Abstract
Protective immunity at the gut-associated mucosal tissue is induced primarily by oral/rectal immunization owing to the need for targeting antigen to the gut-resident dendritic cells (DC). Here we show that an adenovirus type 5 (Ad5) based HIV-1 vaccine can prime a durable antigen-specific CD8 T cell response in the gut following intramuscular immunization in mice. The ability of Ad5 to prime gut homing CD8 T cells in vivo was associated with Ad5-induced expression of retinal dehydrogenase (RALDH) enzymes in conventional DC. The Ad5-mediated induction of RALDH did not require signaling through toll-like receptors, DNA-dependent activator of IRFs and several MAP kinases, or replication capacity of the virus, but was dependant on NF-κB and granulocyte-macrophage colony-stimulating factor. These results provide an innate mechanism through which Ad5-stimulated DC prime gut homing CD8 T cells and have implications for the development of novel mucosal adjuvants for subunit vaccines administered via the intramuscular route.
INTRODUCTION
Pathogenic HIV/SIV infections are characterized by the rapid depletion of CD4 T cells in the gastrointestinal tract within 2 weeks following infection and vaccination strategies that induce high levels of anti-viral immunity at the gut-associated mucosal tissue can significantly enhance protection. Oral/rectal routes of vaccination is the best way to induce immunity at the gut-associated mucosal tissue 1,2. However, both routes of vaccinations are limited by requiring multiple high doses of the vaccine and the need to use adjuvants for induction of an optimal immune response.
The need for oral/rectal immunizations to elicit protective immunity at gut-associated mucosal tissue was attributed to the findings that gut-resident dendritic cells (DCs) have an intrinsic capacity of metabolizing vitamin A to Retinoic Acid (RA) that is required for imprinting gut homing potential on T and B lymphocytes 3,4. Synthesis of RA depends on the oxidative metabolism of retinol to retinal that requires alcohol dehydrogenases and then conversion of retinal to RA that requires retinal dehydrogenases (RALDH). The gut-resident DCs possess the property to synthesize RA because they constitutively express RALDH enzymes 3. It was previously thought that peripheral DCs do not constitutively express RALDH enzymes and thus are incapable of imprinting gut homing phenotype on T and B cells. A recent study has however shown that, under steady-state conditions, RA-producing DCs can also be found in the skin, lungs and the corresponding draining lymph nodes of these tissues 5.
In recent past, some studies have demonstrated that intramuscular immunizations with live replication defective/attenuated recombinant viral vectors such as adenovirus type 5 (Ad5) and modified vaccinia Ankara (MVA) can elicit immune responses in the gut-associated mucosal tissue in the murine 6,7 and macaque 8 models suggesting that a potent gut mucosal immunity is achievable with a parenteral route of immunization. Similarly, acute lymphocytic choriomeningitis virus (LCMV) infection of mice has been shown to induce anti-viral CD8 T cells capable of trafficking to gut within days after infection 9. However, the mechanisms by which parenteral immunizations induce antigen-specific CD8 T cells with gut homing potential are not understood. It is possible that these viruses modulate the function of peripheral DCs such that they acquire the capacity to induce gut homing potential on antigen-specific CD8 T cells. Furthermore, recently the STEP trial evaluating the efficacy of an Ad5 based HIV-1 vaccine was halted because of the presumed increased risk of HIV-1 acquisition in men that were baseline Ad5 seropositive and uncircumcised 10. It was hypothesized that this increased risk could be due to increased frequency of virus target cells at the mucosa primed by Ad5. Thus, defining the mechanisms by which Ad5 induces gut homing potential on T cells is important for the understanding of viral vector-based HIV vaccines.
Here we investigated the mechanisms by which Ad5 can modulate the function of peripheral DCs to induce gut homing potential on CD8 T cells. Our results demonstrate that Ad5 rapidly upregulates the expression of RALDH enzymes in conventional DCs (cDCs) that results in priming of antigen-specific CD8 T cells that co-express gut homing marker α4β7. Impressively, this function of Ad5 is independent of signaling through Toll-like receptors (TLRs), DNA-dependent activator of IRFs (DAI; previously also known as Z-DNA binding protein 1 or ZBP-1) and some MAP kinases, but was dependant on granulocyte-macrophage colony-stimulating factor (GM-CSF) and NF-κB in DC.
RESULTS
Parenteral immunization with Ad5/Env-Gag vaccine can induce gut homing potential on antigen-specific CD8 T cells
To determine whether intramuscularly (i.m.) administered recombinant Adenovirus serotype 5 vaccine vector can induce gut homing potential on antigen-specific CD8 T cells, BALB/c mice were primed and boosted with 1×106 pfu of the Ad5 expressing HIV-1 clade B Env and Gag (Ad5/Env-Gag) at weeks 0 and 4, respectively. The Gag-specific CD8 T cell responses in peripheral blood and lamina propria lymphocyte (LPL) population in the small intestines (will be referred to as ‘Gut’ here after) were evaluated using a H-2Kd restricted Gag tetramer at various time points following the boost (Fig. 1A, 1B). Expansion of gut resident Gag-specific CD8 T cells was detected as early as day 4 following the boost, peaked at day 7, contracted and persisted as late as day 150. At the peak response, the frequency (arithmetic mean) of these cells was about 15% of total CD8 T cells and was also 15-fold higher compared to pre-boost levels. Similar expansion/contraction kinetics was also observed in blood. However, on day 4 following boost, the mean percentage of tetramer-specific CD8 T cells in peripheral blood was 3.7-fold lower compared to the gut suggesting a preferential early homing of antigen-specific CD8 T cells to the gut upon i.m. immunization with Ad5/Env-Gag. By 2 weeks following the boost, the frequencies of Gag-specific CD8 T cells were similar in both compartments. These Gag-specific LPLs were also functional as evident from IFN-γ production upon ex vivo stimulation with a Gag peptide pool that encompasses the entire Gag protein, albeit at a lower level compared to tetramer+ cells (Fig.1C). Interestingly, this low IFN-γ response with respect to tetramer frequency was only true for the LPLs and not for other tissues (spleen and liver) analyzed on day 7 post boost (data not shown) as shown previously 8. Gag-specific CD8 T cells were also present in the intraepithelial lymphocyte (IEL) population of the gut as early as day 7 following the boost (data not shown). However, the frequency of these cells was about 14-fold lower compared to their frequency in the lamina propria.
Figure 1. Parenteral immunization with Ad5/Env-Gag vaccine can induce gut homing potential on antigen-specific CD8 T cells.
