Abstract
Background
Despite the known benefit of intensive statin therapy for reducing future cardiovascular events, its effectiveness in women has been questioned by some.
Methods and Results
In the Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial, 911 (21.9%) women and 3251 (78.1%) men were randomized to intensive statin (atorvastatin 80 mg) versus standard therapy (pravastatin 40 mg) for a median duration of 2.1 years. The primary endpoint was death, myocardial infarction (MI), unstable angina (UA), revascularization (occurring after 30 days) or stroke. Safety endpoints included elevations in liver function tests, creatine kinase and myalgias/myositis. Women had a reduction in LDL of 42.8% from baseline at 30 days (to a median of 60 mg/dL) in the intensive therapy arm with 88.8% reaching LDL goal of <100 mg/dL and 65.0% with <70 mg/dL as compared with a 16.8% reduction in LDL (to a median of 88 mg/dL) in the standard therapy. Women on intensive statin therapy had a significant 25% relative reduction over standard-dose (hazard ratio [HR] 0.75; 95% confidence interval [CI] 0.57-0.99; p=0.04) for primary composite endpoint, compared with a 14% reduction for men (HR 0.86; 95% CI 0.75-0.99; p=0.04), p-interaction=0.38. No differences were observed between genders for safety (all p-interaction ≥0.11).
Conclusions
This trial provides evidence that both women and men derived benefit from intensive statin therapy following ACS and thus gender should not be a factor in determining who should be treated with intensive statin therapy.
Keywords: statin, gender, acute coronary syndrome, prognosis, secondary prevention
Introduction
Cardiovascular disease (CVD) remains the foremost cause of mortality in women in the United States and throughout much of the world.1-2 The contemporary approach to prevention of CVD in women includes lifestyle modification for all and medical therapy for those with CVD risk factors or known disease.1 Several large, randomized, controlled trials which included men and women have documented that lipid-lowering therapy with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) reduces the risk of death or cardiovascular events across a wide range of cholesterol levels, with or without coronary artery disease.3-8 The benefit from intensive statin therapy has extended to the early time period after acute coronary syndrome (ACS).9-12
The Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) trial showed that intensive lipid lowering therapy (atorvastatin 80 mg) that achieved a median low-density lipoprotein (LDL) level of 62 mg/dL was superior to standard-dose lipid lowering therapy (pravastatin 40 mg) that achieved a median LDL level of 95 mg/dL after ACS in reducing clinical events.10
Although the effects of statins on reducing future cardiovascular events are well established in men, the generalizability to women are less certain due to the relatively few number of women included these large randomized secondary prevention trials (<20% in each trial).4-5, 13-15 In addition, the observed risk reductions in women varied widely in these trials. Thus, the goal of this analysis was to determine the efficacy and safety of intensive statin therapy in women in the PROVE IT-TIMI 22 trial.
Methods
Study Population
The PROVE IT-TIMI 22 trial was a multicenter, multinational trial consisting of 4,162 patients who were hospitalized for ACS, defined as acute myocardial infarction or high-risk unstable angina within the previous 10 days. The study design, inclusion/exclusion criteria, and primary results have been reported previously.10, 16 (http://www.clinicaltrials.gov/ registration number NCT00382460) To be eligible, patients not previously on lipid lowering therapy, must have had baseline total cholesterol levels (measured within 24 hours of ACS event) to be less than 240 mg/dL, and those on long-term lipid-lowering therapy less than 200 mg/dL. All patients received aspirin and standard medical therapy and were then randomized to receive either intensive (atorvastatin 80 mg/day) or standard (pravastatin 40mg/day) lipid lowering and to gatifloxacin or placebo in a 2×2 factorial design.17 In addition, patients received dietary counseling and follow-up blood samples collected at 30 days, 4 months, and every 4 months thereafter until their final visit. Patients were followed for 18 to 36 months, with a median duration of 2.1 years. This analysis focused on comparing benefit in women and men, and included subgroup analyses performed for pre- and post-menopausal women, defined from the investigator’s designation of women of child bearing potential from the electronic case report form.
Endpoints
The primary endpoint was a composite endpoint defined as death, myocardial infarction (MI), documented unstable angina (UA) requiring hospitalization, revascularization (> 30 days after randomization), or stroke. Secondary efficacy endpoints included hospitalization for heart failure, the components of the various endpoints individually and in combination; changes in LDL, high-density lipoprotein (HDL), high-sensitivity C-reactive protein (hs-CRP); as well percentages of patients with achieved target LDL of <70 mg/dL and <100 mg/dL, HDL >50 mg/dL, and hs-CRP <2 mg/L at 30 days and final visit. Additionally, rates of increase in alanine aminotrasferase (ALT) or aspartate aminotransferase (AST) levels > 3 times normal range, elevated creatine kinase (CK) > 3 times the upper limit of normal, and myalgias/myositis were assessed for tolerability of therapy.
