Abstract
Objective
In osteoarthritis (OA), subchondral bone changes alter the joint’s mechanical environment and potentially influence progression of cartilage degeneration. Joint distraction as a treatment for OA has been shown to provide pain relief and functional improvement through mechanisms that are not well understood. This study evaluated whether subchondral bone remodeling was associated with clinical improvement in OA patients treated with joint distraction.
Method
Twenty-six patients with advanced post-traumatic ankle OA were treated with joint distraction for three months using an Ilizarov frame in a referral center. Primary outcome measure was bone density change analyzed on CT scans. Longitudinal, manually segmented CT datasets for a given patient were brought into a common spatial alignment. Changes in bone density (Hounsfield Units (HU), relative to baseline) were calculated at the weight-bearing region, extending subchondrally to a depth of 8 mm. Clinical outcome was assessed using the ankle OA scale.
Results
Baseline scans demonstrated subchondral sclerosis with local cysts. At one and two years of follow-up, an overall decrease in bone density (−23% and −21%, respectively) was observed. Interestingly, density in originally low-density (cystic) areas increased. Joint distraction resulted in a decrease in pain (from 60 to 35, scale of 100) and functional deficit (from 67 to 36). Improvements in clinical outcomes were best correlated with disappearance of low-density (cystic) areas (r=0.69).
Conclusions
Treatment of advanced post-traumatic ankle OA with three months of joint distraction resulted in bone density normalization that was associated with clinical improvement.
Keywords: osteoarthritis, joint distraction, bone density, ankle, CT-scan
OA in general
Osteoarthritis (OA) is a degenerative joint disease characterized by cartilage destruction and changes in subchondral bone. Joints most affected are spine, hip and knee1. Ankle OA is less common, but responsible for a significant part of the total (financial) burden of OA. The etiology of ankle OA often includes a history of joint trauma and thereby occurs at a relatively young age2. Treatment options for end-stage ankle OA are limited; both arthrodesis and joint replacement often lead to severe complications and adjacent joint degeneration3.
Bone changes in OA
Subchondral bone changes are a distinctive feature in OA development, and they include sclerosis, cyst formation, bone attrition, bone marrow lesions (evidenced by MRI), and osteophytes. Radiographic imaging generally shows an increase in bone density-commonly referred to as subchondral sclerosis-beneath the weight-bearing joint surface. An increase in bone turnover results in higher bone volume and hypo-mineralization4. Locally, flattening or depression of the subchondral bony surface, also known as bone attrition, has been observed and likely represents bone remodeling in an area of increased loading5. An MRI-DXA study has shown that increased bone density (sclerosis) coinciding with excessive loading is associated with bone marrow lesions6. These MRI-apparent lesions are marked by bone marrow necrosis, fibrosis, and trabecular abnormalities7. Bone marrow lesions may play a role in the pathogenesis of subchondral cysts, as cysts have been observed to arise within regions of marrow edema-like signal8. Subchondral cysts can communicate with the joint space, and are usually lined with fibrous connective tissue containing adipocytes and osteoblasts9.
The role of this variety of subchondral bone changes in the development of OA is not yet clear4, but inevitably the mechanical integrity of the joint surface is eventually disrupted and cartilage responds10. The relationship of subchondral bone changes with clinical outcome seems clearer than for the pathological changes of other damaged tissues in OA11. MRI studies have emphasized the importance of large bone marrow lesions12 and the combination of bone marrow lesions and bone surface attrition13 and their relationship with clinical features in knee OA. Subchondral cysts in the knee joint have been associated with an increased risk of knee replacement14. Consequently, subchondral bone has been identified as an attractive target for treatment in OA.
Joint distraction and other treatment strategies influencing bone
There are a number of treatments that give long-term clinical improvement in severe OA by influencing bone to widely varying degrees. At one extreme is joint replacement15, where the whole joint (including subchondral bone) is removed and with that the (unknown) source of pain. Another treatment is arthrodesis of the affected joint, where the joint surface is removed and joint function is sacrificed16. Other treatment methods resulting in (more or less) clinical improvement include pharmacological bone stimulation17, osteotomy18, and, less widely applied, joint distraction19, 20. Joint distraction is a surgical treatment for advanced OA involving the use of an external fixator to unload the cartilage and underlying bone for a certain period. Joint distraction has been shown to provide long term pain relief and improve joint function20, though mechanisms leading to clinical improvement are not well understood.
Study goal
We aimed to determine the relationship between bone density changes and clinical improvement upon treatment with joint distraction in ankle OA. In this exploratory clinical study the long term effects of joint distraction on longitudinal subchondral bone density changes were studied and related to clinical improvement in young patients with severe ankle OA.
Patients and Methods
Patients
Twenty-six patients (mean age 41±9 years; 17 males) with severe post-traumatic ankle OA were included in this prospective clinical study, taken from a larger trial of 40 patients investigating clinical and other effects of joint distraction21. Suitable CT-scans were unavailable for 14 of those 40 patients (five patients withdrew, one fused before one year of follow-up, three CT-scans had severe metal artifacts, and five baseline CT-scans had technical errors).
