Abstract
BACKGROUND
Reduced P3b event-related potentials (ERP) amplitude during visual target detection in alcoholics is a robust phenomenon. However, this finding is based primarily on samples of treated alcoholics, who comprise only about 25% of alcoholics. We studied visual target detection in a treatment-naïve alcohol dependent sample (TNAD) vs. age and gender comparable non-alcoholic controls (NAC) to investigate whether reduced P3 amplitudes generalize to TNAD.
METHODS
EEGs were recorded from 74 TNAD and 63 age and gender comparable NAC during visual target detection. ANOVA was applied at midline electrodes to amplitudes and latencies of N2 and P3 ERP components during target and rare non43 target conditions.
RESULTS
TNAD had a modestly lower P3b amplitude (p=.05) and a more robustly lower N2b amplitude (p=0.29). In the target condition, TNAD showed a significant reduction in P3b amplitude and a larger reduction in N2b amplitude, with these phenomena being independent of each other. Latencies to P3b, N2b and P3a were earlier in TNAD than NAC, with this effect correlating with our reported effect of better attention in TNAD vs. NAC.
CONCLUSION
The significant reduction of P3b amplitude in TNAD suggests that this phenomenon is present in TNAD, but dramatically smaller than that observed in treated samples (we reported an effect over 5 times as large in treated long-term abstinent alcoholics). The N2b amplitude reduction (not present in long-term abstinent alcoholics) may reflect the effects of active alcohol abuse. Finally, the shorter latencies of these components in TNAD is associated with better scores on tests of attention, and may reflect compensatory attentional effort in the context of active drinking.
Keywords: event-related potentials, alcoholism, genetic markers, effects of drinking
INTRODUCTION
One of the most robust electrophysiological findings in alcoholism research is the reduced amplitude of the P3b event-related potential in alcoholics and individuals at high risk for alcoholism. The P3b ERP component occurs when an individual attends or responds to an infrequent but task-relevant target stimulus. It is defined as a centro-parietal positive voltage peak in the ERP waveform occurring between 300 to 500 milliseconds after target stimulus presentation. Reductions in P3b amplitudes (more prominent in visual compared with auditory paradigms) have consistently been reported in chronic alcoholics (Cohen et al., 2002; 1997; 1995; Emmerson et al., 1987; Glenn et al., 1996; Kamarajan et al., 2005; Pfefferbaum et al., 1991; 1987; Porjesz et al., 1987a; Prabhu et al., 2001; Suresh et al., 2003), and in individuals at high genetic risk for alcoholism. It has been proposed that P3b reduction is not a consequence of the effects of chronic excessive alcohol exposure but, rather, an endophenotypic marker reflecting the genetic predisposition toward alcoholism (Begleiter et al., 1984; Cohen et al., 1997; Hill and Steinhauer, 1993; O'Connor et al., 1986; Porjesz and Begleiter, 1990; Van der Stelt et al., 1998).
The finding of reduced P3b in alcoholics derives mostly from clinical populations of treated alcoholics (and from their offspring). The danger of generalizing from clinical samples to all alcoholics has been demonstrated repeatedly by our group and others (di Sclafani et al., 2008; Fein et al., 2002; Fein and Landman, 2005; Fein et al., 2006b; O'Neill et al., 2001). Treatment-naïve actively drinking alcohol dependent individuals (TNAD) are active drinkers who meet criteria for current alcohol dependence, but who have neither sought nor received any form of alcoholism treatment. Since such untreated individuals make up the majority of alcoholics (U.S. Alcohol Epidemiologic Data Reference Manual Volume 8, 2006), further studies on TNAD are critical for advancing our understanding of alcoholism.
TNAD are not simply treated alcoholics observed early in their alcohol use histories but, rather, come from a different population than (later) treated alcoholics. In fact, they drank 50% less than long-term abstinent treated alcoholics (LTAA) in the period immediately after meeting criteria for heavy drinking (Fein and Landman, 2005). They have a lower family history density of alcohol problems than do LTAA (di Sclafani et al., 2008). They have less psychiatric comorbidity than LTAA, but more comorbidity than NAC (di Sclafani et al., 2008). TNAD showed less severe brain structural differences from NAC than do LTAA samples, with the primary finding in TNAD being a greater age-related decline in cortical gray matter when compared to non-alcoholic controls (Fein et al., 2002).
On a simulated gambling task (SGT), designed to examine the mechanisms behind skewed decision-making of substance abusers, Fein and colleagues’ (2006a) show that, in contrast to LTAA, TNAD showed normal decision-making. This is consistent with only moderate elevation of externalizing symptoms, social deviance proneness and family density of alcoholism in the TNAD sample compared to large elevations of these measures in LTAA (di Sclafani et al., 2007). Finally, we have recently shown intact cognition in TNAD vs. NAC, with an unanticipated finding of better performance of TNAD in the attention domain, possibly involving compensatory attentional strategies in heavy drinking functional alcoholics (Smith and Fein, 2010).
The studies summarized above, showing differences between treated and untreated alcoholics, motivated our examination of P3b amplitudes in treatment-naïve alcoholics. In the current study, we examine the visual P3b, N2b and N2a components of the event-related potential (ERP) response to target and rare non-target stimuli in a treatment-naïve alcohol dependent (TNAD) sample compared to age and gender comparable non-alcoholic controls (NAC). We examined the degree to which this comparison was modulated by the family history of alcoholism, the severity of alcohol abuse and the presence and severity of comorbid psychiatric disorders.
