Abstract
Background
Although there are multiple indications that alcohol can alter many physiological brain functions, including cerebral blood flow (CBF), studies of the latter have generally used small or modest sized samples. Few investigations have yet evaluated how CBF changes after alcohol relate to subsets of subjects with elevated alcoholism risks, such as those with lower levels of response (LR) to alcohol. This study used arterial spin labeling (ASL) after alcohol administration to evaluate a large sample of healthy young men and women with low and high alcohol responses, and, thus, varying risks for alcohol use disorders (AUD).
Methods
Healthy young adult social drinkers with low and high LR (N=88, 50% female) matched on demography and drinking histories were imaged with whole brain resting ASL ~1 hour after ingesting ~3 drinks of ethanol and after a placebo beverage (i.e., 178 ASL sessions). The relationships of CBF changes from placebo to alcohol for subjects with low and high LR were evaluated.
Results
CBF increased after alcohol as compared to placebo in five frontal brain regions. Despite identical BACs, these increases with alcohol were less prominent in individuals who required more drinks to experience alcohol-related effects (i.e., had a lower LR to alcohol). The LR group differences remained significant after covarying for recent drinking quantities.
Conclusions
The results confirm that alcohol intake is associated with acute increases in CBF, particularly in frontal regions. Less intense CBF changes were seen in subjects with a genetically influenced characteristic, a low LR to alcohol, that relates to the future risk of heavy drinking and alcohol problems.
Keywords: alcohol, arterial spin labeling, level of response to alcohol, alcoholism risk
Introduction
The majority of people in Western countries drink alcohol at some time during their lives (Teesson et al., 2006), and the lifetime risk for severe repetitive alcohol problems is 8–10% for women and over 15% for men (Grucza et al., 2008). The physiological correlates of the effects of alcohol on the brain have been examined with a range of techniques, with most suggesting that acute alcohol consumption results in numerous brain changes, including event-related potential latency, the release of several hormones, and increases in cerebral blood flow (CBF) (De et al., 2002; Roberto et al., 2010; Schuckit et al., 1988a). However, while the relationship of some of these effects of alcohol to the risk for future heavy drinking and alcohol use disorders (AUDs) have been reported (e.g., Hill and Shen, 2002; Leggio et al., 2008), no such studies have been published regarding CBF.
Vulnerabilities toward AUDs correspond with several preexisting characteristics, one of which involves a low level of response (LR) to alcohol, perhaps reflecting a lower sensitivity to this drug (Chung and Martin, 2009; Schuckit, 2009). A lower LR is genetically influenced (Heath et al., 1999; Joslyn et al., 2010), and is a robust marker of an enhanced risk for future alcohol problems (e.g., Chung and Martin, 2009; Heath et al., 1999; Schuckit et al., 2007; Volavka et al., 1996), even in lighter drinking subjects as young as age 12 (Schuckit et al., 2007, 2008). The LR can be measured by a retrospective report of the need for a greater number of drinks for effects during a typical drinking session, or by evaluating the subjective and physiologic effects of alcohol at a specific blood level in a laboratory alcohol challenge (Chung and Martin, 2009; Eng et al., 2005). Physiological measures have included observations of less change in standing steadiness after drinking, and less change in a range of more objective CNS measures of alcohol’s effects including less increases in adrenocorticotropin (ACTH) and prolactin hormones, latency measures in event-related potentials, and levels of change in background cortical electroencephalograms (Ehlers and Schuckit, 1988; Schuckit et al., 1987, 1988a, 1988b). Additional evidence of brain differences between subjects with low and high LR to alcohol comes from functional magnetic resonance imaging (fMRI). Using methods focusing on contrast in Blood Oxygen Level Dependent (BOLD) response during a visual working memory task, after placebo (or no drug challenge) those with low LR demonstrated greater response contrast in brain regions relevant to the task, but LR group differences disappeared or reversed in direction when the subjects were imaged after consuming ~0.7 ml/kg of alcohol (Paulus et al., 2006; Tapert et al., 2004; Trim et al., 2010).
Regarding CBF, using positron emission tomography (PET), Volkow and colleagues (1988) demonstrated significant increases in blood flow in the prefrontal and temporal regions and reductions to the cerebellum in six social drinkers after consuming 1.0g/kg of ethanol. 133Xenon inhalation studies also revealed CBF increases in cortical gray matter after a moderate dose of alcohol (Mathew and Wilson, 1986; Newlin et al., 1982), prefrontal regions after a low dose of alcohol, and temporal regions after a higher dose (1.5g/kg) (Sano et al., 1993). Using single photon emission computed tomography (SPECT), Tiihonen and colleagues (1994) observed similar patterns of changes in brain blood flow in the right prefrontal cortex after rapid alcohol ingestion, with support for the findings from a transcranial Doppler study (Blaha et al., 2003). However, all these studies involved relatively small samples and CBF has not generally been compared across non-alcoholic groups at higher and lower risk for future alcohol problems. Until the recent more widespread use of arterial spin labeling (ASL), another informative dynamic brain imaging technique, magnetic resonance imaging (MRI), was not able to directly measure alcohol-related blood flow changes.
