8]. Central to this review, cytosolic Ca2+ is a key contributor to a number of signaling systems. Ca2+-activated calcineurin (CaN), and calcium-calmodulin dependent kinase (CaMKII) are two examples of Ca2+-effectors in cardiomyocyte adaptive signaling. Alterations in cytosolic Ca2+-entry are upstream of these signaling cascades, and thus represent a potentially sensitive target for regulation of this diverse signaling. In this review we summarize findings that are consistent with LTCC sensing changes in Ca2+-entry, and then coupling such changes to reflexive adjustments in LTCC expression via a mobile segment derived from the LTCC.
1. LTCC structure
LTCC exists as part of a multi-protein complex in cardiomyocytes[9]. The main pore-forming subunit of the LTCC in the myocardium is CaV1.2. CaV1.2 is also expressed in vascular smooth muscle, pancreatic β-cells, neurons, and developing skeletal muscle. Closely related CaV1.1 is expressed in mature skeletal muscle. CaV1.3 is expressed in atrial myocytes, neurons, and chromaffin cells. CaV1.4 channels, largely expressed in retina complete the CaV1 family. This review focuses mainly on the CaV1.2 channel. Additional core components that are shared among a variety of cell types include a β-subunit, α2δ-subunit, and in some tissues a γ-subunit, including possibly the heart[10].
The CaV1.2 channel contains four homologous repeating units joined by cytosolic linkers. Each homologous repeat consists of six α-helical transmembrane segments, a β-sheet pore region, and a voltage sensor comprised of the four S4 transmembrane segments (reviewed by[11]). An amino-terminal cytosolic domain is immediately upstream of homologous repeat I, and a cytosolic domain is immediately downstream of homologous repeat IV, transmembrane segment 6 (IVS6) forming the carboxyl-terminus of CaV1.2.
The CaV1.2 carboxyl-terminus extends >600 amino acids from the end of IVS6. There are some discrepancies in size depending on splice variants, species, and tissue-specific expression (see below). The CaV1.2 carboxyl-terminus consists of two broad regions demarcated by a consensus calpain cleavage site[12]. Following the nomenclature presented by the Catterall lab, the upstream carboxyl-terminus is the proximal C-terminus (PCT), and the carboxyl end is the distal C-terminus (DCT; Figure 1). The PCT contains an IQ domain that serves as a calmodulin (CaM) interaction site [13, 14]. Multiple CaM interact with and modulate CaV1.2 function [15, 16] consistent with additional CaM binding sites on PCT [14, 17, 18]. Moreover, calmodulin kinase II (CaMKII) and the PCT IQ domain facilitates ICa,L[19, 20]. PCT also mediates interactions with RGK proteins[21]. A decade ago Bertil Hille in his classical text book noted that CaV1.n and CaV2.n channels have 7 known interacting proteins[11]. A recent quantitative proteomic study of CaV2 channels discovered ~200 candidate interacting proteins [22]. It is expected that a similar number of candidate interacting proteins would be found for CaV1-family channels. Thus, the CaV1.2 channel is a hub of protein-protein interactions.
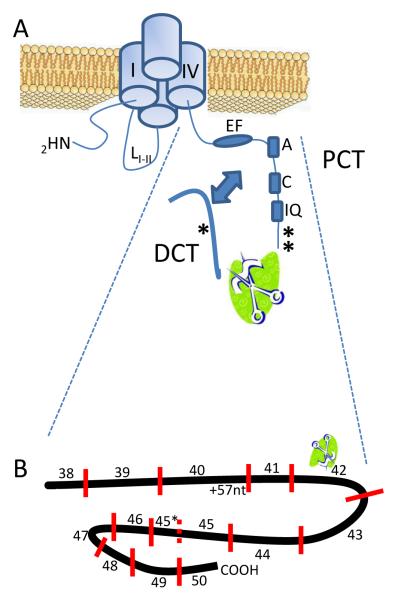
Figure 1.
A) Organization of cytosolic domains of the L-type calcium channel. The carboxyl-terminal domain is cleaved in approximately ½ resulting in a mobile distal C-terminus (DCT) separate from proximal C-terminus (PCT) of the pore-forming domains of channel. The arrow represents akap (ref) aiding dCT – PCT interaction. Asterisks (*) show phosphorylation sites.
B) Relationship of exon usage to PCT and DCT. The consensus calpain cleavage site is encoded within exon42.
A critical protein-protein interaction central to this review is the CaV1.2 – DCT interaction. The identification of CaV1.2 truncation at a consensus calpain substrate site [12, 23, 24], coupled with the presence of ~37kD protein recognized by a DCT-antibody [25] suggests that DCT is generated by proteolytic cleavage of the full-length CaV1.2 protein. A requirement for Ca2+ and calpain activity for PCT cleavage is inferred from sequence data – as yet there is no direct evidence for either a Ca2+ or calpain requirement for DCT liberation.