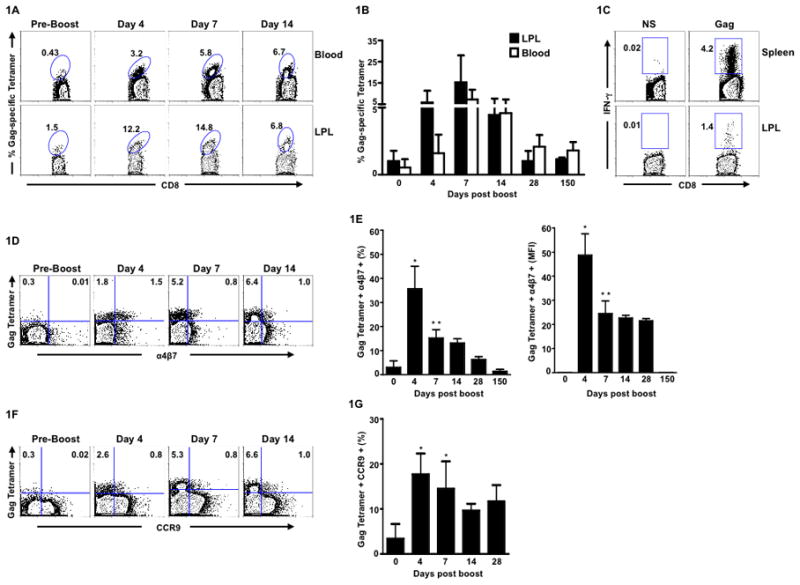
BALB/c mice were primed and boosted with Ad5/Env-Gag vector at week 0 and 4 respectively. (A) Representative FACS plot showing the frequency of Gag-tetramer specific CD8 T cells in blood and in lamina propria lymphocytes (LPL) at different time points following the boost. Numbers on the FACS plots indicate Gag tetramer-specific CD8 T cells expressed as a percent of total CD8 T cells. (B) Kinetics of Gag tetramer-specific CD8 T cell response in blood and LPL following Ad5/Env-Gag boost. (C) Representative FACS plots showing Gag-specific CD8 T cell responses in spleen and LPL at one week following the Ad5/Env-Gag boost as assessed by intracellular cytokine staining. Numbers on the FACS plots indicate Gag-specific IFN-γ+ CD8 T cells expressed as a percent of total CD8 T cells. NS, no stimulation. (D) Representative FACS plot showing α4β7 expression on Gag-tetramer specific CD8 T cells in blood (gated on total CD8 lymphocytes). (E) Mean frequency (left panel) and mean fluorescence intensity (MFI; right panel) of α4β7+ Gag-tetramer+ CD8 T cells in blood. (F) Representative FACS plot showing CCR9 expression on Gag-tetramer specific CD8 T cells in blood (gated on total CD8 lymphocytes). (G) Mean frequency of CCR9+ Gag-tetramer+ CD8 T cells in blood. Three to five mice were analyzed at each time point. Error bars indicate SD. Data is representative of one of two independent experiments. * P < 0.05; ** P < 0.01. P values indicate significantly higher responses compared to day 0.
We next analyzed the expression of the integrin α4β7 on Gag tetramer-specific cells in blood to determine whether the early gut homing potential of these cells can be predicted from the cell surface expression of this gut homing marker (Fig. 1D and 1E). The α4β7 ligand, mucosal addressin cell adhesion molecule-1 (MAdCAM-1), is expressed by the high endothelial venules in the lamina propria and is known to mediate T cell recruitment to the gut 11. About 30– 40% of Gag-tetramer-specific CD8 T cells in blood expressed α4β7 as early as day 4 following the boost. However, the α4β7 expression on these cells decreased rapidly by day 7, and only a small fraction (less than 10%) of tetramer positive cells retained expression on day 28. A similar pattern was also observed for the per-cell expression (as determined by the mean fluorescence intensity) of α4β7 on the tetramer positive cells, which was highest on day 4 following the boost (Fig. 1E, right panel). This high level of α4β7 expression on tetramer positive cells in blood on day 4 following the boost is consistent with the preferential homing of Gag-specific T cells to the gut and thus may be used as a marker to predict the gut homing potential of CD8 T cells following parenteral immunization. We also observed an increase in Gag-tetramer-specific CD8 T cells co-expressing CCR9 (homing marker for small intestine) on day 4 following the boost (Fig. 1F and 1G). However, the percentage of cells co-expressing α4β7 was 2-fold higher in comparison to those co-expressing CCR9. Our results thus suggest that both α4β7 and CCR9 play a role in Ad5-mediated induction of gut trafficking CD8 T cells.
Ad5 endows splenic cDC with the capacity to imprint gut homing potential on antigen-specific CD8 T cells via a retinoic acid dependent pathway
Upon i.m. immunization, Ad5 can either modulate peripheral DC function or target gut resident DC such that they can prime antigen-specific CD8 T cells with gut homing potential. However, it is less likely that the latter is true for this route of antigen delivery. So, we investigated whether the Ad5 can modulate peripheral DC function with regards to priming antigen-specific CD8 T cells with gut homing potential using an in vitro assay system. We co-cultured the ovalbumin-specific OT-I transgenic T cells with either uninfected or Ad5 (non-recombinant) infected splenic cDCs in the presence of ovalbumin peptide (SIINFEKL) for 72 hrs and analyzed the expression of α4β7 on these T cells. To avoid any extraneous virus-induced dendritic cell death during the co-culture, Ad5 infection of cDCs was carried out for 8hrs and cells were then washed to remove cell free virus before co-culturing with the OT-I T cells. The percentage of α4β7 expressing OT-I CD8 T cells was 2.8-fold higher when primed with Ad5- infected cDCs (MOI 0.15) in comparison to the T cells primed with uninfected cDCs (Fig. 2A, middle panel) demonstrating that Ad5 vector alone can enhance the ability of cDC to imprint gut homing potential on antigen-specific CD8 T cells. However, at higher MOI of Ad5 infection, the percentage of CD8 T cells co-expressing α4β7 was diminished (Fig. 2A, middle panel). Importantly, splenic cDCs infected with higher MOIs failed to prime CD8 T cells as efficiently as those infected with a lower MOI (0.15) or uninfected cDCs as evident from the CD8 T cell viability data after 72hrs of co-culture (Fig. 2A, right panel), suggesting that higher MOI of Ad5 may be reducing cDC viability. The Ad5-mediated effect on induction of α47 was also true for CCR9, as following in vitro infection of splenic cDCs (MOI 2), the percentage of CD8 T cells co-expressing CCR9 was 2.5-fold higher in comparison to CD8 T cells that were primed with uninfected cDCs (Fig. 2B). As expected, co-culture of T cells with retinoic acid (RA) induced highest levels of α4β7 and CCR9 on OT-I T cells.
Figure 2. Ad5 endows splenic conventional DC (cDC) with the capacity to imprint gut homing potential on antigen-specific CD8 T cells via a retinoic acid (RA) dependent pathway.