ASL is a non-invasive, reliable MRI method for obtaining quantitative measures of regional CBF that can be used in functional MRI (fMRI) analyses (Liu and Brown, 2007). It is important for the interpretation of fMRI studies, as changes in baseline CBF can influence the magnitude of the BOLD response signal that is key to fMRI results (Brown et al., 2003; Cohen et al., 2002). In ASL, water protons in arterial blood are magnetically labeled as they flow into brain tissue, fMRI images are acquired both with and without tagged blood, and CBF is measured by taking the difference between tag and control images. Although ASL has been used to examine brain blood perfusion in alcohol dependent individuals in a non-intoxicated state (Clark et al., 2007), regional changes in CBF following acute alcohol exposure have not been previously investigated using ASL with a large sample of healthy, non-alcoholic subjects. Nor has the effect of alcohol on CBF using MRI-based imaging techniques been compared in subgroups at higher risk for future problems, including those with a lower level of response (LR) (sensitivity) to alcohol.
The current paper reports results of an additional potential biological correlate of LR, the effect of alcohol on CBF. We hypothesized that ASL in healthy non-alcoholic young adult males and females would demonstrate: 1) greater brain blood perfusion after ingesting a moderate dose of alcohol as compared to after consuming a placebo beverage, consistent with other measures described above; 2) also consistent with other measures, alcohol/placebo differences will be apparent in frontal brain regions; and, most importantly, 3) consistent with a lower intensity of BOLD response contrast after alcohol and less change in EEG, prolactin and cortisol, CBF changes with alcohol will be less intense in subjects with an enhanced risk for future problems, those with diminished LRs to alcohol.
Materials and Methods
Participants and Measures
Possible participants were originally identified from a brief screening questionnaire mailed to students at a local university, using procedures approved by the UCSD Human Research Protections Program. The mailing included questions about demography and drinking history, as well as 4 questions regarding the number of standard alcoholic drinks (~10gm ethanol) required to experience a range of effects the ~first 5 times of drinking and before chronic tolerance was likely to have developed. This Self-Report of the Effects of Alcohol (SRE) questionnaire has good reliability and validity, and a greater number of drinks for effects (or a lower level of response per drink) is associated with a higher future risk for heavy drinking and alcohol problems (Chung and Martin, 2009; Schuckit et al., 2010).
Inclusionary criteria for the study were being age 18–25 and having consumed at least one full standard alcoholic drink in the past. Exclusionary criteria were a lifetime or current diagnosis of alcohol or other drug dependence, bipolar or schizophrenia disorders (American Psychiatric Association, 1994; 1987); a current medical condition or use of a medication that might interfere with an alcohol challenge or brain blood flow; previous head trauma with loss of consciousness >3 minutes; current pregnancy; left handedness; and MRI contraindications (e.g., claustrophobia, irremovable metal).
Procedures
Potential subjects with SRE results in the ~upper and lower thirds on LR were interviewed in person with the Semi-Structured Assessment for the Genetics of Alcoholism instrument (SSAGA; Bucholz et al., 1994; Hesselbrock et al., 1999). These subjects were the respondents most appropriate for generating pairs of participants likely to demonstrate clearly high and low LRs on alcohol challenges, and were selected as matched as closely as possible on age, sex, recent drinking histories, and recent use of nicotine and illicit drugs. The validated and reliable SSAGA was administered by trained research personnel to review criteria for 17 Axis I DSM IV diagnoses as well as the antisocial personality disorder (American Psychiatric Association, 1994; 1987).
The LR likely to be observed during an alcohol challenge carried out in a brain scanner was then directly evaluated with an alcohol challenge after consuming 0.70 ml/kg (for females) or 0.75 ml/kg (for males) of laboratory grade ethanol over ~10 minutes. This was given as a 20%-by-volume dose ingested from a straw attached to a reservoir covered by ethanol-saturated gauze inserted into a sealed container to disguise the beverage (Mendelson et al., 1984; Schuckit and Gold, 1988). LR scores were generated through the Subjective High Assessment Scale (SHAS), a self-report measure of 13 subjective effects of alcohol, each evaluated on a 39-point scale, scored every 15 to 30 minutes after the drink for ~3 hours (Schuckit and Gold, 1988). The analyses focused on the seven items with the greatest validity and coherence in prior work, the SHAS-7 (Eng et al., 2005).