Subjects for the primary study were selected from patients presenting with painful end-stage ankle arthritis to a U.S. tertiary medical center. The criteria for selection of subjects included: symptomatic isolated, unilateral Kellgren-Lawrence22 (KL) grade 3 or 4 ankle OA, skeletally mature and age ≤ 60 years, failure of non-operative treatment > 1 year, and capacity to maintain extremity non-weight-bearing using ambulatory aids. Excluded from the study were patients who met any of the following criteria: history of inflammatory arthritis, the presence of other symptomatic joints on the ipsilateral lower extremity, contralateral ankle arthritis (KL grade 2–4), ankle or hindfoot malalignment, lived greater than 300 miles away from treatment center, current history of alcohol or drug abuse. Written, witnessed consent was obtained from all subjects using IRB approved forms.
Surgical procedure
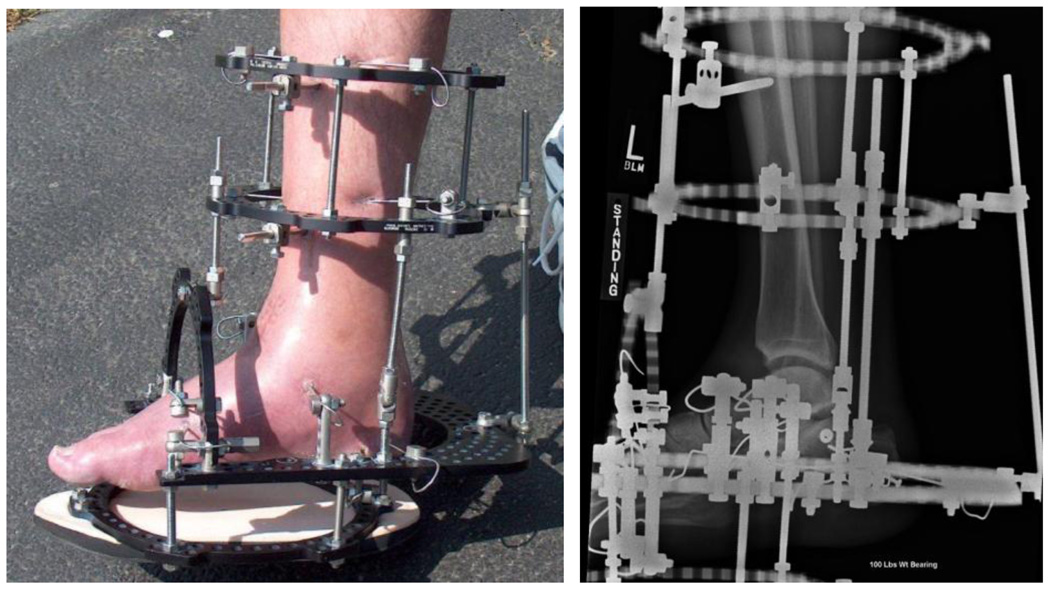
All procedures were performed by one of two attending surgeons (AA, CS). First, an arthroscopic ankle joint lavage was performed, with removal of any extra-articular anterior bony osteophytes. If the anterior osteophytes were too large to remove arthroscopically, they were removed by open means through an extension of the arthroscopic portals. No intra-articular joint debridement was performed. A circumferential external fixator was applied in a standardized fashion (Figure 1). The tibial frame was put on with the rings perpendicular to the tibia, and the foot frame was put on in-line with the foot. The upper tibial ring was secured with two 5 mm half-pins, the lower one with 5 mm half pins and a crossing 1.8 mm (“thin”) wire tensioned to nominally 600 N. The foot frame was then attached with a smooth thin wire transversely across the talus, two crossing thin wires across the calcaneus, and two crossing thin wires across the metatarsals, all tensioned to nominally 360 N.
Figure 1.
An Ilizarov external fixator was used to apply distraction to the ankle (left). Radiographic view of the distracted ankle (right).
Two distraction rods bridging the upper ankle joint were then secured medially and laterally to the fixator. Intra-operatively the ankle was distracted 5 mm. The 4.8 mm internal diameter of the threaded rods connecting the rings, visualized using intra-operative fluoroscopy, was used as a radiographic guide to ensure that this amount of distraction was obtained. As the procedure was done with no tissue dissection and limited (< 1 cm) incisions, it was typically done on a short-stay inpatient admission or outpatient basis.
Follow-up protocol
The fixators were removed between 85 and 95 days after application. The patients were gradually returned to full weight-bearing without boot immobilization by 6 months. After fixator removal, patients returned for study evaluations at 12 months and 24 months post removal.
Outcome parameters
Bone
Double-contrast (systemically and intra-articular) axial CT scans (Siemens Emotion 6/Sensation 16) were obtained at baseline (before treatment), and at one- and two-year follow-ups after treatment to analyze joint space width23 and bone density. Scanning parameters included 120–130 kVp, 55–68 mAs, 750–1000 exposure time, 0.3 mm pixel spacing, 0.63 mm slice thickness, and images were reconstructed with a B31 kernel on 512×512 matrix.