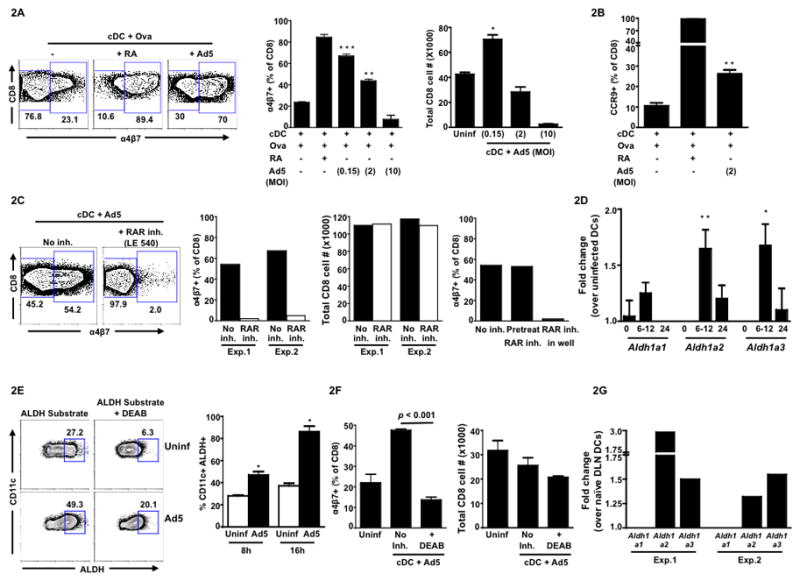
Splenic cDCs isolated from C57BL/6 mice were either left uninfected or infected with Ad5 (non-recombinant) for 8hrs. Following infection, cells were washed and co-cultured with OT-I T cells (CD8) in the presence of the OVA peptide for 72 hrs. (A) Representative FACS plots (left panel) showing cell surface expression of α4β7on OT-I T cells 3 days after co-culture. Cells were gated on total CD8 T cells. Middle panel, mean frequency of α4β7+ cells (gated on total live CD8 lymphocytes) following co-culture with either uninfected cDCs or Ad5-infected cDCs at indicated MOIs. Right Panel, total live (Via-Probe negative) CD8 T cell number at the end of the co-culture primed by cDCs infected with different MOIs. (B) Mean frequency of CCR9+ cells (gated on total live CD8 lymphocytes) following co-culture with either uninfected cDCs or Ad5-infected cDCs at MOI 2. (C) Representative FACS plots (left panel) showing α4β7 expression on total live CD8 T cells following co-culture with Ad5-infected cDCs (MOI 0.15) in the absence or presence of retinoic acid receptor (RAR) inhibitor LE540. Middle panel, frequency of α4β7+ CD8 T cells in the absence or presence of LE540 (inhibitor included in the co-culture). Total live (Via-Probe negative) CD8 T cell number in the absence or presence of LE540 at the end of the co-culture. Data from two independent experiments (Exp.1 and Exp.2) are shown. Right panel, frequency ofα4β7+ CD8 T cells with RAR inhibitor included either only during 8hrs of Ad5 infection and washed before co-culture (pre-treat) or left in the well during co-culture for 72hrs (in well). (D) Quantitative RT-PCR showing the fold change (mean of six independent experiments) in the expression of mRNA encoding retinal dehydrogenase (RALDH) enzymes (Aldh1a1, Aldh1a2 and Aldh1a3) in Ad5-infected cDCs over uninfected cDCs at 0, 6–12 and 24 hrs following in vitro infection. (E) Representative FACS plots (left panel) showing ALDH+ cells (gated on total CD11c+ cells) at 8hrs following in vitro Ad5 infection. The ALDH+ gate was determined based on the DEAB treated uninfected sample. Right panel, cumulative data (mean) for three independent experiments. (F) Mean frequency of α4β7+ CD8 T cells following co-culture with either uninfected cDCs or Ad5-infected cDCs in the absence or presence of a RALDH inhibitor, DEAB (left panel). Right panel, total live (Via-Probe negative) CD8 T cell number in the absence or presence of DEAB at the end of the co-culture. (G) C57BL/6 mice were immunized with Ad5 (5X107 pfu per mouse) intramuscularly and draining lymph nodes (inguinal and popliteal pooled) were harvested at 12hrs post immunization. Quantitative RT-PCR showing the fold change in the expression of mRNA encoding retinal dehydrogenase (RALDH) enzymes (Aldh1a1, Aldh1a2 and Aldh1a3) in cDCs from Ad5-infected mice DLN over naïve mice DLN. Results from two independent experiments are shown (Exp.1 and Exp.2). Error bars indicate SEM. All data (except Fig. 2G) are representative of at least three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001. P values indicate significantly higher responses compared to the respective uninfected DC controls.
Previous studies 3,4 have shown that GALT (gut-associated lymphoid tissue) DCs, have the intrinsic capacity to metabolize vitamin A (retinol) to RA that in turn imprints gut homing potential on naïve/responding T and B cells. So, we investigated whether induction of gut homing potential on antigen-specific CD8 T cells by Ad5-infected cDC was a RA-dependent or an independent effect. Intracellularly, RA signals through either the heterodimeric (RAR/RXR) or homodimeric (RXR/RXR) retinoic acid receptors and function as ligand-induced transcription factor by interacting with retinoic acid response elements (RARE) or RX response elements (RXRE) located in the promoter regions of target genes to regulate transcription 12,13. Inclusion of the retinoic acid receptor (RAR) inhibitor LE540 in the co-cultures of Ad5-infected peripheral cDC and OT-I T cells abrogated the α4β7 expression on OT-I T cells (Fig. 2C), suggesting that Ad5-mediated induction of α4β7 on antigen-specific CD8 T cells is RA dependant. The viable OT-I T cell number at the end of the co-culture was similar in the absence and presence of inhibitor, indicating that loss of α4β7 expression on OT-I cells in the presence of inhibitor was not due to cell death. Furthermore, inclusion of LE540 during the Ad5 infection period but not in co-culture, did not diminish the percent of α4β7 positive OT-I T cells suggesting that inhibition of RAR in Ad5-infected cDCs may not significantly affect their ability to induce gut homing potential on OT-I T cells (Fig. 2C, right panel). Thus, our working hypothesis is that upon Ad5 infection, peripheral cDCs acquire the ability to synthesize RA and the RA acts on T cells to induce α4β7 up regulation.
Previous studies have indicated that it is difficult to measure retinol metabolites in the supernatants of DC cultures as they are made in very small quantities14. So, we resorted to measure the levels of RALDH, a key enzyme that is required for the synthesis of RA. There are three isozymes for RALDH that are encoded by Aldh1a1, Aldh1a2 and Aldh1a3 genes. We quantitated the effect of Ad5 infection on the induction of mRNA for these 3 genes in splenic cDC and found a 1.3 to 1.7-fold increase of Aldh1a1, Aldh1a2 and Aldh1a3 message at 6–12hrs post infection in Ad5-infected over uninfected cDCs (Fig. 2D). We further validated the activity of ALDH protein in individual cells by using a fluorescent substrate (ALDEFLUOR) for ALDH. Following in vitro infection with Ad5, the percentage of ALDH+ cells in Ad5-infected cDCs was 1.7 and 2.3-fold higher in comparison to the uninfected cells at 8hrs and 16hrs respectively (Fig. 2E). Also, in line with our hypothesis, inclusion of a ALDH inhibitor DEAB (4-diethylamino benzaldehyde) in co-cultures, abrogated the ability of Ad5-infected cDC to induce gut homing potential on OT-I T cells (Fig.2F). Viable OT-I T cell numbers at the end of culture confirmed that the DEAB-mediated abolishment of α4β7 expression is not an artifact because of excessive cell death. To confirm that Ad5 induces expression of RALDH genes in vivo, we sorted cDC from the draining lymph nodes of mice following intramuscular immunization with Ad5. Consistent with in vitro data in splenic cDCs, we observed an up regulation of Aldh1a2 by 1.3 to 2.9-fold and Aldh1a3 by 1.5-fold in Ad5-immunized DLN cDCs over naïve control mice DLN cDCs at 12hrs post immunization (Fig. 2G).