Participants whose LR on the alcohol challenge continued to fall into the ~upper and lower thirds of subjective responses to alcohol were then scheduled, in random order, for two imaging sessions with alcohol or a placebo beverage using otherwise identical protocols. After abstaining from food and drink for 12 hours, all subjects were established to have a zero breath alcohol concentration (BrAC) using the Alco-Sensa IV (Intoximeters Inc., St. Louis, MO) when they arrived in the laboratory, and a cannula was inserted in an antecubital vein for blood samples to assess blood alcohol concentration (BAC) during scanning using a photometric enzymatic approach generated from a kit from Roche Pharmaceuticals, as the breathalyzer device was not permitted in the scan room. Subjects then took ~10 minutes to consume either a placebo beverage (caffeine-free diet soda) with a small amount of alcohol in the straw, or the same dose of ethanol noted above. They were placed into the scanner 22 minutes after the start of beverage administration, with testing over the next ~ 1 hour that included blood samples for BAC. After scanning, the cannula was removed, participants were debriefed, and were permitted to go home by taxi or with a driver once BrAC levels were <.01 g/dL.
Imaging data were collected at the UCSD fMRI Center with a 3-Tesla General Electric (Milwaukee, WI) Signa Excite HD scanner using an 8-channel head array coil. Each scan session began with a sagittally acquired high-resolution spoiled gradient recalled (SPGR) anatomical sequence (25 cm field of view; 256 × 256 matrix; 124 slices each 1.0 mm thick covering the whole brain; 4.8 ms echo time; and 20 ms repetition time). To assess CBF differences during each session, three sequences were acquired to obtain absolute CBF measurements. Resting brain blood perfusion was measured with pulsed arterial spin labeling using a modified flow-sensitive alternating inversion recovery (FAIR) sequence with both presaturation pulses and PICORE QUIPSS 2 post-inversion saturation pulses and a spiral readout with 4 interleaves to reduce signal dropout due to susceptibility effects (Liu and Wong, 2005; Wong et al., 1998). Imaging parameters of the ASL scan were: 22 × 22 cm field of view, a 64 × 64 matrix, 3.2 ms echo time, 2500 ms repetition time, post-saturation and inversion times of TI1 = 600 ms and TI2 = 1600 ms, tag thickness 10 cm, tag to proximal slice gap 1cm, 20 5 mm axial slices, and 40 volumes for 20 tag+control image pairs (Wong, 2005). A scan with the inversion pulses turned off was acquired to obtain an estimate of the equilibrium magnetization of cerebral spinal fluid, and a minimum contrast image was acquired to adjust for coil inhomogeneities (Restom et al., 2007). ASL data collection began approximately 60 minutes after beverage consumption began. fMRI tasks, not described here, were also administered (Trim et al., 2010).
Data Processing & Analyses
Analyses focused on contrasting participants’ whole brain resting ASL data obtained after consuming a placebo beverage to whole brain resting ASL results after consuming a moderate dose of alcohol. Imaging data were processed using Analysis of Functional NeuroImages (AFNI; afni.nimh.nih.gov; Cox, 1996), FMRIB Software Library (FSL, Oxford, United Kingdom; Smith et al., 2004), and locally created MatLab scripts. Each ASL dataset was first reconstructed using the SENSE algorithm (Pruessmann et al., 1999; Weiger et al., 2002) to reduce sensitivity to the modulations that occur between shots caused by physiological fluctuations or motion. Second, ASL data were processed with an automated MatLab script that used AFNI and FSL tools. Third, the ASL time series was co-registered to the middle time point to minimize the effects of participant motion. Fourth, surround subtraction of the tag-control time series was performed to create an uncorrected perfusion time series and slice timing delays were accounted for, making the inversion time (TI2) slice specific. Each high-resolution dataset was spatially standardized, then skull stripped using FSL’s BET algorithm, and then segmented using FSL’s FAST algorithm to define cerebral spinal fluid (CSF), gray matter (GM) and white matter (WM) regions. The high-resolution T1-weighted image and partial volume segmentations were registered in ASL space, and partial volume segmentations were down-sampled to the resolution of the ASL data. CBF was calculated from the signal difference between tag and control images (Wong, 2005) and converted to absolute units (ml/100g/min) using the CSF image as a reference signal (Chalela et al., 2000). This resulted in a calibrated perfusion value for each voxel, at each session. A 4.0 mm full-width, half-maximum Gaussian filter was applied to the CBF data in AFNI. Voxels with negative intensities were replaced with zero (Brown et al., 2003). CBF data were warped to standard space and resampled to a 4×4×4 mm resolution grid. Data from both sessions were screened for data quality and outlying values. Seven participants were deleted on the basis of potential artifact or outlying values during one or both sessions, resulting in 44 matched LR pairs with valid data in both sessions (i.e., 176 valid scans).
