Abstract
Background
Alcohol exposure in the rat on Postnatal Days [PD] 4-9 is known to partially damage the hippocampus and to impair hippocampus dependent behavioral tasks. We previously reported that PD4-9 alcohol exposure eliminated the context preexposure facilitation effect (CPFE) in juvenile rats, a hippocampal-dependant variant of contextual fear conditioning. In the CPFE, context exposure and immediate shock occur on successive occasions and this produces conditioned freezing relative to a control group that is not preexposed to the training context. Here we extend our earlier findings by examining the effects of neonatal alcohol administered at multiple doses or over different neonatal exposure periods.
Method
Rat pups (male and female) were exposed to a single binge dose of alcohol at one of three doses (2.75, 4.00, or 5.25g/kg/day) over PD4-9 (Experiment 1), or to 5.25g over PD4-6 or PD7-9 (Experiment 2). Sham intubated (SI) and undisturbed (UD) rats served as controls. On PD31, rats were preexposed to either the training context (Pre) or an alternate context (No-Pre). On PD32, rats received an immediate unsignaled footshock (1.5 mA, 2s) in the training context. Finally, on PD33 all rats were returned to the training context and tested for contextual freezing over a five-minute period.
Results
UD- and SI-Pre rats showed the CPFE, i.e., context preexposure facilitated contextual conditioning, relative to their No-Pre counterparts. The immediate shock deficit was present in all No-Pre groups, regardless of previous alcohol exposure. In Experiment 1, blood alcohol level was negatively correlated with contextual freezing. Group 2.75g-Pre did not differ from controls. Group 4.00g-Pre froze significantly less than Groups UD- and SI-Pre but more than Group 5.25-Pre, which showed the immediate shock deficit. In Experiment 2, alcohol exposure limited to PD7-9, but not PD4-6, disrupted the CPFE.
Conclusions
This is the first demonstration of dose-related impairment on a hippocampus-dependent task produced by neonatal alcohol exposure in the rat. Exposure-period effects support previous studies of alcohol and spatial learning. The CPFE is a more sensitive behavioral task that can be used to elucidate developmental alcohol-induced deficits over a range of alcohol doses that are more relevant to human exposure levels.
Keywords: Fetal alcohol spectrum disorder, hippocampus, amygdala, context conditioning, spatial cognition, dose-response effect, blood alcohol level
INTRODUCTION
Neurobehavioral impairments resulting from developmental alcohol exposure are broadly classified under the rubric of Fetal Alcohol Spectrum Disorders (FASD), which range in severity from cases of Fetal Alcohol Syndrome (FAS) at one end to more subtle learning and memory deficits in the absence of a distinct FAS facial dysmorphology at the other (Sokol, et al., 2003; Manning & Hoyme, 2007). The neurobehavioral impairments present in children with FASD may include attention deficits and poorly adapted social behavior (Mattson, et al., 2001), significant learning impairments (Mattson, et al. 2001), lower IQs (Mattson & Riley, 1998), and spatial memory disruption (Uecker & Nadel, 1996; Hamilton, et al. 2003). Neuroanatomical abnormalities commonly seen in FASD involve structures with known roles in various forms of learning and memory, including the basal ganglia, the cerebellum, and the hippocampus (Spadoni, et al. 2007; Riikonen, et al. 1999; Willoughby, et al., 2008; for review see Riley & McGee, 2005). For example, children with FAS/FASD demonstrate learning deficits in eyelid conditioning and virtual water maze tasks, suggestive of cerebellar and hippocampal dysfunction respectively (Jacobson, et al., 2008; Hamilton, et al. 2003). The use of animal models of developmental alcohol exposure has greatly improved our understanding of the teratogenetic effects of alcohol on the developing nervous system and resulting neurobehavioral dysfunction; important factors contributing to the adverse effects of alcohol include the pattern, level, and developmental period of alcohol exposure (Bonthius & West, 1988, 1990, 1991; Hamre & West, 1993; Tran & Kelly, 2003; Livy, et al., 2003).
A number of studies have examined these factors in relation to the adverse effects of alcohol on the developing hippocampus, a structure implicated in many forms of learning and memory (for recent reviews see Moscovitch, et al., 2005; Morris, 2006; Rudy, 2009). Although the hippocampus remains vulnerable to the adverse effects of alcohol throughout gestation, alcohol exposure limited to the neonatal period in the rat damages the hippocampus and impairs hippocampal-dependent behavior (for reviews see Berman & Hannigan, 2000; Gil-Mohapel, et al. 2010). For example, alcohol exposure encompassing all of gestation (gestational days [GD]1-22) and the brain growth spurt (postnatal days [PD]4-9), or just limited to PD4-9 causes significant reductions in CA1 pyramidal cells over a range of high blood alcohol concentrations (BACs; 268-457 mg/dl; Bonthius & West, 1990, 1991; Livy, et al. 2003; Tran & Kelly, 2003). CA1 pyramidal cell loss also results when alcohol is administered over PD7-9 (Marino, et al. 2004), suggesting hippocampal vulnerability to alcohol over a narrow window of exposure.
Neonatal alcohol exposure resulting in high blood alcohol concentrations (BACs) produces behavioral deficits on tasks disrupted by hippocampal lesions, such as spatial conditional alteration in the T-maze, trace fear conditioning, or the spatial water maze (Thomas, et al. 1996; Hunt, et al. 2009; Goodlett & Johnson, 1997). These studies indicate that high alcohol doses (≥ 5.00g/kg day) produce behavioral deficits on these tasks (Goodlett & Johnson, 1997; Tomlinson, et al. 1998; Hunt, et al. 2009). The developmental window of alcohol exposure also differentially affects performance. For example, Goodlett & Johnson (1997) demonstrated spatial water maze deficits when alcohol was administered over PD4-9 or PD7-9, but not when exposure was limited to PD4-6. In contrast, Hunt, et al. (2009) showed trace fear conditioning deficits following alcohol exposure on PD4-9 or PD4-6, but not PD7-9. The differential disruption of these behaviors may reflect the adverse effects of alcohol on both hippocampal and non-hippocampal brain areas known to be engaged in these tasks (Goodlett & Johnson, 1997; Hunt, et al., 2009). Importantly, information concerning dose-response effects of neonatal alcohol exposure on these tasks is limited. For example, place learning and escape latency in the water maze are disrupted only by neonatal alcohol doses exceeding 4g/kg (Goodlett & Johnson, 1997; Tomlinson, et al. 1998) while a PD4-9 4g/kg alcohol dose resulted in trace fear conditioning deficits relative to only one of two control groups (Hunt, et al., 2009). The lack of data on dose-response relationships likely reflects the relatively modest behavioral impairments that are seen with most of these tasks when high doses (4.5 – 6.6 g/kg) are used (Girard, et al. 2000; Goodlett & Johnson, 1997; Goodlett & Peterson, 1995; Johnson & Goodlett, 2002; Marino, et al. 2004; O’Leary-Moore, et al. 2006; Thomas, et al. 1996). Demonstrating effects at lower doses that reflect the full range of BACs associated with FASD in human subjects remains an important challenge for animal model research (Murawski & Stanton, 2010). This may be accomplished by examining dose-response effects of neonatal alcohol exposure on hippocampal-dependent tasks that are more sensitive to neonatal alcohol exposure (Murawski & Stanton, 2010).
