Abstract
Select lipid-anchored proteins such as glycosylphosphatidylinositol (GPI)-anchored proteins and nonreceptor tyrosine kinases may preferentially partition into sphingomyelin-rich and cholesterol-rich plasmalemmal microdomains, thereby acquiring resistance to detergent extraction. Two such domains, caveolae and lipid rafts, are morphologically and biochemically distinct, contain many signaling molecules, and may function in compartmentalizing cell surface signaling. Subfractionation and confocal immunofluorescence microscopy reveal that, in lung tissue and in cultured endothelial and epithelial cells, heterotrimeric G proteins (Gi, Gq, Gs, and Gβγ) target discrete cell surface microdomains. Gq specifically concentrates in caveolae, whereas Gi and Gs concentrate much more in lipid rafts marked by GPI-anchored proteins (5′ nucleotidase and folate receptor). Gq, apparently without Gβγ subunits, stably associates with plasmalemmal and cytosolic caveolin. Gi and Gs interact with Gβγ subunits but not caveolin. Gi and Gs, unlike Gq, readily move out of caveolae. Thus, caveolin may function as a scaffold to trap, concentrate, and stabilize Gq preferentially within caveolae over lipid rafts. In N2a cells lacking caveolae and caveolin, Gq, Gi, and Gs all concentrate in lipid rafts as a complex with Gβγ. Without effective physiological interaction with caveolin, G proteins tend by default to segregate in lipid rafts. The ramifications of the segregated microdomain distribution and the Gq-caveolin complex without Gβγ for trafficking, signaling, and mechanotransduction are discussed.
INTRODUCTION
In cellular membranes, cholesterol, glycolipids, and select lipid-anchored proteins appear to organize into domains that are resistant to nonionic detergent solubilization. Although two such microdomains, caveolae and lipid rafts, share various biochemical properties, including a requirement for cholesterol (Rothberg et al., 1990; Schnitzer et al., 1994; Murata et al., 1995; Monier et al., 1996; Schroeder et al., 1998) and similar low buoyant densities (Gorodinsky and Harris, 1995; Schnitzer et al., 1995b), they are actually distinct morphologically, biochemically, and functionally (Schnitzer et al., 1995b; Liu et al., 1997). Lipid rafts are distinguished as flat domains rich in glycosylphosphatidylinositol (GPI)-anchored proteins that rely primarily, maybe even solely, on lipid-lipid interactions for their formation and detergent resistance (Ahmed et al., 1997; Schroeder et al., 1998; Brown and London, 2000). In contrast, caveolae are smooth, flask-shaped, cell-surface invaginations (Schnitzer et al., 1995b) that appear to depend on caveolin oligomerization for their formation (Fra et al., 1995; Monier et al., 1996; Lipardi et al., 1998). Caveolin is palmitoylated (Dietzen et al., 1995; Monier et al., 1996), and it binds cholesterol (Monier et al., 1996), which appears to be required for its role in maintaining caveolar structure (Schnitzer et al., 1994). Caveolae and lipid rafts may sometimes associate with each other, but they appear to exist predominately at the cell surface as independent structures (Schnitzer et al., 1995b). Lipid rafts may exist in cells without caveolae (Fra et al., 1994; Gorodinsky and Harris, 1995). Many subfractionation techniques that sort based on detergent-resistance and/or low buoyant densities (Sargiacomo et al., 1993; Chang et al., 1994; Lisanti et al., 1994; Smart et al., 1995) tend to co-isolate caveolae and lipid rafts (Schnitzer et al., 1995b; Oh and Schnitzer, 1999). However, more sophisticated subfractionation techniques can isolate caveolae and lipid rafts separately from the same plasma membranes (Schnitzer et al., 1995b; Oh and Schnitzer, 1999). Unlike caveolae, with their distinct, readily observed, invaginated morphology, lipid rafts are more difficult to observe, and their existence is still the subject of some debate (Mayor and Maxfield, 1995; Kenworthy and Edidin, 1998; Kenworthy et al., 2000).
Caveolae function in mediating endocytosis and transcytosis of select macromolecules (Schnitzer et al., 1994; Schnitzer et al., 1995a; Oh et al., 1998; McIntosh and Schnitzer, 1999). Caveolae are also rich in many signaling molecules, including platelet-derived growth factor receptor (PDGF-R) (Liu et al., 1996; Liu et al., 1997), endothelial nitric oxide synthase (eNOS) (Feron et al., 1996; Garcia-Cardena et al., 1996; Rizzo et al., 1998a), and nonreceptor tyrosine kinases (NRTK) (Liu et al., 1997), many of which may interact directly with caveolin via its scaffolding domain (Li et al., 1996; Garcia-Cardena et al., 1997). The removal of cholesterol from the plasma membrane by cholesterol binding agents such as filipin affects caveolin organization (Rothberg et al., 1990; Rothberg et al., 1992). These compounds disassemble caveolae which disperses caveolar molecules to a more random distribution over the cell surface, thereby disrupting both transport and signaling functions (Schnitzer et al., 1994; Liu et al., 1997). For example, filipin treatment prevents both PDGF-induced downstream signaling past initial receptor autophosphorylation (Liu et al., 1997) and mechanical stress-induced protein tyrosine phosphorylation and activation of the Ras/Raf/MAP kinase pathway (Rizzo et al., 1998b). Like caveolae, lipid rafts contain various signaling molecules, including NRTK, immunoglobulin E receptor, T cell receptor (TCR), and GPI-anchored proteins (Stefanova et al., 1991; Shenoy-Scaria et al., 1992; Field et al., 1995; Gorodinsky and Harris, 1995; Liu et al., 1997; Montixi et al., 1998; Xavier et al., 1998). Lipid raft structure is also affected by cholesterol binding agents which cause the dispersal of raft molecular constituents throughout the plasma membrane (Rothberg et al., 1990). Filipin treatment of cultured lymphocytes inhibits TCR-mediated Ca2+ mobilization and protein tyrosine phosphorylation (Xavier et al., 1998). Thus, the compartmentalization of key signaling molecules in caveolae and lipid rafts appears necessary to provide rapid, efficient, and specific propagation of extracellular stimuli to intracellular targets.
Heterotrimeric G proteins, which are composed of three distinct subunits, α, β, and γ, mediate intracellular signaling by various receptors induced by specific ligands (Morris and Malbon, 1999). Although there is evidence that various G proteins concentrate in detergent-resistant membrane domains (Moffett et al., 2000), it is unclear whether they localize to caveolae and interact with caveolin. Some groups report significant enrichment of Gi, Gs, Go, and Gβγ in caveolin-rich fractions (detergent-resistant buoyant membranes or low density, sonication-released vesicles) (Sargiacomo et al., 1993; Chang et al., 1994; Lisanti et al., 1994; Li et al., 1995; Smart et al., 1995). Others have observed G proteins (Gi, Gs, and Gβγ) present, but not enriched, in isolated caveolae (Schnitzer et al., 1995a). In contrast, another group (Stan et al., 1997) concluded that caveolae cannot function as signaling compartments based on their inability to detect G proteins (and other signaling molecules such as eNOS) in immuno-isolated caveolae. However, more recent work (Oh and Schnitzer, 1999) demonstrates that this past failure in detection was an artifact of the methodology and that G proteins and other signaling molecules are indeed at least present in immuno-isolated caveolin-coated caveolae. Like other caveolar signaling molecules, G proteins may interact with caveolin. In binding assays using recombinant proteins, one group observed a direct interaction between Gi and Go subunits and the caveolin scaffolding domain (Li et al., 1995). This interaction may be regulatory, because synthetic peptides corresponding to the scaffolding domain inhibit GTPase activities of Gi and Go (Li et al., 1995) and GTPγS binding to Go (Li et al., 1995). More recently, however, another group (Huang et al., 1997) detected little Gi and Gs within caveolae or coated pits and did not observe either G protein-caveolin interactions or an inhibitory effect of caveolin or its scaffolding domain peptide on GTPase activity or GTPγS binding.