The tibia and talus bones were manually segmented at each time point using OsiriX Imaging Software (OsiriX Project; Geneva, Switzerland) with an interactive pen display (Cintiq 21UX; Wacom Technology, Vancouver, Canada). Segmentation data were then processed into continuous 3D surfaces (Figure 2A) using Geomagic Studio software (Geomagic Inc., Research Triangle Park, NC, USA). The spatial transformations for registering baseline and follow-up datasets were calculated by aligning bone surfaces using an iterative closest point algorithm in the Geomagic software. Baseline and follow up surfaces aligned with an average signed distance error of 0.21±0.9 mm and an unsigned distance error of 0.73±0.7 mm (mean±SD). Then, utilizing ITK and purpose-written MATLAB code, the CT datasets for a given patient were transformed into a common spatial alignment.
Figure 2.
A. Surface created from a cloud of segmented points, with the patch placed on the weight-bearing area (upper left). Bone density calculations were performed at 1 mm intervals beneath the bone surface, along the surface normals and extending subchondrally up to 8 mm. Adjacent to the joint surface was a high density area where the cortical plate was located, gradually extending to trabecular bone with lower density (schematic drawing in the upper right). B. Mean density (+/− SD) was measured up to 8 mm from joint surface at the different time points in all patients (n=26). Density gradually decreased further from the joint surface. At 1 and 2 years of follow-up, overall density decreased in both tibia and talus. All time points differed statistically significantly from baseline (all p<0.001).
Changes in bone density (in Hounsfield Units (HU), measured relative to baseline) were queried at over 30,000 discrete locations beneath the tibial and talar weight-bearing regions (Figure 2A). The measurement grid covered a subchondral patch of nominally 650 mm2, with typically 4000 point measurements per surface (~0.17 mm2/point). Bone density was measured at 1 mm intervals beneath the bone surface, along the surface normals and extending subchondrally up to 8 mm.
Point-by-point comparison
Baseline and follow-up data were compared point-by-point over the measurement grids. For each surface point, the bone density at every 1 mm interval was compared between the two time points. Further analysis bracketed data based upon the supposition that subchondral bone density in healthy joints would be expected to be >400 HU within the first 3 mm (subchondral plate) and between 100 and 400 HU in the deeper trabecular bone (4–8 mm beneath surface; Figure 2A). Any densities outside of these putative normal density ranges were considered to be abnormal (pathological). The densities of regions of bone with low density (ostensibly cystic, defined as <400 HU for the first 3 mm closest to the joint surface and <100 HU for 4–8 mm from the joint surface) at baseline were compared to the densities at corresponding locations in follow-up scans, and reported as per point changes in density.
Clinical parameter
The primary clinical outcomes were changes in the Ankle Osteoarthritis Scale (AOS) score24; consisting of pain and disability subscales. The AOS questionnaire was completed at baseline and at one and two years after fixator removal.
Statistics
Baseline and follow-up data for bone density at 1 to 8 mm from joint surface showed a normal distribution and parametric statistics were applied. Statistical significance in changes over time were determined using the paired samples T-test (the data at baseline and follow-up per patient served as a pair). In case of point-by-point comparison of bone density, the 95% confidence interval for mean change over time was given (mean change per patient per point). Clinical data also showed normal distribution and significant improvement was determined by using the paired samples T-test. Spearman correlations of the sum of change in bone density for tibia and talus (mean change per point in high and low density areas) were used to identify significant correlations with clinical improvement (percentage change compared to baseline).
Results
Recruitment and Follow-up
The study was opened for enrollment in December, 2002 and closed in October, 2006. Follow-up was completed for the last patient in March, 2009.
Bone outcome
At one year following completion of joint distraction, the mean subchondral bone density for the study group was decreased (Figure 2B). Immediately beneath the joint surface, density was approximately 700 HU at baseline, and gradually decreased with greater distance from the surface. The follow-up data followed a similar trend, but at substantially lower densities. Mean density over the area of 1 to 8 mm from the joint surface decreased 23±12% (mean±SD; p<0.001) for the tibia and from 18±15% (p<0.001) for the talus. Two years after distraction, the overall decrease in bone density compared to baseline was still present (−21±12%; p<0.001, −16±15%; p<0.001 for tibia and talus respectively) and for the tibia statistically indistinguishable (p=0.35) from that observed at one year of follow-up.