Contextual fear conditioning has been used to demonstrate both pre- and postnatal alcohol-induced learning deficits in rodent models of FASD (Weeber, et al., 2001; Allan, et al. 2003; Murawski & Stanton, 2010). A variant of contextual fear conditioning that is consistently sensitive to anterograde hippocampal insult is the context preexposure facilitation effect (CPFE), where preexposure to a context facilitates conditioning to an immediate shock given on a subsequent occasion (Fanselow, 1990; Rudy & O’Reilly, 2001). In the standard CPFE design, rats are exposed to either the training context or an alternate context for a 5 minute preexposure phase. Twenty-four hours later all rats receive an immediate shock in the training context. During the testing phase, those rats preexposed to the context show enhanced contextual freezing relative to those rats preexposed to the alternate context. This difference defines the CPFE (Fanselow, 1990; Rudy & O’Reilly, 2001). The hippocampus makes important contributions during each of the three phases of the CPFE. Hippocampal activity supporting the CPFE during preexposure is NMDA receptor and protein synthesis dependent (Schiffino, et al., submitted; Barrientos, et al. 2002; Matus-Amat, et al. 2007). Hippocampal activity (but not plasticity) is also required during the training and testing phases of the CPFE paradigm (Matus-Amat, et al. 2004). The sensitivity of this task to hippocampal insult over the various phases of training makes it an attractive task to examine the effects of neonatal alcohol exposure on hippocampal-dependent learning, especially at lower alcohol doses (Murawski & Stanton, 2010).
We recently reported that exposure to a high binge-dose (5.25g/kg/day) of alcohol over PD4-9 abolishes learning in the CPFE variant of contextual fear conditioning in juvenile rats (Murawski & Stanton, 2010). In contrast, the same alcohol exposure regimen failed to alter fear conditioning to a discrete auditory cue, and only modestly impaired conventional contextual fear conditioning. The present study sought to extend these observations by determining how impairment of the CPFE is affect by different doses of alcohol (Experiment 1) and different developmental periods of exposure (Experiment 2).
EXPERIMENT 1: Dose Response Effects of Neonatal Alcohol on the CPFE
The current experiment was designed to explore dose response effects of neonatal alcohol exposure on the context preexposure facilitation effect (CPFE) paradigm. Rats were exposed to one of three alcohol doses (2.75, 4.00, or 5.25g/kg/day), sham intubated, or left undisturbed over PD4-9 and began CPFE training as juveniles (PD31-2). We predicted a linear decrease in the CPFE with increasing neonatal alcohol dose, compared to control groups (UD and SI).
MATERIALS AND METHODS
Subjects
The subjects were 146 Long Evans rats (79 female and 67 male) derived from 24 litters and were the offspring of time-mated female housed with breeder males overnight at the animal housing colony of the Office of Laboratory Animal Medicine at the University of Delaware. The following morning, rats were examined for an ejaculatory plug and, if found, that day was designated Gestational day (GD) 0. Pregnant females were housed in clear polypropylene cages (45 × 24 × 21 cm) with standard bedding and ad lib access to water and rat chow. The animal housing facility was maintained on a 12:12 hour light/dark cycle with lights on at 7:00 am. The date of birth was designated as postnatal day (PD) 0 (all births occurred on GD22). On PD3, litters were culled to 8 pups per litter (usually 4 males and 4 females) and pups received subcutaneous injections of a non-toxic black ink into one or more paws to aid in identification. Pups were weaned from their mother on PD21 and housed with same-sex litter mates in 45 × 24 × 17 cm cages until PD29 when they were individually house in small white polypropylene cages (24 × 18 × 13 cm) with ad lib access to water and rat chow for the remainder of the experiment. All subjects were treated in accord with a protocol approved by the Institutional Animal Care and Use Committee at the University of Delaware following guidelines established by the National Institutes of Health.
Alcohol Dosing
Rat pups from 17 litters were randomly assigned to receive one of three alcohol doses (2.75, 4.00, or 5.25g/kg/day) or to received sham intubations (SI), with an equal number of males and females in each group whenever possible. Within a given litter (of 8 pups), one male and one female pup were assigned to receive a given alcohol dose or to be sham intubated. An additional 7 litters remained undisturbed (Group UD) during the dosing period (PD4-9), except that their body weights were taken on PD4 and PD9. Same-sex littermates assigned to the same dosing condition (2.75g, 4.00g, 5.25g, SI, or UD) were assigned to different behavioral groups (Pre vs. No Pre, see below) so that no more than one same-sex littermate was assigned to a particular experimental condition (dosing condition X behavioral group).
Alcohol was administered via intragrastric intubation over PD4 through PD9 as described previously (Brown, et al., 2007, 2009; Murawski & Stanton, 2010). Briefly, on PD4 pups were separated from their mothers and placed as a litter into one of two clear Lexan containers set over a heating pad (GD model %E12107) that provided warmth during separation. Pups were weighed prior to the first intubation (usually around 10:00 am). The intubation process involved passing PE10 tubing lubricated with corn oil down the esophagus and into the stomach of the rat pup. For alcohol-exposed pups, alcohol was delivered in a custom milk formula solution (see Kelly & Lawrence, 2008 for details) in a single binge dose administered at 12.53% (Group 2.75g), 18.19% (Group 4.00g), or 23.94% (Group 5.25g) v/v. The formula was delivered in a volume of 0.02778 ml/g body weight. Group SI received an intubation without an infusion of formula. After dosing/intubation was completed (< 20 minutes per litter), pups were returned as a litter to their mothers. Approximately two hours (± 5 min) after the ethanol dose, pups were again briefly separated for a second dosing session. Pups received a small tail-clip and a 20 μl blood sample was collected using a heparinized capillary tube. Blood samples from Group SI were discarded; those from alcohol-exposed pups were saved from further blood alcohol analysis (see below). The second dosing on PD4 involved an infusion of milk formula only (without ethanol; Group 2.75g, 4.00g, 5.25g) or a sham intubation (Group SI). A third formula-only or sham intubation was administered two hours (± 5 min) after the second dosing. Dosing occurred in a similar fashion on PD5-9, except that no blood samples were taken and pups received only one additional formula/sham intubation after the first intubation instead of two. Alcohol-exposed pups received additional formula-only intubations in an effort to maintain normal weight gain in ethanol-exposed pups during the dosing period (Marino et al., 2004).
Blood Alcohol Concentration Analysis
Blood samples from alcohol-exposed pups were centrifuged and the plasma was collected and stored at −20 °C. Blood alcohol concentrations (BACs) were determined using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA) as previously described (Brown, et al. 2008). Briefly, the rate of oxidation of alcohol in each plasma sample was measured. BACs (expressed in mg/dl) were calculated based on comparisons to known values of an alcohol standard solution.
Apparatus and Stimuli
The context preexposure facilitation effect (CPFE) paradigm has been previously reported by our lab (Murawski & Stanton, 2010; Burman, et al., 2009; Schiffino et al, submitted) and others (Fanselow, 1990; Rudy, et al. 2004; Rudy & O’Reilly, 2001) and occurred in three successive phases: preexposure; training; and testing (see below). Rats were preexposed to one of two distinct contexts. Context A was a clear Plexiglas chamber measuring 16.5 × 21.1 × 21.6 cm with a grid floor made of 9 stainless steel bars 0.5 cm in diameter spaced 1.25 cm apart. A shock scrambler (Med Associates, Georgia, VT ENV-414S) connected to the grid floor provided a 1.5 mA, 2s footshock unconditioned stimulus (US). Four Context A chambers were positioned in a 2 × 2 formation on a Plexiglas stand within a fume hood that provided both ambient light and background noise. Adjacent walls of two chambers were made opaque. Context B involved modifications to Context A; these included a wire mesh floor and back wall that protruded into the chamber altering its internal space and a white paper drape obscuring three of the chamber walls (side, back, and ceiling). Animals were run in loads of 4 (see below). During preexposure two of the 4 chambers were styled as Context A, while the other two as Context B. The specific chambers acting as Context A or Context B were equally balanced across groups. A video camera recorded activity from the four chambers simultaneously. The video camera fed into a Dell computer that ran FreezeFrame software (Actimetrics, Wilmette, IL). Animal movement was determined by measuring changes in pixel luminance, where freezing was defined as a bout of 0.75 s or longer without changes in pixel luminance.