2. L-type Calcium Channel (cacna1c) Promoter
CaV1.2 is encoded by the cacna1c gene which is located on chromosome 6 in mouse and 12 in human. By all accounts it is a very complex locus. Databases list as many as 35 isoforms. In human there are 7 predicted alternative promoters with variations in the mRNA including 5′ and 3′ truncations[26]. CaV1.2 expression has been shown to be regulated by β–adrenergic stimulation[27, 28], α adrenergic stimulation[27-29], androgens[30, 31], elevated blood pressure[32], atrial tachycardia[33], endothelin via mitochondrial signaling[34], inflammation[35], and calcium entry through the channel[25, 36].
Much of the work examining the cacna1c promoter has been done in rat. Initial work demonstrated an approximately 2KB 5′ flanking region as a member of the TATA-less class of core promoters [37]. In this work binding sites for transcription factors such as NKX2.5, Mef2c, AP-1, a cAMP response element, and hormone binding sites were identified. In addition, this work demonstrated the tissue specificity of this promoter as well as identified a region critical for promoter activity, a minimal promoter.
The cacna1c gene in human and rat has two confirmed alternative promoters [38, 39] that result in differential initial exon usage[40]; reviewed by[41] . The distinct promoters drive the expression of two isoforms of CaV1.2, one predominates in cardiac tissue, and the other expressed in vasculature and other muscle tissues. The majority of the CaV1.2 in human cardiac tissue expresses a longer first exon[42]. Greater than 90% of human DCT appears as a single expressed set of exons [43]. Nonetheless, down-stream splicing variations have been noted. To date only a limited number of exon-usage patterns have been attributed with specific functional consequences. These include an exon8/8a variant that contributes to DHP sensitivity[44]. Carboxyl-terminus differential exon usage includes, in PCT an alternative 57 nucleotides following exon 40, and, in DCT a mutually exclusive exon45 versus 45* (Figure 1B). Differential exon usage in the carboxyl terminus influences L-type Ca2+ current (ICa,L). The shorter the DCT becomes, the greater the current amplitude. Exons 41-42 encode the distal end of the PCT, and influences voltage- and Ca2+-dependent inactivation [45-47].
Hormones, specifically testosterone, have been shown to alter the expression of CaV1.2 mRNA. Experiments examining coronary tissue from swine demonstrated enhanced expression of CaV1.2 in the male population in vivo, with an increased expression of CaV1.2 in response to testosterone in vitro. This in vitro response was blocked by an androgen antagonist suggesting testosterone may be a regulator of expression through interaction with hormone binding sites in the CaV1.2 promoter[48].
More recent work in HL-1 cells showed increased expression of CaV1.2 mRNA, protein and current in response to Angiotensin II treatment (AngII) [49]. Moreover, AngII increased CaV1.2 promoter activity [49]. A combination of mutation analysis and truncation of the promoter suggested that the upstream cAMP response element (cre) was the ANG II interaction site [49].
3. DCT in the nucleus regulates transcription
LTCC couples membrane depolarization to gene expression [50]. Several mechanisms have been proposed to explain excitation-transcription coupling: 1) signaling by Ca2+ secondary to LTCC-mediated cytosolic Ca2+-entry; 2) signaling by CaM / CaMKII; and 3) signaling by DCT.
1) LTCC activity is linked to transcriptional regulation of a variety of genes such as c-fos in hypoxia[51], RhoA/Rok, myocardin, and SRF pathways in smooth muscle[52], and in cardiac memory[53]. Cardiac memory describes electrical pacing induced ionic remodeling, leading to changes in action potential duration[54]. The transcription factor CREB (cAMP-responsive element binding protein) is decreased during cardiac memory induction. In contrast, long-term potentiation in neurons is associated with increased CREB. Regardless, in both cardiac memory and LTP, pharmacological blockade of LTCC prevents stimulus-transcriptional signaling (reviewed by [50]). The details relating nuclear signaling to LTCC Ca-entry are less clear. Ventricular pacing induced ionic remodeling requires CaMKII and NFAT activity [55]. Though this signaling requires ICa,L, alternative mechanisms for NFAT activation exist, including direct activation by IP3[56]. In this vein, agonist stimulated excitation-transcription coupling in cardiac myocytes can involve Ca2+-CaMKII- signaling that is apparently independent of beat to beat Ca-signaling [57]. These results are consistent with the notion that micro-domains of intracellular Ca2+ dictate downstream responses. Micro-domains of intracellular Ca2+ can arise from heterogeneous distributions of LTCC complex components, or temporal and spatial heterogeneity in local Ca2+. Restricted populations of LTCC show ICa,L modulation due to membrane sub-domains containing appropriate signaling complexes[58, 59]. By extension, it is plausible to speculate that heterogeneous LTCC complexes, and /or specialized sub-domain localization of LTCC are restricted for excitation-transcription coupling. A mutually exclusive hypothesis is that heterogeneous Ca2+ sub-domains form discrete upstream signals for transcriptional regulation. Cardiac myocytes show well-established temporal and spatial cytosolic Ca2+ heterogeneities. Temporally, Ca2+rises >10-fold from diastole to systole, and spatially the cleft between T-tubules and SR forms a restricted space. Increasing LTCC activity in cardiac myocytes is not necessarily sufficient to elicit transcriptional responses manifested as cardiac hypertrophy [60, 61]. This is consistent with the notion that peak (systolic) cleft Ca2+ is saturated at baseline. This raises the idea that diastolic Ca2+ is an important determinant for myocardial transcriptional signaling. To date, interrogation of cleft diastolic Ca2+ has been hampered by sufficient spatial and temporal resolution.