Statistical Analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) or median with interquartile range [IQR] for continuous variables and as frequency and percentages for nominal variables. Differences in baseline characteristics between women and men were assessed by χ2-test for categorical variables and Student’s t test or Wilcoxon rank sum test for continuous variables, as appropriate. When comparing the difference between treatment groups as stratified by gender, we used Wilcoxon rank sum test to compare the differences in lipid profile and χ2-test to compare the proportions of patients who reached target lipid goal and side effect profiles. We used logistic regression to test the interaction between gender and side effects. Cumulative event rates stratified by intensive versus standard-dose lipid lowering therapy were estimated using the product limit (Kaplan-Meier) methods and log-rank test for each gender separately. Cox proportional hazards models were used to evaluate the effect of treatment strategy for women and men separately. Cox regression was utilized to test for effect modification by evaluating the interaction terms for gender and treatment strategy for each outcome, as well as the interaction for gender and gatifloxacin for the primary endpoint. In the subanalysis of women, we used Cox proportional hazards models to compare the effect of treatment strategy on the primary endpoint in each group as stratified by menopausal status and by estrogen replacement therapy (ERT). We also tested the interaction between treatment strategy and both menopausal status and ERT for the primary endpoint using Cox regression. We used Schoenfeld’s residual to verify that the proportional hazards assumption was not violated. A two-sided p-value of <0.05 was considered to be statistically significant. All analyses were performed using STATA 10 (StataCorp LP, College Station, Texas).
Results
Patient Characteristics
There were 911 (21.9%) women and 3251 (78.1%) men. The number of women randomized to high-dose atorvastatin was 465 while 446 received standard-dose pravastatin. Table 1 depicts the baseline characteristics of the PROVE IT-TIMI 22 cohort as stratified by gender. Women were on average 2.6 years older and more often had diabetes, hypertension, and a history of heart failure, while more men smoked, had a history of prior myocardial infarction (MI), and coronary artery bypass grafting (CABG). With respect to ACS type, more women had unstable angina while more men had ST elevation MI, with no difference in the percentage of non-ST elevation MI. Importantly, there was no difference in the percentage of prior statin use between women (26.3%) and men (24.9%, p=0.37). At the time of randomization, the proportions of men and women on beta-blockers and aspirin were similar. There were no major differences in baseline characteristics by randomization group in the subgroup of men and the subgroup of women.
Table 1.
Baseline Characteristics of Patients by Gender
| Women (n=911) | Men (n=3251) | p-value | |
|---|---|---|---|
| Age (mean ± SD) | 60.2 ± 11.7 | 57.6 ± 11 | <0.001 |
| White (%) | 776 (85.2%) | 3000 (92.3%) | <0.001 |
| Diabetes (%) | 212 (23.3%) | 522 (16.1%) | <0.001 |
| Smoking | |||
| Current (%) | 357 (39.2%) | 1172 (36.1%) | 0.08 |
| Past (%) | 236 (25.9%) | 1312 (40.4%) | <0.001 |
| Hypertension (%) | 557 (61.1%) | 1534 (47.2%) | <0.001 |
| Prior MI (%) | 133 (14.6%) | 636 (19.6%) | 0.001 |
| Prior PCI (%) | 129 (14.2%) | 513 (15.8%) | 0.23 |
| Prior CABG (%) | 77 (8.5%) | 377 (11.6%) | 0.007 |
| Peripheral Vascular Disease (%) | 56 (6.2%) | 185 (5.7%) | 0.60 |
| History of CHF (%) | 50 (5.5%) | 87 (2.7%) | <0.001 |
| History of Angina (%) | 203 (22.3%) | 703 (22.0%) | 0.65 |
| Type of ACS | |||
| UA (%) | 312 (34.2%) | 906 (27.9%) | <0.001 |
| Non STEMI (%) | 325 (35.7%) | 1179 (36.3%) | 0.73 |
| STEMI (%) | 274 (30.1%) | 1164 (35.8%) | 0.001 |
| PCI for index event (%) | 596 (65.4%) | 2272 (68.9%) | 0.01 |
| TIMI Risk Score for UA/NSTEMI | |||
| 0-2 (%) | 264 (41.4%) | 1047 (50.2%) | |
| 3-4 (%) | 332 (52.1%) | 926 (44.4%) | <0.001 |
| 5-7 (%) | 41 (6.4%) | 112 (5.4%) | |
| TIMI Risk Score for STEMI | |||
| 0-2 (%) | 156 (56.9%) | 827 (71.1%) | |
| 3-4 (%) | 81 (29.6%) | 270 (23.2%) | <0.001 |
| ≥5 (%) | 37 (13.5%) | 67 (5.8%) | |
| Medications Prior to ACS Event | |||
| Statins (%) | 240 (26.3%) | 809 (24.9%) | 0.37 |
| Beta blockers (%) | 236 (25.9%) | 769 (23.7%) | 0.16 |
| Calcium channel blockers (%) | 189 (20.8%) | 505 (15.5%) | <0.001 |
| ACEI/AII antagonists (%) | 261 (28.7%) | 736 (22.7%) | <0.001 |
| ASA (%) | 314 (34.5%) | 1137 (35%) | 0.78 |
| Medications At Randomization | |||
| Beta blockers (%) | 763 (83.8%) | 2679 (82.4%) | 0.35 |
| Calcium channel blockers (%) | 192 (21.1%) | 612 (18.8%) | 0.13 |
| ACEI or AII antagonists (%) | 521 (57.2%) | 1867 (57.5%) | 0.89 |
| ASA (%) | 829 (91.0%) | 2961 (91.1%) | 0.94 |
Gender differences in lipid profile and biomarkers
The baseline median LDL levels were similar in men and women: 106 [88, 129] mg/dL in women and 106 [88, 127] mg/dL in men (p=0.65). The baseline median triglyceride levels were also similar: 157 [121, 218] mg/dL in women and 155 [116, 209] mg/dL in men (p=0.13). Not surprisingly, the baseline HDL was higher in women (44 [37, 53] mg/dL) than men (37 [32, 43] mg/dL, p<0.0001). The baseline median hs-CRP level in women was 13.5 [5.7, 29.3] mg/L and in men was 11.9 [4.7, 28.9] mg/L (p=0.02).