METHODS
Participants
Treatment-naïve alcohol dependent individuals (TNAD) were recruited from the community by cafe´ postings, newspaper advertisements, a local Internet site and subject referrals. The group consisted of TNAD individuals (37 women and 49 men, average age 31.2 years) who met DSM-IV (American Psychiatric Association, 2000) criteria for current alcohol dependence. The NAC group consisted of 30 women and 40 men (mean = 32.3 years of age). The inclusion criterion for NAC was a lifetime drinking average of less than 30 standard drinks per month, with no periods of drinking more than 60 drinks per month. Both groups had comparable years of education. Exclusion criteria for both groups were: (1) lifetime or current diagnosis of schizophrenia or schizophreniform disorder; (2) history of lifetime or current drug (other than nicotine or caffeine) dependence or abuse; (3) history of significant neurological disease, head trauma or cranial surgery; (4) history of diabetes, stroke, or hypertension that required emergent medical intervention; or (5) clinical evidence of the Wernicke–Korsakoff syndrome. TNAD and NAC samples were the same as reported on in Fein et al. (2010), with the NAC group of our current study being the same as the NAC group in our previous study, with a few subjects in both groups being excluded because of less than 20 artifact free EEG trials in each condition.
Procedure
Participant screening was initially conducted by phone interview, which assessed alcohol use/dependence, use/dependence of other drugs, medical history, and mental-health history. Participants who met all inclusion criteria and no medical or substance-related exclusion criteria were invited to complete a total of 4 sessions that included clinical, neuropsychological, electrophysiological, and neuroimaging assessments. All participants were informed of the study’s procedures and signed a consent form before their participation. The participants were asked to abstain from using alcohol for at least 24 hours before any lab visits, and a Breathalyzer test was administered to all subjects before each session. Subjects who completed testing were paid for time and travel and, those who completed the entire study, were also given a completion bonus.
During the first session, alcohol use history was assessed using the timeline follow-back methodology of the Lifetime Drinking History questionnaire (Skinner and Allen, 1982; Sobell and Sobell, 1990). Alcohol use variables for lifetime use and peak use phases were recorded with this methodology. The density of a family history of alcohol problems was assessed using the family history drinking questionnaire (Mann et al., 1985). The family history density (FHD) measure was the proportion of first-degree relatives who had alcohol problems. Although corroboration of these measures from relatives was not sought, the FHD measure has been shown to have high test–retest reliability and overall accuracy (Stoltenberg et al., 1998; Vogel-Sprott et al., 1985). Psychiatric diagnoses were assessed using the computerized Diagnostic Interview Schedule (c-DIS) (Robins et al., 1998). Participants were examined for a total of 6 anxiety disorders (social phobia, agoraphobia, panic disorder, PTSD, obsessive disorder, and compulsive disorder), 3 mood disorders (depression, dysthymia, and mania), and 2 externalizing disorders (conduct disorder and antisocial personality disorder). Lifetime symptom counts for all positive symptoms were obtained for each disorder, using the DSM-IV (American Psychiatric Association, 2000) symptom guidelines. The result of neuropsychological testing on a matched subset of the samples studied here was recently published (Smith and Fein, 2010) showing intact cognitive function in TNAD, with an unanticipated effect of better attention domain scores in TNAD, possibly reflecting compensatory attentional strategies in heavy drinking functional alcoholics. We examined the possible association between reduced ERP latencies reported below and attention domain scores. The attention domain score consisted of the average Z-score for the Stroop (Golden, 2002) Color condition, and the MicroCog (Powell et al., 1993) Numbers Forward, Numbers Reversed, Alphabet, and Word List 1 subtests.
VP3 Experimental Paradigm
All stimuli were presented on a computer monitor using the E-Prime software system (Psychology Software Tools Inc., Pittsburgh, PA). Stimuli were presented on a black screen for 200 ms, followed by a delay varying between 1,000 and 1,100 ms before the next stimulus. Three different types of visual stimuli were presented: (1) standard stimuli; a small hollow white square, (2) target stimuli; a small white X and (3) novel rare nontarget stimuli; different shapes of various colors. Participants were instructed to press a response box button only when they saw target stimuli. Stimuli were presented in a predetermined order, with standard stimuli appearing 210 times, target stimuli appearing 35 times, and rare non-target stimuli appearing 35 times over approximately 6.5 minutes. Each participant was shown an example of the target stimulus before the task began.
EEG Acquisition
EEG was acquired on three EEG acquisition systems (with two different amplifiers) during the course of the study. The first two were a 32-channel system and a 40-channel system, both of which used the NuAmps single-ended, 32/40-channel amplifier and Scan 4.2 Acquisition software (Compumedics Neuroscan Inc., El Paso, USA). The third was a 64-channel system which used the SynAmps2 amplifier and Scan 4.3 acquisition software (Compumedics Neuroscan Inc., El Paso, USA). Electrode sites Fz, FCz, Cz, CPz and Pz, which were the main electrode sites analyzed in this study, were common to all three systems. A right ear reference electrode was used for all recordings. The ground electrode was placed 4 cm above the nasion for the 32-channel and 40-channel caps, and 8 cm above the nasion for the 64-channel caps. Electrode site impedances were kept below 10 kΩ for all recordings. A vertical electro-oculogram (EOG) was recorded from bipolar electrodes placed above and below the left eye for use in offline reduction of ocular artifacts. The EEG and EOG channels were sampled at 250 Hz and stored for offline analysis. To ensure that between-amplifier comparisons were valid, data from control participants recorded on both amplifier systems (NuAmps, SynAmps2) were examined and revealed no differences associated with the different acquisition amplifiers (Andrew and Fein, 2010; Fein and Chang, 2008).