2) Additional interest in ICa,L CREB signaling stems from the observation that CREB, acting on a cre element on the CaV1.2 promoter transcriptionally regulates CaV1.2[49]. In neurons, LTCC function has been implicated in CREB signaling [62-65]. LTCC activity stimulates nuclear localization of phosphorylated CREB (pCREB) via CaM/CaMKII signaling [66]. LTCC-induced activation of CaMKII parallels CREB signaling leading to the conclusion that CaMKII localized to LTCC PCT domains may provide a link from LTCC to nucleus. DCT may also be involved in transducing LTCC – CREB signaling. Anchoring recombinant CaV1.2 DCT inhibits CREB-dependent transcription in heterologous expression systems[67].
3) Direct DCT transcriptional signaling. A mutually exclusive alternative signal transduction mechanism is that DCT links LTCC activity to transcriptional signaling. Our first clue that the DCT was more than a traditional ion channel regulator was the observation that a substantial fraction of DCT localizes to the nucleus of cardiomyocytes (photo Figure 2). Dolmetsch’s laboratory reported DCT nuclear localization in neurons from the brain, and over-expressed in HEK 293T cells[68]. In neurons DCT regulates transcription of a variety of genes including up-regulation of connexin31.1 and down-regulation of NaCa-exchanger 1 [68]. In addition, DCT interacts with nuclear proteins, and stimulates neurite outgrowth[68]. Thus DCT function is multi-faceted. At the cell membrane DCT regulates LTCC current, and in the nucleus DCT is a regulator of transcription.
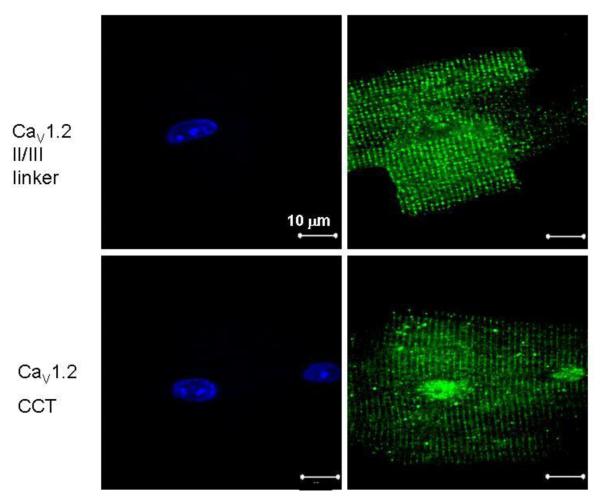
Figure 2.
CaV1.2, upstream of DCT is localized in a T-tubule restricted pattern whereas DCT also localizes to the nucleus in adult mouse ventricular myocytes. Confocal micrographs: Left panels: DAPI; right panels – antibody staining. Negative controls of no primary antibody yielded no detectable fluorescence.
On general terms homeostatic feedback loops require a sensor, a signal transducer or transfer component, and a resulting output signal. Pharmacological and genetic chronic blockade of ICa,L resulted in up-regulation of CaV1.2 protein[69, 70]. This suggested the intriguing possibility that LTCC serve as sensor, and via unknown transfer component, was also the target of its own function. There are numerous signaling cascades activated by LTCC Ca2+-entry each of which has numerous intermediaries. DCT, by contrast, represents a privileged pathway for communication between LTCC function and transcription. One requirement for DCT homeostatic auto-regulation of LTCC (including DCT) is that DCT interacts with the CaV1.2 promoter. Chromatin immunoprecipitation assays established DCT – CaV1.2 promoter interaction with elements containing NKX2.5/MEF, C/EBP, and CRM1 sites, but not with a cre-containing element [25]. Moreover, nuclear localized DCT restricted CaV1.2 transcription, CaV1.2 mRNA, CaV1.2 protein, and LTCC current [25]. This raises the possibility that dependent on sub-cellular localization DCT can function as part of a sensor, a transfer element, and an effector mechanism in LTCC regulation (Figure 3). Hence, we use the terminology ‘LTCC auto-regulation.’