Design and Procedures
Preexposure
All behavioral training occurred during the light cycle (between 2–5pm). Starting on PD31-2, rats were weighed and transported (four at a time) to the testing room in distinctive transport cages made of clear Lexan (11 × 11 × 18 cm) but covered with orange construction paper to obscure visual cues during transport. The rats waited (<3 min) in an adjacent room as the experimenter cleaned out the chambers (both Context A and B) with a 5% ammonium solution. Rats were then brought into the testing room and were placed in either Context A or Context B. The rats were allowed to explore the context for a 5 minute period and were immediately returned to their home cage at the end of the preexposure session.
Training
On PD32-3 (24 h after preexposure), rats were again weighed and transported to a waiting room adjacent the testing room for 3–5 minutes while the experimenter set up for the training session. Again, the chambers (now consisting of only Context A) were wiped down with a 5% ammonium solution prior to training. All rats were trained in Context A. For those rats preexposed to Context A (Group Pre), training occurred in the same chamber where preexposure occurred 24 h earlier. Rats preexposed to Context B (Group No Pre) were trained in a chamber placed in a different spatial location to the chamber they had been preexposed in (e.g., if an animal was preexposed to Context B on the top left chamber it was trained in Context A in the bottom right chamber). Rats were brought into the testing room one at a time, placed into their respective training context, and received an immediate (<5 s) 1.5mA 2s footshock. Rats were immediately removed after US offset and returned to their home cages.
Testing
On PD33-34 rats were returned to the chamber where, 24 h earlier, they had received an immediate shock and were tested for contextual freezing over a 5 minute period. The procedures were the same as those described for preexposure to Context A.
Data Analysis
The data were analyzed using FreezeFrame software (Actimetrics, Wilmette IL) with the bout length set at 0.75 s. The freezing threshold (change in pixels/frame) was initially set as described in the instructions. A human observer blind to subjects’ groups verified the setting by watching the session and adjusting the threshold if necessary to ensure that small movements were not recorded as freezing. Freezing behavior was scored as the total percent time spent freezing over a 5 minute session for both preexposure and testing sessions.
Animal data were imported into Statistica 8 data analysis software. Weight gain over the dosing period (PD4-9) was analyzed with a repeated measures ANOVA with the between-subjects factors of sex and dosing condition and the within-subjects factors of age. Additionally, body weights during preexposure (PD31-2) were examined with a factorial ANOVA with sex and dosing condition as factors. A 2 (sex; M vs. F) X 3 (dosing condition; 2.75g, 4.00g, and 5.25g) factorial ANOVA assessed differences between group BACs. Freezing behavior was analyzed with a 2 (sex; M vs. F) X 5 (dosing condition; UD, SI, 2.75g, 4.00g, and 5.25g) X 2 (preexposure group; Pre vs. No Pre) factorial ANOVA. Post hoc analyses (Newman-Keuls) were used to characterize treatment and interaction effects. Finally, BAC/Freezing correlations were assessed with regression analysis only on alcohol-exposed rats preexposed to the training context. Statistical significance was set to p ≤0.05.
Outliers were defined a priori as having scores ± 2 standard deviations from the mean of the other rats in their respective group and were excluded from analysis. Eleven rats met the outlier criterion based on their freezing scores during testing. These included one rat from each of the 10 groups [2 (preexposure) X 5 (dosing condition)] with the addition of a second rat from Group SI-Pre. An additional rat from Group 4.00g was removed because its BAC meet the criterion as a statistical outlier. Behavioral analysis was conducted on the remaining 134 subjects (minimum n=10 per group). BAC analysis was conducted on 73 alcohol-exposed rats (Group 2.75g: n=26; Group 4.00g: n=23; and Group 5.75g: n=24), while BAC/Freezing correlations were conducted on 46 alcohol-exposed rats preexposed to the training context (Group 2.75g: n=16; Group 4.00g: n=15; and Group 5.25g: n=15). Only alcohol-exposed pups preexposed to the training context were included in this analysis because Group No Pre was not expected to learn and therefore no correlation between freezing and BAC was expected.
RESULTS
Body Weights and BACs
Table 1 summarizes body weights of Groups UD, SI, 2.75g, 4.00g, and 5.25g at PD4, PD9, and PD31-32. All groups gained a significant amount of weight over the dosing period (PD4-9). A 5 (dosing condition) X 2 (sex) X 2 (days) repeated measures ANOVA revealed significant main effects of sex [F(1, 124)=4.2, p<0.05], of dosing condition [F(4, 124)=9.1, p<0.01], and of days [F(1, 124)=2211.4, p<0.01], in addition to a significant Days X Dosing Condition interaction [F(4, 124)=46.4, p<0.01]. The main effect of sex demonstrated that females had reduced body weights compared to males (14.3g ± 2.2 vs. 15.0g ± 2.1, respectively). Newman-Keuls post-hoc tests of the Days X Dosing Condition interaction showed that weights did not differ across groups on PD4 (ps>0.70). On PD9, body weights for Groups UD, SI, and 2.75g did not differ from one another (ps>0.08); Group 4.00g (17.8g ± 2.7) weighed significantly less than Groups UD (19.7g ± 2.9; p<0.01) but did not differ from Group SI (18.8g ±2.7; p>0.10); and Group 5.25g weighed significantly less than all other groups (14.2g ± 2.1; ps<0.01). A 5 (dosing condition) X 2 (sex) factorial ANOVA on PD31-2 body weight revealed a main effect of sex [F(1, 124)=31.4, p<0.01], with females weighing significantly less than males on PD31-2 (94.8g ± 1.3 vs. 105.8g ± 1.4, respectively). No other main effects or interactions were significant (Fs<2.1). Although Groups 4.00g and 5.25g showed growth retardation at PD9 compared to controls, this retardation was transient because there were no weight differences between dosing conditions at the time of testing (PD31-2).
Table 1.
Body weights and BACs for Experiments 1, 2a, and 2b. Average body weights (in grams ± SE) are given from five dosing conditions in Experiment 1(UD=undisturbed; SI=sham intubated; 2.75g; 4.00g; and 5.25g) taken from the first and last day of the dosing period (PD4 and PD9, respectively). For Experiment 2, body weights were recorded over PD7 and PD9 (Exp 2a) or PD4 and PD6 (Exp 2b) for the three dosing conditions (UD=undisturbed; SI=sham intubated; and 5.25g=ethanol exposed). Significant differences in body weight compared to UD are noted with an ‘*’. Body weights taken at the beginning of preexposure (PD31-2) are also summarized for each experiment. In all experiments, PD31-2 weights for females were significantly lower than males and reported separately. BACs were taken from blood samples collected on the first day of dosing (PD4 or 7) from all ethanol-exposed groups (reported in mg/dl).