Because this is the first study to our knowledge to examine acute effects of a moderate dose of alcohol using ASL, group-level analysis centered on a whole brain, voxel-level paired t-test (AFNI 3dttest) to contrast perfusion values between the placebo and alcohol sessions. Type I error was controlled based on Monte Carlo simulation results using AFNI’s AlphaSim with a voxel-wise alpha of 0.05 and cluster-wise alpha of 0.001. This resulted in a minimum cluster volume threshold of 1344μl (21 contiguous voxels, each with effects at p<.05), which yielded an overall 0.1% chance of finding an effect under the null hypothesis. To ensure that CBF values were not influenced by known decreased perfusion in white matter (e.g., Hermes et al., 2007; Parkes et al., 2004; Shin et al., 2007), we applied an averaged gray matter mask that was created by obtaining each individual participant’s gray matter volumes using FSL FAST and then averaging across participants using AFNI 3dmerge. The averaged gray matter mask was then applied to the t-test results.
To evaluate if results might have reflected errors related to low detection of brain perfusion, temporal signal to noise ratio (tSNR) was evaluated for each subject in each voxel by dividing the mean signal with the standard deviation from ASL tag-control difference time series values. The mean tSNR was extracted for each participant in each region where a significant session (alcohol versus placebo) effect was found.
Follow-up analyses were conducted to evaluate if CBF changes from placebo to alcohol related to the level of response to alcohol. Here, the SHAS-7 scores during the alcohol challenges were correlated with a CBF change score (alcohol minus placebo) for each cluster showing a significant alcohol versus placebo session effect (α=.05). Finally, characteristics related to LR or CBF (e.g., past drinking) were covaried in all evaluations of the CBF change score (alcohol minus placebo) for each cluster showing a significant session effect (α=.05).
Results
Main effects for alcohol vs. placebo condition differences
The demographic characteristics and substance use histories of the subjects with high and low LRs are shown in Table 1. Consistent with matching procedures, the two groups were similar on age, gender, education, drinking frequency, tobacco and cannabis use characteristics, as well as peak BAC during the imaging alcohol session. Also consistent with their manner of selection, the low LR group needed significantly more drinks to achieve the effects on the SRE and showed lower SHAS values during the alcohol challenge. Consistent with the impact of LR on drinking behavior even as young as age 12 (Schuckit et al., 2008), those with low LR typically consumed ~1 drink more per drinking occasion. Therefore, this background characteristic is used as a covariate in relevant analyses. The sample excluded 7 subjects because of imaging related problems. Their data were similar to the 88 participants described in Table 1 for age (19.6 year), % female (43%), years of education (13.6), days per month drank (4.8), drinks per occasion (3.2), SRE score (3.7), peak BAC (.06), as well as patterns of use of tobacco (e.g., 0.3 units per occasion) and cannabinols (25.3 lifetime occasions).
Table 1.
Participant Characteristics as Mean (and Standard Deviations) for 88 Subjects
| Low LR (n=44) | High LR (n=44) | t-statistic | |
|---|---|---|---|
| Age | 19.7 (1.39) | 20.2 (1.48) | −1.63 (p=0.11) |
| % Female | 50 | 50 | -- |
| Years of education completed | 13.5 (1.00) | 13.8 (1.12) | −1.00 (p=0.32) |
| Days/month used alcohol | 4.6 (4.70) | 3.3 (3.59) | 1.39 (p=0.17) |
| Typical drinks consumed/occasion | 4.3 (1.71) | 3.3 (1.89) | 2.63 (p=0.01) |
| Days/month of tobacco use | 0.48 (1.09) | 1.4 (3.74) | −1.62 (p=0.11) |
| Tobacco units/occasion | 0.3 (0.55) | 0.5 (1.55) | −0.64 (p=0.52) |
| Lifetime cannabis use occasions | 28.6 (86.41) | 29.0 (95.70) | −0.21 (p=0.98) |
| SRE units | 4.1 (1.35) | 2.8 (1.40) | 4.49 (p<0.001) |
| SHAS-7 units (60 min) | 4.3 (3.13) | 14.3 (6.06) | −9.74 (p<0.001) |
| Peak BAC in g/dL (60 min) | 0.06 (0.02) | 0.06 (0.02) | 0.11 (p=0.91) |
Note that alcohol doses were given as gm/kg of weight.
Regarding CBF, in the whole brain analysis, five frontal regions showed significant differences between alcohol and placebo conditions in brain blood perfusion (corrected p<.001, clusters ≥ 1344μl). In all five clusters, perfusion was higher after alcohol compared to placebo (see Table 2 and Figure 1). There were no differences in motion adjustments applied to the ASL time series data between alcohol and placebo conditions for any of the six motion parameters evaluated.