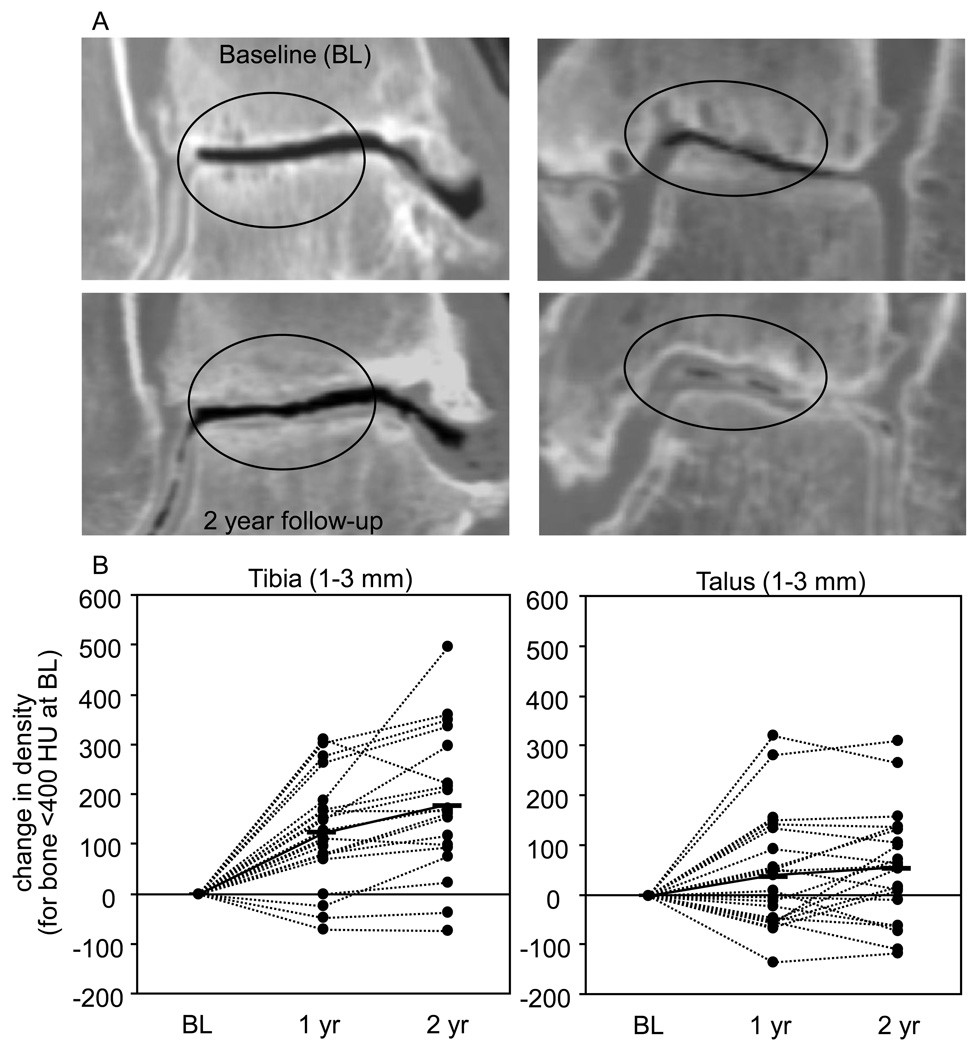
A point-by-point comparison (Table 1 shows mean ± SD and the 95% confidence interval) revealed that regions of bone from 1 to 3 mm beneath the joint surface with abnormally low density (< 400 HU) at baseline showed an increase in density; individual results are presented in Figure 3B. Also, more dense regions (>400 HU) over that same volume saw a decline, and returned to more normal densities at both one and two year time points (Table 1).
Table 1.
Change in density (mean ± SD in HU, 95% confidence interval, lower limit, upper limit) compared to baseline for tibia and talus at one and two years of follow-up (n=26).
| 1 year | 2 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% | CI | Mean | SD | 95% | CI | ||||
| 1–3 mm | |||||||||||
| > 400 HU | tibia | −237 | ± | 111 | −186 | −288 | −192 | ± | 117 | −139 | −244 |
| talus | −156 | ± | 93 | −188 | −194 | −136 | ± | 82 | −101 | −171 | |
| < 400 HU | tibia | 123 | ± | 108 | 77 | 169 | 180 | ± | 143 | 116 | 244 |
| talus | 41 | ± | 111 | −3 | 84 | 58 | ± | 114 | 9 | 107 | |
| 4–8 mm | |||||||||||
| > 400 HU | tibia | −184 | ± | 92 | −141 | −226 | −193 | ± | 102 | −147 | −239 |
| talus | −138 | ± | 77 | −107 | −170 | −127 | ± | 80 | −93 | −161 | |
| < 100 HU | tibia | 144 | ± | 133 | 83 | 206 | 153 | ± | 131 | 94 | 212 |
| talus | 92 | ± | 100 | 51 | 133 | 78 | ± | 144 | 17 | 140 | |
Figure 3.
A. Representative CT scans of two patients before and two years after distraction, showing severe bone pathology at baseline (upper panels) with cysts and sclerosis in the weight bearing area. At 2 years of follow up (lower panels) there is a decrease of density in the sclerotic area while subchondral cysts diminished (within ovals) and the cartilage layer seemed to increase B. Change in density of the low density areas (<400 HU at baseline) for individual patients. A mean increase (solid lines) can be observed at one and two years of follow-up.