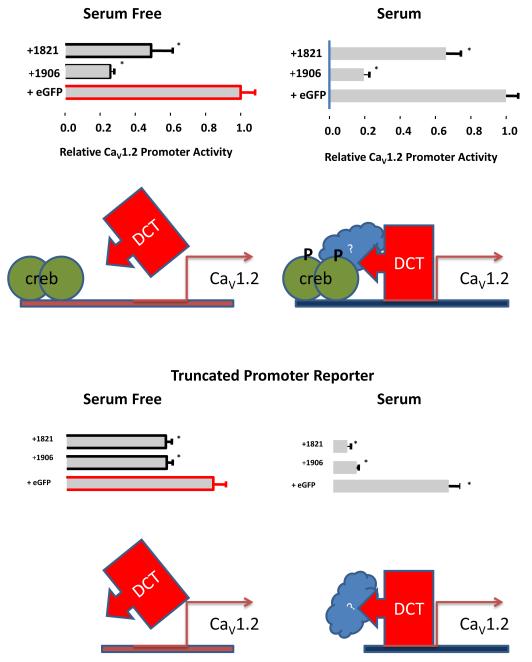
Figure 3.
Schematic diagram of DCT - CaV1.2 promoter regulation.
4. DCT signaling of cardiac myocyte growth
There is overwhelming evidence that LTCC pharmacological blockade reduces cardiac mass in patients. Animal studies suggest that LTCC pharmacological blockade can be mediated by direct myocardial actions, rather than activity secondary to relief of hypertension. In mice LTCC-mediated Ca2+ entry is a proximal signal for cardiac hypertrophy[71], and subpressor doses of nifedipine inhibit cardiac hypertrophy induced experimentally by pressure overload via aortic constriction[72]. LTCC blockade also inhibits cardiac hypertrophic signaling in vitro [73]. However, the linkage between LTCC signaling and hypertrophic growth is not entirely clear. Here we focus on potential contributions by DCT. Exogenous over-expressed DCT inhibits fetal bovine serum induced cardiac myocyte hypertrophy [25]. Atrial natriuretic factor (ANF) is marker for pathological cardiac hypertrophy (reviewed by [5]), and DCT over-expression also blocks serum-induced ANF expression. This is consistent with DCT interactions with multiple genes in cardiac myocytes as previously noted for neurons[68]. Closer examination of DCT repression of CaV1.2 transcription revealed a potentially complex mechanism of action (summarized in Figure 4). Deletion of the cre element from the CaV1.2 promoter reduces promoter activity [37, 38, 49], and mutation of the cre interaction site eliminates angiotensin II regulation of CaV1.2 expression [49]. DCT does not apparently directly interact with the upstream cre element, but the cre element interacting proteins may influence DCT regulation of CaV1.2 promoter activity. In absence of growth factor, DCT inhibition of CaV1.2 promoter activity is significantly blunted. Thus, DCT may contribute to Ca homeostasis by governing CaV1.2 increases of expression.
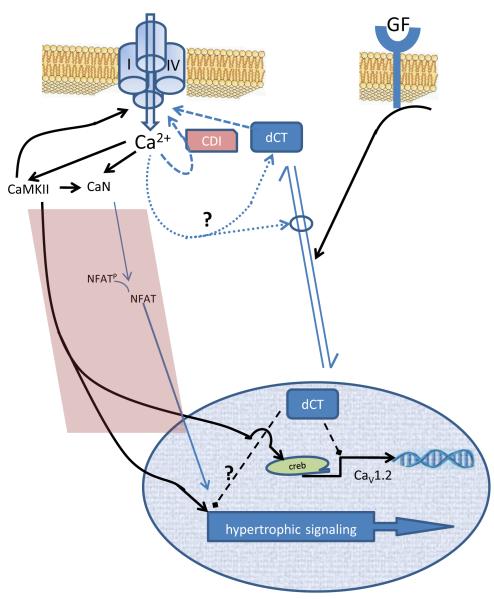
Figure 4.
The LTCC - dCT signaling axis. DCT confers signaling in distinct domains of the cell (right side of diagram). In the vicinity of the channel DCT inhibits LTCC current. Ca 2+-dependent inactivation is the major modulator of ICa,L whereby local LTCC Ca-entry leads to Ca 2+-CaM interaction on the PCT driving channel closure/inactivation. In contrast, DCT inhibits LTCC Ba2+ current suggesting Ca 2+-independent current blockade. In the nucleus DCT regulates multiple genes including an interaction with the CaV1.2 promoter resulting in inhibition of transcription. In neurons, elevated cytosolic Ca2+ favors increased cytosolic / nuclear localization. Though speculative, this suggests a negative feedback loop whereby excessive ICa,L is countered by additional DCT to provide additional ICa,L blockade. The left side of the diagram (shaded area) depicts feed-forward loops. Increased ICa,L can increase CaMKII and directly or indirectly increase calcineurin (CaN). CaMKII and CaN may lead to increased hypertrophic signaling. The caveat, however, is that it is unclear whether specific sub-domains of Ca2+ are required for hypertrophic signaling. Within the nucleus DCT may also interfere with hypertrophic signaling; although, the effect of dCT on hypertrophy may be secondary to DCT effects on Ca2+ signaling. The right side of the diagram represents data supporting growth factors increasing nuclear localization of DCT.