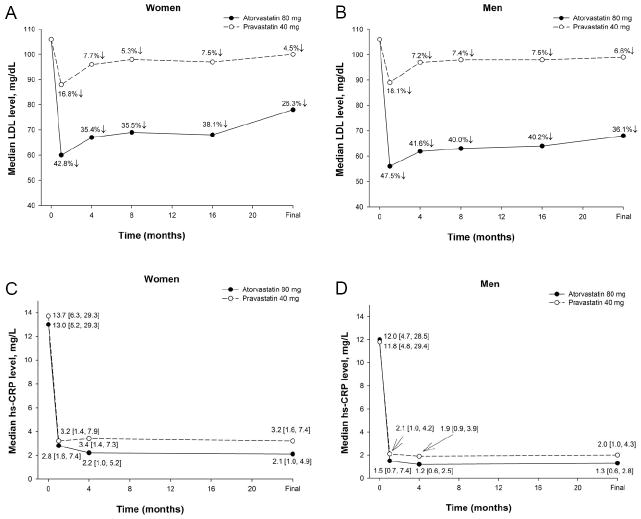
Figure 1 shows the changes in median LDL and hs-CRP levels by treatment group when stratified by gender. At 30 days, women had a reduction in LDL levels by 42.8% (to 60 mg/dL) in the atorvastatin 80 mg arm at 30 days compared with 16.8% (to 88 mg/dL) in the pravastatin 40 mg arm. The difference was statistically significant (p<0.0001). Comparably, men had a reduction of 47.5% (from 106 to 56 mg/dL) in LDL at 30 days in the atorvastatin 80 mg arm compared with 18.1% (from 106 to 89 mg/dL) in the pravastatin 40 mg arm (p<0.0001). This LDL reduction with atorvastatin was greater in men than women (p<0.0001), whereas the LDL reduction with pravastatin did not differ between gender (p=0.32). For both women and men, the hs-CRP levels drastically fell from baseline and were persistently lower when treated with high-dose atorvastatin than with standard-dose pravastatin (all p≤0.003).
Figure 1.
Changes in LDL levels in women (A) and men (B) as stratified by randomization treatment group from baseline to 30-days, 4-, 8-, 16-months, and final visit. A greater percent reduction in LDL was observed in the high-dose atorvastatin group as compared to the standard-dose pravastatin group at all follow-up time points (all p<0.0001). Changes in hs-CRP levels in women (C) and men (D) as stratified by randomization treatment group from baseline to 30-days, 4-month, and final visit. The hs-CRP levels were significantly lower in the high-dose atorvastatin than standard-dose pravastatin arm at all follow-up time points (all p≤0.003).