In this study, we focus in detail on the distribution of G proteins at the cell surface in vivo using dual immunofluorescence microscopy and subcellular fractionation techniques that allow reliable and consistent purification of caveolae and lipid rafts separately from each other and from complicating, contaminating membranes (i.e. Golgi) (Schnitzer et al., 1995b; Oh and Schnitzer, 1999). We show that Gi and Gs at the cell surface preferentially target lipid rafts whereas Gq concentrates in caveolae through its specific interaction with caveolin. This differential distribution may help explain the basis of many of the discrepancies between studies reported in the literature.
MATERIALS AND METHODS
Materials
Antibodies against caveolin were purchased from Transduction Labs (Lexington, KY) (rabbit polyclonal (pAb) and mouse monoclonal (clone # 2234)), Zymed Laboratory (South San Francisco, CA) (mouse monoclonal (clone #Z034)), and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) (rabbit pAb). Polyclonal antibodies recognizing common subunits of Gq (Gq/11, cat# sc-392), Gi (Gi/o/t/z, sc-386), Gs (Gs/olf, sc-383), and Gβ (Gβ1–4, sc-261), as well as specifically Gq (sc-393), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Both Gq/11-common and Gq-specific antibodies gave similar results. Polyclonal antibodies to Gs, Gi, and Gq were a gift from Dr. David Manning (University of Pennsylvania, Philadelphia, PA). Antibodies to 5′NT were a gift from Dr. Paul Luzio (University of Cambridge). The folate receptor antibodies, MOV18 and MOV19, were a gift from Dr. John Ghrayeb (Centocor). Texas Red anti-mouse IgG and Bodipy anti-rabbit IgG were purchased from Molecular Probes (Eugene, OR). M-450 Dynabeads (anti-mouse conjugated magnetic beads) and M-280 Dynabeads (anti-rabbit conjugated magnetic beads) were purchased from Dynal (New Hyde Park, NY). The N2a mouse neuroblastoma cells were obtained from the American Type Culture Collection. All other reagents/supplies were obtained as in our past work (Schnitzer et al., 1995a; Schnitzer et al., 1995b; Schnitzer et al., 1995c; Schnitzer et al., 1996).
Isolation of Plasma Membrane- and Caveolae-enriched Fractions from Rat Lung Homogenates
Homogenates of rat lung tissues were subjected to Percoll gradient centrifugation to isolate a plasmalemmal fraction (PM) as described (Smart et al., 1995; Oh and Schnitzer, 1998; Oh and Schnitzer, 1999). Briefly, rat lungs were flushed free of blood, homogenized in buffer (0.25 M sucrose/1 mM EDTA/20 mM Tricine, pH 7.8), filtered through a Nytex filter, and subjected to centrifugation (1000 × g) for 10 min. The post-nuclear supernatant was mixed with Percoll and subjected to centrifugation (84,000 × g) for 45 min. A single membranous band, readily visible ∼ 2/3 from bottom of the tube (PM), was collected. PM is enriched in plasmalemmal markers such as caveolin, angiotensin-converting enzyme (ACE), and 5′ nucleotidase (5′NT), but it also contains endosomal and Golgi membrane markers, such as ε-COP, and possibly other intracellular membranes (Oh and Schnitzer, 1999). A caveolin-rich low density fraction (AC) was separated from PM by sonication, followed by Optiprep density centrifugation. AC is significantly enriched in caveolin (and thus caveolae) but also contains lipid rafts, Golgi/endosomal, and possibly other membranes (Oh and Schnitzer, 1999).
Isolation of Luminal Endothelial Cell Plasma Membranes, Caveolae, and Lipid Rafts
The luminal endothelial cell plasma membranes and their caveolae were isolated directly from rat lung tissue using an in situ silica-coating procedure, as described (Schnitzer et al., 1995b; Oh and Schnitzer, 1998). Briefly, rat lungs were perfused via the pulmonary artery with a colloidal silica solution to coat the endothelial cell luminal surface and allow selective isolation of the luminal endothelial cell plasma membranes (P) from the lung homogenate (H) by density centrifugation. P is enriched in the endothelial cell surface proteins caveolin, 5′NT, and ACE but is markedly depleted in markers for other cell types or intracellular organelles such as ε-COP (Schnitzer et al., 1995b; Oh and Schnitzer, 1998). The caveolae (V) were separated from P by homogenization and isolated from the silica-coated membrane pellet stripped of caveolae (P-V) by sucrose density centrifugation in a low buoyant density fraction (Schnitzer et al., 1995b; Schnitzer et al., 1995c). V contains a homogeneous population of morphologically distinct caveolar vesicles enriched in caveolin, while being markedly depleted in noncaveolar proteins including ACE, β-actin, 5′NT, and uPAR (Schnitzer et al., 1995b; Oh and Schnitzer, 1999). To isolate lipid rafts (LR), P-V was incubated in 2 M K2HPO4, to separate membranes from silica coating before their homogenization in 1% Triton X-100 at 4°C, followed by sucrose gradient centrifugation, as described previously (Schnitzer et al., 1995b; Oh and Schnitzer, 1998). LR was collected as a visible, membranous band between 10 and 15% sucrose, and it is enriched in GPI-anchored proteins but essentially devoid of caveolin.
Immuno-affinity Isolation of Caveolae
Magnetic immuno-isolations were performed as described (Oh and Schnitzer, 1999). Briefly, M450 Dynal beads conjugated to caveolin mAb (clone 2234) (2 × 107 M450 beads and 25 μg IgG) were incubated for 1 h at 4°C with 25 μg of the starting membrane fraction (SM), then washed and magnetically separated to isolate two fractions: material bound to the beads (B) versus unbound material (U). Testing V by this method shows nearly complete binding of the membranes and proteins in V to the beads, indicating effective quantitative isolation of a reasonably homogeneous fraction of caveolae (Oh and Schnitzer, 1999).
Immunofluorescence Microscopy
Bovine lung microvascular endothelial cells (BLMVEC), monkey kidney epithelial cells (MA104), or N2a mouse neuroblastoma cells were grown on coverslips for dual immunofluorescence confocal microscopy as described in our past work (Oh et al., 1998). Briefly, cells were fixed with methanol, blocked with 2% goat serum, then stained with antibodies to caveolin (clone Z034), 5′NT, folate receptor plus antibodies to specific G protein subunits (1:250 dilution). The bound primary antibody was detected with a reporter IgG conjugated to Texas Red (anti-mouse IgG) or Bodipy (anti-rabbit IgG) (Molecular Probes, Eugene, OR). The immunofluorescence signal was visualized and photographed using a confocal fluorescence microscope (Perkin Elmer-Cetus Wallac, Gaithersburg, MD) The ratio of overlapping signals was quantified using Metamorph Software (Universal Imaging, Chesterfield, PA) and was confirmed by drawing a line through a digital image of the cells and by counting the total number of red, green, or overlapping (yellow) signals contacting the line and calculating the ratio of yellow to green or red pixels, as in past work (Liu et al., 1997)(our unpublished results). At least five lines were used in each calculation, and each line had at least 50 signals associated with it. Methanol fixation was used after a comparative evaluation of fixatives showed the equivalence of methanol and glutaraldehyde in preventing subsequent sequestration of GPI-anchored proteins by antibodies.