| Experiment | Dose | Body Weight |
BACs (mg/dl) PD4 | |||
|---|---|---|---|---|---|---|
| PD4 | PD9 | PD31-2 (males) | PD31-2 (females) | |||
| Exp 1 | UD | 11.4 ± 1.4 | 19.7 ± 2.9 | 105.8 ± 11.7 | 98.2 ± 12.2 | N/A |
| SI | 10.8 ± 0.9 | 18.9 ± 2.3 | 104.2 ± 08.7 | 95.9 ± 09.7 | N/A | |
| 2.75g | 10.9 ± 1.4 | 19.9 ± 3.3 | 105.0 ± 11.9 | 95.8 ±09.7 | 230.5 ± 5.6 | |
| 4.00g | 10.7 ± 1.1 | 17.6 ± 2.8* | 108.4 ± 12.6 | 94.5 ± 10.2 | 369.2 ± 8.4 | |
| 5.25g | 11.0 ± 1.3 | 14.2 ± 2.1* | 102.0 ± 13.2 | 87.5 ± 10.6 | 476.5 ± 10.3 | |
| Dose | PD7 | PD9 | PD31 (males) | PD31 (females) | BACs (mg/dl) PD7 | |
| Exp 2a | UD | 16.3 ± 2.3 | 19.9 ± 2.7 | 100.5 ±3.8 | 98.3 ± 1.5 | N/A |
| SI | 16.4 ± 2.6 | 21.1 ± 3.0 | 107.1 ±3.6 | 96.5 ± 2.0 | N/A | |
| 5.25g | 16.5 ± 3.1 | 16.8 ± 2.9* | 103.8 ±3.2 | 93.1 ± 2.3 | 419.4 ± 9.3 | |
| Dose | PD4 | PD6 | PD31 (males) | PD31 (females) | BACs (mg/dl) PD4 | |
| Exp 2b | UD | 11.5 ± 1.6 | 16.6 ±5.0 | 101.1 ±5.7 | 92.1 ± 5.0 | N/A |
| SI | 10.6 ± 1.0 | 14.0 ± 1.3* | 108.0 ±2.2 | 96.4 ± 2.4 | N/A | |
| 5.25g | 10.7 ± 1.0 | 10.9 ± 1.1* | 100.3 ±2.3 | 91.6 ± 2.0 | 480.6 ± 9.9 | |
Table 1 also summarizes BACs for Groups 2.75g, 4.00g, and 5.25g. BACs were obtained from 73 of 80 alcohol-exposed rats (technical difficulties caused 2 samples to be lost from Group 2.75g, 3 from Group 4.00g, and 1 from Group 5.25g). As noted previously, an additional BAC (from group 4.00g) was removed as a statistical outlier prior to analysis. A 3 (dosing condition) X 2 (sex) factorial ANOVA on BACs revealed a main effect of dosing condition [F(2, 67)=237.9, p<0.01]; sex did not affect BAC levels (p>0.08).
Behavioral Measures
Preexposure
On PD31-2, rats from each dosing condition were preexposed to either Context A or Context B for a 5 minute preexposure period. Preexposure data from 12 rats were lost due to equipment failure (data lost did not include more than 2 rats in any group). Subsequent preexposure analysis involved the remaining 123 subjects. A 5 (dosing condition) X 2 (sex) X 2 (preexposure) factorial ANOVA failed to reveal any significant differences among the factors (ps>0.34). Rats preexposed to Context A (Pre) froze 2.5 ± 0.7% while those preexposed to Context B (No Pre) froze 2.9 ± 7% demonstrating low levels of baseline freezing during preexposure for all subjects, regardless of dosing condition, sex, or preexposure context.
Testing
Testing for context conditioning occurred on PD33-4 (Figure 1). Alcohol produced dose-related decreases in test performance in those rats preexposed to the training context. A 5 (dosing condition) X 2 (sex) X 2 (preexposure) factorial ANOVA revealed main effects of dosing condition [F(4, 114)=5.67, p<0.01] and of preexposure [F(1, 114)=54.62, p<0.01], as well as a significant Dosing Condition X Preexposure interaction [F(4. 114)=3.63, p<0.01). Newman-Keuls post-hoc tests demonstrated the context preexposure facilitation effect (CPFE) in Groups UD, SI, and 2.75g, where freezing was significantly increased in groups preexposed to Context A (Pre) over those preexposed to Context B (No Pre; ps<0.05). In contrast, Groups 4.00g and 5.25g failed to show the CPFE, where total freezing did not differ significantly across the Pre vs. No Preconditions. Freezing for Groups UD-, SI-, and 2.75g-Pre did not differ from one another (ps>0.31). Groups 4.00g and 5.25g preexposed to Context A differed significantly from Groups UD, SI, and 2.75g also preexposed to Context A (ps<0.05), but did not differ from their No Pre counterparts (ps>0.12) or from one another (p>0.13). Finally, there were no significant differences in freezing between (control) groups preexposed to Context B (ps>0.90).
Fig. 1.
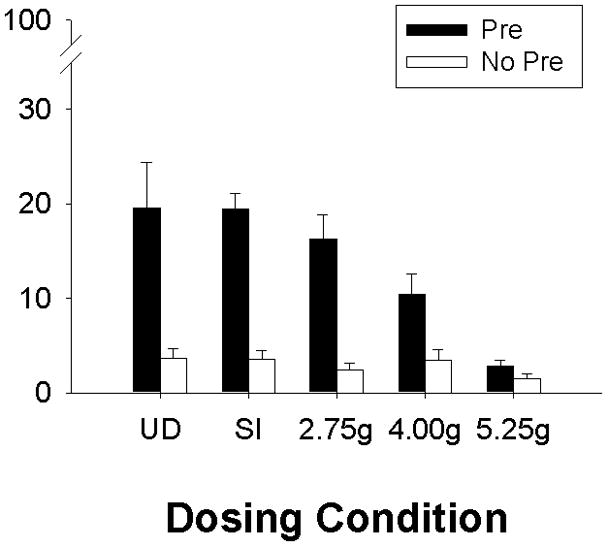
Mean (+ SE) percent freezing during testing for the context preexposure facilitation effect (CPFE) in Experiment 1. Dosing conditions include Groups UD (undisturbed), SI (sham-intubated), 2.75g, 4.00g, and 5.25g. Rats from each group were preexposed to either Context A (Pre, filled bars) or Context B (No Pre, clear bars). All rats were given an immediate shock 24h later in Context A, and tested 24 h following immediate shock for contextual freezing in Context A. Preexposure to Context A facilitated freezing during the test in Groups UD, SI, 2.75g, and 4.00g. Exposure to the highest ethanol dose (5.25g) over the neonatal period (PD4-9) disrupted the CPFE. Groups UD-Pre (n=13) and – No-Pre (n=12); Groups SI-Pre (n=15) and – No-Pre (n=14); Groups 2.75g-Pre (n=17) and – No-Pre (n=11); Groups 4.00g-Pre (n=16) and – No-Pre (n=11); and Groups 5.25g-Pre (n=15) and – No-Pre (n=10).
To further examine the relationship between dosing condition and the CPFE, trend analysis (planned orthogonal comparisons) using a one-way ANOVA (dosing condition) on rats preexposed to the training context (Group Pre) was performed. The only significant trend was the linear relationship [F(1, 72)=22.73, p<0.01]. In addition, regression analysis examined the correlation between BAC levels and test freezing for alcohol exposed rats (Groups 2.75g, 4.00g, & 5.25g) preexposed to Context A. Only data from Group Pre were included in this analysis because Group No Pre is a control condition that exhibits no learned freezing [see above] and so no correlation with BAC was expected. In addition, the restricted range of scores in Groups No-Pre violates the assumptions of regression analysis. One BAC was excluded for meeting the criteria previously set for an outlier. A correlation analysis performed on the remaining 45 rats revealed a significant negative correlation between test freezing and BAC (r=−0.53; p<0.01). This correlation is illustrated in Figure 2.
Fig. 2.
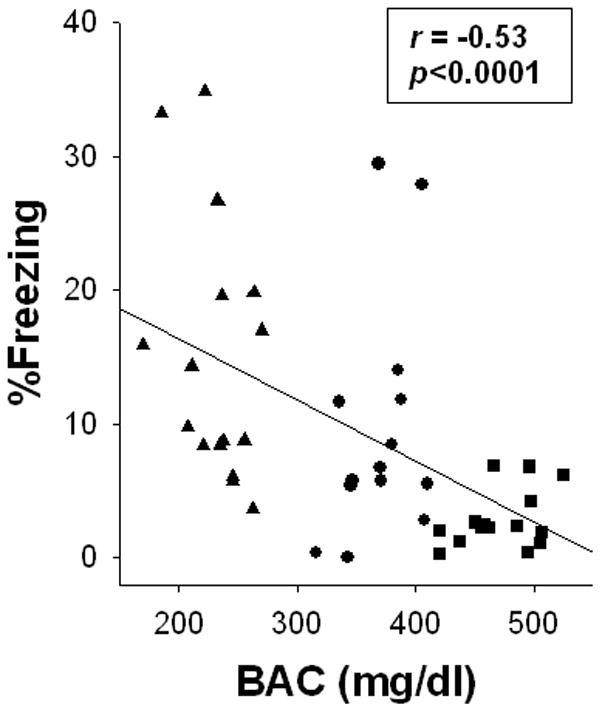
Scatter plot showing the relationship between blood alcohol concentration (BAC) obtained on postnatal day (PD) 4 and percent freezing during testing on PD31-2 for Experiment 1. BACs and % freezing (during testing) are obtained from alcohol-exposed rats (Group 2.75g= filled triangles; 4.00g= filled circles; and 5.25g= filled squares) preexposed to the training context (Group Pre). As BAC increases % freezing decreases (r=-0.53, p < .01).