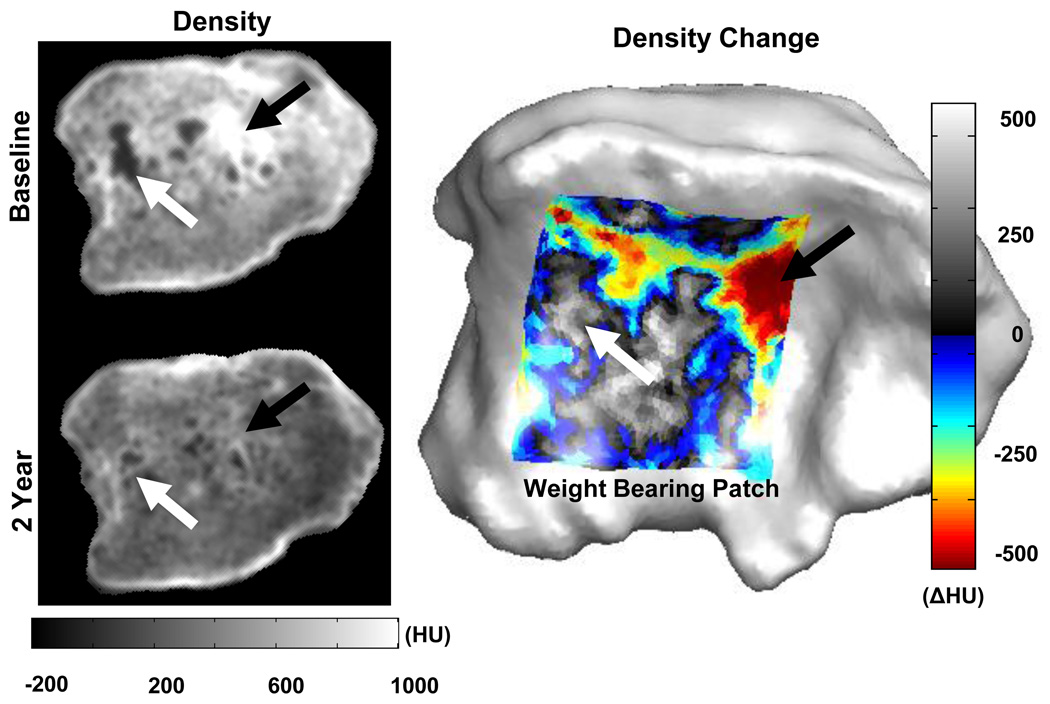
Further away from the joint surface (from 4–8 mm), a density increase at low density areas was also observed, as well as a decrease in density in regions of bone with baseline densities >400 HU (Table 1) .We observed that at baseline, clustered low-density areas were surrounded by high-density areas. At two years of follow up, normalization toward a more homogenous density distribution was seen (Figure 4).
Figure 4.
After two years of distraction, the normalization of bone densities is evident on CT (left). The change in bone density (2yr-baseline) in the weight bearing area of the tibia (mean from 1–3 mm depth), right, depicts the increase in density near cysts (white arrow), and a decrease in density in sclerotic regions (black arrow).
To investigate reproducibility, two patients with CT scans that had been taken approximately two weeks apart (for reasons unrelated to the study) were analyzed using this methodology, with the hypothesis that bone densities would not have changed over that short time-period. The reproducibility analysis showed an average measured difference in distal tibia bone density of only 32±30 (mean±SD) HU and 35±19 HU. While two subjects cannot definitively confirm the validity of these methods, they do support the validity of this technique.
Clinical outcome
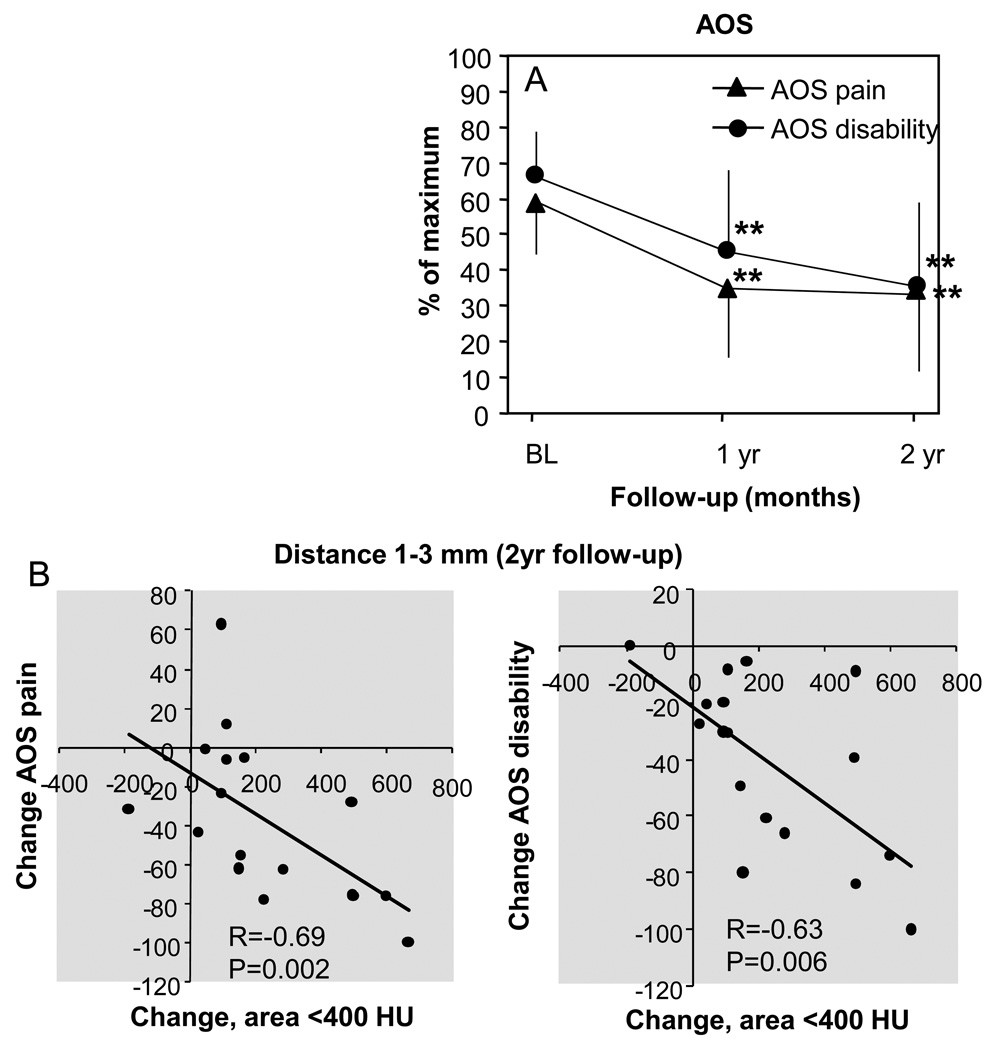
Clinical outcome was measured by use of the AOS (Figure 5A). At baseline, AOS pain was 60±3% (mean±SD) of the maximum score and decreased to 35±4% (p<0.001) of the maximum score at one year of follow-up and to 35±5% (p<0.001) of maximum score at two years follow-up. AOS disability showed comparable results. A baseline score of 67±2% of the maximum score decreased to 46±5% (p<0.001) of the maximum score at one year of follow-up and to 36±5% (p<0.001) of the maximum score at two years of follow-up.