Immunoprecipitation of Caveolin and G Protein Complexes
Purified luminal endothelial cell membranes (P) (100 μg of total protein) or N2a cells that had been scraped from the plate, washed, and pelleted by centrifugation were solubilized for 1 h at 4°C with 20 mM CHAPS in TBS (50 mM Tris pH 7.6, 135 mM NaCl), then incubated for 1–2 h at 4°C with magnetic beads coated with antibodies to either caveolin or specific G-protein subunits, as in our past work (Rizzo et al., 1998a). Magnetic separation was used to isolate two fractions—insoluble material bound to the beads (I) and soluble material not bound to the beads (S). Western analysis was performed as described using caveolin or G protein antibodies (Rizzo et al., 1998b)
Preparation of Rat Lung Cytosols and Immunoprecipitation of Caveolin and G Proteins
Sprague Dawley rat lungs were perfused with Ringer's solution, followed by sucrose/HEPES solution containing protease inhibitors, as in past work (Schnitzer et al., 1995b; Oh et al., 1998). The lungs were minced, homogenized in 5 ml cytosolic buffer (25 mM KCl, 2.5 mM Mg(C2H3O2)2, 5 mM EGTA, 150 mM KC2H3O2, 25 mM HEPES, pH7.4), and filtered through Nytex (53 μ then 33 μ). The filtered material was subjected to centrifugation at 100,000 × g for 60 min at 4°C in a SW55 rotor using an Optima Max-E Ultracentrifuge (Beckman Coulter). The supernatant was subsequently respun at 300,000 × g for 60 min at 4°C to generate membrane-free cytosol. Our testing of this material in sucrose gradients shows no detectable floating membranes or caveolin in the floating fractions. Fifty microliters cytosol (5 mg/ml) was incubated for 60 min at room temperature with M450 Dynal magnetic beads coated with antibodies (2 × 107 beads per 25 μg antibodies) either to caveolin or to specific G protein subunits, as described (Oh and Schnitzer, 1999). After magnetic separation of the bound material, the beads were split into four equal aliquots, and each was subjected to Western analysis.
RESULTS
Select G Proteins at the Cell Surface in Caveolae
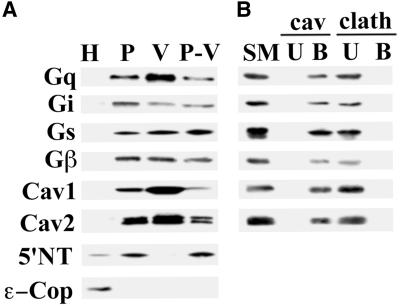
To assess the G protein content in the plasma membrane and caveolae, we isolated luminal endothelial cell plasma membranes (P) from rat lung homogenates (H) using the colloidal silica coating technique (Schnitzer et al., 1995b; Schnitzer et al., 1995c; Oh and Schnitzer, 1998). The caveolae were stripped from the plasma membrane by homogenization, then isolated by sucrose density centrifugation to yield isolated caveolae (V) well separated from the pellet containing resedimented silica-coated membranes stripped of caveolae (P-V) (Schnitzer et al., 1995b; Schnitzer et al., 1995c; Oh and Schnitzer, 1998). Western analysis of 5 μg of each fraction revealed significant enrichment in P relative to H for Gq, Gi, Gs, and Gβ (Figure 1A). In addition, all were found, to varying degrees, in caveolae (V). Only Gq was enriched along with caveolin in V compared with both P and P-V. Gi, Gs, and Gβ, although present, were not concentrated in V. Their level in isolated caveolae did not correlate with the increased caveolin concentration but, rather, was equivalent to the rest of the plasma membrane, consistent with the substantial signal for Gi, Gs, and Gβ but not caveolin remaining in P-V. This is in agreement with past work demonstrating the presence of Gi, Gs, and Gβ in both V and P-V fractions with very little detected in the Triton-soluble membrane fraction (Schnitzer et al., 1995a). In contrast, Gq appeared to follow caveolin, to concentrate in V, and to be markedly depleted, though not absent, in P-V.
Figure 1.
Distribution of G proteins in isolated membranes and caveolin-coated caveolae. (A) Subcellular fractionation was performed on rat lung tissues to obtain whole lung homogenates (H), silica-coated luminal endothelial cell plasma membranes (P), caveolae (V), and repelleted silica-coated membranes stripped of caveolae (P-V). Western analysis of 5 μg of each these fractions is shown using antibodies to Gi, Gs, Gq, and Gβ, 5′ NT, ε-Cop, and caveolin-1 and 2. (B) The caveolae fraction (V) was subjected to immuno-affinity isolation using magnetic beads attached to antibodies to caveolin-1 (cav) or clathrin heavy chain (clath). Five micrograms of the V fraction starting material (SM) and the entire amount of antibody bound (B) or unbound (U) material was subjected to Western analysis using antibodies to the indicated proteins.
Because G proteins are also extensively present in Golgi membranes (Denker et al., 1996), we assessed the quality of each fraction by Western analysis using antibodies against caveolin-1 and -2, the GPI-anchored protein, 5′NT, and the intracellular endosomal and Golgi marker, ε-COP. P displayed ample enrichment for 5′NT, caveolin-1, and -2 relative to H, while being markedly depleted in ε-COP (Figure 1A). V was enriched in caveolin-1 and –2, whereas 5′NT and ε-COP appeared absent.
To confirm further that the G proteins detected in V are indeed in caveolin-coated caveolae and not from any possible contaminating membranes, we performed immuno-isolation on V using caveolin-1α antibody-conjugated magnetic beads, as described previously (Oh and Schnitzer, 1999). The caveolin antibody bound (B) and unbound (U) fractions were subjected to Western analysis using Gq, Gi, Gs, and Gβ antibodies. As shown in Figure 1B, all of the G proteins detected in V were found in the caveolin-coated caveolae bound to the magnetic beads (B), with no signal detected in U, thereby confirming their existence in caveolin-1-coated caveolae. Both caveolin-1 (α and β) and -2 were found exclusively in B. Control immuno-isolations using an antibody to clathrin gave no signal in B with all of the G protein and caveolin remaining in U. These data are consistent with past reports (Oh and Schnitzer, 1999) in which essentially all of the material from V fractionates into B, indicating that V contains a rather homogeneous population of caveolin-coated caveolae.
Immunomicroscopy of Plasmalemmal G Proteins in Cultured Cells
One reason for Gi and Gs lacking enrichment in V may be that they are only weakly associated and become separated from the caveolae during the membrane subfractionation procedure. To avoid this possibility and to determine whether select G proteins also concentrate in caveolae of cultured cells, we examined their plasma membrane distribution in BLMVEC by dual immunofluorescence confocal microscopy, using antibodies to caveolin and either Gi, Gs, Gq, or Gβ (Figure 2). Consistent with past reports (Liu et al., 1997; Oh et al., 1998), caveolin-1 antibodies revealed a punctate staining pattern marking the caveolae on the cell surface (Figure 2, left panels, red labeling). Likewise, all G proteins displayed significant punctate cell surface staining, indicating their preferential targeting and elevated concentration within discrete plasmalemmal microdomains (Figure 2, middle panels; green labeling). When the images were overlaid, Gq showed the greatest degree of colocalization with caveolin (mean of 88%; Figure 2, right panels; yellow signal), whereas the signal for Gi (18 ± 6%), and Gs (32 ± 9%) showed considerably less overlap with caveolin. Gβ was a composite of the Gq, Gi and Gs signals, exhibiting marked colocalization with caveolin as well as significant nonoverlapping signals (our unpublished results). Similar results were obtained using other cell types including MA104 (see Figure 4 and our unpublished results). These data are in agreement with the membrane subfractionation data demonstrating substantial enrichment of Gq, but not Gi, Gs, and Gβγ in caveolae of lung microvascular endothelium in tissue. Using techniques that detected caveolar localization of dynamin, eNOS, VAMP, and other proteins (Oh et al., 1998; Rizzo et al., 1998a; McIntosh and Schnitzer, 1999), we also attempted to localize G proteins by performing electron microscopy on immunogold labeled ultra-thin cryo-sections of rat lung tissue. We detected ample signal for caveolin coating the bulb of caveolae but no signal for G proteins (our unpublished results), suggesting that the antibodies are not suitable for such electron microscopy studies.