An assessment of Post-alcohol withdrawal hyper-excitability (PAWH) was implemented partway through the study, after which it was administered to 26 controls and 51 TNAD subjects. PAWH was measured using a self-report questionnaire where subjects estimated (on a 0 to10 point scale) the frequency and distress caused by physical and psychological symptoms experienced during alcohol withdrawal. For the frequency estimate, a 0 meant never, 1 corresponded to 10 % of the times one ceased drinking, up to a 10 which indicated the symptom was experienced 100% of the time one ceased drinking. For the degree of distress caused by the presence of the symptom, a 0 meant not at all distressing, a score of 5 meant somewhat distressing, and a 10 meant “unbearable.” The symptoms were compiled from the Diagnostic Interview Schedule (DIS) (Robins et al., 1998), the alcohol dependence scale (Skinner and Allen, 1982), and SSAGA interviews (Bucholz et al., 1994). We computed the average frequency and intensity over eight symptoms that measure PAWH: i) shakes (hands tremble, shake inside); ii) feel tense, nervous or anxious; iii) feel fidgety or restless; iv) have trouble concentrating v) heart pound or beat rapidly; vi) feel hypersensitive to stimuli (e.g. light, sound, touch); vii) have difficulty sleeping; and viii) have memory problems.
EEG Analysis
The Brain Vision Analyzer package (BVA, Brain Products, Munich, Germany) was utilized for processing of ERPs. Artifacts were removed using the Gratton and Coles method implemented in BVA (Gratton et al., 1983). Data were then band-pass filtered between 0.5 and 10 Hz using a zero-phase lag filter at 48 dB/octave. Within the time window from 200 to 500 ms (covering the N2-P3 complex), 0.5 – 10 Hz accounts for approximately 95% evoked power in the full bandwidth recordings. Stimulus-locked trials were extracted for all instances where there was a correct behavioral response for the target and rare non-target experimental conditions, with each trial comprising 100ms of data pre-stimulus and 750ms post-stimulus. The trials were baseline corrected using the 100ms pre-stimulus interval. Any trials with out of range voltages (± 75 mV) were rejected as artifact and excluded from further processing. If any subject had less than 20 trials per condition (target and rare non-target), that subject was eliminated from the analysis. On this basis, 10 TNAD and 5 NAC subjects were eliminated, leaving 76 TNAD (33 women and 43 men) and 65 NAC (28 women and 37 men) for further analyses (these are the subjects included in the various tables).
ERP waveforms were extracted by averaging all trials for each condition. Grand average ERP waveforms were produced by averaging the ERPs within a group by condition factor. Grand average topographical maps were also computed at each time point to display the ongoing spatial distribution of ERP amplitudes. Based on the grand average topographic maps, amplitudes and latencies of the ERP components at electrode Pz (P3b), Cz (P3a) and FCz (N2a and N2b) were extracted for each subject using a semi-automated peak detection algorithm. P3a and N2a were measured on each subject’s rare non-target grand average while P3b and N2b were measured on each subject’s target condition average. Peak locations were checked and adjusted manually where necessary and, if no discernable peak was present or the locations were ambiguous, that particular ERP component for that subject was coded as missing. Statistical analysis was then applied to the N2 and P3 amplitudes and latencies.
Statistical Analysis
All statistical analyses were performed using SPSS (SPSS Inc., Chicago, USA). Two-way group × gender analysis of variance (ANOVA) was conducted separately for the target and rare non-target conditions. For ERPs, separate ANOVAs were conducted for each component (N2a and b, P3a and b) and separately for amplitude and latency. Independence of P3 and N2 findings were examined via analysis of covariance. The associations between demographic, alcohol use and clinical variables, and the ERP measures, were examined using correlations and partial correlations.
RESULTS
Demographics and Subject Variables
Table 1 shows demographic, alcohol use and clinical measures for the TNAD and NAC groups. Both groups included subjects encompassing the entire range from 20 to 50 years; neither age nor years of education differed by group or gender. TNAD, on average, had a density of first degree relatives with alcohol problems that was 50% higher than NAC (F(1, 136) = 4.823, p = 0.030), but was markedly less than our previously reported results for long-term abstinent alcoholics (LTAA) (di Sclafani et al., 2008), about forty percent for LTAA vs. about twenty percent for TNAD (F(1, 119) = 21.080, p < 0.001, with group (LTAA vs. TNAD) accounting for 15 percent of the variance of family history density). As anticipated, alcohol consumption was dramatically greater in the TNAD vs. NAC, as these variables were associated with group selection criteria. TNAD drank over 12 times as much as NAC. We did not assess cigarette smoking in the study, but routinely do so in our current studies. As reported previously, there was also evidence of greater psychiatric comorbidity in the TNAD group, as reflected in higher Antisocial Personality Disorder and Depression symptom counts (di Sclafani et al., 2008).
Table 1.