Inactivated Ad5 also endows splenic cDC with the capacity to imprint gut homing potential on antigen-specific CD8 T cells
To investigate whether live Ad5 is required for the ability of cDCs to induce gut homing potential on antigen-specific CD8 T cells, we infected splenic DCs either with UV inactivated or heat inactivated Ad5. UV inactivation is known to inhibit viral transcription and heat inactivation is known to inhibit viral entry 15. UV or heat inactivation of the virus resulted in a minimum of 5-log reduction in the viral titer (data not shown). Interestingly, neither UV nor heat inactivation diminished the ability of Ad5 to modulate cDC function to imprint gut homing potential on OT-I T cells (Fig. 3A). In fact, we observed a marginally higher percentage of α4β7+ CD8 T cells primed with DCs pulsed with either of the inactivated Ad5 in comparison to those primed with live Ad5-infected DCs. Consistent with the priming of α4β7+ CD8 T cells, we also observed induction of ALDH activity in cDCs by 16–24 hrs following stimulation with UV or heat inactivated Ad5 (Fig. 3B). Notably, in contrast to live Ad5, the inactivated Ad5s induced ALDH+ cells with a delayed kinetics.
Figure 3. Inactivated Ad5 also endows splenic conventional DC (cDC) with the capacity to imprint gut homing potential on antigen-specific CD8 T cells.
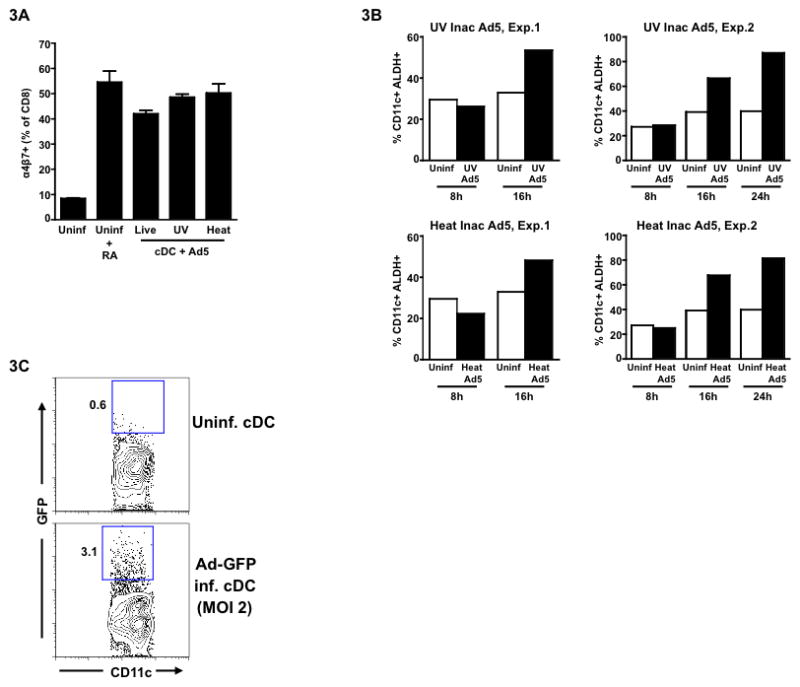
Following isolation of splenic cDCs from wild-type C57BL/6 mice, they were either left uninfected or infected with Ad5 (live) or UV/heat inactivated Ad5 (MOI 0.15) for 8hrs. After infection, cells were washed and cultured with OT-I T cells (CD8) in the presence of OVA peptide for 72 hrs. (A) Mean frequency of α4β7+ CD8 T cells following co-culture with either uninfected cDCs or live or inactivated Ad5- infected/stimulated cDCs. (B) Frequency of ALDH+ cells. Data from two independent experiments (Exp.1 and Exp.2) are shown. (C) Splenic cDCs from wild-type C57BL/6 mice were infected with Ad5-GFP. After 24 hrs of infection, cells were harvested and analyzed by FACS to detect relative (to uninfected cDC) levels of expression of GFP (y-axis on flow plot). Error bars indicate SEM. Data from one of the three independent experiments (except for Fig.3B) is shown.
Murine DCs lack the Coxsackievirus and Adenovirus Receptor (CAR) that is needed for Ad5 infectivity suggesting that Ad5 does not efficiently infect murine cDC. To confirm this, we infected splenic cDC with an Ad5 expressing GFP (Ad5-GFP) at a MOI of 2 for 24 hrs and found that less than 3% of the total cDC population was positive for GFP (Fig. 3C). The low level of infection could be due to CAR-independent rAd5 tropism that has been reported previously 16–18. These results demonstrate that productive Ad5 infection per se may not be important since UV or heat inactivated Ad5 could also modulate DC function. We speculate that mere Ad5 binding to the DC is probably engaging multiple pattern recognition receptors (PRRs) and/or cell surface integrins such as α5β1, αvβ3 and αvβ5 19, and that downstream signaling through these up regulates RALDH genes.
Signaling through multiple Pattern Recognition Receptors (PRRs) is dispensable for Ad5 infected cDCs to imprint gut homing potential on CD8 T cells
Stimulation through TLR2 on DC by zymosan can induce Aldh1a2 20 and Ad5 is known to engage multiple PRRs, including the TLRs 21. Thus, we investigated whether signaling through the TLRs on cDC is required for Ad5-mediated enhancement of cDC function to prime gut homing potential on CD8 T cells. DCs from both MyD88−/− as well as TRIF −/− mice were as efficient as DCs from wild-type B/6 mice to induce α4β7 expression on CD8 T cells following infection with Ad5 demonstrating that TLR signaling is not required for the ability of Ad5 to modulate cDC function (Fig. 4A). In addition, we detected the Ad5-mediated induction of RALDH message by qRT-PCR in cDCs from these knockout mice (data not shown), thus suggesting against any synergism between TLR signaling and RA metabolism in Ad5-infected cDCs. Interestingly, in the absence of Ad5, we noted a marked decrease in proliferation of OT-I T cells in cultures with cDC from the knockout mice (Supplementary Fig. 1), however, Ad5 infection restored this defect.
Figure 4. Signaling through multiple Pattern Recognition Receptors (PRRs) is dispensable for Ad5-infected conventional DCs (cDCs) to imprint gut homing potential on CD8 T cells.