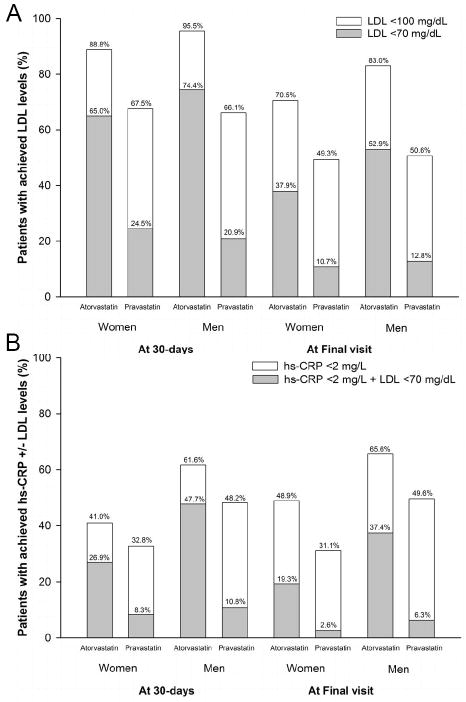
Figure 2 shows that at 30 days, 88.8% of women reached the goal LDL level <100 mg/dL in the atorvastatin 80 mg arm as compared to 67.5% in the pravastatin 40 mg arm, with the goal LDL of <70 mg/dL reached in 65.0% as compared to 24.5%, respectively. Similarly, in men at 30 days, the goal LDL of <100 mg/dL was achieved in 95.5% of the atorvastatin arm as compared to 66.1% in the pravastatin arm, with 74.4% reaching the goal of <70 mg/dL as compared to 20.9%, respectively. When comparing the achievement of goals between men and women, fewer women than men achieved these two target levels of LDL when treated with high-dose atorvastatin (all p<0.001), while there were no differences between the gender groups to reach targeted LDL levels when treated with standard-dose pravastatin (all p≥0.13). Moreover, for both men and women, a greater proportion of patients achieved a hs-CRP level <2 mg/L and a dual goal of hs-CRP <2 mg/L with LDL <70 mg/dL with high-dose atorvastatin than standard-dose pravastatin (all p≤0.015). However, within both high-dose atrovastatin and standard-dose pravastatin groups, more men reached dual goal than women at 30 days (p<0.001)
Figure 2.
Percentage of patients with (A) achieved LDL target levels of <100 mg/dL and <70 mg/dL and with (B) achieved hs-CRP <2 mg/L and/or LDL <70 mg/dL by randomized treatment group as stratified by gender at 30 days and at final visit.
A. All p-values <0.001 when comparing proportion of patients with achieved target LDL levels with atorvastatin versus pravastatin as stratified by women and men. More proportion of men reached their target LDL levels with atorvastatin than women (all p-values <0.001). In patients on pravastatin, the proportion of men and women who achieved target LDL levels were not different (all p-values ≥0.13). B. All p-values ≤0.015 when comparing proportions of patients with achieved hs-CRP +/- LDL levels with treatment group as stratified by gender.
The increase in HDL levels at 30 days was slightly higher in the standard-dose pravastatin group than high-dose atorvastatin in both gender groups (women: 5.3% increase in HDL levels with pravastatin compared to 0% with atorvastatin; men: 2.9% increase with pravastatin compared to 0% with atorvastatin; both p<0.0001). When comparing the effects of statin therapy to achieve the HDL target levels of >50 mg/dL at 30 days, both women and men who received pravastatin reached the target HDL levels more frequently than atorvastatin (women: 39.9% [pravastatin] vs 27.0% [atorvastatin], p<0.001; men: 13.7% vs 9.3%, p<0.001). These differences persisted throughout the study (all p<0.001).
For effects on triglycerides, the baseline median levels were not different between treatment group in women (159 mg/dL [atorvastatin] vs 156 mg/dL [pravastatin], p=0.72) while slightly higher in men on intensive dose statin than standard dose statin (157 mg/dL vs 153 mg/dL, p=0.05). There was greater decrease at 30 days in the intensive dose over the standard dose statin for both women (114 mg/dL vs 138 mg/dL) and men (107 mg/dL vs 139 mg/dL), both p<0.0001. The median triglyceride levels remained lower in the intensive dose group than the standard dose group at study termination (women: 131 mg/dL [atorvastatin] vs 153 mg/dL [pravastatin]; men: 122 mg/dL [atorvastatin] vs 149 mg/dL [pravastatin]; both p<0.0001).
Gender differences in side effects of high versus standard-dose statin
Table 2 depicts the difference in rates of discontinuation of statin and side effects observed during the study using the safety cohort of patients who received at least one dose of study drug. There were no differences in the rate of statin discontinuation between men and women, or between atorvastatin versus pravastatin (p-interaction=0.77). While more women and men had an increase in ALT or AST levels > 3 times normal in the high-dose atorvastatin arm as compared to the pravastatin arm (both p≤0.001), no gender differences were detected with either ALT or AST elevations for either drugs (p-interaction=0.11). Both women and men had similar rates of elevated CK > 3 times the upper limit of normal and myalgias or myositis (all p≥0.20), with no gender differences between statin groups and the increase in CK (p-interaction=0.62) and the presence of myalgias or myositis (p-interaction=0.63).
Table 2.
Rate of premature discontinuation of statin therapy and side effects of high versus standard-dose statin as stratified by gender.