Demographics, Symptom Counts and Alcohol Use Measures
| NAC |
TNAD |
Effect Sizes (% of Variance) |
|||||
|---|---|---|---|---|---|---|---|
| Variables | Male | Female | Male | Female | Group | Gender | Group × Gender |
| (n=37) | (n=28) | (n=43) | (n=33) | ||||
| Demographics | |||||||
| Age (y) | 32.1±8.4 | 32.4±8.4 | 31.7±8.1 | 30.8±7.9 | 0.4 | 0 | 1 |
| Years of education (y) | 16.0±1.8 | 16.4±1.4 | 16.2±1.9 | 15.8±1.7 | 0.8 | 0 | 1.4 |
| Family Drinking Densitya | 0.11±0.15 | 0.15±0.25 | 0.16±0.21 | 0.26±0.30 | 3.4* | 2.3 | 0.4 |
| (n=42) | |||||||
| Symptom Counts | |||||||
| Major Depressive Disorder | 3.6±6.4 | 7.8±8.5 | 8.7±9.0 | 8.2±7.1 | 3.0* | 1.3 | 2.2 |
| Anti-social Personality Disorder | 4.2±4.4 | 1.8±1.9 | 7.0±4.7 | 4.6±4.7 | 9.9*** | 7.5*** | 0 |
| Alcohol use variables | (n=35) | (n=26) | |||||
| Age started drinking (y) | 19.3±4.0 | 19.4±5.5 | 16.0±1.9 | 15.8±2.2 | 20.0*** | 0 | 0.1 |
| Average lifetime drinking dose (std. drinks/mo) | 6.7±6.5 | 5.8±5.1 | 94.9±43.5 | 64.7±30.6 | 62.5b | 6.9** | 6.2b |
| Duration of use (y) | 12.05±7.5 | 11.98±8.8 | 15.86±8.2 | 14.71±7.4 | 4.0b | 0.1 | 0.1 |
| Average peak dinking dose (std. drinks/mo) | 14.9±16.1 | 12.7±11.3 | 173.3±124.1 | 118.8±66.0 | 42.9b | 3.3* | 2.9 b |
| Duration of peak use (y) | 4.6±5.9 | 4.3±5.3 | 4.6±4.4 | 4.6±4.6 | 0.0b | 0 | 0.0 b |
| Post-Alcohol Witdrawal Hyperexcitability (PAWH) | |||||||
| Frequencyc | 0.45±0.50 | 0.49±0.67 | 1.7±1.0 | 2.4±1.7 | 29.5*** | 1.9 | 1.4 |
| Severity of Typical Withdrawal Episode | 0.44±1.0 | 0.79±0.93 | 2.4±1.4 | 3.0±1.9 | 33.4*** | 2.9 | 0.3 |
Demographic and alcohol measures are reported as mean values.
Proportion of first-degree relatives who are problem drinkers
Significance levels for group comparisons of alcohol use variables are not valid as alcohol use was part of the group selection criteria.
Percent of drinking occasions in which withdrawal is experienced: 1=10%, 2=20%, etc…
As described above, PAWH was measured using a self-report questionnaire where participants estimated (on a 10 point scale) first, the frequency and then, the distress level of physical and psychological symptoms experienced during alcohol withdrawal. Although neither group experienced a high frequency or intensity of PAWH, TNAD experienced both a greater frequency and intensity of PAWH than did NAC. For TNAD, the mean score (± sd) for the frequency of withdrawal symptoms was 2.06 ± 1.4 meaning that, on average, they experienced withdrawal symptoms after drinking 20% of the time. On the distress level scale (10 point scale), a zero indicated that the withdrawal symptoms bothered the participant “not at all”, a three indicated that the symptoms were “a little bothersome” and five indicated that the symptoms were “somewhat bothersome”. For TNAD, the mean score for distress was 2.70 ± 1.68, indicating that the participants typically found the distress of withdrawal symptoms less then “a little bothersome”. There were no significant associations between N2 or P3 measures and PAWH measures within either group.