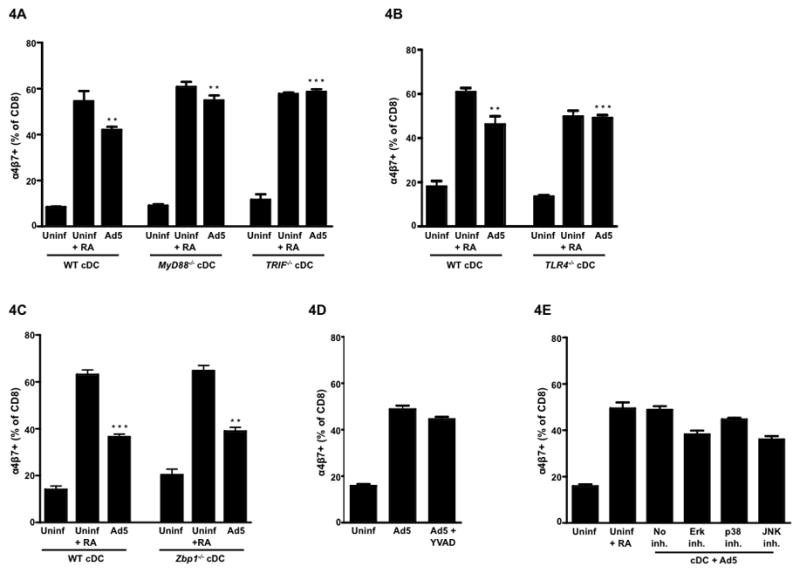
Splenic cDCs were isolated from either wild-type C57BL/6 mice or different gene knockout mice and infected with Ad5 (MOI 0.15) for 8hrs, after which, infected cells were washed and co-cultured with OT-I T cells (CD8) in the presence of the ova peptide for 72hrs. (A) Mean frequency of α4β7+ CD8 T cells following co-culture with either uninfected cDCs or Ad5-infected cDCs isolated from either wild-type C57BL/6 mice or MyD88−/− or TRIF −/− mice. (B) Mean frequency of α4β7+ CD8 T cells following co-culture with either uninfected cDCs or Ad5-infected cDCs isolated from either wild-type C57BL/6 mice or TLR4−/− mice. (C) Mean frequency of α4β7+ CD8 T cells following co-culture with either uninfected cDCs or Ad5-infected cDCs isolated from either wild-type C57BL/6 mice or Zbp1−/− mice. (D) Mean frequency of α4β7+ CD8 T cells following co-culture with Ad5-infected cDCs from wild-type C57BL/6 mice in the presence or absence of caspase-1 inhibitor YVAD-cmk. (E) Mean frequency ofα4β7+ CD8 T cells following co-culture with Ad5-infected cDCs from wild-type C57BL/6 mice in the presence or absence of ERK inhibitor (UO126), p38 MAPK inhibitor (SB203580) or JNK inhibitor (SP600125). All inhibitors were included only during the 8 hrs Ad5 infection and were excluded from the 72 hrs of co-culture. Error bars indicate SEM. Data from one of the three (for Fig.4A) or one of two (for Fig. 4B–E) independent experiments is shown. ** P < 0.01; *** P < 0.001. P values indicate significantly higher responses compared to the respective uninfected DC controls.
Only TLR4 is known to activate both the TIRAP/MyD88 and the TRAM/TRIF signaling pathways 22,23, unlike all other known TLRs that use either MyD88 or TRIF as a downstream adaptor molecule 24,25. To rule out any role of TLR4 signaling in RA metabolism following Ad5 infection, we used cDC from TLR4−/− mice. Ad5-infected splenic cDCs from TLR4−/− mice were as efficient as cDCs from wild-type B/6 mice in inducing α4β7 expression on CD8 T cells (Fig. 4B), thus ruling out a role for TLR4.
Ad5 is a non-enveloped double stranded DNA virus and viral DNA recognition is known to trigger host innate immune responses. Intracellularly, viral DNA can be recognized either by endosomal TLR9 or by a cytosolic DNA sensor called the DAI (previously known as ZBP1) 26. To investigate the role of Ad5 DNA recognition by splenic cDC for induction of gut homing potential on CD8 T cells, we used cDCs from Zbp1−/− mice in our in vitro assay. Ad5-infected cDCs from Zbp1−/− mice could prime α4β7+ CD8 T cells with similar efficiency as those from wild-type B/6 mice (Fig. 4C). Similar results were also obtained when cDCs from TLR9−/− mice were used (data not shown). These results demonstrate that Ad5-mediated modulation of cDC function to imprint gut homing potential on CD8 T cells is independent of TLR and DAI signaling.
Also, the inclusion of YVAD-cmk (N-Ac-Tyr-Val-Ala-Asp-chloromethyl ketone, a caspase-1 inhibitor) during Ad5 infection did not attenuate the ability of cDCs to imprint gut homing potential on OT-I T cells (Fig. 4D). This may rule out any possible involvement of caspase-1 inflammasomes (particularly AIM2 – absent in melanoma 2, a cytoplasmic inflammasome for dsDNA) or pro-inflammatory IL-1β 27,28. However, further studies in ASC (apoptosis-related speck-like protein; an adaptor molecule that links many upstream NOD-like receptors, NLRs) knockout mice can conclusively delineate the role of inflammasomes in Ad5-mediated effect on cDCs with respect to RA metabolism.
DC function can be regulated by multiple MAP kinase pathways such as ERK, p38 and JNK, and Ad5 infection is known to activate these kinases 29. To investigate any role of MAPK activation in modulating splenic cDC function, we treated Ad5-infected cDCs with specific MAPK inhibitors UO126 (for MEK1/2), SB203580 (for p38 MAPK) and SP600125 (for JNK). Interestingly, inclusion of MAPK inhibitors during Ad5 infection did not affect the ability of cDC to imprint gut homing specificity on OT-I T cells (Fig. 4E), thus demonstrating the lack of involvement of these MAPKs.
NF-κB activation in cDCs is crucial for the priming of gut homing CD8 T cells following Ad5 infection
Several studies have shown that DC activation and maturation is closely associated with NF-κB induction 30,31. Ad5 infection (both in mice and humans) is known to trigger rapid production of pro-inflammatory cytokines such as TNF-α that in turn is a potent activator of NF-κB 32,33. We next sought to determine if intracellular activation of NF-κB had any role in modulating Ad5-infected cDC function to prime gut homing CD8 T cells. Inclusion of a potent NF-κB inhibitor NAC ( N-acetyl-L-cysteine) 34 during the Ad5 infection of cDCs (but not in co-culture) significantly reduced the percent of α4β7 positive OT-I cells (Fig. 5A, left panel) suggesting that Ad5-mediated enhancement of cDC function to imprint gut homing potential is dependent on activation of the NF-κB. Viable OT-I T cell number at the end of co-culture suggested that the NAC-mediated reduction in α4β7 expression was not an erroneous observation because of excessive cell death (Fig. 5A, right panel). To further confirm the role of NF-κB, we used cDCs from Nfkb1−/− mice in our in vitro assay. Consistent with the results obtained with the NF-κB inhibitor, Ad5 infection failed to enhance priming of α4β7+ CD8 T cells by cDCs from Nfkb1−/− mice (Fig. 5B, left panel). Interestingly, in the absence of Ad5, we noted a marked decrease in proliferation of OT-I T cells in cultures with cDC from Nfkb1−/− mice (Fig. 5B, right panel). However, Ad5 infection restored this defect suggesting that the failure to up regulate α4β7 by CD8 T cells in Nfkb1−/− cDC co-cultures was not due to diminished ability of these DC to get activated and present antigen.