Figure 2.
Immunofluorescence microscopy of G protein and caveolin-1 colocalization. Bovine lung microvascular endothelial cells (BLMVEC) were fixed and incubated with antibodies, either to caveolin (left panel, red labeling) or to the indicated G proteins (middle panel, green labeling). Signal overlap for caveolin and G protein generates yellow in the overlay of the two images (right panel).
Figure 4.
Immunofluorescence microscopy of lipid rafts for colocalization with G proteins and caveolin. MA104 cells (panels A-G) were grown on coverslips, fixed, and incubated with monoclonal antibodies to either folate receptor (panels A, C, E, green labeling) or caveolin (panels B, D, F; green labeling), and polyclonal antibodies to Gq (panels A and B, red/orange), Gi (panels C and D, red/orange), Gs (panel E and F, red/orange), and caveolin (G, red/orange). (Panel H) BLMVEC labeled with 5′NT antibodies (green) and caveolin antibodies (red/orange). Images were overlaid, and the signal overlap is indicated in yellow. Inset: higher magnification of a representative area of the image showing overlapping signals. Magnification of insets, 3×.
Segregation of G Proteins between Isolated Caveolae and Lipid Rafts
Because Gi and Gs were primarily concentrated in discrete, caveolin-free microdomains, as detected by immunofluorescence microscopy, we tested whether select G proteins differentially targeted lipid rafts. Caveolae and lipid rafts were isolated separately from each other from the same plasma membranes, namely P, as described previously (Schnitzer et al., 1995b). Western analysis under equivalent protein conditions revealed that Gi and Gs were indeed enriched in the lipid rafts (LR), compared with caveolae (V) (Figure 3). Densitometric analysis revealed a 2- to 4-fold enrichment of Gi and Gs in lipid rafts. Note that the signal for these G proteins was always equivalent in P, V, and P-V, so that LR was the first subfraction of P to show any significant enrichment. Conversely, Gq (24-fold enrichment in V over LR) and caveolin (35-fold) were highly concentrated in caveolae and minimally or not detected in LR. Consistent with previous reports (Schnitzer et al., 1995b), the GPI-anchored protein and lipid raft marker, 5′NT, was concentrated in LR (34-fold more concentrated in LR versus V) (Figure 3). These data demonstrate that G proteins can be differentially distributed at the cell surface between caveolae and lipid rafts with Gq selectively segregating and concentrating in caveolae, while Gi and Gs preferentially target lipid rafts.
Figure 3.
Differential distribution of G proteins in lipid rafts. Caveolae (V) and lipid rafts (LR) were isolated from the silica-coated endothelial cell plasma membranes (P) derived from lung. Western analysis of V and LR (5 μg each) is shown using antibodies to the indicated G proteins, caveolin-1, and the lipid raft marker, 5′NT.
Gi and Gs Target Lipid Rafts in Cultured Cells
To extend the subfractionation data demonstrating the selective concentration of Gi and Gs in, and apparent exclusion of Gq from, lipid rafts, we performed dual immunofluorescence confocal microscopy on cultured endothelial and epithelial cells using antibodies to caveolin-1, Gi, Gs, Gq, and the lipid raft markers, folate receptor, and 5′NT. Figure 4 shows an overlay of G protein (red label) and folate receptor (green label) images (see inset), displaying little overlap of Gq with folate receptor on the surface of MA104 cells (Figure 4A, 17 ± 6%, yellow label), whereas Gs (Figure 4E, 80 ± 5%) and Gi (Figure 4C, 61 ± 10%) exhibited significant overlap with folate receptor. Studies using BLMVEC colabeled with 5′NT and G proteins gave similar results (our unpublished results). Figure 4, panels B, D, and F, shows an overlay of caveolin (green label) and G protein (red/orange label) staining in MA104 cells. Similar to the BLMVEC (Figure 2), Gq exhibited significant overlap with caveolin-containing caveolae (88 ± 8%, yellow label), while Gi and Gs demonstrated much less colocalization with caveolin, at 17 ± 8% and 31 ± 9% (yellow label, see inset), respectively. We also observed little colocalization of caveolin with folate receptor (15 ± 3% in MA104) and 5′NT (3 ± 2% in BLMVEC), on the cell surface, consistent with caveolae and lipid rafts being distinct membrane domains (Figure 4G, H) (Schnitzer et al., 1995b). These data support the above subfractionation and caveolin colocalization data demonstrating the rather selective segregation of Gq to caveolae and Gi and Gs to lipid rafts at the cell surface.
G Proteins in Other Caveolin-enriched Fractions
The above data are quite consistent with past studies showing the presence but not enrichment of Gs and Gi in caveolae (Liu et al., 1996; Oh and Schnitzer, 1999). In contrast to the data presented here, other past studies reported that Gi, Gs, and Gβ are enriched in caveolar membrane fractions (Sargiacomo et al., 1993; Chang et al., 1994; Lisanti et al., 1994; Li et al., 1995; Smart et al., 1995; Song et al., 1997). Many factors could contribute to this difference, including the cell type and the membrane subfractionation technique used. Therefore, we examined G protein distribution utilizing a popular, alternative, detergent-free subfractionation method used in many of these studies. We isolated plasma membranes from rat lung tissue using Percoll gradient centrifugation (PM). A caveolin-enriched fraction (AC) was isolated from PM by sonication followed by density centrifugation. Western analysis revealed significant enrichment in AC for caveolin, Gq, Gi, Gs, and Gβ as compared with PM (see Figure 5), thereby confirming the results reported in past studies.
Figure 5.
Western analysis of sonicated low-density caveolin-rich fractions and immuno-isolated caveolae. (A) Rat lung tissue homogenates were fractionated on a Percoll gradient to isolate plasma membranes (lane PM), followed by sonication and sucrose gradient centrifugation to isolate a caveolin-rich fraction (lane AC). Subsequently, 25 μg of AC was subjected to immuno-affinity isolation using caveolin-1 antibody conjugated magnetic beads separate caveolin-coated vesicles bound to the beads (B) from noncaveolar unbound material (U) in the supernatant. Western analysis was performed on 5 μg of each fraction using antibodies to the indicated proteins. (B) The level of specific proteins in the bound versus the unbound fraction was quantified, and the ratiowas plotted for caveolin and for each of the indicated G proteins.
Because AC has been shown to contain noncaveolar markers, including ε-COP and 5′NT (Figure 5A and past work (Oh and Schnitzer, 1999)), we subjected AC to immuno-isolation using caveolin antibodies. This procedure isolates caveolin-coated vesicles away from other possible contaminating membranes in AC (Oh and Schnitzer, 1999), including lipid rafts and Golgi membranes, a site known to be rich in G proteins (Denker et al., 1996). Caveolin antibody bound (B) and unbound (U) fractions were examined by Western analysis and, although ample signals for each of the G proteins tested (Gq, Gi, Gs and Gβ) were seen in the starting material (AC), only Gq was enriched in the caveolae bound to the caveolin antibody-conjugated beads (B) (Figure 5A). The signal from the bound versus unbound fractions was quantified densitometrically, and the ratio was calculated for each G protein and caveolin (Figure 5B). As expected, caveolin was enriched 5-fold in B (Oh and Schnitzer, 1999), whereas ε-COP apparently remained completely in U. In addition, Gq was enriched 3-fold while Gi, Gs, and Gβ exhibited no enrichment in B. These findings are consistent with the data obtained from Western analysis of the H, P, V, P-V, and LR subfraction analysis presented above. Thus, an alternative analysis not utilizing the silica-coating procedure also detected the enrichment of Gq, but not Gi, Gs, and Gβ, in caveolae. It appears that the uniform enrichment observed in AC compared with PM for each of the G proteins may reflect their high concentration in either caveolae, lipid rafts, and/or Golgi membranes, all of which are present to a great extent in this heterogeneous low density membrane fraction.