Figure 5.
A. Clinical outcome presented by the AOS score (mean±SD). Subscales pain and disability are shown on a scale of 0–100 (100 being the worst outcome). ** indicate p-values <0.001 compared to baseline. B. Correlations between percentage change in AOS subscale and change in density in low density areas (sum of change of the tibia and talus).
Correlations
No statistical significant correlation was detected between the decrease in sclerotic regions and decrease in pain despite a relatively high coefficient (r=−0.24; p=0.36). A modest correlation was found between the lack of improvement of disability and more decrease of bone density in high density areas(r=−0.52; p=0.03), but only in the area close to the joint surface (1–3 mm). Interestingly, the increase in density in cystic regions near the joint surface did strongly correlate with clinical improvement as assessed by the AOS pain and AOS disability after two years (Figure 5B).
Discussion
Summary
The present study demonstrates that treatment of advanced post-traumatic ankle OA with joint distraction produced an overall decrease of subchondral bone density, which persisted for at least two years. Subchondral bone at baseline consisted of varied regions of relatively low density (cystic) and high density (sclerotic) areas. While overall density decreased, density in cystic lesions actually increased (normalization of bone density). In addition, a correlation was found between clinical improvement and the resolution of subchondral bone cysts.
Method limitations
Patients included in this study suffered from end-stage post-traumatic ankle OA characterized by severely damaged subchondral bone. In a few cases, bone boundaries were challenging to segment. Not only were some of these ankles subject to degenerative morphological changes, but bone remnants from previous fracture and hardware were also present. For these cases, registration was performed using only undamaged segments of the bone surface. While the presence of metal objects and/or fracture lines away from the joint may corrupt some volume of the CT data, no artifacts were visually apparent near the joint surface.
Analysis was done on a selected region of the joint surface that corresponded to the weight-bearing area. The baseline surface served as the datum for bone density analyses at all three time points. Over time, the contour of the joint surface may have slightly changed (due to attrition), and therefore density measurements relative to the joint surface would not perfectly coincide between baseline and follow up datasets. However, we presumed that using a single surface with concurrent vector normals as datum for each dataset in the point-by-point comparison, will provide the most appropriate means for consistent analyses of the same location (e.g. cystic regions) at different longitudinal time points.
Although these methods demonstrated sufficient precision in the reproducibility analysis, the imaging protocol was not optimized for bone densitometry. Initially, these CT studies were designed to extract bone and cartilage geometries for computational stress analysis. For that reason, each scan did not include a calibration phantom. Although Quantitative CT (QCT) would have been a better modality, the methods used appear to have provided satisfactory precision and accuracy for this work. The CT scanners used in this study were tested for accuracy, homogeneity, and geometric distortions with phantom calibrations at least once per week. Nonetheless, we acknowledge that some variability occurred, however, the significant changes in bone density were much greater than any expected variation in scanner performance. As with all CT, partial volume effect and beam hardening artifacts were present. However their effects are believed to be minimal considering the ankle’s size, geometry, and thin soft tissue envelope. Despite those limitations, clinical CT provided sufficiently reliable high-resolution data volumes that enabled point-by-point comparisons to be made in 3D space.
Bone changes due to distraction
While normal trabecular bone usually exhibits a density less than 400 HU within 10 mm of the joint surface, these patients had an average density greater than 400 HU at baseline. Increased subchondral density (sclerosis) was expected in these OA ankles. Within the sclerotic area, low density areas were observed (the presence of cysts, either communicating or not with the joint space), with densities <400 HU in the subchondral plate and < 100 HU in the deeper trabecular bone considered pathological25
One year after treatment with joint distraction, overall density had decreased. At 4–5 mm below the joint surface, density had decreased below 400 HU, a density level expected in subchondral trabecular bone not affected by OA. In addition, discrete pockets of low density bone decreased. The results from this study suggest that joint distraction may lead to a normalization of OA-induced pathological subchondral bone changes for a period of at least two years.