For DCT to function as a transcriptional regulator it must be present in the nucleus. DCT is a predicted 37-40 kDa protein and thus requires regulated cellular localization rather than simple passive diffusion. To date, only two publications document DCT sub-cellular localization [25, 68]. Exposure of neurons to a depolarizing, high-K+ bath solution induces relatively less nuclear localization, and this effect is reversed by LTCC blockade or chelation of bath Ca2+[68]. In cardiac myocytes, serum promotes nuclear localization [25]. Thus, the simplest explanation for serum inhibition of CaV1.2 expression is that serum-signaling promotes nuclear localization (Figure 4), or perhaps inhibits nuclear exclusion. DCT is a repressor of CaV1.2 expression, therefore, more nuclear DCT results in less CaV1.2 transcription. These ideas require further examination.
5. LTCC, DCT, and acute feedback regulation by Ca2+
The unambiguous contribution of LTCC to function is to permit cytosolic Ca2+-entry. Depolarization opens LTCC, Ca2+-entry occurs, and LTCC inactivation commences. Two major mechanisms limit Ca2+-entry: voltage-dependent inactivation (VDI), and Ca2+-dependent inactivation (CDI)[74]. VDI may dominate in basal state, whereas CDI becomes a dominate mechanism following β-adrenergic stimulation[75]. CDI occurs when Ca permeating via the LTCC interacts with CaM that is pre-bound to the LTCC. Mutagenesis, functional, and structural studies show that the CaV1.2 PCT region is a major contributor to CDI. CaV1.2 truncation mutants lacking DCT continue to exhibit CDI [76, 77]; however, immobilization of DCT inhibits CDI [78]. Thus, DCT is not required, but contributes to ICa,L kinetics – including local feedback signaling by permeating Ca2+.
6. Modulation of LTCC Involves DCT
ICa,L via LTCC triggers a greater ryanodine receptor-based Ca2+ release in cardiomyocytes. Thus subtle changes in LTCC function lead to greater downstream changes. Sympathetic stimulation of cardiomyocytes causes activation of the β-adrenergic signaling axis, ultimately increasing protein kinase A (PKA) activity. LTCC are a key substrate for β-adrenergic activated protein kinase A. Thus, a critical element of understanding the β-adrenergic signaling axis is a more detailed understanding of LTCC modulation. Investigations aimed at elucidating PKA-phosphorylation-substrate sites on CaV1.2 have been an area of intensive examination yet remain unsettled in some important respects. Early studies showed that a PKA consensus site on DCT, Ser1928 (numbering using the rabbit ortholog) is phosphorylated by PKA following β-adrenergic stimulation [79-81]; however, follow-up studies convincingly argued against an ICa,L-modulation effect resulting from DCT Ser1928 phosphorylation. Recombinant channels expressed in non-excitable cells do not reconstitute the β-adrenergic – LTCC signaling axis. To overcome this limitation, a clever approach was designed to assess CaV1.2 point mutations in cardiomyocytes. The nifedipine-binding site was previously localized to domain III transmembrane segment 5 (IIIS5) and IVS6 [82]. Point mutations in these domains rendered CaV1.2 fully functional but resistant to dihydropyridines (DHP) such as nifedipine. Thus, DHP-resistant channels over-expressed in cardiomyocytes could be distinguished from endogenous LTCC simply by recording ICa,L in the presence of a dose of DHP to provide maximal native ICa,L blockade. A DCT point mutation at Ser1928 to Ala on this DHP-resistant channel over-expressed in cardiomyocytes resulted in channel modulation in response to β-adrenergic stimulation[83]. Similarly, a genetic-engineered mouse carrying a knock-in of a Ser1928-CaV1.2 channel retained β-adrenergic modulation of ICa,L[84]. Truncation of DCT at position 1905 significantly blunted modulation[83]. Thus, DCT is necessary for the sympathetic stimulation induced increase of ICa,L. Even though DCT is phosphorylated by PKA, apparently phosphorylation of DCT is not required for channel modulation.
More recently, Catterall’s laboratory reported a comprehensive series of recombinant channel studies. PKA modulation of ICa,L can be reconstituted with recombinant channels with the co-expression of A-kinase anchoring protein (AKAP) along with LTCC subunits[85]. Site directed mutagenesis experiments established that Ser1700 and Ser1704 on the proximal CT (PCT) are substrates for AKAP-localized PKA, and that these PCT phosphorylation-substrates are required for ICa,L modulation by PKA[86]. Although DCT was required for modulation, DCT phosphorylation was not necessary. Therefore, DCT contributes to ICa,L modulation, but the functional consequence of DCT phosphorylation is unknown. It should be noted here that DCT is also a substrate for PKC[10], and functional consequences require further exploration.
7. Perspective
LTCC Ca2+-entry regulates numerous effector pathways that ultimately regulate transcription. In turn, LTCC are regulated by a variety of physiological and pathophysiological stimuli. DCT represents a possible privileged signaling pathway between functioning LTCC and the nucleus. A major question that needs attention is the relative importance of DCT among LTCC-dependent signaling cascades. Further complexity, or perhaps opportunities, for signal transduction crosstalk may arise from the known interactions among DCT – AKAP – CaN – and LTCC-PCT domains.