Taken together, the findings of Experiment 1 indicate a dose-response function for the effect of neonatal alcohol on context conditioning. As alcohol dose increases, disruption of the CPFE increases, with maximal disruption occurring at the 5.25 g/kg dose.
EXPERIMENT 2: EtOH Exposure Window and the CPFE
In addition to alcohol dose, a second important variable influencing the impact of alcohol on the developing nervous system is the timing of alcohol exposure. For example, the craniofacial abnormalities associated with FAS result from the teratogenic effects of 1st trimester (or equivalent) alcohol exposure (Sulik, et al, 1981, Sulik, 2005), while alcohol exposure limited to the 3rd trimester equivalent, but not at earlier times, results in reductions of CA1 pyramidal cell number (Livy, et al. 2003; Tran & Kelly, 2003). In the current series of experiments rat pups were exposed to a high binge dose of alcohol (5.25g/kg/day), sham intubated (SI), or left undisturbed (UD) over two distinct neonatal periods (PD4-6 or PD7-9). Rats were then examined for context learning using the CPFE paradigm starting on PD31. Spatial learning in the water maze is disrupted by a high binge dose of alcohol over PD4-9 and PD7-9, but not PD4-6 (Goodlett & Johnson, 1997). In contrast, trace fear conditioning is disrupted by alcohol exposure over PD4-9 and PD4-6, but not PD7-9 (Hunt, et al. 2009). The CPFE is sensitive to hippocampal inactivation at any of the three phases of the paradigm (Rudy, et al. 2004) suggesting that it may be a more sensitive assay of hippocampal dysfunction induced by neonatal alcohol exposure than other behavioral tasks (Murawski & Stanton, 2010). We therefore predicted alcohol-induced CPFE deficits over both PD4-6 and PD7-9 exposure windows.
MATERIALS AND METHODS
Subjects
The subjects for Experiment 2 were 133 Long Evans rats (66 females and 67 males) derived from 24 litters (11 dosed and 13 undisturbed). All materials and methods for the current experiment (e.g., animal housing and care, apparatus and stimuli, and design and procedures) are as in Experiment 1 except as described below.
Alcohol Dosing
Litters to undergo alcohol dosing were randomly assigned to one of two dosing periods (PD4-6 or PD7-9). Rat pups from litters assigned to a specific dosing period (PD4-6 or PD7-9) were further separated to either receive 5.25g/kg/day (Group 5.25g-PD4-6 or Group 5.25g-PD7-9) of alcohol in a single binge dose or sham intubations (Group SI-PD4-6 or Group 5.25g-PD7-9). Separate litters were left undisturbed (Group UD) except that their body weights were taken on PD4 and PD6 (UD-PD4-6) or PD7 and PD9 (UD-PD7-9). No more than one same-sex litter mate was assigned to a particular experimental condition.
Alcohol dosing proceeded as in Experiment 1 except that dosing began on the first day of a particular dosing period (PD4 for PD4-6 and PD7 for PD7-9). In addition, blood samples were collected 2 hr (±5 min) after alcohol exposure on the first day of dosing (either PD4 or PD7) and were processed as before. A second milk-only dose was also given on the first day of dosing with a single milk-only dose given the following two days.
Procedure
All rats were preexposed to either Context A (Pre) or Context B (No Pre) starting on PD31. Training (PD32) and Testing (PD33) occurred as in Experiment 1.
Data Analysis
Body weight and BAC analysis for the two dosing periods occurred separately and were assessed as in Experiment 1. Analysis of freezing during Preexposure and during Testing involved a 2 (dosing period; PD4-6 vs. PD7-9) X 2 (sex; M vs. F) X 3 (dosing condition; UD vs. SI vs. 5.25g) X 2 (preexposure; Pre vs. No Pre) factorial ANOVA. Statistical significance was set to p ≤0.05.
As in Experiment 1, outliers were excluded from analysis. These included 7 rats from Exp 2a (one rat per group plus an additional rats from UD-No Pre) and 6 rats from Exp 2b (1 rat from UD-Pre and UD-No Pre, 2 rats from SI-Pre, and one rat from SI-No Pre and 5.25g-No Pre). An additional 3 rats were excluded due to equipment failure. Statistics were run on the remaining 117 rats.
RESULTS
Body weights and BACs
Experiment 2a
The body weights on PD7, PD9, and PD31 of the three dosing conditions (UD, SI, and 5.25g) in Experiment 2a are summarized in Table 1. A 2 (sex) X 3 (dosing condition) X 2 (days; PD7 vs. PD9) repeated measures ANOVA revealed a main effect of dosing condition [F(2, 58)=3.31, p<0.05], a main effect of days [F(1, 58)=752.2, p<0.01], and a dosing condition X days interaction [F(2, 58)=56.5, p<0.01]. A Newman-Keuls analysis of the dosing condition X days interaction revealed no group differences on PD7 (ps>0.7); on PD9, Group 5.25g (16.8g ± 2.9) weighed significantly less than either Group UD (19.9g ± 2.7) or SI (21.1g ± 3.0), which did not differ from one another. A 3 (dosing condition) X 2 (sex) factorial ANOVA on PD31 weights revealed a main effect of sex [F(1, 58)=10.43, p<0.01], with females weighing significantly less than males (95.9g ± 1.2 vs. 104.3g ± 2.0, respectively). The lack of a main effect of dosing condition (p>0.4) or sex X dosing condition interaction (p>0.2) indicates that the early growth retardation seen in Group 5.25g at PD9 did not persist to the time of behavioral testing at PD31. Blood samples obtained from the 22 subjects in Group 5.25g on PD7 resulted in an average BAC of 419.4 ± 9.3 mg/dl (Table 1).
Experiment 2b
Table 1 also summarizes the PD4, PD6, and PD31 body weights of subjects in the three dosing conditions (UD, SI, and 5.25g) in Experiment 2b. A 2 (sex) X 3 (dosing condition) X 2 (days) repeated measures ANOVA revealed a main effect of dosing condition [F(2, 47)=10.8, p<0.01], a main effect of days [F(1, 47)=92.5, p<0.01], and a dosing condition X days interaction [F(2, 47)=24.2, p<0.01]. The interaction was further analyzed with a Newman-Keuls post-hoc that revealed no weight differences on PD4, but significant differences between all three dosing conditions on PD6 (ps<0.02). These group weight differences did not persist up to the age of testing. A 2 (sex) X 3 (dosing condition) factorial ANOVA on PD31 body weights only revealed a main effect of sex [F(1, 47)=10.8, p<0.01], with females weighing significantly less than males (f=93.2g ± 2.0; m=102.9g ± 2.2). Blood samples obtained from the 19 subjects in Group 5.25g on PD4 resulted in an average BAC of 480.6 ± 9.9 mg/dl (Table 1).
Behavioral Measures
Analysis of behavioral measures combined data across Experiments 2a and 2b in order to include dosing period as a factor in the ANOVA. The close agreement across experiments of body weights and of behavioral data in the control groups (UD and SI) minimizes the potential problems with cross-experiment comparisons that could arise in the combined ANOVA.