| Atorvastatin | Pravastatin | P-value | P-interaction | |
|---|---|---|---|---|
| Premature discontinuation of statin therapy | ||||
| Women | 179 (38.9%) | 191 (43.0%) | 0.21 | 0.77 |
| Men | 509 (31.3%) | 548 (34.0%) | 0.10 | |
| Increase in ALT or AST > 3x normal | ||||
| Women | 18 (4.1%) | 2 (0.5%) | <0.001 | 0.11 |
| Men | 51 (3.3%) | 23 (1.4%) | <0.001 | |
| Elevated CK > 3x ULN | ||||
| Women | 3 (0.7%) | 3 (0.7%) | 0.94 | 0.62 |
| Men | 29 (1.9%) | 20 (1.3%) | 0.20 | |
| Myalgias/myositis | ||||
| Women | 15 (3.3%) | 16 (3.6%) | 0.78 | 0.63 |
| Men | 48 (3.0%) | 43 (2.7%) | 0.63 |
Gender differences in outcomes with high versus standard-dose statin
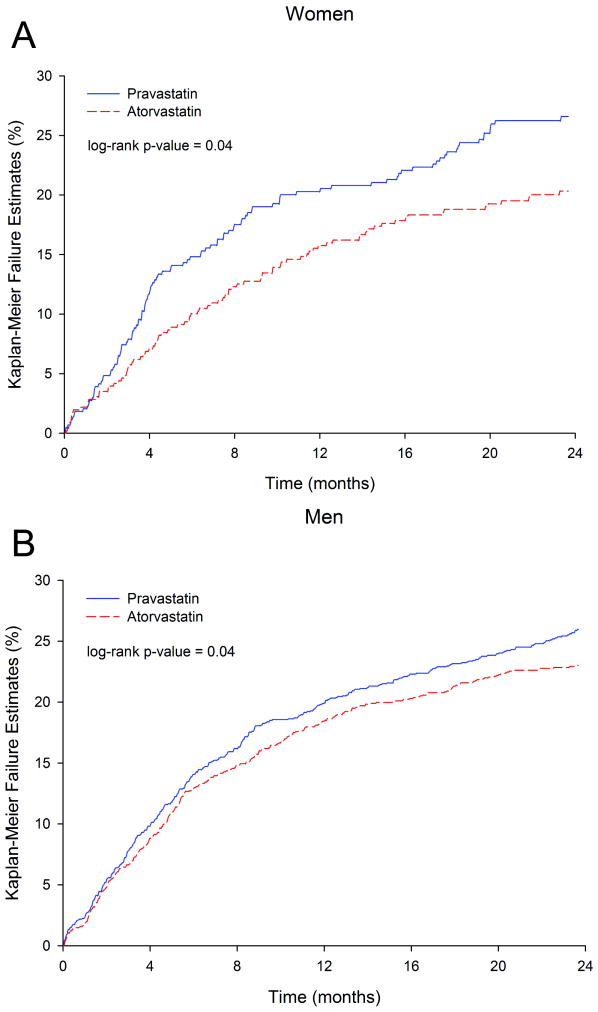
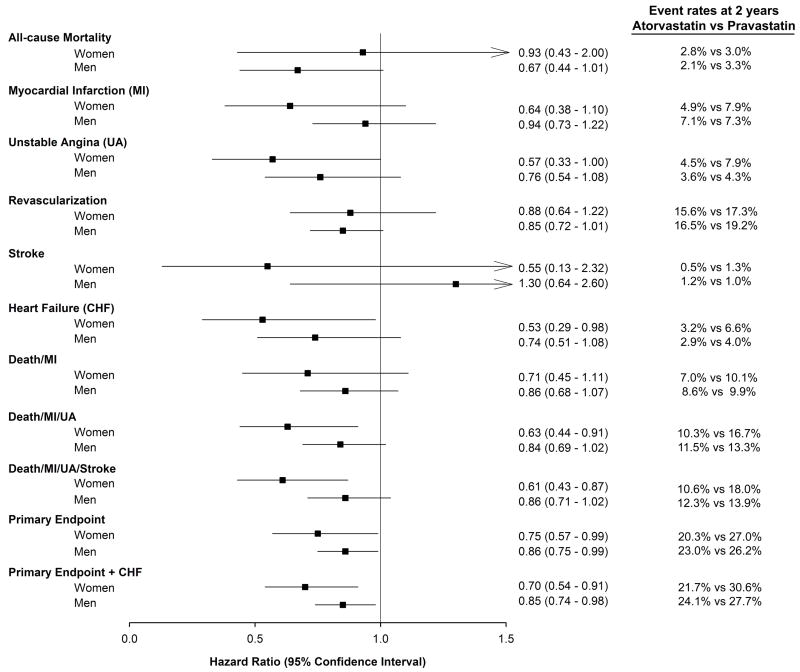
Figure 3 illustrates the Kaplan-Meier curves for the primary endpoint when stratified by treatment group for women and men separately with fewer events in the high-dose statin arm than the standard-dose statin arm in both genders. As shown in Figure 4, the event rates at 2 years for the primary endpoints were lower both in women (20.3% atorvastatin versus 27.0% pravastatin, log-rank p-value=0.04) and men (23.0% atorvastatin versus 26.2% pravastatin, log-rank p-value=0.04) when treated with intensive than standard-dose statin. Although there was no gender difference between treatment group and the primary endpoint (p-interaction=0.38), women benefited from intensive statin therapy with a 6.7% absolute reduction in events and a 25% relative risk reduction (RRR) in hazard over standard statin therapy (p=0.04), while men had a 3.2% absolute reduction in events and 14% RRR in hazard (p=0.04). Results of the interaction test are consistent when adjusting for differences in baseline characteristics between men and women (p-interaction=0.34). Based on a mean LDL delta between treatment groups at 30 days of 25.6 mg/dl in women and 32.1 mg/dl in men and a RRR of 24.5% (95% CI 0.95-42.5%) in women and a RRR of 13.6% (95% CI 0.7-24.9%) in men for the primary endpoint event, a mean change of 1.0 mg/dl (95% CI 0.6-27.0 mg/dl) would produce a 1% reduction in events in women whereas in men a mean change of 2.4 mg/dl (95% CI 1.3-45.9 mg/dl) was needed to produce the same 1% reduction in events.