Gq in Lipid Rafts of Cells without Caveolae
To determine the cell surface distribution of Gq in the absence of caveolae, we examined the localization of Gq in N2a neuroblastoma cells, which lack detectable caveolin and caveolae (Gorodinsky and Harris, 1995). We isolated Triton-insoluble membrane fractions (TIM) from N2a cells for Western analysis. Gq, Gi, and Gs were all detected in TIM (our unpublished results). Because TIM may contain intracellular membrane contaminants in addition to lipid rafts, we performed dual immunofluorescence microscopy on these cells with antibodies to Gq, Gi, and Gs, as well as caveolin and the lipid raft marker, 5′NT. Figure 6 shows that Gq and Gi exhibited a punctate cell surface staining distribution with significant signal overlap with 5′NT (73 ± 3% for Gq, 76 ± 9% for Gi), indicating their presence primarily in lipid rafts at the cell surface. A similar distribution was observed for Gs (79 ± 3%, our unpublished results). As expected (Gorodinsky and Harris, 1995), the caveolin antibody only gave a very faint diffuse background signal (our unpublished results). Thus, in the absence of caveolin and caveolae, Gq can target lipid rafts.
Figure 6.
Immunofluorescence microscopy of Gq and Gi concentrated in lipid rafts of N2a cells lacking caveolin and caveolae. N2a cells were fixed and incubated with monoclonal antibodies to 5′NT (panels A, D, green labeling) and polyclonal antibodies to Gq (panel B, red) and Gi (panel E, red). Images were overlaid (panels C and F), and the signal overlap is indicated in yellow. Inset: higher magnification of a representative area of the image showing overlapping signals.
Gq Specifically Forms a Complex with Caveolin But Not Gβγ
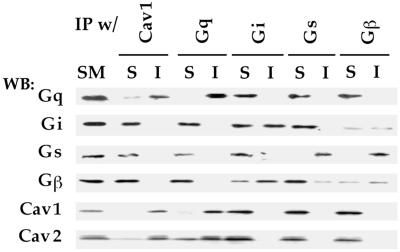
Caveolin is a structural protein of caveolae but not of lipid rafts. Because Gq appears to be specifically restricted to caveolae and not lipid rafts in cells with both microdomains, we investigated whether the basis for this preferential distribution involved an interaction with caveolin, which is known to associate with a variety of signaling molecules. Using a coimmunoprecipitation assay developed to explore eNOS interactions with caveolin (Rizzo et al., 1998a), we mildly solubilized silica-coated plasma membranes (P) from rat lung and then immunoprecipitated with caveolin-1 or G-protein antibodies. The starting material (SM), immunoprecipitated proteins (I), and nonprecipitated, soluble fraction (S) were each examined by Western analysis using antibodies against Gi, Gs, Gq, Gβ, and caveolin. SM represents an equivalent amount of the solubilized protein fraction used in each immunoprecipitation experiment. When the immunoprecipitates were probed with the same antibody used for the precipitation, Gq, Gs, and caveolin were found nearly completely in I with a signal intensity comparable to that seen in SM (Figure 7). Little or no signal was found in S, indicating reasonably quantitative immunoprecipitation (>95% immunoprecipitated). Gi and Gβ were not as efficiently precipitated, with the signal divided approximately equally between I and S. When the immunoprecipitation was performed using caveolin antibodies, Gq was the only G-protein subunit detected in the immunoprecipitated caveolin complexes. Nearly all of the Gq and caveolin signal was in I, with little to none remaining in S. In contrast, neither Gi, Gs, nor Gβ was detectably associated with caveolin-1 in this assay and remained in S. Likewise, when the immunoprecipitation was performed using each of the G-protein antibodies, only Gq, but not Gi, Gs, nor Gβ, associated with caveolin. Notably, Gi and Gs, but not Gq or caveolin, appeared to associate with Gβγ subunits. Western analysis of the Gβ immunoprecipitated complex detected the presence of both Gi and Gs but not caveolin nor Gq. Given that Gi and Gs both interact with Gβ in this assay, one can conclude that Gq does not associate equivalently, or at least not as avidly, with Gβγ as do the others. Taken together, these data indicate that, in the plasma membrane under physiological conditions found in tissue, caveolin associates differentially with G proteins with a substantial preference for Gq but apparently not Gi, Gs, nor Gβ1–4. The specificity of this interaction provides a clear mechanism to concentrate Gq preferentially within caveolae at the cell surface.
Figure 7.
Coimmunoprecipitation of G proteins with membrane-bound caveolin. Proteins solubilized from plasma membranes (P) were subjected to immunoprecipitation (IP) using antibodies to the indicated proteins bound to magnetic beads. The starting material (SM) (5 μg) solubilized from P and equal volumes of the material bound to the beads (I) or unbound in the supernatant (S) were subjected to Western analysis using antibodies to the indicated proteins.
We (K.R. Solomon and J.E. Schnitzer, unpublished data) and others (Uittenbogaard et al., 1998) have been able to detect the presence of soluble caveolin in cytosol from tissue or cultured cells. To investigate the possibility of Gq-caveolin complexes outside of membranes, we performed coimmunoprecipitation assays on membrane-free cytosols prepared from rat lung homogenates. Although it is clear that these molecules are found in small quantities in the cytosol relative to membranes, we were still able to detect the presence of caveolin, Gq, Gi, and Gs but not Gβγ subunits in the cytosolic fraction (Figure 8). When the immunoprecipitation was performed using caveolin antibodies, only Gq, and not Gs, Gi, or Gβ, was found in the immunoprecipitated caveolin complexes (Figure 8). Likewise, when the various G protein antibodies were used, caveolin was found only in Gq complexes and not in association with Gs, Gi, or Gβ. In contrast to the immunoprecipitation assay performed on solubilized membranes, cytosolic Gi and Gs was not associated with Gβγ subunits. This is consistent with GPR and Gαβγ complexes being completely membrane-associated (Morris and Malbon, 1999). Thus, in the cytosol and in the absence of detergent, Gq, but not Gi, Gs, or Gβ, remained specifically bound to caveolin, even when not embedded in the lipid membrane.
Figure 8.
Coimmunoprecipitation of Gq with soluble caveolin. Cytosols were prepared from rat lung homogenates and subjected to immunoprecipitation (IP) with magnetic beads coated with caveolin-1 or G protein antibodies as indicated. Equal volumes of the material bound to the beads was then subjected to Western analysis (WB), using antibodies to caveolin-1 or the indicated G proteins. HC, immunoglobulin heavy chain; LC, immunoglobulin light chain.
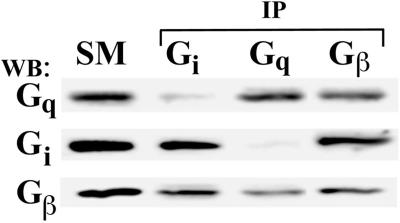
Last, we investigated whether Gq, in the absence of caveolin, would interact with Gβγ. We again used N2a cells in this case to immunoprecipitate Gq, Gi, and Gβγ for Western analysis of the SM, I, and S, as above. Figure 9 shows that both Gq and Gi associated with Gβγ in N2a cells. Thus, in cells lacking caveolin expression, Gq can form a stable complex with Gβγ. Taken together, these data suggest that Gq, but not Gi nor Gs, preferentially associates with caveolin over Gβγ.
Figure 9.