Acknowledgements
We are grateful for the many insightful discussions with Dr Douglas Andres. This work was supported by NIH HL074091 (JS), and an NIH Interdisciplinary Cardiovascular Sciences Training Grant HL-072734 (SMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 2.Dibb KM, Graham HK, Venetucci LA, Eisner DA, Trafford AW. Analysis of cellular calcium fluxes in cardiac muscle to understand calcium homeostasis in the heart. Cell Calcium. 2007;42:503–12. doi: 10.1016/j.ceca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42:345–50. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Zak R. Growth of the Heart in Health and Disease. Raven Press; New York: 1984. [Google Scholar]
- 5.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006;116:623–6. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn GW, 2nd, Robbins J, Sugden PH. Phenotyping hypertrophy: eschew obfuscation. Circ Res. 2003;92:1171–5. doi: 10.1161/01.RES.0000077012.11088.BC. [DOI] [PubMed] [Google Scholar]
- 8.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 9.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–52. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Katchman A, Morrow JP, Doshi D, Marx SO. Cardiac L-type calcium channel (Cav1.2) associates with {gamma} subunits. FASEB J. doi: 10.1096/fj.10-172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hille B. Ion Channels of Excitable Membranes. Sinauer Associates, Inc.; Sunderland, MA: 2001. [Google Scholar]
- 12.Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG, 2nd, Bigelow DJ, Catterall WA. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:5274–9. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuhlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the(alpha)1C subunit. J Biol Chem. 2000;275:21121–9. doi: 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]
- 14.Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of CaM tethering and Ca{super2+}-dependent inactivation of L-type Ca{super2+} channels. J. Biol. Chem. 2001 doi: 10.1074/jbc.M104959200. M104959200. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Rumpf CH, Van Petegem F, Arant RJ, Findeisen F, Cooley ES, Isacoff EY, Minor DL., Jr. Multiple C-terminal tail Ca(2+)/CaMs regulate Ca(V)1.2 function but do not mediate channel dimerization. EMBO J. 29:4062. doi: 10.1038/emboj.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fallon JL, Baker MR, Xiong L, Loy RE, Yang G, Dirksen RT, Hamilton SL, Quiocho FA. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2+ calmodulins. Proc Natl Acad Sci U S A. 2009;106:5135–40. doi: 10.1073/pnas.0807487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pate P, Mochca-Morales J, Wu Y, Zhang JZ, Rodney GG, Serysheva II, Williams BY, Anderson ME, Hamilton SL. Determinants for calmodulin binding on voltage-dependent Ca2+ channels. J Biol Chem. 2000;275:39786–92. doi: 10.1074/jbc.M007158200. [DOI] [PubMed] [Google Scholar]
- 18.Mouton J, Feltz A, Maulet Y. Interactions of Calmodulin with Two Peptides Derived from the C-terminal Cytoplasmic Domain of the Cav1.2 Ca2+ Channel Provide Evidence for a Molecular Switch Involved in Ca2+-induced Inactivation. J. Biol. Chem. 2001;276:22359–22367. doi: 10.1074/jbc.M100755200. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y, Dzhura I, Colbran RJ, Anderson ME. Calmodulin kinase and a calmodulin-binding ‘IQ’ domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism. J Physiol. 2001;535:679–87. doi: 10.1111/j.1469-7793.2001.t01-1-00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–47. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang C, Crump SM, Jin L, Correll RN, Finlin BS, Satin J, Andres DA. Rem GTPase interacts with the proximal Ca(V)1.2 C-terminus and modulates calcium-dependent channel inactivation. Channels (Austin) :4. doi: 10.4161/chan.4.3.11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller CS, Haupt A, Bildl W, Schindler J, Knaus HG, Meissner M, Rammner B, Striessnig J, Flockerzi V, Fakler B, Schulte U. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc Natl Acad Sci U S A. 107:14950–7. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Jongh KS, Colvin AA, Wang KK, Catterall WA. Differential proteolysis of the full-length form of the L-type calcium channel alpha 1 subunit by calpain. J Neurochem. 1994;63:1558–64. doi: 10.1046/j.1471-4159.1994.63041558.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic Processing of the C Terminus of the alpha 1C Subunit of L-type Calcium Channels and the Role of a Proline-rich Domain in Membrane Tethering of Proteolytic Fragments. J. Biol. Chem. 2000;275:8556–8563. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- 25.Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104:1373–81. doi: 10.1161/CIRCRESAHA.108.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7(Suppl 1):S12, 1–14. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maki T, Gruver EJ, Davidoff AJ, Izzo N, Toupin D, Colucci W, Marks AR, Marsh JD. Regulation of calcium channel expression in neonatal myocytes by catecholamines. J Clin Invest. 1996;97:656–63. doi: 10.1172/JCI118462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan IQ, Chen B, Marsh JD. Transcriptional regulation of L-type calcium channel expression in cardiac myocytes. J Mol Cell Cardiol. 2000;32:1841–9. doi: 10.1006/jmcc.2000.1217. [DOI] [PubMed] [Google Scholar]