ERP Amplitude and Latency
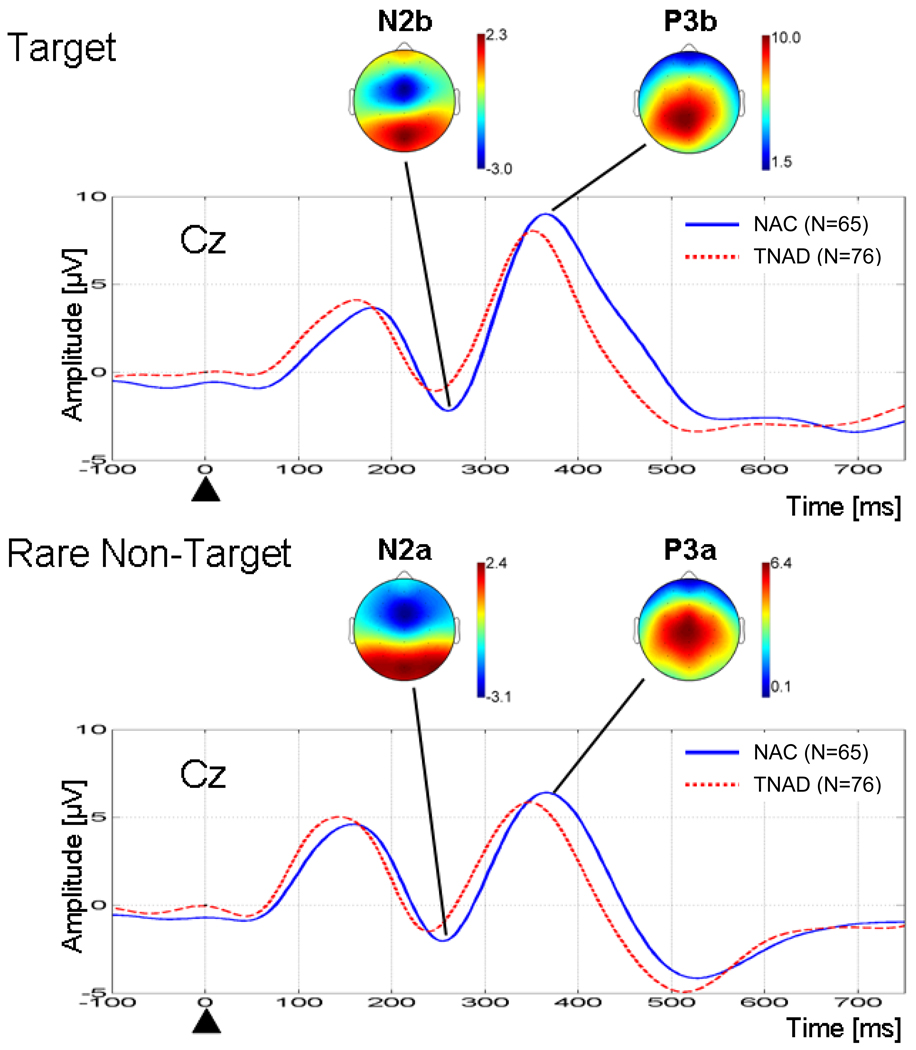
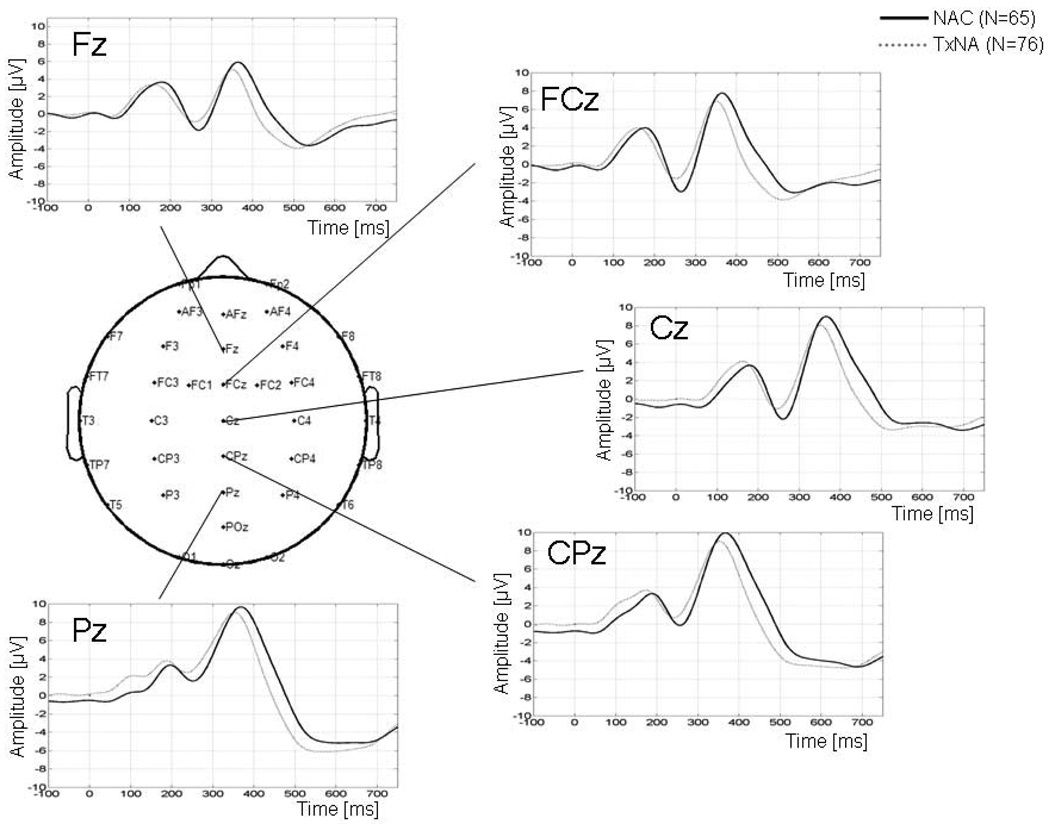
The comparison of grand average ERP waveforms, across groups for the target and rare non-target conditions at Cz, are shown in Figure 1. The spatial distribution for the N2 and P3 ERP components is shown for NAC (TNAD showed similar spatial distributions). P3b shows a parietal maximum around Pz, while P3a shows a more anterior localization over Cz. N2 shows a frontal maximum around FCz for both conditions, with a more focal distribution for the target vs. the rare non-target condition. Analyses of these components were carried out at these electrodes, where the component was maximal. Figure 2 shows the ERP waveforms for TNAD and NAC for the target condition for all midline electrodes.
Figure 1.
Grand average ERP waveforms for TNAD and NAC at electrode Cz for the target and rare non-target conditions. The black triangle indicates stimulus onset. For each condition, the NAC grand average topographic map at the latency of the N2 and P3 peak are shown.
Figure 2.
ERP waveforms across midline electrodes for the target condition for TNAD and NAC.
Table 2 shows the results of the group × gender ANOVA applied separately to the amplitudes and latencies of the N2 and P3 components. For the target condition, P3b amplitude was lower in TNAD (F(1, 135) = 3.914, p = 0.050), with the effect accounting for 2.8 % of the variance. The P3b latency was shorter in TNAD (F(1, 135) = 4.850, p = 0.029), with the effect accounting for 3.5% of the variance. TNAD had a larger reduction of N2b amplitude vs. NAC (F(1, 133) = 8.920, p = 0.003) than was present for P3b amplitude, with the effect accounting for 6.3% of the variance. The latency of the N2b component did not significantly differ between groups. For the rare-non target condition, both N2a and P3a latency were shorter in TNAD (F(1, 127) = 4.770, p = 0.031; and F(1, 134) = 14.5, p < 0.001), with the effects accounting for 3.6% and 9.8% of variance, respectively. The amplitudes of the N2a and P3a did not differ between groups. There were no significant gender, or group × gender, effects for effects in either the target or the rare non-target conditions. The group comparisons were repeated with total ASPD symptoms as a covariate and with total MDD (Major Depressive Disorder) as a covariate. A higher ASPD symptom count was associated with reduced P3b amplitude (accounting for 3.1 % of the variance), and this covariate effect accounted for the group difference in P3b amplitude. None of the other variables were affected by either covariate.
Table 2.