Table 2.
Regions Showing Brain Blood Perfusion Differences between Placebo and Alcohol Arterial Spin Labeling Sessions for 88 Subjects
| Anatomic Region* | Brodmann Areas | Volume (μl) | Talairach | Placebo Mean (ml/100g/min) | Alcohol Mean (ml/100g/min) | % Change | Effect size Cohen’s d | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| R middle frontal gyrus, extending to R inferior and superior frontal gyri | 10, 9, 8, 6 | 10,944 | −42 | −47 | 12 | 58.48 | 66.31 | 16.58 | 1.34 |
| Bilateral cingulate gyri, extending to bilateral medial frontal and superior frontal gyri | 6,32 | 7,552 | 6 | 11 | 40 | 81.31 | 88.57 | 10.98 | 1.07 |
| L middle frontal gyrus, extending to L superior frontal gyrus | 8, 10, 9 | 4,224 | 38 | −27 | 40 | 62.06 | 68.82 | 13.62 | 1.01 |
| Bilateral medial frontal gyrus, extending to bilateral anterior cingulate | 10, 9, 32 | 3,712 | 10 | −47 | 8 | 64.34 | 71.56 | 13.79 | 1.03 |
| R precentral gyrus, extending to R inferior and middle frontal gyri | 6, 9 | 3,520 | −46 | 1 | 44 | 70.43 | 77.80 | 12.68 | 1.13 |
Talairach coordinates refer to peak effect group difference within the cluster.
Abbreviations: R right; L left.
Figure 1.
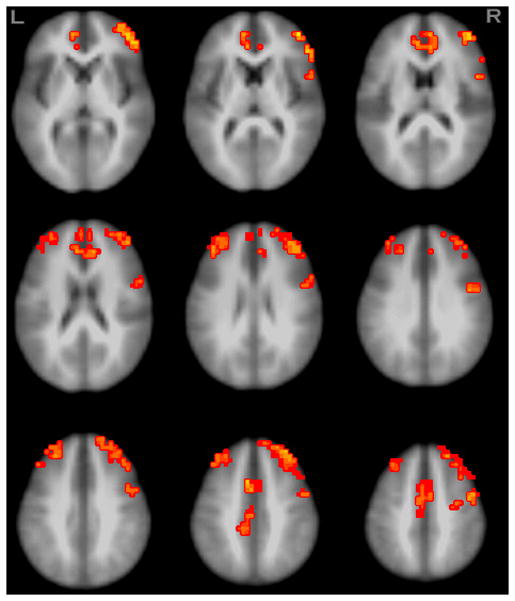
Regions showing brain blood perfusion differences between placebo and alcohol. Orange colors indicate regions with greater brain blood perfusion under the alcohol condition compared to the placebo (N=88, p<.05, clusters ≥1344μl).
A step was taken to help ensure that alcohol vs. placebo differences were not attributable to decreased perfusion in white matter voxels resulting from the use of an averaged gray matter mask (Hermes et al., 2007; Parkes et al., 2004; Shin et al., 2007). Here, individually ascertained gray matter masks were applied to each participant’s CBF dataset prior to group averaging, and the condition effect was again examined in a paired t-test. Results were consistent with those reported above, with the same regions showing higher ASL values in the alcohol as compared to placebo session (corrected p<.01, clusters ≥896 μl).
Steps were also taken to evaluate if results might have reflected errors related to low detection of brain perfusion by using tSNR analyses for the five clusters with alcohol-placebo condition effects. tSNR ratios for each significant cluster ranged from 1.16–1.57, with no significant differences in temporal signal to noise ratios between alcohol and placebo conditions.