Coimmunoprecipitation of Gαβγ complexes in N2a cells lacking caveolae. Proteins solubilized from N2a cells were subjected to immunoprecipitation (IP) using antibodies to the indicated proteins bound to magnetic beads. The starting material (SM) (5 μg) and equal volumes of the material bound to the beads (I) or unbound in the supernatant (S) were subjected to Western analysis using antibodies to Gq.
Dissociation of G Proteins from Caveolae
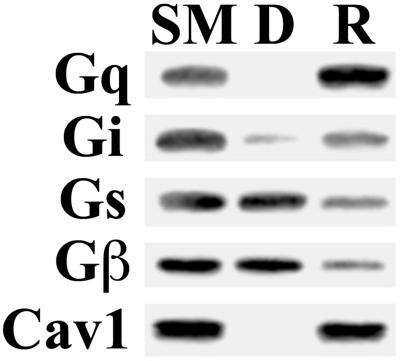
The preferential interaction of caveolin with Gq suggests that caveolin, acting as a scaffolding protein, retains and thereby concentrates Gq in caveolae. Gi, Gs, and Gβ may still have a preference for the specialized lipid milieu of the caveolae but may be freer to diffuse into and out of caveolae and thus do not attain or maintain enrichment. To examine this possibility more closely, we studied the relative dissociation rates of G proteins from caveolae. In past work (Oh and Schnitzer, 1999), we observed dissociation of signaling molecules, including Gs, from caveolae after extended incubation in detergent-free buffer. We incubated an aliquot of V in MBS for 4 h at 4°C, then separated the pelleted caveolar membranes (R) from any dissociated proteins remaining in solution (D) by centrifugation. The starting caveolar membranes (SM), D, and R were examined by Western analysis for caveolin and G protein subunits (Figure 10). The caveolar membranes sedimented readily, as indicated by equivalent caveolin signals in SM and R with little to no signal detected in D. Likewise, Gq was also found exclusively in R with no signal in D. This is consistent with Gq remaining bound to caveolin. In contrast, Gi, Gs and Gβ, although still present in R, were readily detected in D, indicating that they can dissociate from the caveolar membrane. This is consistent with our previous observation that, over time, Gs can dissociate from the caveolar membrane fraction to a soluble, nonsedimenting fraction (Oh and Schnitzer, 1999). Note as previously reported (Oh and Schnitzer, 1999), little to no dissociation was detected with shorter 1-h incubations; in this case all G-protein subunits and caveolin sedimented with the membranes found in R and were not in D (our unpublished results). It appears that the preferential interaction of Gq with caveolin stabilizes this subunit to remain bound in the caveolar membrane, whereas those G-protein subunits not avidly associated with caveolin can move more freely out of caveolae.
Figure 10.
Dissociation of G proteins from caveolae. Isolated caveolae (V) (20 μg) were diluted in MBS and gently mixed at 4°C for 4 h followed by centrifugation to separate the repelleted caveolar membranes (R) from the soluble phase containing any dissociated proteins (D). An equal volume of starting caveolae (lane SM), R, and D were subjected to Western analysis using antibodies to the indicated proteins.
DISCUSSION
Heterotrimeric G proteins are important cell surface molecules located on the inner leaflet of the lipid bilayer and are required to link select receptor-ligand interactions to intracellular signaling cascades (Morris and Malbon, 1999). There are multiple isoforms of each type of G-protein subunit. Gα contains at least 20 isoforms divided between four subfamilies, Gi/o/t/z, Gs/olf, Gq/11, and G12/13, while Gβ and Gγ contain at least five isoforms each (Morris and Malbon, 1999). Here, we report a detailed analysis of the localization of several Gα-isoforms and Gβ-subunits in the plasma membrane, revealing that G proteins can target caveolae and lipid rafts differently. Cell surface Gq preferentially targets caveolae via its specific ability to associate with caveolin. Conversely, without equivalent effective association with caveolin, Gi, Gs, and Gβ, apparently by default, tend to concentrate preferentially within lipid rafts. The differential microdomain distribution was verified by four independent lines of evidence: 1) Gq is enriched, whereas Gi, Gs, and Gβ are present but not enriched, in caveolae purified from rat lung tissue using two different subfractionation methodologies yielding homogeneous populations of caveolin-coated caveolae; 2) Gq extensively colocalizes with caveolin-marked caveolae, whereas Gi and Gs primarily reside in lipid rafts rich in GPI-anchored proteins (5′NT and folate receptor) at the surface of cultured endothelial and epithelial cells as visualized by dual immunofluorescence confocal microscopy; 3) Gq, but not Gi, Gs, or Gβ, specifically coimmunoprecipitates with caveolin, both from the membrane in caveolae and when free in the cytosol; and 4) Gi, Gs, and Gβ, but not Gq, dissociate and readily move out of caveolae. Last, not all cells have caveolae. In cells lacking caveolin, we find that all three G proteins (Gq, Gi, and Gs) concentrate in lipid rafts as a complex with Gβγ subunits. Thus, caveolin forms an oligomeric coat structure around the bulb of caveolae, which appears to act as a multimeric binding scaffold that traps, concentrates, and stabilizes Gq within caveolae.
Gα-subunits interact with membrane lipids via saturated acyl chains, typically myristate and/or palmitate, covalently attached at the amino terminus (Morris and Malbon, 1999). Our data reveal that G proteins at the cell surface can preferentially target and concentrate in lipid rafts of various intact cells grown in culture and in vivo in tissue. How and why they target the special lipid milieu of lipid rafts is less clear. One possibility is that certain GPI-anchored proteins, such as CD59, CD48, and Thy1 can somehow associate with select Gα subunits (Solomon et al., 1996), possibly through an unknown bridging molecule. Alternatively, G-protein subunits may target lipid rafts directly via their lipid chain modification. Lipid rafts are thought to arise when highly saturated sphingolipids, in the presence of cholesterol, self-assemble to create detergent-resistant domains within the plasma membrane (Schroeder et al., 1998; Brown and London, 2000). Proteins modified with saturated acyl chains, such as GPI and palmitate, have a higher affinity for this environment due to their ability to pack well into the highly ordered lipid milieu of lipid rafts (Melkonian et al., 1999) and thus may selectively partition into these domains, thereby acquiring detergent resistance (Schroeder et al., 1998; Moffett et al., 2000). Other proteins modified by prenyl groups, which have a bulky, branched structure, do not pack well and appear to be excluded from rafts (Melkonian et al., 1999). Yet differences have been observed in the relative detergent insolubility between GPI-linked proteins (Schnitzer et al., 1995b; Melkonian et al., 1999), which, with a similar lipid anchor should theoretically partition to lipid rafts in equal proportions, suggesting that there may be additional contributing factors that determine membrane targeting.
Our data suggest that another factor affecting targeting of lipid-anchored proteins may be their ability to interact stably with caveolin. Although many labs have found G proteins in detergent-resistant membrane fractions (Sargiacomo et al., 1993; Chang et al., 1994; Lisanti et al., 1994; Li et al., 1995; Schnitzer et al., 1995a; Smart et al., 1995; Solomon et al., 1996), we present here an example of preferential segregation between two distinct subtypes, caveolae and lipid rafts. Like GPI-anchored proteins, G-protein subunits are modified with saturated fatty acids that, in the absence of an interaction with caveolin (or other caveolar protein) to tightly hold them in caveolae, may cause them to target by default lipid rafts. This seems to be the case for cells that do not express caveolin where Gq also targets lipid rafts similarly to Gs and Gi. Of course, Gi and Gs may segregate to lipid rafts via direct binding to a yet unknown protein. Last, an interesting consequence of differential segregation is that it may provide a basis for compartmentalization of upstream and downstream signaling molecules on the cell surface—that is, the G protein coupled receptors (GPCR) may be targeted to specific microdomains based on their association with G proteins found there. Of course, there may be an as-yet-undiscovered GPCR component that targets the receptor to specific membrane microdomains, which may then draw its G-protein effectors to that region.