Preexposure
A 2 (dosing period; PD4-6 vs. PD7-9) X 2 (sex) X 3 (dosing condition) X 2 (preexposure) factorial ANOVA performed on preexposure freezing revealed a main effect of dosing period [F(1, 93)=9.17, p<0.01], with PD7-9 subjects freezing more during preexposure than PD4-6 subjects (6.4 ± 0.8% vs. 2.6 ± 0.9%, respectively). No other main effects or interactions approached significance (ps>0.06). This difference in preexposure freezing between the two dosing periods had little, if any, effect on subsequent behavioral performance, as a function of alcohol treatment group or behavioral condition. Analysis of difference scores (%freezing during testing minus %freezing during preexposure; data not shown) did not differ significantly from analysis of testing scores (see below), and therefore only test-score analysis is described.
Testing
On PD31, subjects from all groups were tested for contextual conditioning over a five minute period in the context (Figure 3). Alcohol exposure from PD7-9 disrupted the CPFE whereas exposure from PD4-6 did not. A 2 (dosing period) X 2 (sex) X 3 (dosing condition) X 2 (preexposure) factorial ANOVA revealed main effects of dosing condition [F(2, 93)=5.6, p<0.01] and preexposure [F(1, 93)=70.4, p<0.01]. A significant interaction of dosing period X dosing condition X preexposure [F(2, 93)=5.2, p<0.01] was further analyzed using Newman-Keuls. Control groups (UD and SI) did not differ across dosing periods (ps>0.5). Freezing in control subjects preexposed to the training context (Group Pre) did significantly differ from their counterparts preexposed to an alternate context (Group No Pre; ps<0.05). This significant difference between Group Pre vs. Group No Pre in the control groups across exposure periods is indicative of the context preexposure facilitation effect (CPFE), with those animals preexposed to the training context showing facilitated contextual conditioning to an immediate shock compared those preexposed to an alternate context. A different pattern of results occurred for animals neonatally exposed to alcohol. Rats exposed to 5.25g of alcohol over PD7-9 failed to show the CPFE, with Group Pre not differing significantly from any of the Group No Pre animals (ps>0.5) while at the same time differing significantly from all Group Pre animals (ps<0.03). In contrast, rats exposed to 5.25g of alcohol over PD4-6 did demonstrate the CPFE, where preexposure to the training context resulted in a significant increase in contextual freezing compared to counterparts preexposed to an alternate context.
Fig. 3.
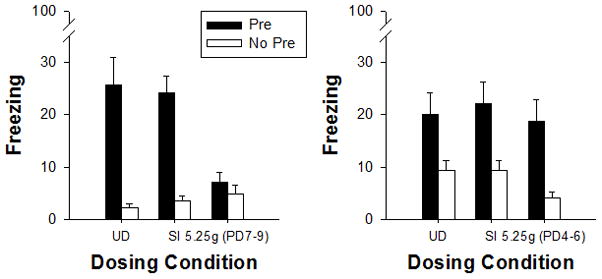
Mean (±SE) percent freezing during the 5 minute test for the context preexposure facilitation effect (CPFE) in Experiments 2a and 2b. In Experiment 2a (PD7-9 exposure, left panel), Groups UD (undisturbed) and SI (sham intubated) demonstrate the CPFE whereas Group 5.25g does not. In the UD and SI control groups, rats preexposed to the training context (Pre, filled bars) show enhanced contextual freezing compared to their counterparts preexposed to an alternate context (No Pre, clear bars). In contrast, rats exposed to 5.25g of ethanol over PD7-9 [5.25g (PD7-9)] show little contextual freezing regardless of preexposure condition. In Experiment 2b (PD4-6 exposure, right panel), all treatment groups (Groups UD, SI & 5.25g) preexposed to the training context (Pre, filled bars) show the CPFE compared to No Pre controls. Thus, alcohol exposure over PD7-9 disrupts the CPFE, while exposure over PD4-6 does not. For PD7-9, Groups UD-Pre (n=8) and UD-No-Pre (n=9); Groups SI-Pre (n=13) and SI-No-Pre (n=12); Groups 5.25g-Pre (n=11) and –No-Pre (n=11). For PD4-6, Groups UD-Pre (n=9) and –No-Pre (n=9); Groups SI-Pre (n=7) and –No-Pre (n=9); and Groups 5.25g-Pre (n=10) and –No-Pre (n=9).
Summary of Findings
Rat pups exposed to a high binge dose of alcohol over PD7-9 failed to demonstrate the CPFE, where preexposure to the testing context 24h prior to receiving an immediate shock did not facilitate contextual fear conditioning. In contrast, those rat pups exposed to alcohol over PD4-6 did demonstrate the CPFE, not differ from control rats. Alcohol exposure limited to PD7-9 is sufficient to account for the CPFE disruption demonstrated after exposure to 5.25g of alcohol over PD4-9 (Exp. 1).
GENERAL DISCUSSION
The current series of experiments examined the impact of neonatal alcohol on subsequent contextual learning in juvenile rats by manipulating dose and timing of alcohol exposure. In agreement with our previous studies (Schiffino, et al. submitted; Murawski & Stanton, 2010; Burman, et al. 2009), juvenile rats from control groups (Groups UD & SI) preexposed to the testing context showed enhanced contextual fear conditioning to an immediate shock received 24h later in the testing context relative to those preexposed to an alternate context. Those rats preexposed to an alternate context (regardless of dosing condition) failed to condition to an immediate shock, demonstrating an immediate shock deficit (Fanselow, 1990). Under our parameters the context preexposure facilitation effect (CPFE) did not differ between control groups. Alcohol exposure at the 5.25g/kg dose produced a complete disruption the CPFE, while rats exposed to a lower dose (4.00g/kg) showed impaired contextual conditioning. Group 2.75g did not differ significantly from either control group. The dose-related impairment of contextual learning was supported by a significant negative correlation between BAC and contextual freezing. We also demonstrated that the timing of alcohol exposure significantly affected alcohol-induced deficits in the CPFE. Rats exposed to a high binge dose of alcohol over PD7-9 demonstrated contextual learning deficits through an absence of the CPFE whereas the CPFE was present in those rats exposed to alcohol over PD4-6, which did not differ from controls (Experiment 2). Developmental alcohol exposure is known to result in hyperactivity in both humans and in animal models (Mattson & Riley, 1998; Thomas, et al. 2004), and could interfere with freezing behavior used to assess contextual learning in our current studies. We have previously reported that alcohol-exposed rats demonstrate control-level post-shock and delay fear conditioning to a discrete tone CS (Murawski & Stanton, 2010), ruling out performance effects. These results are consistent with other studies showing no deficits in conditioned freezing to a discrete CS in rats exposed to alcohol either prenatally (Allan, et al. 2003; Weeber, et al. 2001) or during the early neonatal period (Wagner & Hunt, 2006; Hunt, et al. 2009).
Rats administered an alcohol dose of 5.25g over PD4-9 show a complete absence of the CPFE in agreement with our earlier findings (Murawski & Stanton, 2010). We extend this finding by further demonstrating disruption of the CPFE at a lower alcohol dose (4.00g/kg). Rats given 4.00g of alcohol over PD4-9 show a reduction in trace fear conditioning (Hunt, et al., 2009), where this group differs significantly from one of two control groups. Group 2.75g (BACs: 230 mg/dl) showed a CPFE comparable to control groups. BACs within the range of 230 mg/dl have been demonstrated to negatively affect learning and memory on some behavioral tasks. For example, our lab has previously reported neonatal alcohol-induced learning deficits on complex task variants of eyeblink conditioning with BACs at or below 200 mg/dl (Brown, et al. 2007, 2009). Learning impairments in a spatial water maze task have been reported when neonatal alcohol exposure produced BACs of 265 mg/dl (Goodlett & Johnson, 1997). Different methods of alcohol administration produce different BACs (Gil-Mohapel, et al. 2010). In the studies of Brown, et al. (2007, 2009) and in Goodlett & Johnson (1997), alcohol was administered over two feedings. In the current studies we administered alcohol in a single bolus (Tran & Kelly, 2003; Marino, et al. 2004), and therefore direct comparisons of BACs between experiments is confounded by dosing method. Using our current dosing method and behavioral task we demonstrate a complete absence of learning in rats that received 5.25g of alcohol, whereas this same dose produces only modest acquisition deficits in other hippocampal-dependant tasks (Goodlett & Johnson, 1997; Johnson & Goodlett. 2002; Marino, et al. 2004). To our knowledge this is also the first paper to report a regression effect between blood alcohol level and a hippocampal-dependent behavior.