ERP Amplitude and Latency ANOVA
| NAC |
TNAD |
Effect Sizes (% of variance) |
||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Group | Gender | Group × Gender |
||
| ERPs | ||||||||
| Target Condition | ||||||||
| N2b | (n=36) | (n=27) | (n=42) | (n=32) | ||||
| Amplitude at FCz (µV) | −4.2±3.3 | −5.5±3.9 | −3.2±4.0 | −2.9±3.1 | 6.3** | 0.5 | 1.2 | |
| Latency at FCz (ms) | 266.4±27.1 | 267.3±20.9 | 262.1±32.6 | 258.5±35.2 | 1.2 | 0.1 | 0.1 | |
| P3b | ||||||||
| (n=43) | (n=33) | |||||||
| Amplitude at Pz (µV) | 12.3±5.2 | 13.1±5.3 | 10.4±4.9 | 11.8±4.2 | 2.8* | 1.2 | 0.1 | |
| Latency at Pz (ms) | 377.1±40.3 | 366.1±34.0 | 361.5±34.8 | 354.6±33.9 | 3.5* | 1.6 | 0.1 | |
| Rare Non-Target Condition | ||||||||
| (n=36) | (n=25) | (n=40) | (n=30) | |||||
| N2a | ||||||||
| Amplitude at FCz (µV) | −4.8±3.5 | −5.4±3.8 | −3.8±3.7 | −4.5±2.8 | 1.8 | 0.8 | 0.0 | |
| Latency at FCz (ms) | 258.4±26.7 | 267.7±33.4 | 249.3±30.9 | 254.1±26.2 | 3.6* | 1.4 | 0.1 | |
| P3a | ||||||||
| (n=36) | (n=27) | (n=42) | (n=42) | |||||
| Amplitude at Cz (µV) | 8.7±4.8 | 7.8±3.9 | 8.0±4.1 | 8.0±4.1 | 0.3 | 0.8 | 0.0 | |
| Latency at Cz (ms) | 368.4±35.8 | 378.8±37.6 | 351.6±42.7 | 351.6±42.7 | 9.8*** | 0.1 | 0.1 | |
Table 3 shows the correlations among the latency and amplitude measures for the target and rare non-target conditions. The four latency measures were highly correlated with each other with between 25 and 38% of the variance being shared between measures. The P3a and P3b amplitudes were also highly correlated, as were the N2a and N2a amplitude, but the P3 amplitudes were not correlated with the N2 amplitudes. The lack of association between P3 and N2 amplitudes suggests that the group effects on these measures likely are independent of each other. Nonetheless, analyses of covariance were carried out to determine whether the P3 and N2 group effects characterized overlapping variance (i.e., using one variable as the dependent variable and the other as the covariate). The first analyses showed that the N2b amplitude difference between groups was independent of the P3b amplitude difference. The group effect went from accounting for 6.3% to 5.3% of N2 variance when P3 amplitude was entered as a predictor. The N2 group effect was still significant (F(1, 132) = 7.42, p = 0.007), while P3 amplitude did not account for significant N2 variance (F(1, 132) = 1.84, p = 0.177). Conversely, the P3 target condition difference between groups was largely independent of N2 target condition amplitude. N2 amplitude did not account for significant P3 variance (F(1, 132) = 1.84, p = 0.177), but adding N2 as a predictor reduced the P3 effect from borderline significance to a trend (i.e., from group accounting for 2.8% to its accounting for 1.8% of P3 amplitude variance). For the latency measures, analysis of covariance showed that P3b and N2b group latency effects disappeared when P3a latency was used as a covariate, while the P3a latency effect remained (although reduced from 9.8% to about 7.5–7.9 % of variance accounted for when P3b or N2a latency was used as a covariate).
Table 3.
Correlations Among Dependent Variables
| P3bAmpPz | N2bLatFCz | N2bAmpFCz | P3aLatCz | P3aAmpCz | N2aLatFCz | N2aAmpFCz | |
|---|---|---|---|---|---|---|---|
|
P3bLatPz |
−0.109 (N=139) | 0.645*** (N=137) | −0.137 (N=137) | 0.613*** (N=138) | −0.047 (N=138) | 0.488*** (N=131) | −0.007 (N=131) |
|
P3bAmpPz |
−0.140 (N=137) | −0.155 (N=137) | −0.110 (N=138) | 0.563*** (N=138) | −0.193* (N=131) | −0.070 (N=131) | |
|
N2bLatFCz |
−0.027 (N=137) | 0.536*** (N=136) | −0.179* (N=136) | 0.598*** (N=131) | 0.073 (N=131) | ||
|
N2bAmpFCz |
−0.200* (N=136) | −0.181* (N=136) | −0.006 (N=131) | 0.626*** (N=131) | |||
|
P3aLatCz |
−0.108 (N=138) | 0.641*** (N=131) | −0.093 (N=131) | ||||
|
P3aAmpCz |
−0.232** (N=131) | −0.258** (N=131) | |||||
|
N2aLatFCz |
0.156 (N=131) |
Correlation analyses revealed significant associations between demographic / alcohol use variables and N2 and P3 measures. For NAC, P3b and N2a amplitudes decreased with increasing age (r = −0.300, p = 0.017; and r = −0.301, p = 0.019). For TNAD, increasing age was associated with longer P3b, N2a, and P3a latencies (r = 0.34, 0.32, and 0.31, all p’s < 0.01), and higher family drinking density was associated with longer P3b latencies (r =0.24, p = 0.043). Across all subjects, P3b latency, but not P3a nor N2b latency, was negatively correlated with the attention domain neuropsychological test score (r = −0.17, p = 0.05). Other than these associations, there were no significant or trend (p< 0.10) associations.