- 29.Golden KL, Ren J, Dean A, Marsh JD. Norepinephrine regulates the in vivo expression of the L-type calcium channel. Mol Cell Biochem. 2002;236:107–14. doi: 10.1023/a:1016112617817. [DOI] [PubMed] [Google Scholar]
- 30.Golden KL, Marsh JD, Jiang Y. Castration reduces mRNA levels for calcium regulatory proteins in rat heart. Endocrine. 2002;19:339–44. doi: 10.1385/ENDO:19:3:339. [DOI] [PubMed] [Google Scholar]
- 31.Golden KL, Marsh JD, Jiang Y. Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res. 2004;36:197–202. doi: 10.1055/s-2004-814445. [DOI] [PubMed] [Google Scholar]
- 32.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 33.Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–54. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan D, Xi Q, Pfeffer LM, Jaggar JH. Mitochondria control functional CaV1.2 expression in smooth muscle cells of cerebral arteries. Circ Res. 107:631–41. doi: 10.1161/CIRCRESAHA.110.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi XZ, Pazdrak K, Saada N, Dai B, Palade P, Sarna SK. Negative transcriptional regulation of human colonic smooth muscle Cav1.2 channels by p50 and p65 subunits of nuclear factor-kappaB. Gastroenterology. 2005;129:1518–32. doi: 10.1053/j.gastro.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 36.Davidoff AJ, Maki TM, Ellingsen O, Marsh JD. Expression of calcium channels in adult cardiac myocytes is regulated by calcium. J Mol Cell Cardiol. 1997;29:1791–803. doi: 10.1006/jmcc.1997.0406. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, Fan QI, El-Zaru MR, Vanderpool K, Hines RN, Marsh JD. Regulation of DHP receptor expression by elements in the 5′-flanking sequence. Am J Physiol Heart Circ Physiol. 2000;278:H1153–62. doi: 10.1152/ajpheart.2000.278.4.H1153. [DOI] [PubMed] [Google Scholar]
- 38.Pang L, Koren G, Wang Z, Nattel S. Tissue-specific expression of two human Ca(v)1.2 isoforms under the control of distinct 5′ flanking regulatory elements. FEBS Lett. 2003;546:349–54. doi: 10.1016/s0014-5793(03)00629-x. [DOI] [PubMed] [Google Scholar]
- 39.Saada NI, Carrillo ED, Dai B, Wang WZ, Dettbarn C, Sanchez J, Palade P. Expression of multiple CaV1.2 transcripts in rat tissues mediated by different promoters. Cell Calcium. 2005;37:301–9. doi: 10.1016/j.ceca.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Blumenstein Y, Kanevsky N, Sahar G, Barzilai R, Ivanina T, Dascal N. A novel long N-terminal isoform of human L-type Ca2+ channel is up-regulated by protein kinase C. J Biol Chem. 2002;277:3419–23. doi: 10.1074/jbc.C100642200. [DOI] [PubMed] [Google Scholar]
- 41.Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res. 2005;68:197–203. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Dai B, Saada N, Echetebu C, Dettbarn C, Palade P. A new promoter for alpha1C subunit of human L-type cardiac calcium channel Ca(V)1.2. Biochem Biophys Res Commun. 2002;296:429–33. doi: 10.1016/s0006-291x(02)00894-x. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Papp AC, Binkley PF, Johnson JA, Sadee W. Highly variable mRNA expression and splicing of L-type voltage-dependent calcium channel alpha subunit 1C in human heart tissues. Pharmacogenet Genomics. 2006;16:735–45. doi: 10.1097/01.fpc.0000230119.34205.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–32. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- 45.Klockner U, Mikala G, Eisfeld J, Iles DE, Strobeck M, Mershon JL, Schwartz A, Varadi G. Properties of three COOH-terminal splice variants of a human cardiac L-type Ca2+-channel alpha1-subunit. Am J Physiol. 1997;272:H1372–81. doi: 10.1152/ajpheart.1997.272.3.H1372. [DOI] [PubMed] [Google Scholar]
- 46.Soldatov NM, Zuhlke RD, Bouron A, Reuter H. Molecular structures involved in L-type calcium channel inactivation. Role of the carboxyl-terminal region encoded by exons 40-42 in alpha1C subunit in the kinetics and Ca2+ dependence of inactivation. J Biol Chem. 1997;272:3560–6. doi: 10.1074/jbc.272.6.3560. [DOI] [PubMed] [Google Scholar]
- 47.Soldatov NM, Bouron A, Reuter H. Different voltage-dependent inhibition by dihydropyridines of human Ca2+ channel splice variants. J Biol Chem. 1995;270:10540–3. doi: 10.1074/jbc.270.18.10540. [DOI] [PubMed] [Google Scholar]
- 48.Bowles DK, Maddali KK, Ganjam VK, Rubin LJ, Tharp DL, Turk JR, Heaps CL. Endogenous testosterone increases L-type Ca2+ channel expression in porcine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2091–8. doi: 10.1152/ajpheart.00258.2004. [DOI] [PubMed] [Google Scholar]
- 49.Tsai CT, Wang DL, Chen WP, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, Lai LP, Tseng YZ, Chiang FT, Lin JL. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–85. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Premkumar DR, Mishra RR, Overholt JL, Simonson MS, Cherniack NS, Prabhakar NR. L-type Ca(2+) channel activation regulates induction of c-fos transcription by hypoxia. J Appl Physiol. 2000;88:1898–906. doi: 10.1152/jappl.2000.88.5.1898. [DOI] [PubMed] [Google Scholar]