Although Gi and Gs appear to be concentrated primarily in lipid rafts, our data show that they may still be present to some degree in caveolae. Gs appears to localize to a more substantive subset of caveolae than Gi (see Figure 2) and thus may actually be associated specifically with a small subpopulation of caveolae. This subset-specific enrichment may not be detected in the general population of caveolae isolated by subfractionation. Unfortunately, our attempts to perform subset analysis by immuno-isolating caveolae in V using various G-protein antibodies have failed because the available antibodies do not efficiently immuno-isolate caveolae. Given that we can detect complexes of Gi and Gs with Gβγ subunits but not caveolin by coimmunoprecipitation, Gs may be sequestered in caveolae indirectly as part of a GPCR complex. Gs-containing GPCR complexes may enter caveolae via another mechanism, for instance, as part of an endocytic pathway functioning in GPCR internalization. Alternatively, the apparent overlap with caveolae may reflect instances in which caveolae and lipid rafts are closely associated with, yet still distinct from each other in the plasma membrane as first described in our past work (Schnitzer et al., 1995b). Caveolae can be attached by their necks to flat, detergent-resistant microdomains that form an annular region surrounding the caveolar ostia and are rich in GPI-anchored proteins (Schnitzer et al., 1995b). Molecules in lipid rafts located in this region immediately adjacent to caveolae would, at the level of resolution obtainable by immunofluorescence microscopy, appear to overlap with the caveolin punctate signals. Such localization of Gs near the neck of the caveolae may be an intermediate state in a normal translocation process for G proteins into caveolae, for example, as part of a recycling signaling pathway. Further investigation of these possibilities will require careful examination of the membrane localization by immunogold electron microscopy with other G-protein antibodies (our attempts at immunolabeling ultrathin frozen tissue sections with currently available antibodies failed), the development of new antibodies, and/or additional refinements to the purification procedure that would allow individual subsets of caveolae to be isolated. Note that the interaction of Gq with caveolin in caveolae likely places Gq not at or near the neck, but rather at the bulb of caveolae.
Because Gi and Gs do not form strong interactions with caveolin as does Gq, and because they can dissociate from caveolae into solution and thus presumably out into the noncaveolar membrane, it is possible that their presence in caveolar membrane subfractions was underestimated due to loss during the isolation procedure. Yet the isolated lipid rafts, but not caveolae, were enriched in Gs and Gi. This concern is also minimized by the confirmation using immunomicroscopy of intact cells that Gq colocalizes with caveolin to a much greater degree than Gi and Gs. If differential dissociation from membranes or other movement during subfractionation contributes to low Gi and Gs signals in caveolae, then significant differences between caveolae and lipid rafts are likely to exist, possibly in the lipid milieu responsible for G protein targeting to these domains. Although caveolae and lipid rafts may have common lipids, such as cholesterol and sphingolipids, the full complement of lipids in each domain is presently unknown. Any lipid differences may explain the differential G-protein partitioning, which may be quite dynamic within each microdomain. The experiments presented herein represent only a snapshot of protein distribution at equilibrium. Because the lipid composition of caveolae may be similar but not necessarily identical to lipid rafts (Brown and Rose, 1992; Liu et al., 1997), Gi and Gs may actually be moving in and out of caveolae and back to the rest of the plasma membrane at rates faster than in lipid rafts.
It is noteworthy that Gq appears to have a higher affinity for caveolin than for Gβγ. In our assays, we cannot detect an interaction between Gq and Gβγ in caveolae in situ or in cytosolic caveolin:Gq complexes. Yet Gq-Gβγ complexes were readily detected in N2a cells, which lack caveolin expression. Gq-Gβγ interactions have also been detected in detergent extracts of S. frugiperda cells overexpressing various G proteins (Fletcher et al., 1998), again showing that Gq can stably interact with Gβγ under conditions where caveolin is absent. When caveolin is expressed, Gq appears to preferentially form a complex with it rather than any Gβγ subunits, causing Gq to selectively partition to caveolae. In cells lacking caveolin and caveolae, Gq may, by default, stably associate with Gβγ and partition to lipid rafts. Interestingly, the lack of Gq association with Gβγ in caveolae suggests that Gq may reside in caveolae not as part of a Gαβγ complex or perhaps even a GPCR complex. The exclusive nature of the Gq-caveolin complex formation, i.e. Gq-Gβγ complexes were not detected at all in the presence of caveolin, suggests that Gβγ and caveolin probably bind to the same region of Gq. Thus, Gq activity may be negatively regulated independently of Gβγ by caveolin in a manner analogous to other caveolin-bound signaling molecules, such as eNOS (Garcia-Cardena et al., 1997; Ju et al., 1997; Ghosh et al., 1998; Rizzo et al., 1998a). In a sense, caveolin may be functioning similarly to GPCR and/or Gβγ. Finally, this Gq-caveolin complex also may have a unique role in caveolae, perhaps functioning in mediating acute cellular responses to mechanical stress (see below) or in regulating the trafficking of caveolae and perhaps select signaling molecules. For instance, we find that the Gq-coupled receptor for endothelin (ETB) is concentrated in endothelial caveolae, and endothelin stimulates both the budding of caveolae and internalization of the ETB (Oh et al., 2000).
In addition to its possible role in compartmentalized signaling, caveolae in vascular endothelium play a key role in sensing and responding to mechanical stressors, such as fluid shear and pressure, acting externally on the cell surface (Rizzo et al., 1998b; Rizzo and Schnitzer, 1999). Caveolae can function as mechanosensitive organelles and may contain many of the signaling molecules, including eNOS and heterotrimeric G proteins, that mediate acute responses to mechanical stress (Rizzo et al., 1998a; Rizzo and Schnitzer, 1999). Gq is activated in cultured endothelial cells in response to fluid shear (Gudi et al., 1996; Gudi et al., 1998). We have proposed that caveolae may be mechanosensing organelles and that caveolin may be a mechanosensor on the surface of vascular endothelial cells (Rizzo et al., 1998b; Rizzo and Schnitzer, 1999), whereas Frangos, Gudi, and colleagues propose that a G protein may constitute the mechanosensor (Gudi et al., 1998). Given that Gq is concentrated specifically in caveolae, and because Gq interacts avidly with caveolin, the cell surface mechanosensor may actually be the Gq-caveolin complex rather than Gq alone (found minimally in caveolae). We suggest that caveolin oligomers may act as loaded tension-bearing coiled springs responding acutely to changes in membrane tension (Rizzo et al., 1998b; Rizzo and Schnitzer, 1999). Recently, we found that mechanical stressors may place a strain on caveolae, resulting in conformational changes in caveolin and its oligomers (Oh and Schnitzer, 2000) to cause the release of key signaling molecules such as eNOS (Rizzo et al., 1998a) and Gq (Oh and Schnitzer, 2000). Likewise, caveolin oligomers may inhibit Gq activation until stressor changes induce Gq release for activation and downstream signaling. In some ways, caveolin oligomers may function analogously to the Gβγ subunit and/or the GPCR by replacing ligand induction with stress induction.