Figure 5. NF-κB activation in conventional DCs (cDCs) is crucial for the priming of gut homing CD8 T cells following Ad5 infection.
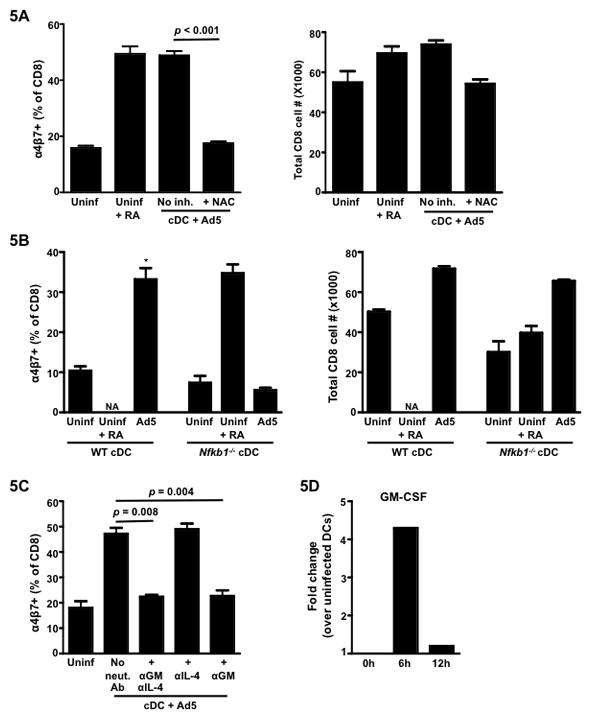
Splenic cDCs from either wild-type C57BL/6 mice or Nfkb1−/− mice were infected with Ad5 (MOI 0.15) for 8hrs, after which, infected cells were washed and co-cultured with OT-I T cells (CD8) in the presence of the ova peptide for 72hrs. (A) Mean frequency of α4β7+ CD8 T cells following 72-hrs of co-culture with Ad5- infected cDCs (from wild-type C57BL/6 mice) in the presence or absence of NF-κB inhibitor, NAC (left panel). Right panel, total live (Via-Probe negative) CD8 T cell number at the end of the co-culture. The inhibitor was included only during the 8 hrs of Ad5 infection and was excluded from the co-culture. (B) Mean frequency of α4β7+ cells (gated on total live CD8 lymphocytes) following 72-hrs co-culture is shown on the left and total live (Via-Probe negative) CD8 T cell number at the end of the co-culture is shown on the right. (C) Mean frequency of α4β7+ CD8 T cells following co-culture with Ad5-infected cDCs from wild-type C57BL/6 mice in the presence or absence of neutralizing Ab to GM-CSF (MP1-22E9) or IL-4 (11B11) or both. Neutralizing Abs were included in the 72 hrs of co-culture. (D) Quantitative RT-PCR showing the fold change in the expression of mRNA encoding GM-CSF (Csf2) in Ad5-infected cDCs over uninfected cDCs at 0, 6 and 12 hrs following in vitro infection. Error bars indicate SEM. Data from one of the two (for Fig. 5A–C) or one of three (for Fig.5D) independent experiments is shown. * P < 0.05. P values indicate significantly higher responses compared to the respective uninfected DC controls. NA, Not available.
GM-CSF, in synergism with IL-4, has been shown to induce RA producing capacity in bone-marrow derived and splenic cDCs in mice 35. NF-κB can also regulate GM-CSF expression 36. We thus further investigated the role of GM-CSF in Ad5-mediated up regulation of α4β7 on CD8 T cells. Interestingly in co-cultures with Ad5-infected cDCs and OT-I T cells, neutralization of GM-CSF resulted in reduction of α4β7 expression on OT-I T cells to a level that was comparable to uninfected cDC (Fig. 5C). Neutralization of IL-4 did not diminish Ad5-mediated effect, either alone or in combination with GM-CSF. In addition, the levels of GM-CSF message increased by 4.3-fold in the infected splenic cDCs over uninfected controls at 6 hours following infection (Fig. 5D). Our data thus suggests that Ad5-mediated effect can be either directly through up regulation of RALDH enzymes in cDCs or indirectly through GM-CSF or maybe both synergistically involving NF-κB.
DISCUSSION
Tissue-specific lymphocyte trafficking is regulated by the expression of different homing receptors on these cells 11 and the tissue resident DCs have been shown to imprint this property on them 37. The gut resident DC possess the property of metabolizing vitamin A to RA that in turn regulates expression of the gut homing receptor α4β7 on T and B cells 3. Our results demonstrate that the live replication deficient vector Ad5 can induce expression of vitamin A metabolizing enzymes in peripheral draining lymph node DCs in vivo and also in splenic DCs in vitro, and that correlates with the ability of this virus to prime gut homing CD8 T cells. These results provide an innate mechanism by which Ad5 modulates draining lymph node and splenic DC function to prime gut homing T cells. To our knowledge, this is the first study that shows induction of vitamin A metabolizing enzymes in non-gut associated DC by a replication-deficient viral vector. These results have important implications for developing mucosal vaccines.
Our results demonstrate that NF-κB activation in cDC is essential for Ad5-mediated induction of their ability to prime gut homing CD8 T cells. Ad5 is a potent inducer of NF-κB38 and studies have demonstrated the presence of NF-κB binding sites both in the murine Aldh1a2 39 as well as in the GM-CSF (Csf2) 36 promoter region, thus suggesting that NF-κB is a transcription activator for both genes. Our data with neutralizing Ab to GM-CSF also showed that this cytokine can modulate Ad5-infected splenic cDC’s ability to induce expression of α4β7 on CD8 T cells. As reported earlier 35, GM-CSF made by lamina propria macrophages or granulocytes is known to positively regulate Aldh1a2 expression in gut DCs. The same study also showed that RA in-turn can up regulate the Csf2 expression in mesenteric lymph node resident non-DCs. As evident from the qRT-PCR data in our study, Ad5 infection can induce both RALDH enzymes as well as Csf2 in the splenic cDCs by as early as 6–12 hrs following infection. Thus in the context of Ad5, as is true for the gut microenvironment, we can speculate that RA and GM-CSF have a synergistic effect on modulating cDC function with respect to priming of gut homing CD8 T cells. Ad5 immunization studies in GM-CSF knockout mice (Beta-c−/−) should further validate the role of GM-CSF in Ad5-mediated induction of gut homing of CD8 T cells.