Figure 3.
Kaplan Meier Curves of Primary Endpoint by study termination in women (A) and men (B).
Figure 4.
Hazard ratios between intensive-dose Atorvastatin versus standard-dose Pravastatin therapy in women and men. All p-interactions were non-significant.
Women had a 37% relative reduction in hazard in death/MI/UA (p=0.01), a 43% reduction in unstable angina (p=0.05), a 47% reduction in hospitalization for heart failure (p=0.04), and a 30% reduction in the combination of primary endpoint or heart failure (p=0.007) when treated with high-dose atorvastatin. Figure 4 depicts the results of the five individual components that comprise to make the pre-specified primary endpoint. No significant difference in all-cause mortality was seen in women with intensive vs. standard statin therapy (2.8% vs 3.0%; hazard ratio [HR] 0.93, 95% CI 0.43 – 2.00, p=0.85) while there was a trend toward reduced mortality in men (2.1% vs 3.3%; HR 0.67, 95% CI 0.44 – 1.01, p=0.06), although the power for these comparisons is low given the low rates of death in both groups. Similarly, there was no difference in revascularizations in women (15.6% intensive vs 17.3% standard; HR 0.88, 95% CI 0.64 – 1.22, p=0.45) with a trend towards fewer revascularizations in men (16.5% vs 19.2%; HR 0.85, 95% CI 0.72 – 1.01, p=0.06). However, there were no gender differences between high versus standard-dose dose statin therapy for two year outcomes evaluated individually or in various combinations (all p-interaction>0.18). No interaction was seen between gender and treatment with gatifloxacin (p-interaction=0.22).
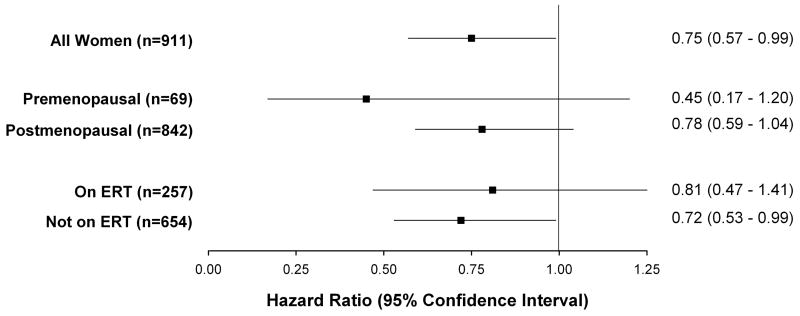
Subgroup analysis of women
Figure 5 depicts the relative risk reduction of the primary endpoint when treated with intensive versus standard-dose statin in women only, when stratified based on pre- and post-menopausal status, and when stratified by ERT. No differences in primary endpoint between women on intensive and standard dose lipid therapy were seen based on menopausal status (p-interaction=0.27) or ERT (p-interaction=0.75), though women not on ERT did achieve a 26% reduction in hazard (p=0.04). The non-significant 22% reduction in primary endpoint for the post-menopausal group remained unchanged after adjustment for ERT (adjusted HR 0.78, 95% CI: 0.58-1.02; p=0.08).
Figure 5.
Hazard ratios of the primary endpoint in women subgroup treated with intensive lipid therapy (Atorvastatin 80) as compared to standard lipid lowering therapy (Pravastatin 40mg) therapy. ERT denotes estrogen replacement therapy. P-interaction=0.27 for menopausal status and lipid therapy; p-interaction=0.75 for ERT and lipid therapy.
Discussion
In this subgroup analysis of PROVE IT-TIMI 22, use of intensive statin therapy with high-dose atorvastatin post-ACS led to a significant reduction in cardiovascular events in women (as well as in men), and with similar safety profiles when compared to standard-dose pravastatin. For a 1% reduction in events, the mean reduction in LDL level needed in women was 1.0 mg/dL while in men a reduction of 2.4 mg/dL was needed to achieve that benefit. With a 6.7% absolute reduction and 25% relative reduction of the primary endpoint with intensive-dose atorvastatin in women compared with a 3.2% absolute reduction and 14% relative reduction seen in men, the clinical impact of intensive statin therapy is quite robust in women and reinforces the value of treating women with intensive statin therapy post-ACS to reduce cardiovascular events.