In the end, the data presented here may help to clarify the relationship between caveolae and lipid rafts as specific distinct plasma membrane microdomains. Because both caveolae and lipid rafts may have somewhat similar lipid compositions and are thus resistant to solubilization by Triton X-100, there has been a propensity in the field to equate caveolae with lipid rafts. However, there is evidence indicating that caveolae constitute a separate microdomain from lipid rafts. First, detergent-insoluble membranes can be isolated from cells that lack caveolae and caveolin expression (Fra et al., 1994; Gorodinsky and Harris, 1995). Second, electron microscopy studies on homogeneous caveolae preparations reveal that caveolae have a distinct morphology as compared with the other membranes found in the detergent-insoluble fraction (Schnitzer et al., 1995b). Third, new techniques have been devised that allow lipid rafts to be purified away from caveolae, permitting each of these fractions to be studied independently (Schnitzer et al., 1995b; Oh and Schnitzer, 1998). These studies have revealed that molecules previously detected in detergent-insoluble membranes and thus assumed to reside in caveolae, such as several GPI-anchored proteins, actually segregate to noncaveolar lipid rafts. Moreover, structural proteins of caveolae such as caveolin (Rothberg et al., 1992) and dynamin (Oh et al., 1998) are present in caveolae but not in lipid rafts. Here, we have extended these observations to another set of molecules, select heterotrimeric G proteins. We have demonstrated both biochemically and by immunofluorescence microscopy that various G protein α-subunits can differentially segregate to distinct plasma membrane microdomains—Gq to caveolae, and Gi and Gs to lipid rafts. The molecular mechanism for this segregation appears to be a replacement of Gq interaction with its Gβγ-subunit by a physiologically effective and stable association of Gq with caveolin. In cells without both microdomains and without caveolin expression, all three G proteins form a complex with Gβγ and target lipid rafts. G proteins tend to exist not randomly on cell surfaces, but rather concentrated in specialized distinct microdomains.
ACKNOWLEDGMENTS
We thank Dr. David Manning for the G protein antibodies, Dr. Paul Luzio for the 5′NT antibodies, and Dr. John Ghrayeb for the folate receptor antibodies. We thank Lucy A. Carver for writing assistance. This work was supported in part by National Institutes of Health grants HL-52766 and HL-58216. This work was presented in part at the FASEB meeting in San Diego, CA (April, 2000) and American Society for Cell Biology in Washington, D.C. (December, 1999).
REFERENCES
- Ahmed SN, Brown DA, London E. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochem. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Ying YS, Rothberg KG, Hooper NM, Turner AJ, Gambliel HA, De Gunzburg J, Mumby SM, Gilman AG, Anderson RG. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994;126:127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, Mc Caffery JM, Palade GE, Insel PA, Farquhar MG. Differential distribution of alpha subunits and beta gamma subunits of heterotrimeric G proteins on Golgi membranes of the exocrine pancreas. J Cell Biol. 1996;133:1027–1040. doi: 10.1083/jcb.133.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzen DJ, Hastings WR, Lublin DM. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem. 1995;270:6838–6842. doi: 10.1074/jbc.270.12.6838. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Field KA, Holowka D, Baird B. FcεRI-mediated recruitement of p53/p56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci USA. 1995;92:9201–9205. doi: 10.1073/pnas.92.20.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JE, Lindorfer MA, DeFilippo JM, Yasuda H, Guilmard M, Garrison JC. The G protein beta5 subunit interacts selectively with the Gq alpha subunit. J Biol Chem. 1998;273:636–644. doi: 10.1074/jbc.273.1.636. [DOI] [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA. 1995;92:8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the NOS caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gachhui R, Crooks C, Wu C, Lisanti MP, Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Hepler JR, Chen LT, Gilman AG, Anderson RGW, Mumby SM. Organization of G proteins. and adenylyl cyclase. at the plasma membrane. Mol Biol Cell. 1997;8:2365–2378. doi: 10.1091/mbc.8.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of < 100A using imaging fluorescence resonance energy transfer. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of choler toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Li S, Couet J, Lisanti MP. Src tyrosine kinases, G-alpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, Zurzolo C. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, Cook RF, Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- Mayor S, Maxfield FR. Insolubility and redistribution of GPI-anchored proteins at the cell surface after detergent treatment. Mol Biol Cell. 1995;6:929–944. doi: 10.1091/mbc.6.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DP, Schnitzer JE. Caveolae require intact VAMP for targeted transport in vascular endothelium. Am J Physiol. 1999;277:H2222–2232. doi: 10.1152/ajpheart.1999.277.6.H2222. [DOI] [PubMed] [Google Scholar]
- Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- Monier S, Dietzen DJ, Hastings WR, Lublin DM, Kurzchalia TV. Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett. 1996;388:143–149. doi: 10.1016/0014-5793(96)00519-4. [DOI] [PubMed] [Google Scholar]
- Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–5348. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC. Physiological regulation of G-protein linked signaling. Physiol Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Isolation and subfractionation of plasma membranes to purify caveolae separately from glycosyl-phosphatidylinositol-anchored protein microdomain. In: Celis J, editor. Cell Biology: A Laboratory Handbook. Vol. 2. Orlando, FL: Academic Press; 1998. pp. 34–36. [Google Scholar]
- Oh P, Schnitzer JE. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. Toward understanding the basis of purification [published erratum appears in J Biol Chem 1999 Oct 8;274:29582] J Biol Chem. 1999;274:23144–23154. doi: 10.1074/jbc.274.33.23144. [DOI] [PubMed] [Google Scholar]
- Oh P, Schnitzer JE. Role of caveolin mechanoscaffold in the regulation of mechanotransduction by caveolae. Mol Biol Cell. 2000;11:A636. [Google Scholar]
- Oh P, Reimoneq RD, Schnitzer JE. Endothelin-1 stimulates the fission and internalization of caveolae. FASEB J. 2000;14:A449. [Google Scholar]
- Rizzo V, McIntosh DP, Oh P, Schnitzer JE. In situ flow activates endothelial nitric oxide synthase in luminal caveolae of endothelium with rapid caveolin dissociation and calmodulin association. J Biol Chem. 1998a;273:34724–34729. doi: 10.1074/jbc.273.52.34724. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Sung A, Oh P, Schnitzer JE. Rapid mechanotransduction in situ at the luminal cell surface of vascular endothelium and its caveolae. J Biol Chem. 1998b;273:26323–26329. doi: 10.1074/jbc.273.41.26323. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Schnitzer JE. Role of caveolae on mechanotransduction. In: Catravas J D, Callow AD, Ryan US, editors. Vascular Endothelium: Mechanisms of Cell Signaling. Vol. 308. Amsterdam, Netherlands: IOS Press; 1999. pp. 97–116. [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995a;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995b;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Jacobson BS, Dvorak AM. Caveolae from luminal plasmalemma of rat lung endothelium: microdomains enriched in caveolin, Ca(2+)-ATPase, and inositol trisphosphate receptor. Proc Natl Acad Sci USA. 1995c;92:1759–1763. doi: 10.1073/pnas.92.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, McIntosh DP. Role of GTP hydrolysis in fission of caveolae directly from plasma membranes [published erratum appears in Science 1996 Nov 15;274:1069] Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. [DOI] [PubMed] [Google Scholar]
- Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored protein by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Kwong J, Fujita T, Olszowy MW, Shaw AS, Lublin DM. Signal transduction through decay-accelerating factor: Interaction of glycosyl-phosphatidylinositol anchor and protein tyrosine kinases p56lck and p59fyn1. J Immunol. 1992;149:3535–3541. [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon KR, Rudd CE, Finberg RW. The association between glycosylphosphatidylinositol-anchored proteins and heterotrimeric G protein alpha subunits in lymphocytes. Proc Natl Acad Sci USA. 1996;93:6053–6058. doi: 10.1073/pnas.93.12.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Sargiacomo M, Galbiati F, Parenti M, Lisanti MP. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol (Noisy-le-grand) 1997;43:293–303. [PubMed] [Google Scholar]
- Stan RV, Roberts WG, Predescu D, Ihida K, Saucan L, Ghitescu L, Palade GE. Immunoisolation and partial characterization of endothelial plasmalemmal vesicles (caveolae) Mol Biol Cell. 1997;8:595–605. doi: 10.1091/mbc.8.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Ying Y, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. Involvement in cholesterol trafficking. J Biol Chem. 1998;273:6525–6532. doi: 10.1074/jbc.273.11.6525. [DOI] [PubMed] [Google Scholar]
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–732. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]