Though Ad5 is known to induce NF-κB 38, yet, the mechanism/s leading to NF-κB activation are yet to be defined. NF-κB can be induced by many PRRs including TLRs, retinoic acid-inducible gene I (RIG-I) and DAI. Of these, being a double stranded DNA enveloped virus, it is likely that Ad5 could target TLRs and DAI. However, our results demonstrated that signaling through TLRs and DAI in splenic cDC is not necessary for the Ad5-mediated induction of their ability to prime gut homing CD8 T cells. It is interesting to note that the cDCs from these knockout mice were poor at stimulating OT-I T cells in comparison to cDCs from wild-type B/6 mice and Ad5 restored this defect (Supplementary Fig 1), suggesting that Ad5 can activate cDC in a TLR and DAI independent manner. A similar situation was also observed with cDC from Nfkb1−/− mice demonstrating that the failure of these DCs to prime α4β7 positive OT-I T cells was not due to lack of DC activation (Fig. 5B).
Vitamin A can be metabolized by any one of the three isoforms of the RALDH enzyme. Our results showed that Ad5 induces expression of primarily Aldh1a2 and Aldh1a3 genes, and it is not clear whether both these isoforms are needed for the Ad5-mediated induction of gut homing CD8 T cells by splenic DC. The TLR2 ligand, zymosan, is also known to induce Aldh1a220, however, Ad5-mediated induction of RALDH enzymes was not dependant on TLR signaling. These results suggest that Ad5 is working through a pathway that is different from previously reported TLR dependant induction of RALDH enzymes in splenic cDC.
Since both DCs as well as T cells are known to have retinoic acid receptors, we investigated whether the endogenously made RA by the Ad5-infected peripheral cDCs is working in an autocrine manner (thus further conditioning these DCs somehow to prime CD8 T cells to up regulate α4β7) or in a paracrine manner on the CD8 T cells. For example, RA made by zymosan stimulated DC has been shown to act on the same DC in an autocrine manner 20. However in our study, the inclusion of RAR inhibitor only during the 8 hours Ad5 infection period did not abrogate the α4β7 expression on CD8 T cells suggesting that RA made by the Ad5-stimulated DC is working mostly in a paracrine manner.
Our results demonstrate that the cytosolic DNA sensor DAI is not required for Ad5-mediated priming of gut homing CD8 T cells by splenic DC suggesting that Ad5 DNA is not required for this function. It is important to note here that the role of DAI as a cytosolic sensor for viral DNA can be redundant as other studies in murine model have shown that DAI-deficient mice exhibit uncompromised immune responses following infection with DNA viruses 40,41. A recent study using a human cell line has however identified the extra-chromosomal histone H2B as biologically crucial for innate immune response to DNA viruses 42. Whether histone H2B plays any role as a cytosolic DNA sensor in the murine model or not needs to be further investigated.
At present it is unclear which part of Ad5 (either protein or nucleic acid) is responsible for it’s ability to induce vitamin A metabolizing enzymes in cDC. We speculate that this could be one of the Ad5 proteins as other studies have shown that even empty capsids derived from Ad5 can induce strong inflammatory responses 43,44. Identification of this key adenoviral protein/s that can endow peripheral cDC with the ability to imprint gut homing potential on antigen-specific CD8 T cells can help in development of novel mucosal adjuvants for subunit vaccines administered via the parenteral route of immunization.
METHODS
Mice and Immunization
For immunization studies, female BALB/c mice of 6–8 weeks of age were purchased from Charles River Laboratories (Willmington, MA). Mice were immunized with 1×10^6 pfu of recombinant Ad5 expressing HIV-1 clade B Env and Gag (Ad5/Env-Gag) or non-recombinant Ad5 in sterile PBS in a final volume of 100μl with 50μl injected intramuscularly in each of the hind legs at Week 0 (prime) and Week 4 (boost). For in vitro co-culture assays, C57BL/6, OT-I Tg and Nfkb1−/− mice were purchased from Jackson Laboratories (Bar Harbor, Maine). The Zbp1−/− mice were provided by Dr. S. Akira (Osaka University, Japan). The MyD88−/−, TRIF −/− and TLR4−/− mice were bred onsite. All knockout mice were on a C57BL/6 genetic background. Mice were housed in specific pathogen-free conditions in the Emory Vaccine Center vivarium and were cared for under guidelines established by the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals” using protocols approved by the Emory University Institutional Animal Care and Use Committee (IACUC).
Isolation of LPLs
LPLs from small intestines were isolated as described previously 45. Briefly, small intestines were removed and Peyer’s patches were excised. The intestine was opened up longitudinally and cleaned of all fecal contents. Intestines were cut into small pieces and were transferred into 50ml conical tubes and shaken at 250 rpm for 20 mins. at 37°C in HBSS media (Life Technologies, Carlsbad, CA) supplemented with 5% FBS (Cellgro, Manassas, VA) and 2mM EDTA (Promega, Madison, WI). After a total of two rounds of EDTA treatment, cell suspensions were passed through a strainer and the remaining intestinal tissue was washed and minced, transferred into 50ml conical tubes and shaken for 20 mins. at 37°C in HBSS media supplemented with 5% FBS and type VIII collagenase (1.5mg/ml; Sigma, St. Louis, MO). Cell suspensions were collected and passed through a strainer and were pelleted by centrifugation at 1500 rpm. Mononuclear cells were then isolated by use of a discontinuous density gradient procedure (45% and 70%) with Percoll (GE Heathcare Bio-Sciences, Uppsala, Sweden).
Tetramer and intracellular cytokine staining
Tetramer and intracellular cytokine staining was performed as described before 46. For tetramer analysis, cells were stained using allophycocyanin (APC)-conjugated MHC class I (H-2Kd) peptide (AMQMLKETI) tetramer (NIH tetramer core facility), CD4-FITC (clone L3T4), CD19-FITC (clone 1D3), α4β7-PE (clone DATK32), CCR9 (clone 242503, R&D Systems) and CD8-PerCP (clone 53-6.7). CD8+ CD4− CD19- and tetramer+ cells were scored as tetramer positive cells. All antibodies were purchased from BD Pharmingen (San Diego, CA).
Analysis of ALDH activity
Aldehyde dehydrogenase (ALDH) activity at a single cell level was quantified using purified CD11c+ cells and ALDEFLUOR staining kit (StemCell Technologies, Vancouver, Canada), according to the manufacturer’s protocol with modifications. Briefly, cells (1X106/ml) were suspended in ALDEFLUOR assay buffer containing the activated ALDEFLUOR substrate, with or without the ALDH inhibitor DEAB, and incubated for 45 mins. at 37°C. ALDEFLUOR reacted cells were scored in FITC channel of BD FACSCalibur. Cell viability was determined using the Via-Probe dye (BD Pharmingen).