LR Group differences in change in CBF from placebo to alcohol
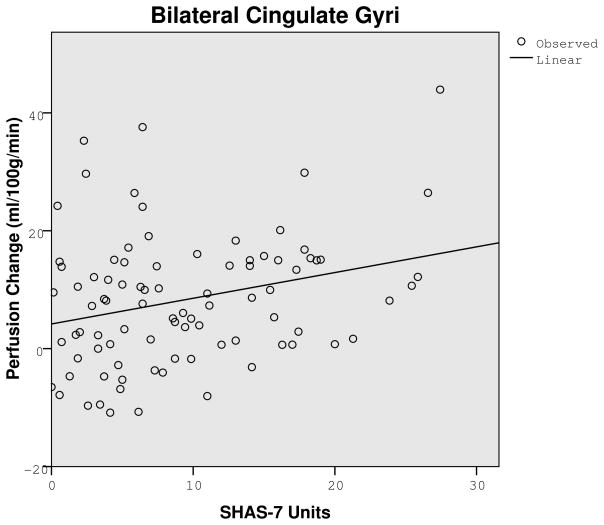
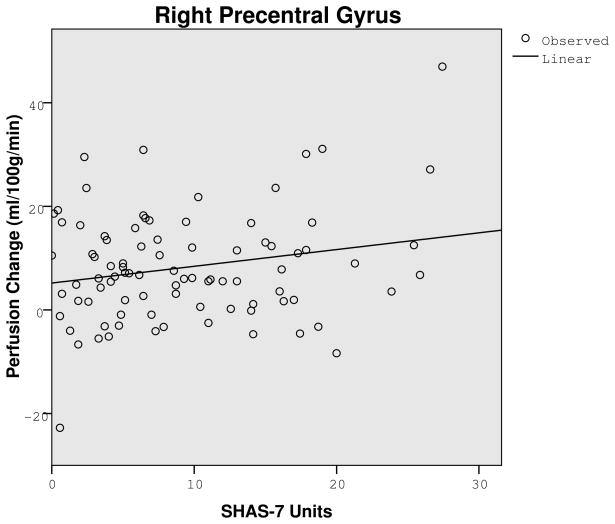
A key analysis evaluated if a low LR to alcohol related to the CBF contrast from placebo to alcohol. Following placebo there were no significant LR group differences. Using ml/100g/minute for low and high groups (with 86 df for each), CBF values were: 59.20 versus 57.77, t=.52, p=.61 for the right middle gyrus; 84.62 versus 77.99, t=1.96, p=.054 for the bilateral cingulate gyri; 63.72 versus 60.40, t=1.65, p=.25 for the left middle frontal gyrus; 65.57 versus 63.11, t=.74, p=.46 for the bilateral medial frontal gyrus; and 73.32 versus 67.53, t=1.95, p=.055 for the right precentral gyrus. However, after alcohol, all five regions in Table 2 showed at least a trend for lower alcohol-related change in CBF after alcohol for the low LR group. Here, those with a lower score on the SHAS (i.e., a lower LR) demonstrated significantly less perfusion change from placebo to alcohol in the two regions shown in Figure 2: bilateral cingulate gyri (r = .27, p=.01) and right precentral gyrus (r = .21, p<.05), with data from the left middle frontal gyrus approaching significance (r = .19, p=.08). Findings in the remaining two regions in Table 2 were in the same direction, but not significant. Similar conclusions were generated for lower vs. higher LR subjects using data from the SRE. The data presented here remained unchanged after controlling for the number of drinks per occasion in recent months.
Figure 2.
Graphs showing correlation between perfusion change (alcohol session minus placebo session) and the Subjective High Assessment Scale (SHAS-7) after alcohol in the bilateral cingulate (r = .27, p=.01) and right precentral gyri (r = .21, p<.05), showing less cerebral blood flow change after alcohol for individuals reporting few subjective changes following a moderate dose of alcohol.
Discussion
This study confirmed and extended prior brain imaging results regarding the increase in CBF following alcohol intake (Newlin et al., 1982; Tiihonen et al., 1994; Volkow et al., 1988). In this sample of 88 healthy young adults, a moderate dose of alcohol was found to increase brain blood flow up to 17% as compared to placebo in the first report of this phenomenon in an MRI paradigm. This is also the first study to document that subjects with a low LR to alcohol, a genetically influenced characteristic that predicts future heavy drinking and AUDs (e.g., Chung and Martin, 2009; Trim et al., 2009; Volavka et al., 1996), demonstrated less increase in CBF after alcohol compared to matched subjects with high LR.
Regarding LR, the results indicating that those with a lower LR showed less effects of this drug on CBF are consistent with prior studies reporting lesser effect of alcohol on several other CNS physiological measures in those with a low LR. These include lesser alcohol-related impact on ACTH and prolactin, as well as less change in background cortical electroencephalograms and event-related potentials (Ehlers and Schuckit, 1988; Schuckit et al., 1987, 1988a, b).
Our group is currently evaluating how several additional neuroimaging findings relate to LR in young, drinking and not yet alcoholic subjects. The goal is to enhance our understanding of differences in more specific physiological CNS effects of alcohol across subjects with lower and higher LR. A recent paper documented LR group differences in a subset of 60 of the subjects (30 LR matched pairs) reported here, where, consistent with the current analyses, the most prominent findings were seen in frontal regions (Trim et al., 2010). Using a visual working memory task during alcohol and placebo sessions, and despite similar performance on the cognitive task for low and high LR subjects, the LR by alcohol/placebo condition effect remained significant even after controlling for BAC, changes in CBF, and drinking history. In that study, subjects with a low LR had higher functional activation in frontal and cingulate regions during placebo, results that are similar to our earlier pilot study (Paulus et al., 2006). However, that LR group placebo difference disappeared after consuming alcohol, largely as a result of lower BOLD response contrast with alcohol compared to placebo for those with a low LR. Additional evaluations in progress will expand our understanding of the LR differences through imaging results in the context of recognition of emotional faces (the Hariri task) and an impulsivity measure (a stop signal task).