DISCUSSION
The central finding of this study, with regard to its primary goal, is of a marginally significant reduction of P3b amplitude in 20–50 year old TNAD compared to age and gender comparable NAC. The magnitude of the P3b reduction was much smaller than we previously observed in treated long-term abstinent alcoholics (LTAA) (averaging 6.7 years abstinence and just over 46 years of age) compared to their age and gender comparable NAC (Fein and Chang, 2006). The effect size in the study of treated LTAA was about five-times that observed in the current study. This dramatic difference in effect size was the result of two factors: there was both a greater variability of P3b amplitude in younger vs. older cohorts and a smaller mean difference in P3b amplitude between TNAD and NAC vs. LTAA and their age appropriate NAC.
It’s unclear how to interpret this result in terms of the context presented in the introduction (i.e., whether reduced P3b amplitude primarily reflects an endophenotypic marker for alcoholism and/or is a result of chronic alcohol abuse). We did not observe an association of P3b amplitude with the family history density of alcoholism or with alcohol use measures within TNAD; however, the much lower family history density in TNAD vs. LTAA, and the lower levels of alcohol use in TNAD vs. LTAA, are both associated with a much smaller decrease in P3b in TNAD vs. LTAA. The lower family history density in TNAD together with their having less of a reduction in P3b is consistent with family history density being an important determinant of the P3b reduction in alcoholics. The current study is consistent with the findings of an early COGA (Consortium on the Genetics of Alcoholism) study that compared P3 amplitudes in alcoholics (and their families) from a community sample with the P3s obtained from members of densely affected families ascertained through probands in treatment (Porjesz et al., 1998). In that study it was found that P3 was not reduced in alcohol dependent individuals in the community sample (with low family density) compared to the alcohol dependent individuals from the high density families, despite meeting the same DSM criteria for dependence. Finally, P3b amplitude was negatively correlated with the number of ASPD symptoms, suggesting that the P3b reduction is primarily associated with externalizing symptoms.
In summary, it is not possible to say whether the reduction in P3b observed in the current study is entirely the result of genetic vulnerability in a subset of individuals or has a contribution from the effects of alcohol abuse in TNAD. Either is consistent with our observation of a much smaller decrease in P3b in TNAD compared to the decrease observed in LTAA. TNAD have both a lower family history density of alcoholism than LTAA, less severe externalizing propensity, and less severe alcohol dependence as measured by all indices of alcohol consumption. Longitudinal examination of P3b in TNAD may provide data relevant to the cause of the P3b reduction observed herein.
The observation of shorter latencies of ERP components in TNAD vs. NAC was unexpected. Our finding is of a primary reduction in P3a latency, which was partially reflected in N2b and P3b latency. If anything, most studies have observed P3 latency increases in alcoholics. However, this result is in agreement with our recent report (Smith and Fein, 2010) on neuropsychological testing on a matched subset of the samples studied. That report showed intact cognitive function in TNAD, with an unanticipated effect of better attention domain scores in TNAD, possibly reflecting compensatory attention strategies in heavy drinking functional alcoholics. Correlation analysis showed that the P3b latency was significantly negatively correlated with the neuropsychological assessment attention domain, consonant with the reduced P3 latency observed here being a reflection of the same phenomenon observed in our earlier paper on neuropsychological assessment in these same subjects. Thus, although we acknowledge that this finding may reflect a Type 1 error, it is also consistent with reflecting compensatory attentional strategies in heavy drinking functional alcoholics. Finally, we observed a decrease in N2b amplitude that was about twice as large as the observed P3b amplitude reduction. Although not reported in our prior study of LTAA (Fein and Chang, 2006), we did not observe any N2 amplitude differences in that study – this is consistent with an N2 amplitude reduction being an effect of active alcohol use or abuse. This is analogous to a recent study by Martin and Garfield (2006) where alcohol administration reduced N2 amplitude (while increasing P3 amplitude – also corresponding with our observation of a marginal decrease in P3 amplitude). It is also compatible with Porjesz et al. (1987b) and Kathmann and colleagues’ (1996) observation of no difference in N2 in 14 day abstinent alcoholics. The N2 is associated with stimulus categorization, perceptual closure and attention focusing, and is thought to signal that a completed perceptual representation has been formed (Potts, 2004; Wijers et al., 1997). The more anterior fronto-central N2 is frequently interpreted as an index of response inhibition (Falkenstein, 2006; 1999) and/or conflict monitoring (Donkers and van Boxtel, 2004; Nieuwenhuis et al., 2003; van Veen and Carter, 2002a; 2002b) mediated by the anterior cingulate cortex (ACC) and other prefrontal regions (Bekker et al., 2005; Jonkman et al., 2007; Mathalon et al., 2003; van Veen and Carter, 2002a; van Veen and Carter, 2002b). Watson and colleagues (2009) have recently examined the effects of ketamine and thiopental on N2 amplitude, showing that drugs that affect glutamate and GABA neurotransmission reduce N2 amplitude, indexing a disruption of ACC activity required for the detection and processing of infrequent target stimuli. It is possible that alcohol has comparable effects but, given the novelty of our observation, replication of our observation should precede additional study of mechanisms involved.
Acknowledgments
This work was supported by National Institutes for Health, NIH Grants #1R21AA017311, #1R01AA016944 and #2R01AA013659.