The benefit of statin therapy for women remains controversial with some authors questioning the effectiveness of statins in the primary prevention of coronary artery disease in this subgroup of patients claiming insufficient data,18-20 while advocates recommend their use in women even in primary prevention.21-23 Despite equally high 30-day mortality rates post-ACS in men and women,24 gender bias has been observed in the management of women with fewer treated with statin therapy after such events as compared to men.25-26 Our study examines the efficacy and safety of intensive statin therapy for secondary prevention and provides further evidence to support its use in women. In addition to the 25% reduction in the pre-specified primary endpoint of the PROVE IT-TIMI 22 trial in women, we found a consistent beneficial effects of high-dose atorvastatin on cardiovascular events in women with 30-47% relative reductions in the risks for death/MI/UA, unstable angina, heart failure, and the combination of primary endpoint with heart failure.
When the five individual components of the primary endpoint were analyzed separately, we did not observe a significant difference in all-cause mortality between the statin groups in women (or men), which has been reinforced by the meta-analysis of four trials of intensive vs. standard dose statin therapy.27 While not powered to analyze the individual endpoints separately, this subanalysis provides some insight on the driving forces behind the favorable response of intensive statin therapy over standard dose statin therapy in the reduction of the primary endpoint. Reassuring is that neither the softer endpoints of revascularization or stroke were the driving forces, and that UA and MI were the predominant factors leading to our primary results. Although we lacked power for the analysis, the benefit of intensive statin therapy appeared to be similar in pre- and post-menopausal women with suggestion towards greater reduction in the primary endpoint in the pre-menopausal group.
Interestingly, these clinical benefits of intensive statin therapy were seen in women despite less favorable achieved LDL levels. Fewer women on intensive statin therapy reached targeted LDL levels of <100 mg/dL and <70 mg/dL as recommended by the National Cholesterol Education Program Adult Treatment Panel III (ATP-III)28 when compared to men. With regards to the effect of the two statin treatment strategies on HDL levels, more women reached their HDL target level of >50 mg/dL than men. This small advantage for women in reaching HDL targets might have contributed to the modestly greater risk reduction in the primary endpoint in women. We also observe a reduction in triglyceride levels for both women and men on intensive therapy over standard dose therapy. Furthermore, for both women and men, the LDL and hs-CRP levels achieved were lower in the high-dose atorvastatin treated group than the standard-dose pravastatin group. This improvement in both lipid and inflammatory profiles in conjunction with lower event rates confirm prior reports that reduction in both LDL and hs-CRP levels are predictors to therapy response and outcomes.29-33
The dramatic benefit of intensive statin therapy in women in our study of a 25% relative reduction in the primary endpoint, 37% reduction in death/MI/UA, and 43% reduction in unstable angina is supported by other trials.34 In the Treating to New Targets (TNT) study, 1902 women with stable coronary heart disease with median follow-up of 4.9 years had a relative reduction of 27% in cardiovascular events when treated with intensive-dose atorvastatin 80 mg as compared to low-dose atorvastatin 10 mg (HR 0.73, p=0.049), while men had relative reduction of 21% (HR 0.79, p=0.001) a pattern which was similar to our study.15 Multiple trials have demonstrated efficacy of statins, in both primary and secondary prevention of coronary events and death in women with both elevated and normal levels of cholesterol.7-8, 15 It seems appropriate to continue to apply to women preventive strategies shown to be successful in men, while recognizing that optimal classification and management of women with dyslipidemia and encouraging the enrollment of more women in lipid lowering trials.
Heart failure is common in women.2 An additional finding in this analysis was a benefit in reducing the risk of hospitalization for heart failure in women (and men). We found a striking benefit of intensive statin therapy for reducing not only the primary endpoint of cardiovascular events but also of heart failure, and the greatest relative risk reduction in women for any outcome was for heart failure, with a 47% reduction.
Limitations
Several limitations are noteworthy of this analysis. Despite the lack of interaction found in our analysis, each subgroup was significant on its own, thus demonstrating the benefit of intensive statin therapy in both gender groups. While we cannot conclude that women benefit more than men, our results may be constrained by power, as it is uncertain what constitutes a meaningful power for subgroup analysis of interaction term with survival analytical data. The seemingly paradoxical effect of less LDL lowering in women vs. men with high-dose atorvastatin, yet with a trend to greater absolute reduction in cardiovascular events in women over men is intriguing. However the LDL reductions with intensive statin therapy were large in both men and women. Our analysis is underpowered for the comparison between pre- and post-menopausal women, although trends were seen for the reduction of cardiovascular events in premenopausal women. Future randomized trials should include more women, both pre-and post-menopausal and in numbers robust enough to assess the effects of interventions on clinical outcomes, including not only primary and secondary prevention, but all levels of risk along the cardiovascular continuum.
Conclusions
Despite having less significant LDL lowering with intensive compared to standard lipid lowering therapy than men, women had dramatic and significant reductions in clinical events. Both women and men post-ACS benefit from high-dose statin therapy and they should be treated with intensive regimens.