The specific neurological deficits induced by developmental alcohol exposure are due in part to the timing of alcohol exposure. We demonstrate that alcohol exposure over PD4-9 or PD7-9 results in contextual learning deficits in juvenile rats, while exposure limited PD4-6 failed to disrupt this learning. Evaluating the effects of alcohol on spatial learning using these same exposure periods, Goodlett and Johnson (1997) report a similar pattern of alcohol-induced deficits: a high binge dose of alcohol (5.25g/kg) administered over PD4-9 or PD7-9, but not PD4-6, resulted in spatial learning deficits in the water maze in juvenile rats (PD26-31; Goodlett & Johnson, 1997). The CPFE requires spatial exploration during the preexposure phase (McHugh & Tonegawa, 2007) and therefore the neural mechanisms involved in spatial learning may be differentially susceptible to the effects of alcohol over the two neonatal exposure periods, with greater deficits induced with a PD7-9 exposure relative to a PD4-6 exposure. In contrast to these findings, Hunt, et al. (2009) report trace fear conditioning deficits in juvenile rats exposed to 5.00g/kg of alcohol over PD4-9 and PD4-6, but not when alcohol exposure occurred over PD7-9. In trace conditioning, the conditioning stimulus (CS) is separated from the unconditioned stimulus (US) by a stimulus free period. Trace fear conditioning is mediated by interactions of multiple brain structures (e.g., hippocampus, mPFC, amygdala [Burman, et al. 2006; Gilmartin & Helmstetter, 2010]) and neurochemical systems (e.g., glutamatergic, cholinergic [Gilmartin & Helmstetter, 2010; Fontan-Lozano, et al. 2005]), all of which, to varying degrees, are affected by developmental alcohol exposure (Tran & Kelly, 2003; Whitcher & Klintsova, 2008; Kelly, et al. 2009; Norman, et al. 2009; O’Leary-Moore, et al., 2008). Although many of the neuroanatomical and neurochemical substrates supporting contextual, spatial, and trace conditioning are shared (D’Hooge & De Deyn, 2001; Fanselow & Poulos, 2005; Czerniawski, et al. 2009), further research is needed to examine how alcohol exposure over discrete neonatal periods affects these systems and produces these seemingly discrepant behavioral results.
The hippocampus makes important contributions during each of the three phases of the CPFE. A number of studies demonstrate that the CPFE can be disrupted by dorsal hippocampus (DH) blockade of NMDA receptors prior to or inhibition of protein synthesis following preexposure (Schiffino, et al., submitted; Barrientos, et al. 2002; Matus-Amat, et al. 2007). In addition, anterograde lesions or muscimol inactivation of the DH prior to training or to testing disrupt the CPFE (Matus-Amat, et al. 2004). The CPFE deficits that result from neonatal alcohol exposure in the current series of experiments may reflect disruptions of hippocampal function. Alcohol exposure limited to the neonatal period results in significant CA1 pyramidal cell loss (Tran & Kelly, 2003; Livy, et al. 2003; Marino, et al., 2004), with some studies showing CA3 and dentate gyrus reductions as well (Livy, et al. 2003). Using unbiased stereological methods, Bonthius, et al. (2001) demonstrated dose-dependent CA1 pyramidal cell loss when alcohol was administered over PD4-9. It is probable that the correlation between contextual freezing and BACs in the current study may reflect varying degrees of cell loss within the hippocampus. Anatomical studies are planned to address this question explicitly.
Mechanisms underlying synaptic plasticity within the hippocampus may be compromised by neonatal alcohol exposure. Acute exposure to alcohol in PD7-9 hippocampal slices inhibits glutamatergic transmission and LTP induction (Puglia & Valenzuela, 2010b), while alcohol exposure via vapor inhalation over PD2-9 (BACs 232–295 mg/dl) inhibited LTP induction in CA1 (Puglia & Valenzuela, 2010a). Alcohol induced disruptions in LTP over PD7-9 may contribute to abnormal hippocampal development (Puglia & Valenzuela, 2010a, 2010b). PD4-9 alcohol exposure (BACs=351 mg/dl) has also been reported to reduce synaptic efficacy in hippocampal slices taken from PD45 and 60 rats, although in this study LTP induction was not affected by alcohol exposure (Bellinger, et al., 1999). Further, pre- and/or neonatal alcohol results in reduced 3H-MK-801 binding in the hippocampus, suggestive of reductions in NMDA receptors (Diaz-Granados, et al., 1997; Savage, et al. 1992). NMDA receptor activation during the preexposure phase of the CPFE is thought to initiate a cascade of events that results in protein synthesis and other plastic changes necessary to support learning about the context (Schiffino, et al. submitted; Barrientos, et al. 2002; Matus-Amat, et al. 2007). Alcohol-induced reductions of hippocampal NMDA receptors or other factors supporting synaptic plasticity may be sufficient to disrupt this process and could account for a disruption in the CPFE.
Neonatal alcohol exposure is known to target other brain structures that, when compromised, may account for deficits in contextual fear conditioning. For example, neonatal alcohol targets the mPFC; alcohol exposure over PD4-9 (5.25g/kg/day) produces reductions of mPFC spine density in juvenile rats (Whitcher & Klintsova, 2008). A recent study demonstrated contextual conditioning deficits through inactivation or NMDA receptor blockade of the mPFC within a context where tone-shock pairings occurred previously (Gilmartin & Helmstetter, 2010). Alcohol-induced disruptions to the mPFC, therefore, may alter contextual conditioning. However, when contextual conditioning occurred in the absence of any discrete stimuli, such as in our experiments, inactivation or NMDA receptor blockade of the mPFC had no effect on context conditioning (Gilmartin & Helmstetter, 2010). The role of the mPFC during the CPFE is currently unknown. A second brain area targeted by neonatal alcohol is the cerebellum (Hamre & West, 1993; Goodlett & Eilers, 1997). There is some evidence that the cerebellar vermis contributes to contextual fear conditioning (Sacchetti, et al., 2002) and therefore the effects of alcohol on the developing cerebellum may negatively affect conditioning to a context. However, our results show CPFE disruptions when alcohol is administered over PD7-9, an exposure window that does not result in Purkinje cell loss (Hamre & West, 1993). Parahippocampal structures are thought to support contextual fear conditioning when the hippocampus is damaged or inactivated prior to training (Rudy, 2009). The parahippocampal system, however, is less efficient at supporting context conditioning through configural processes than is the hippocampus (Rudy, 2009; Fanselow, 2009). The trend toward more moderate CPFE impairment seen in Group 4.00g may reflect context learning through this alternate system, although the structures within this system may be affected by developmental alcohol exposure as well (Angelucci, et al. 1999). Although the results reported here are likely to involve disruptions of hippocampal function induced by neonatal alcohol exposure, further studies examining brain-behavior relationships within the constraints of our protocol are needed to further substantiate that claim.
The context preexposure facilitation effect is disrupted by neonatal alcohol exposure. We demonstrate disruption the CPFE, a hippocampal-dependant task, by neonatal alcohol at a lower dose than previously reported, a disruption that is significantly correlated with blood alcohol concentration. In addition, alcohol exposure limited to the PD7-9 developmental period is sufficient to account for these alcohol-induced deficits. Understanding the consequences of developmental alcohol exposure on the developing nervous system, especially at alcohol doses relevant to those occurring in human populations, is a major goal of FASD research using animal models. The results reported here support this goal and lend themselves to further understanding mechanisms and interventions to mitigate alcohol-induced behavioral impairments (Thomas, et al. 2007, 2008; Wagner & Hunt, 2006; Incerti, et al. 2010).