These findings regarding alcohol’s effects on CBF and the LR group differences in the CBF discussed above did not reflect differences in signal to noise ratios as these did not vary substantially across brain areas. This indicates fairly uniform signal sensitivity where alcohol vs. placebo effects were detected. While it is possible that the frontal vascular territories may be more susceptible to the acute effects of alcohol on CBF, no consistent differences in CBF between vascular territories or anatomic regions have been apparent in healthy younger volunteers (Floyd et al., 2003; Leenders et al., 1990; Parkes et al., 2004). This literature suggests that the effects of alcohol administration were not confounded by regional cerebrovascular differences.
It is interesting to note that there were no relationships between the alcohol condition and CBF changes in the cerebellum, as had been reported with PET by Volkow and colleagues (1988). The reduction in CBF in that region as well as the increases in the temporal region as reported by Sano and colleagues (1993) were only seen after relatively higher doses of alcohol (1.0 to 1.5 g/kg). The lower dosage used in the current investigation (~7 ml/kg) was closer to 0.6 g/kg, and therefore dose effects might have contributed to the differences across studies.
Of course, the current findings should be viewed from the perspective of the methods employed. First, while the study sample (88 subjects generating 176 ASL evaluations) is large compared to most neuroimaging protocols, the power to detect results with small effect sizes is limited. Second, it is important to remember these were young, healthy, well-educated men and women, and results may be different in established alcoholics or other segments of the population. Third, the analyses cannot address whether the less intense increase in CBF after alcohol for subjects with low LR is a result or a cause of the lesser BOLD response contrast in fMRI analyses following alcohol as reported in prior studies.
In summary, this study provides confirmation in a large healthy sample that a moderate dose of alcohol increases gray matter CBF, particularly in frontal regions, but with less CBF increases among individuals with a lower LR (sensitivity) per drink. The increases in CBF following alcohol consumption are also important for interpreting BOLD signal differences associated with alcohol intake, since the BOLD signal reflects a complex balance between changes in CBF, cerebral blood volume (CBV) and the cerebral metabolic rate of oxygen consumption (CMRO2) (Buxton et al. 2004). As such, CBF effects need to be partialled out when interpreting BOLD signal differences.
Acknowledgments
This research was supported by NIH/NIAAA grant R01 015760.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) American Psychiatric Association; Washington, D.C: 1987. [Google Scholar]
- Blaha M, Aaslid R, Douville CM, Correra R, Newell DW. Cerebral blood flow and dynamic cerebral autoregulation during ethanol intoxication and hypercapnia. J Clin Neurosci. 2003;10:195–198. doi: 10.1016/s0967-5868(02)00126-1. [DOI] [PubMed] [Google Scholar]
- Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23:829–837. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage. 2004;23(Suppl 1):S220–33. doi: 10.1016/j.neuroimage.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Chung T, Martin CS. Subjective stimulant and sedative effects of alcohol during early drinking experiences predict alcohol involvement in treated adolescents. J Stud Alcohol Drugs. 2009;70:660–667. doi: 10.15288/jsad.2009.70.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CP, Brown GG, Eyler LT, Drummond SP, Braun DR, Tapert SF. Decreased perfusion in young alcohol-dependent women as compared with age-matched controls. Am J Drug Alcohol Abuse. 2007;33:13–19. doi: 10.1080/00952990601082605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De A, Boyadjieva N, Oomizu S, Sarkar DK. Ethanol induces hyperprolactinemia by increasing prolactin release and lactotrope growth in female rats. Alcohol Clin Exp Res. 2002;26:1420–1429. doi: 10.1097/01.ALC.0000030621.35354.E0. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Schuckit MA. EEG response to ethanol in sons of alcoholics. Psychopharmacol Bull. 1988;24:434–437. [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79:83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- Grucza RA, Bucholz KK, Rice JP, Bierut LJ. Secular trends in the lifetime prevalence of alcohol dependence in the United States: a re-evaluation. Alcohol Clin Exp Res. 2008;32:763–770. doi: 10.1111/j.1530-0277.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Hermes M, Hagemann D, Britz P, Lieser S, Rock J, Naumann E, Walter C. Reproducibility of continuous arterial spin labeling perfusion MRI after 7 weeks. MAGMA. 2007;20:103–115. doi: 10.1007/s10334-007-0073-3. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit MA, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biol Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn G, Ravindranathan A, Brush G, Schuckit M, White RL. Human variation in alcohol response is influenced by variation in neuronal signaling genes. Alcohol Clin Exp Res. 2010;34:800–812. doi: 10.1111/j.1530-0277.2010.01152.x. [DOI] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113 (Pt 1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Malandrino N, Mirijello A, D’Angelo C, Vonghia L, Miceli A, Capristo E, Kenna GA, Gasbarrini G, Swift RM, Addolorato G. Relationship between the hypothalamic-pituitary-thyroid axis and alcohol craving in alcohol-dependent patients: a longitudinal study. Alcohol Clin Exp Res. 2008;32:2047–2053. doi: 10.1111/j.1530-0277.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Liau J, Perthen JE, Liu TT. Caffeine reduces the activation extent and contrast-to-noise ratio of the functional cerebral blood flow response but not the BOLD response. Neuroimage. 2008;42:296–305. doi: 10.1016/j.neuroimage.2008.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc. 2007;13:517–525. doi: 10.1017/S1355617707070646. [DOI] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke. 1986;17:1156–1159. doi: 10.1161/01.str.17.6.1156. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, McGuire M, Mello NK. A new device for administering placebo alcohol. Alcohol. 1984;1:417–419. doi: 10.1016/0741-8329(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Golden CJ, Quaife M, Graber B. Effect of alcohol ingestion on regional cerebral blood flow. Int J Neurosci. 1982;17:145–150. doi: 10.3109/00207458208985916. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magn Reson Med. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcohol Clin Exp Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37:430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Wendt PE, Wirsen A, Stenberg G, Risberg J, Ingvar DH. Acute effects of alcohol on regional cerebral blood flow in man. J Stud Alcohol. 1993;54:369–376. doi: 10.15288/jsa.1993.54.369. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–14. [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch C. Serum prolactin levels in sons of alcoholics and control subjects. Am J Psychiatry. 1987;144:854–859. doi: 10.1176/ajp.144.7.854. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO, Croot K, Finn P, Polich J. P300 latency after ethanol ingestion in sons of alcoholics and in controls. Biol Psychiatry. 1988a;24:310–315. doi: 10.1016/0006-3223(88)90199-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Risch SC, Gold EO. Alcohol consumption, ACTH level, and family history of alcoholism. Am J Psychiatry. 1988b;145:1391–1395. doi: 10.1176/ajp.145.11.1391. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Hesselbrock V, Bucholz KK, Kramer J, Kuperman S, Dietiker C, Brandon R, Chan G. The ability of the Self-Rating of the Effects of Alcohol (SRE) Scale to predict alcohol-related outcomes five years later. J Stud Alcohol Drugs. 2007;68:371–378. doi: 10.15288/jsad.2007.68.371. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Heron J, Horwood J, Davis J, Hibbeln J ALSPAC Study Team. The self-rating of the effects of alcohol questionnaire as a predictor of alcohol-related outcomes in 12-year-old subjects. Alcohol Alcohol. 2008;43:641–646. doi: 10.1093/alcalc/agn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Tolentino NJ, Hall SA. Comparing Structural Equation Models That Use Different Measures of the Level of Response to Alcohol. Alcohol Clin Exp Res. 2010;34:861–868. doi: 10.1111/j.1530-0277.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ. Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med. 2007;58:1232–1241. doi: 10.1002/mrm.21420. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- Teesson M, Baillie A, Lynskey M, Manor B, Degenhardt L. Substance use, dependence and treatment seeking in the United States and Australia: a cross-national comparison. Drug Alcohol Depend. 2006;81:149–155. doi: 10.1016/j.drugalcdep.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T. Acute ethanol-induced changes in cerebral blood flow. Am J Psychiatry. 1994;151:1505–1508. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim RS, Simmons AN, Tolentino NJ, Hall SA, Matthews SC, Robinson SK, Smith TL, Padula CB, Paulus MP, Tapert SF, Schuckit MA. Acute ethanol effects on brain activation in low- and high-level responders to alcohol. Alcohol Clin Exp Res. 2010;34:1162–1170. doi: 10.1111/j.1530-0277.2010.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Goodwin DW, Gabrielli WF, Jr, Penick EC. The electroencephalogram after alcohol administration in high-risk men and the development of alcohol use disorders 10 years later: Preliminary findings. Arch Gen Psychiatry. 1996;53:258–263. doi: 10.1001/archpsyc.1996.01830030080012. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Weiger M, Pruessmann KP, Osterbauer R, Bornert P, Boesiger P, Jezzard P. Sensitivity-encoded single-shot spiral imaging for reduced susceptibility artifacts in BOLD fMRI. Magn Reson Med. 2002;48:860–866. doi: 10.1002/mrm.10286. [DOI] [PubMed] [Google Scholar]
- Wong EC. Quantifying CBF with pulsed ASL: technical and pulse sequence factors. J Magn Reson Imaging. 2005;22:727–731. doi: 10.1002/jmri.20459. [DOI] [PubMed] [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]