Mechanism of bone changes
In joint distraction, both cartilage and subchondral bone are unloaded for a certain period. Since bone becomes osteopenic when unloaded26, it was not surprising to observe a decrease in bone density following distraction, although the duration of two years was somewhat unexpected. The exact mechanism for the disappearance of cysts could lie in the dramatic changes in mechanical and biochemical environment induced by distraction. Cysts represent areas of bone necrosis9, and have the potential to not only increase but also decrease14. Less surrounding sclerosis -and probably subsequently less stiff bone27 may allow mechanical stimuli to reach the cystic areas and induce bone formation. This, in combination with an overall increase in bone turnover, might be the necessary circumstance under which cystic areas can be repaired.
A role in clinical improvement
No positive correlations were found between globally diminished sclerosis and clinical improvement. In contrast, patients with less dramatic bone density decreases saw an improvement in disability scores. Although counterintuitive at first, this could be a result from remodeling that was stimulated by greater loading, made possible by the improvement in function.
The correlation between an increase in density of low density areas and patient-reported outcomes suggest that the resolution of bone cysts was beneficial to clinical outcome. Cyst-related joint pain might be caused by increased pressure and fluid flow in the subchondral bone. During loading, compression of cartilage forces fluid into the bone through the damaged subchondral plate28. The hydraulic conductance of osteochondral tissue has been shown to be higher in osteoarthritis29. When cysts and defects in the subchondral plate diminish, the subchondral bone is less subject to increased fluid flow and pressure responsible for joint pain. Especially in cystic areas (pores) close to the joint surface, within the cortical plate, an increase in hydraulic conductance might be responsible for joint pain. Bone cysts (and bone surface attrition) seem to evolve in regions of bone marrow lesions and might be the next level of bone marrow pathology in OA8. The relationship between bone marrow lesions as seen on MRI and clinical symptoms has already been established, and it could also be explained by increased pressure within the bone in areas of excessive loading and mechanically compromised trabecular structure8, 30.
Bone and cartilage interaction
The results from this study show that subchondral bone density changes in response to joint distraction. Hypotheses can be made with respect to the effect of these bone changes on cartilage. Bone remodeling may lead to a more physiologically normal distribution of mechanical stresses, particularly near regions with less dense bone that may in turn encourage cartilage repair activity and changes the availability of cartilage destructive mediators originating from bone31–33. In addition to bone changes, visual assessment of the CT arthrographic data suggested there to be an increase in cartilage thickness -an observation that deserves additional research.
The current study showed that joint distraction started a process of bone remodeling and a subsequent improvement in clinical outcome in a series of OA patients. While further research is needed to establish efficacy before distraction can be widely implemented, these results underline that joint distraction is potentially an effective method for treating severe OA.
Acknowledgements
We would like to acknowledge Ms. Patty Stolley for her role in guiding patients and coordinating the study. This work benefited from the use of the Insight Segmentation and Registration Toolkit (ITK), open source software developed as an initiative of the U.S. National Library of Medicine and available at www.itk.org. The authors did not have any writing assistance.
Role of funding source
This study was financially supported by grants from the NIH/NIAMS (AR048939 and AR055533).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
F. Intema (f.intema@umcutrecht.nl) takes responsibility for the integrity of the work as a whole, from inception to finished article. This author is responsible for study design, data analysis and interpretation as well as drafting of the manuscript. T. Thomas has participated in data analysis, with an emphasis on method development and validation. This author has also participated in drafting of the article as well as creating figures. D. Anderson was involved in study design, development of analysis methods, data interpretation and drafting of the manuscript. J. Elkins was involved in a pilot study preceding the current work. T. Brown is responsible for obtaining of funding and critical revision of the article for important intellectual content. N. Amendola was responsible for provision and treatment of patients and revision of the article for important intellectual content. F. Lafeber has participated drafting and critical revision of the article for important intellectual content. C. Saltzman was responsible for obtaining of funding, study design, provision and treatment of patients, and critical revision of the article for important intellectual content.
Competing interest statement
None of the authors have competing interests.