REFERENCES
- American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Publishing; 2000. [Google Scholar]
- Andrew C, Fein G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res. 2010;34:669–680. doi: 10.1111/j.1530-0277.2009.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Source analysis of the N2 in a cued Go/NoGo task. Brain Res Cogn Brain Res. 2005;22:221–231. doi: 10.1016/j.cogbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B. Alcohol-related ERP changes recorded from different modalities: a topographic analysis. Alcohol Clin Exp Res. 2002;26:303–317. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biol Psychiatry. 1997;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Cohen RA, O'Donnell BF, Meadows ME, Moonis M, Stone WF, Drachman DA. ERP indices and neuropsychological performance as predictors of functional outcome in dementia. J Geriatr Psychiatry Neurol. 1995;8:217–225. doi: 10.1177/089198879500800404. [DOI] [PubMed] [Google Scholar]
- di Sclafani V, Finn P, Fein G. Psychiatric comorbidity in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2007;31:795–803. doi: 10.1111/j.1530-0277.2007.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Sclafani V, Finn P, Fein G. Treatment-naive Active Alcoholics Have Greater Psychiatric Comorbidity Than Normal Controls but less than Treated Abstinent Alcoholics. Drug Alcohol Depend. 2008;98:115–122. doi: 10.1016/j.drugalcdep.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Emmerson RY, Chamberlin HM, Dustman RE, Shearer DE. EEG, visually evoked and event related potentials in young abstinent alcoholics. Alcohol. 1987;4:241–248. doi: 10.1016/0741-8329(87)90018-8. [DOI] [PubMed] [Google Scholar]
- Falkenstein M. Inhibition, conflict and the Nogo-N2. Clin Neurophysiol. 2006;117:1638–1640. doi: 10.1016/j.clinph.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Visual P300s in long-term abstinent chronic alcoholics. Alcohol Clin Exp Res. 2006;30:2000–2007. doi: 10.1111/j.1530-0277.2006.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug Alcohol Depend. 2008;92:141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcoholism: Clinical & Experimental Research. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Finn P. Sensation Seeking in Long-Term Abstinent Alcoholics, Treatment-Naive Active Alcoholics, and Nonalcoholic Controls. Alcohol Clin Exp Res. 2010;34:1045–1051. doi: 10.1111/j.1530-0277.2010.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B. Treated and treatment-naive alcoholics come from different populations. Alcohol. 2005;36:19–26. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Landman B, Tran H, McGillivray S, Finn P, Barakos J, Moon K. Brain atrophy in long-term abstinent alcoholics who demonstrate impairment on a simulated gambling task. Neuroimage. 2006a;32:1465–1471. doi: 10.1016/j.neuroimage.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006b;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA, Smith LT. ERP responses to target and nontarget visual stimuli in alcoholics from VA and community treatment programs. Alcohol. 1996;13:85–92. doi: 10.1016/0741-8329(95)02018-7. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. 2002 [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroenceph clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Event-related potentials in women at risk for alcoholism. Alcohol. 1993;10:349–354. doi: 10.1016/0741-8329(93)90019-k. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Sniedt FL, Kemner C. Source localization of the Nogo-N2: a developmental study. Clin Neurophysiol. 2007;118:1069–1077. doi: 10.1016/j.clinph.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann N, Soyka M, Bickel R, Engel RR. ERP changes in alcoholics with and without alcohol psychosis. Biol Psychiatry. 1996;39:873–881. doi: 10.1016/0006-3223(95)00289-8. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Martin FH, Garfield J. Combined effects of alcohol and caffeine on the late components of the event-related potential and on reaction time. Biol Psychol. 2006;71:63–73. doi: 10.1016/j.biopsycho.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003;64:119–141. doi: 10.1016/s0301-0511(03)00105-4. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Hesselbrock V, Tasman A. Correlates of increased risk for alcoholism in young men. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:211–218. doi: 10.1016/0278-5846(86)90075-8. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ. Effects of abstinence on the brain: quantitative magnetic resonance imaging and magnetic resonance spectroscopic imaging in chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:1673–1682. [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcohol Clin Exp Res. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Ford JM. Late event-related potential changes in alcoholics. Alcohol. 1987;4:275–281. doi: 10.1016/0741-8329(87)90023-1. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7:465–469. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987a;4:283–287. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. The N2 component of the event-related brain potential in abstinent alcoholics. Electroencephalogr Clin Neurophysiol. 1987b;66:121–131. doi: 10.1016/0013-4694(87)90181-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O'Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcohol Clin Exp Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56:5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual p3 in female alcoholics. Alcohol Clin Exp Res. 2001;25:531–539. [PubMed] [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University School of Medicine; 1998. [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Smith S, Fein G. Cognitive performance in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2010;34:1–9. doi: 10.1111/j.1530-0277.2010.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behaviorial Assessment. 1990;12:77–90. [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Suresh S, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Stimus A, Begleiter H. Auditory P3 in female alcoholics. Alcohol Clin Exp Res. 2003;27:1064–1074. doi: 10.1097/01.ALC.0000075549.49800.A0. [DOI] [PubMed] [Google Scholar]
- U.S. Alcohol Epidemiologic Data Reference Manual Volume 8 N. Alcohol Use and Alcohol Use Disorders in the United States: Main Findings from the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) 2006 [Google Scholar]
- Van der Stelt O, Geesken R, Gunning WB, Snel J, Kok A. P3 scalp topography to target and novel visual stimuli in children of alcoholics. Alcohol. 1998;15:119–136. doi: 10.1016/s0741-8329(97)00106-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002a;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002b;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Vogel-Sprott M, Chipperfield B, Hart DM. Family history of problem drinking among young male social drinkers: reliability of the Family Tree Questionnaire. Drug Alcohol Depend. 1985;16:251–256. doi: 10.1016/0376-8716(85)90049-3. [DOI] [PubMed] [Google Scholar]
- Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers AA, Lange JJ, Mulder G, Mulder LJ. An ERP study of visual spatial attention and letter target detection for isoluminant and nonisoluminant stimuli. Psychophysiology. 1997;34:553–565. doi: 10.1111/j.1469-8986.1997.tb01742.x. [DOI] [PubMed] [Google Scholar]