Acknowledgments
Sources of Funding: The trial was funded by Bristol Myers Squibb, Princeton New Jersey, and Sankyo, Parsippany, New Jersey.
Footnotes
Conflict of Interest Disclosures: Quynh A Truong received research/grant support from NIH grants L30HL093896 and 1K23HL098370. Carolyn H. McCabe received research grants/support from AstraZeneca, Bristol-Myers Squibb/Sanofi Partnership, GlaxoSmithKline, and Merck. Christopher P Cannon reports receiving research/grant support from Accumetrics, AstraZeneca, GlaxoSmithKline, Merck, Intekrin Therapeutics, and Takeda; He has served on advisory boards for Bristol-Myers Squibb/Sanofi-Aventis and Novartis (but funds donated to charity) and having equity ownership in Automedics Medical Systems.
References
- 1.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. J Am Coll Cardiol. 2007;49:1230–1250. doi: 10.1016/j.jacc.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 4.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 5.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 6.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 8.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 9.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 11.de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 12.Sacks RM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun C-C, Davis BR, Braunwald E Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 13.Lewis SJ, Sacks FM, Mitchell JS, East C, Glasser S, Kell S, Letterer R, Limacher M, Moye LA, Rouleau JL, Pfeffer MA, Braunwald E. Effect of pravastatin on cardiovascular events in women after myocardial infarction: the cholesterol and recurrent events (CARE) trial. J Am Coll Cardiol. 1998;32:140–146. doi: 10.1016/s0735-1097(98)00202-2. [DOI] [PubMed] [Google Scholar]
- 14.Herrington DM, Vittinghoff E, Lin F, Fong J, Harris F, Hunninghake D, Bittner V, Schrott HG, Blumenthal RS, Levy R. Statin therapy, cardiovascular events, and total mortality in the Heart and Estrogen/Progestin Replacement Study (HERS) Circulation. 2002;105:2962–2967. doi: 10.1161/01.cir.0000019406.74017.b2. [DOI] [PubMed] [Google Scholar]
- 15.Wenger NK, Lewis SJ, Welty FK, Herrington DM, Bittner V. Beneficial effects of aggressive low-density lipoprotein cholesterol lowering in women with stable coronary heart disease in the Treating to New Targets (TNT) study. Heart. 2008;94:434–439. doi: 10.1136/hrt.2007.122325. [DOI] [PubMed] [Google Scholar]
- 16.Cannon CP, McCabe CH, Belder R, Breen J, Braunwald E. Design of the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE IT)-TIMI 22 trial. Am J Cardiol. 2002;89:860–861. doi: 10.1016/s0002-9149(02)02201-4. [DOI] [PubMed] [Google Scholar]
- 17.Cannon CP, Braunwald E, McCabe CH, Grayston JT, Muhlestein B, Giugliano RP, Cairns R, Skene AM. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 18.Vos E, Rose CP. Questioning the benefits of statins. CMAJ. 2005;173:1207. doi: 10.1503/cmaj.1050120. author reply 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendrick M. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? No BMJ. 2007;334:983. doi: 10.1136/bmj.39202.397488.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redberg R. Why I Don’t Recommend Statins for Primary Prevention in Women. 2010;2010 http://cardioexchange.org/blogs/blogpost?postId=624.
- 21.Mizuno K, Nakaya N, Ohashi Y, Tajima N, Kushiro T, Teramoto T, Uchiyama S, Nakamura H. Usefulness of pravastatin in primary prevention of cardiovascular events in women: analysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA study) Circulation. 2008;117:494–502. doi: 10.1161/CIRCULATIONAHA.106.671826. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM. Should women be offered cholesterol lowering drugs to prevent cardiovascular disease? Yes BMJ. 2007;334:982. doi: 10.1136/bmj.39202.399942.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mora S, Glynn RJ, Hsia J, Macfadyen JG, Genest J, Ridker PM. Statins for the Primary Prevention of Cardiovascular Events in Women With Elevated High-Sensitivity C-Reactive Protein or Dyslipidemia: Results From the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and Meta-Analysis of Women From Primary Prevention Trials. Circulation. 2010;121:1069–1077. doi: 10.1161/CIRCULATIONAHA.109.906479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan SC, Beaver SK, Houck PM, MacLehose RF, Lawson HW, Chan L. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med. 2000;343:8–15. doi: 10.1056/NEJM200007063430102. [DOI] [PubMed] [Google Scholar]
- 25.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Jr, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006;113:490–498. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 27.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 28.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45:1644–1648. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 30.Ray KK, Cannon CP, Cairns R, Morrow DA, Rifai N, Kirtane AJ, McCabe CH, Skene AM, Gibson CM, Ridker PM, Braunwald E. Relationship between uncontrolled risk factors and C-reactive protein levels in patients receiving standard or intensive statin therapy for acute coronary syndromes in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2005;46:1417–1424. doi: 10.1016/j.jacc.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 32.Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 34.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]