- 52.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–78. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 53.Plotnikov AN, Yu H, Geller JC, Gainullin RZ, Chandra P, Patberg KW, Friezema S, Danilo P, Jr., Cohen IS, Feinmark SJ, Rosen MR. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844–9. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 54.Rosen MR, Cohen IS. Cardiac memory … new insights into molecular mechanisms. J Physiol. 2006;570:209–18. doi: 10.1113/jphysiol.2005.097873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, Allen BG, Nattel S. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103:733–42. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 56.Rinne A, Blatter LA. Activation of NFATc1 is directly mediated by IP3 in adult cardiac myocytes. Am J Physiol Heart Circ Physiol. 299:H1701–7. doi: 10.1152/ajpheart.00470.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–82. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 107:747–56. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca2+-Dependent Transgenic Model of Cardiac Hypertrophy : A Role for Protein Kinase C{{alpha}} Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- 61.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–44. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy TH, Worley PF, Baraban JM. L-type voltage-sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–35. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- 63.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–31. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol. 2003;13:354–65. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 65.Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci STKE. 2003;2003:PE4. doi: 10.1126/stke.2003.166.pe4. [DOI] [PubMed] [Google Scholar]
- 66.Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol. 2008;183:849–63. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobrinsky E, Schwartz E, Abernethy DR, Soldatov NM. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J Biol Chem. 2003;278:5021–8. doi: 10.1074/jbc.M211254200. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel ca(v)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crump SM, Correll RN, Schroder EA, Lester WC, Finlin BS, Andres DA, Satin J. L-type calcium channel alpha-subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am J Physiol Heart Circ Physiol. 2006;291:H1959–71. doi: 10.1152/ajpheart.00956.2005. [DOI] [PubMed] [Google Scholar]
- 70.Schroder E, Magyar J, Burgess D, Andres D, Satin J. Chronic verapamil treatment remodels ICa,L in mouse ventricle. Am J Physiol Heart Circ Physiol. 2007;292:H1906–16. doi: 10.1152/ajpheart.00793.2006. [DOI] [PubMed] [Google Scholar]
- 71.Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ago T, Yang Y, Zhai P, Sadoshima J. Nifedipine inhibits cardiac hypertrophy and left ventricular dysfunction in response to pressure overload. J Cardiovasc Transl Res. 3:304–13. doi: 10.1007/s12265-010-9182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A beta1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol. 2006;291:H1299–308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- 74.Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–60. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- 75.Findlay I. Physiological modulation of inactivation in L-type Ca2+ channels: one switch. J Physiol. 2004;554:275–83. doi: 10.1113/jphysiol.2003.047902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron. 2001;31:973–85. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 77.Wahl-Schott C, Baumann L, Cuny H, Eckert C, Griessmeier K, Biel M. Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc Natl Acad Sci U S A. 2006;103:15657–62. doi: 10.1073/pnas.0604621103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kobrinsky E, Tiwari S, Maltsev VA, Harry JB, Lakatta E, Abernethy DR, Soldatov NM. Differential role of the alpha1C subunit tails in regulation of the Cav1.2 channel by membrane potential, beta subunits, and Ca2+ ions. J Biol Chem. 2005;280:12474–85. doi: 10.1074/jbc.M412140200. [DOI] [PubMed] [Google Scholar]
- 79.De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the alpha 1 subunit of the cardiac L-type calcium channel by adenosine 3′,5′-cyclic monophosphate-dependent protein kinase. Biochemistry. 1996;35:10392–402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 80.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during {beta}1-adrenergic regulation. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J. Identification of PK-A phosphorylation sites in the carboxyl terminus of L-type calcium channel alpha 1 subunits. Biochemistry. 1996;35:9400–6. doi: 10.1021/bi960683o. [DOI] [PubMed] [Google Scholar]
- 82.Hockerman GH, Peterson BZ, Johnson BD, Catterall WA. Molecular determinants of drug binding and action on L-type calcium channels. Annu Rev Pharmacol Toxicol. 1997;37:361–96. doi: 10.1146/annurev.pharmtox.37.1.361. [DOI] [PubMed] [Google Scholar]
- 83.Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–8. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–44. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–96. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 86.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]