References
- 1.Moskowitz RW. Osteoarthritis. Fourth Edition. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer Business; 2007. [Google Scholar]
- 2.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 3.Saltzman CL, Kadoko RG, Suh JS. Treatment of isolated ankle osteoarthritis with arthrodesis or the total ankle replacement: a comparison of early outcomes. Clin Orthop Surg. 2010;2:1–7. doi: 10.4055/cios.2010.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burr DB. The importance of subchondral bone in osteoarthrosis. Curr Opin Rheumatol. 1998;10:256–262. doi: 10.1097/00002281-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Neogi T, Nevitt M, Niu J, Sharma L, Roemer F, Guermazi A, et al. Subchondral bone attrition may be a reflection of compartment-specific mechanical load: the MOST Study. Ann Rheum Dis. 2010;69:841–844. doi: 10.1136/ard.2009.110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo GH, Hunter DJ, Zhang Y, McLennan CE, Lavalley MP, Kiel DP, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. 2005;52:2814–2821. doi: 10.1002/art.21290. [DOI] [PubMed] [Google Scholar]
- 7.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 8.Carrino JA, Blum J, Parellada JA, Schweitzer ME, Morrison WB. MRI of bone marrow edema-like signal in the pathogenesis of subchondral cysts. Osteoarthritis Cartilage. 2006;14:1081–1085. doi: 10.1016/j.joca.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Pouders C, De Maeseneer M, Van Roy P, Gielen J, Goossens A, Shahabpour M. Prevalence and MRI-anatomic correlation of bone cysts in osteoarthritic knees. AJR Am J Roentgenol. 2008;190:17–21. doi: 10.2214/ajr.07.2098. [DOI] [PubMed] [Google Scholar]
- 10.Imhof H, Breitenseher M, Kainberger F, Rand T, Trattnig S. Importance of subchondral bone to articular cartilage in health and disease. Top Magn Reson Imaging. 1999;10:180–192. doi: 10.1097/00002142-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14 Suppl A:A87–A94. doi: 10.1016/j.joca.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 12.Hayes CW, Jamadar DA, Welch GW, Jannausch ML, Lachance LL, Capul DC, et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology. 2005;237:998–1007. doi: 10.1148/radiol.2373041989. [DOI] [PubMed] [Google Scholar]
- 13.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14:1033–1040. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Tanamas SK, Wluka AE, Pelletier JP, Martel-Pelletier J, Abram F, Wang Y, et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis Res Ther. 2010;12:R58. doi: 10.1186/ar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saltzman CL, McIff TE, Buckwalter JA, Brown TD. Total ankle replacement revisited. J Orthop Sports Phys Ther. 2000;30:56–67. doi: 10.2519/jospt.2000.30.2.56. [DOI] [PubMed] [Google Scholar]
- 16.Coester LM, Saltzman CL, Leupold J, Pontarelli W. Long-term results following ankle arthrodesis for post-traumatic arthritis. J Bone Joint Surg Am. 2001;83-A:219–228. doi: 10.2106/00004623-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kwan TatS, Lajeunesse D, Pelletier JP, Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol. 2010;24:51–70. doi: 10.1016/j.berh.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brouwer RW, Raaij van TM, Bierma-Zeinstra SM, Verhagen AP, Jakma TS, Verhaar JA. Osteotomy for treating knee osteoarthritis. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004019.pub3. CD004019. [DOI] [PubMed] [Google Scholar]
- 19.Marijnissen AC, Van Roermund PM, Van Melkebeek J, Schenk W, Verbout AJ, Bijlsma JW, et al. Clinical benefit of joint distraction in the treatment of severe osteoarthritis of the ankle: proof of concept in an open prospective study and in a randomized controlled study. Arthritis Rheum. 2002;46:2893–2902. doi: 10.1002/art.10612. [DOI] [PubMed] [Google Scholar]
- 20.Ploegmakers JJ, van Roermund PM, van Melkebeek J, Lammens J, Bijlsma JW, Lafeber FP, et al. Prolonged clinical benefit from joint distraction in the treatment of ankle osteoarthritis. Osteoarthritis Cartilage. 2005;13:582–588. doi: 10.1016/j.joca.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Saltzman C, Hillis S, Stolley M, Amendola N. Prospective RCT of Motion vs. Fixed Distraction in Treatment of Ankle OA. AAOS annual scientific meeting. 2010 [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Khoury GY, Alliman KJ, Lundberg HJ, Rudert MJ, Brown TD, Saltzman CL. Cartilage thickness in cadaveric ankles: measurement with double-contrast multi-detector row CT arthrography versus MR imaging. Radiology. 2004;233:768–773. doi: 10.1148/radiol.2333031921. [DOI] [PubMed] [Google Scholar]
- 24.Domsic RT, Saltzman CL. Ankle osteoarthritis scale. Foot Ankle Int. 1998;19:466–471. doi: 10.1177/107110079801900708. [DOI] [PubMed] [Google Scholar]
- 25.Hounsfield GN. Nobel Award address. Computed medical imaging. Med Phys. 1980;7:283–290. doi: 10.1118/1.594709. [DOI] [PubMed] [Google Scholar]
- 26.Bikle DD, Halloran BP. The response of bone to unloading. J Bone Miner Metab. 1999;17:233–244. doi: 10.1007/s007740050090. [DOI] [PubMed] [Google Scholar]
- 27.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986:34–40. [PubMed] [Google Scholar]
- 28.van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. The natural history of osteochondral lesions in the ankle. Instr Course Lect. 2010;59:375–386. [PubMed] [Google Scholar]
- 29.Hwang J, Bae WC, Shieu W, Lewis CW, Bugbee WD, Sah RL. Increased hydraulic conductance of human articular cartilage and subchondral bone plate with progression of osteoarthritis. Arthritis Rheum. 2008;58:3831–3842. doi: 10.1002/art.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunter DJ, Gerstenfeld L, Bishop G, Davis AD, Mason ZD, Einhorn TA, et al. Bone marrow lesions from osteoarthritis knees are characterized by sclerotic bone that is less well mineralized. Arthritis Res Ther. 2009;11:R11. doi: 10.1186/ar2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2005;13:988–997. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez C, Gabay O, Salvat C, Henrotin YE, Berenbaum F. Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthritis Cartilage. 2009;17:473–481. doi: 10.1016/j.joca.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Westacott CI, Webb GR, Warnock MG, Sims JV, Elson CJ. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 1997;40:1282–1291. doi: 10.1002/1529-0131(199707)40:7<1282::AID-ART13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]