DC:OT-I co-culture
CD11c+ DCs from collagenase type IV (1mg/ml; Worthington Biochemical, Lakewood, NJ) digested spleens were isolated using anti-CD11c-coated magnetic beads (N418 clone, Miltenyi Biotec, Germany). The resulting purity of cDCs was approximately 95%. OT-I T lymphocytes were purified from OT-I spleens using CD8 microbeads (Ly-2 clone, Miltenyi Biotec). The purity of CD8 T cells was > 95%. cDCs and OT-I T cells were co-cultured at a ratio of 1:2 for 72 hrs. To the co-culture, the OVA257–264 SIINFEKL peptide was added (20pM). Where indicated, Retinoic Acid (10nM; Fluka, St. Louis, MO), LE540 (1μM; Wako, Japan), DEAB (10μM; Fluka), UO126 (10μM; LC Laboratories, Woburn, MA), SB203580 (20μM; LC Laboratories), SP600125 (100μM; LC Laboratories), N-Acetyl-L cysteine (30mM; Sigma), Ac-YVAD-CMK (50μM; Bachem, King of Prussia, PA) and neutralizing Ab to murine IL-4 (clone 11B11; 10μg/ml, eBioscience) and GM-CSF (clone MP1-22E9; 10μg/ml, eBioscience) was included during either DC infection period or the co-culture. At the end of co-culture, cell surface expression of α4β7 or CCR9 on total CD8 T cells were analyzed by flow cytometry. The Via-Probe dye (BD Pharmingen) was included in the staining panel to eliminate dead cells.
Adenoviral vectors
The E1/E3 deleted Adenovirus type 5 without a transgene (Ad5), or encoding either the HIV-1 clade B Env and Gag (Ad5/Env-Gag) or a green fluorescent protein (Ad5-GFP) were used. The viral vectors were generated by standard methods as described previously 47. Heat inactivation of the virus was achieved by incubation at 56°C for 1 hour. UV inactivation was achieved by exposing the virus to a 365-nm long UV lamp for 1hr.
Gene expression analysis
The relative quantitative real-time PCR (qRT-PCR) was performed for mRNA expression analyses as described previously 48. Total four individual gene expression assays were used from Applied Biosystems (Foster City, CA) for Mus. musculus: aldehyde dehydrogenase family 1, subfamily A1 (Aldh1a1); aldehyde dehydrogenase family 1, subfamily A2 (Aldh1a2); aldehyde dehydrogenase family 1, subfamily A3 (Aldh1a3); mouse GAPD (GAPDH) Endogenous Control (VIC/MGB Probe). Samples were tested in duplicate. Fold changes were calculated using the comparative CT method 49 using the equation 2−ΔΔCT where ΔΔCT = [(CT gene of interest – CT gene of control) sample A – (CT gene of interest – CT gene of control) sample B].
Statistics
Student’s t-test was used to compare the differences between groups. The Bonferroni method was used to adjust the P values for multiple comparisons. Statistical analyses were performed using TIBCO Spotfire S+ 8.1. A two-sided P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We are grateful to Dr. Shizuo Akira for kindly providing us with Zbp1−/− mice. We thank the veterinary staff at the Yerkes Division of Research Resources for their support and H. Drake-Perrow for administrative support. We also thank the Emory CFAR virology core for qPCR measurements. This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grants R01 AI057029 and R01 AI071852 to RRA; Yerkes National Primate Research Center base grant, P51 RR00165; Emory CFAR grant P30 AI050409.
Footnotes
Competing Interests Statement
The authors have no conflicting financial interests.
Disclosure
The authors have no conflicting financial interests.
References
- 1.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 2.Belyakov IM, Berzofsky JA. Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity. 2004;20:247–253. doi: 10.1016/s1074-7613(04)00053-6. [DOI] [PubMed] [Google Scholar]
- 3.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 5.Guilliams M, et al. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 6.Lin SW, Cun AS, Harris-McCoy K, Ertl HC. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine. 2007;25:2187–2193. doi: 10.1016/j.vaccine.2006.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tatsis N, et al. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology. 2007;367:156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman DR, et al. Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination. J Immunol. 2008;181:4188–4198. doi: 10.4049/jimmunol.181.6.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchbinder SP, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 12.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 13.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Svensson M, et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell WC, Valentine RC, Pereira HG. The effect of heat on the anatomy of the adenovirus. J Gen Virol. 1967;1:509–522. doi: 10.1099/0022-1317-1-4-509. [DOI] [PubMed] [Google Scholar]
- 16.Cheng C, et al. Mechanism of ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 2007;3:e25. doi: 10.1371/journal.ppat.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 18.Nemerow GR. Cell receptors involved in adenovirus entry. Virology. 2000;274:1–4. doi: 10.1006/viro.2000.0468. [DOI] [PubMed] [Google Scholar]
- 19.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 20.Manicassamy S, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman ZC, et al. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto M, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 23.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev. 2004;199:227–250. doi: 10.1111/j.0105-2896.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 26.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 27.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamanini A, et al. Interaction of adenovirus type 5 fiber with the coxsackievirus and adenovirus receptor activates inflammatory response in human respiratory cells. J Virol. 2006;80:11241–11254. doi: 10.1128/JVI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neumann M, et al. Differential expression of Rel/NF-kappaB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood. 2000;95:277–285. [PubMed] [Google Scholar]
- 31.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginsberg HS, et al. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991;88:1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic Res Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- 35.Yokota A, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreck R, Baeuerle PA. NF-kappa B as inducible transcriptional activator of the granulocyte-macrophage colony-stimulating factor gene. Mol Cell Biol. 1990;10:1281–1286. doi: 10.1128/mcb.10.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villablanca EJ, Russo V, Mora JR. Dendritic cell migration and lymphocyte homing imprinting. Histol Histopathol. 2008;23:897–910. doi: 10.14670/HH-23.897. [DOI] [PubMed] [Google Scholar]
- 38.Borgland SL, Bowen GP, Wong NC, Libermann TA, Muruve DA. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-kappaB. J Virol. 2000;74:3941–3947. doi: 10.1128/jvi.74.9.3941-3947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Sperkova Z, Napoli JL. Analysis of mouse retinal dehydrogenase type 2 promoter and expression. Genomics. 2001;74:245–250. doi: 10.1006/geno.2001.6546. [DOI] [PubMed] [Google Scholar]
- 40.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, et al. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobiyama K, et al. Extrachromosomal histone H2B mediates innate antiviral immune responses induced by intracellular double-stranded DNA. J Virol. 2010;84:822–832. doi: 10.1128/JVI.01339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kafri T, et al. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc Natl Acad Sci U S A. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molinier-Frenkel V, et al. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J Virol. 2000;74:7678–7682. doi: 10.1128/jvi.74.16.7678-7682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 46.Ganguly S, Liu J, Pillai VB, Mittler RS, Amara RR. Adjuvantive effects of anti-4-1BB agonist Ab and 4-1BBL DNA for a HIV-1 Gag DNA vaccine: different effects on cellular and humoral immunity. Vaccine. 28:1300–1309. doi: 10.1016/j.vaccine.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawakami Y, et al. Substitution of the adenovirus serotype 5 knob with a serotype 3 knob enhances multiple steps in virus replication. Cancer Res. 2003;63:1262–1269. [PubMed] [Google Scholar]
- 48.Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 49.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