Acknowledgments
The authors would like to thank Sarah A. Jablonski, Felipe L. Schiffino, and Luke W. Ayers for technical assistance and Dr. Jeffrey B. Rosen for providing access to the fear conditioning equipment used in the studies reported here. This work was supported by the University of Delaware and by NIH grants RO1 AA014288-01 and 1F31AA019594-01.
References
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcoholism-Clinical and Experimental Research. 2003;27(12):2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Fiore M, Cozzari C, Aloe L. Prenatal ethanol effects on NGF level, NPY and ChAT immunoreactivity in mouse entorhinal cortex: a preliminary study. Neurotoxicol Teratol. 1999;21(4):415–25. doi: 10.1016/s0892-0362(99)00005-7. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, O’Reilly RC, Rudy JW. Memory for context is impaired by injecting anisomycin into dorsal hippocampus following context exploration. Behav Brain Res. 2002;134(1–2):299–306. doi: 10.1016/s0166-4328(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Bedi KS, Wilson P, Wilce PA. Ethanol exposure during the third trimester equivalent results in long-lasting decreased synaptic efficacy but not plasticity in the CA1 region of the rat hippocampus. Synapse. 1999;31(1):51–8. doi: 10.1002/(SICI)1098-2396(199901)31:1<51::AID-SYN7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Blood alcohol concentration and microencephaly: a dose-response study in the neonatal rat. Teratology. 1988;37(3):223–31. doi: 10.1002/tera.1420370307. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14(1):107–18. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44(2):147–63. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Woodhouse J, Bonthius NE, Taggard DA, Lothman EW. Reduced seizure threshold and hippocampal cell loss in rats exposed to alcohol during the brain growth spurt. Alcohol Clin Exp Res. 2001;25(1):70–82. [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Dev Psychobiol. 2007;49(3):243–57. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- Brown KL, Burman MA, Duong HB, Stanton ME. Neonatal binge alcohol exposure produces dose dependent deficits in interstimulus interval discrimination eyeblink conditioning in juvenile rats. Brain Res. 2009;1248:162–75. doi: 10.1016/j.brainres.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16(2):103–13. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behav Neurosci. 2009;123(5):1148–52. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19(1):20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 2001;36(1):60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Diaz-Granados JL, Spuhler-Phillips K, Lilliquist MW, Amsel A, Leslie SW. Effects of prenatal and early postnatal ethanol exposure on [3H]MK-801 binding in rat cortex and hippocampus. Alcohol Clin Exp Res. 1997;21(5):874–81. [PubMed] [Google Scholar]
- Fanselow MS. Factors Governing One-Trial Contextual Conditioning. Animal Learning & Behavior. 1990;18(3):264–270. [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–34. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2009;14(1):7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan-Lozano A, Troncoso J, Munera A, Carrion AM, Delgado-Garcia JM. Cholinergic septo-hippocampal innervation is required for trace eyeblink classical conditioning. Learn Mem. 2005;12(6):557–63. doi: 10.1101/lm.28105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Res Rev. 2010 doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17(6):289–96. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard TA, Xing HC, Ward GR, Wainwright PE. Early postnatal ethanol exposure has long-term effects on the performance of male rats in a delayed matching-to-place task in the Morris water maze. Alcohol Clin Exp Res. 2000;24(3):300–6. [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiol Learn Mem. 1995;64(3):265–75. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19(6):435–46. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21(4):738–44. [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hamre KM, West JR. The effects of the timing of ethanol exposure during the brain growth spurt on the number of cerebellar Purkinje and granule cell nuclear profiles. Alcohol Clin Exp Res. 1993;17(3):610–22. doi: 10.1111/j.1530-0277.1993.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Jacobson SE, Torok EJ. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects. Alcohol. 2009;43(6):465–74. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incerti M, Vink J, Roberson R, Wood L, Abebe D, Spong CY. Reversal of Alcohol-Induced Learning Deficits in the Young Adult in a Model of Fetal Alcohol Syndrome. Obstetrics and Gynecology. 2010;115(2):350–356. doi: 10.1097/AOG.0b013e3181cb59da. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–72. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Johnson TB, Goodlett CR. Selective and enduring deficits in spatial learning after limited neonatal binge alcohol exposure in male rats. Alcohol Clin Exp Res. 2002;26(1):83–93. [PubMed] [Google Scholar]
- Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods Mol Biol. 2008;447:101–10. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25(4):447–58. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Manning MA, Eugene Hoyme H. Fetal alcohol spectrum disorders: a practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31(2):230–8. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. Int J Dev Neurosci. 2004;22(5–6):363–77. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):279–94. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25(3):185–91. [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24(10):2431–9. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–31. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Tonegawa S. Spatial exploration is required for the formation of contextual fear memory. Behav Neurosci. 2007;121(2):335–9. doi: 10.1037/0735-7044.121.2.335. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23(11):2829–46. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, McAndrews MP, Levine B, Black S, Winocur G, Nadel L. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. Behav Brain Res. 2010;212(2):133–42. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary-Moore SK, McMechan AP, Mathison SN, Berman RF, Hannigan JH. Reversal learning after prenatal or early postnatal alcohol exposure in juvenile and adult rats. Alcohol. 2006;38(2):99–110. doi: 10.1016/j.alcohol.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats. Alcohol. 2010a;44(3):283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia MP, Valenzuela CF. Ethanol acutely inhibits ionotropic glutamate receptor-mediated responses and long-term potentiation in the developing CA1 hippocampus. Alcohol Clin Exp Res. 2010b;34(4):594–606. doi: 10.1111/j.1530-0277.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, Verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Dev Med Child Neurol. 1999;41(10):652–9. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Experimental Biology and Medicine. 2005;230(6):357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Affect Behav Neurosci. 2001;1(1):66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;28(7):675–85. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C. Cerebellar role in fear-conditioning consolidation. Proc Natl Acad Sci U S A. 2002;99(12):8406–11. doi: 10.1073/pnas.112660399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DD, Queen SA, Sanchez CF, Paxton LL, Mahoney JC, Goodlett CR, West JR. Prenatal ethanol exposure during the last third of gestation in rat reduces hippocampal NMDA agonist binding site density in 45-day-old offspring. Alcohol. 1992;9(1):37–41. doi: 10.1016/0741-8329(92)90007-w. [DOI] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiol Learn Mem. submitted doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Delaney-Black V, Nordstrom B. Fetal alcohol spectrum disorder. Jama. 2003;290(22):2996–9. doi: 10.1001/jama.290.22.2996. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, McGee CL, Fryer SL, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Neurosci Biobehav Rev. 2007;31(2):239–45. doi: 10.1016/j.neubiorev.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214(4523):936–8. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Exp Biol Med (Maywood) 2005;230(6):366–75. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29(5):433–52. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology and Teratology. 2004;26(1):35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121(1):120–30. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Sather TM, Whinery LA. Voluntary exercise influences behavioral development in rats exposed to alcohol during the neonatal brain growth spurt. Behav Neurosci. 2008;122(6):1264–73. doi: 10.1037/a0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D, Wilce P, Bedi KS. Spatial learning ability of rats following differing levels of exposure to alcohol during early postnatal life. Physiol Behav. 1998;63(2):205–11. doi: 10.1016/s0031-9384(97)00424-1. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25(5):519–28. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34(3):209–23. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: Reversal by choline. Behavioral Neuroscience. 2006;120(2):482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Savage DD, Sutherland RJ, Caldwell KK. Fear conditioning-induced alterations of phospholipase C-beta1a protein level and enzyme activity in rat hippocampal formation and medial frontal cortex. Neurobiol Learn Mem. 2001;76(2):151–82. doi: 10.1006/nlme.2000.3994. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008 doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J Int Neuropsychol Soc. 2008;14(6):1022–33. doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]


