Abstract
To investigate the dependency of T1 relaxation on mechanical strain in articular cartilage, quantitative MRI T1 imaging experiments were carried out on cartilage before/after the tissue was immersed in gadolinium contrast agent and when the tissue was being compressed (up to ~ 48% strains). The spatial resolution across the cartilage depth was 17.6μm. The T1 profile in native tissue (without the presence of gadolinium ions) was strongly strain-dependent, which is also depth-dependent. At the modest strains (e.g., 14% strain), T1 reduced by up to 68% in the most surface portion of the tissue. Further compression (e.g., 45% strain) reduced T1 mostly in the middle and deep portions of the tissue. For the gadolinium-immersed tissue, both modest and heavy compressions (up to 48% strain) increased T1 slightly but significantly, although the overall shapes of the T1 profiles remained approximately the same regardless of the amount of strains. The complex relationships between the T1 profiles and the mechanical strains were a direct consequence of the depth-dependent proteoglycan concentration in the tissue, which determined the tissue’s mechanical properties. This finding has potential implications in the use of gadolinium contrast agent in clinical MRI of cartilage (the dGEMRIC procedure), when the loading or loading history of patients is considered.
Keywords: MRI, articular cartilage, T1 relaxation, strain, dGEMRIC, gadolinium contrast agent
INTRODUCTION
Magnetic resonance imaging (MRI) (1–3) has been used extensively to study the degradation of articular cartilage, which is a hallmark of osteoarthritis and related joint diseases that affect an increasingly large portion of the human population. The difficulties in a reliable detection of early tissue degradation using imaging are due to several factors. Firstly, articular cartilage is a thin layer of tissue (typically 0.5–2mm) with a number of complex depth-dependent properties, in particular a depth-dependent orientation of collagen fibrils (4,5) and a depth-dependent glycosaminoglycan (GAG) concentration (6,7). Secondly, the degradation of cartilage at the early stages is marked by a set of intertwined changes in its molecular, chemical and enzymatic concentrations and activities; hence any early maker must be sensitive to these molecular level changes (8). Third, the early degradations tend to be focal and small, which requires a high resolution in imaging (9).
Among the MRI parameters that are sensitive to the tissue degradation, T2 relaxation is commonly used in basic research and clinical diagnostics because of its sensitivity to the collagen orientation and water content (5,10,11) (12). In contrast, T1 relaxation in the native tissue has been found mostly homogeneous over the tissue depth and isotropic to the tissue orientation in the magnet (5,13). The clinical potential of T1 in MRI of cartilage was noticed largely due to a MRI procedure known as the dGEMRIC method (delayed Gadolinium Enhanced Magnetic Resonance Imaging of Cartilage) (14,15), which dopes the tissue/patient with paramagnetic ions, gadolinium (Gd). Based on the assumption that charged mobile ions distribute in cartilage in an inverse relation to the concentration of the negatively charged glycosaminoglycan (GAG) molecules, the acquisition of two T1 images, before and after a patient is injected with a charged MRI contrast agent, Gd(DTPA)2-, enables the construction of a GAG image in cartilage. Any local deficiency of GAG can be interpreted as a cartilage lesion (2).
Since articular cartilage is a load-bearing tissue that is being compressed constantly daily, MRI experiments of a compressed cartilage have been carried out at both microscopic resolutions and clinical settings (16–27). This study concerns any strain-dependency in the T1 profiles in the absence and presence of Gd(DTPA)2- in (canine) articular cartilage. A μMRI procedure was used to image the ex vivo tissue blocks at microscopic resolution (17.6 μm pixel resolution). We hypothesized that the T1 profiles in articular cartilage would be depth-dependently sensitive to mechanical strains, which could affect any quantitative determination of GAG concentrations in cartilage.
MATERIALS AND METHODS
Cartilage Preparation
Canine humeral heads were harvested shortly after the sacrifice of two mature (1–2 year old) and musculoskeletally healthy dogs that were used for an unrelated biomedical research that has been ongoing for more than the last ten years. The handling of the animal subjects was approved by the Institutional Review Boards. Two adjacent cartilage-bone blocks (about 1.75 × 2 × 6 mm) were harvested from the central part of one humeral head. A home built compression device was used to compress the tissue inside the rf coil (22). One block was imaged three times under the 0°, 14%, and 45% static compressions, respectively; the other tissue block was also imaged three times (0%, 13%, 48% strains) after it was immersed for 10 hours in 1mM solution of a commercially available Gd(DTPA)2- contrast agent (Magnevist, Berlex, NJ) at the room temperature (about 25 °C). (The experiments were repeated with two more cartilage blocks from a different dog; the results were nearly identical.)
MRI Methods
MRI experiments were conducted at room temperature on a Bruker AVANCE II 300 NMR spectrometer equipped with a 7-Tesla/89-mm vertical-bore superconducting magnet and micro-imaging accessory (Bruker Instrument, Billerica, MA). A homemade 5 mm solenoid coil was used for imaging, where the orientation of the cartilage block with respect to B0 was set at 55° (the magic angle). The echo time (TE) of the imaging sequence was 8.6ms; and the repetition time (TR) of the imaging experiment was 2s and 0.8s for the before- and after-immersion experiments respectively. The 0.8mm-thick imaging slice was transversely located in the middle of the 10mm-long specimen. The 2D in-plane pixel size was 17.6 μm. The measurement of 2D T1 images used the inversion-recovery pulse sequence with 5 inversion points (for the T1before, they were 0, 0.4, 1.1, 2.2, 4.0s; for the T1Gd, they were 0, 0.1, 0.3, 0.5, 1s), which allowed the calculation of T1 relaxation in the tissue through a single exponential equation on a pixel-by-pixel basis. Other experimental details have been documented in literature (7).
Statistical Analysis
Imaging data groups were evaluated for any significant difference using commercial software KaleidaGraph (v4.0, Synergy Software, Reading, PA) in two sets of statistical analysis. First, each set of data was compared with the reference set using the nonparametric Wilcoxon–Mann–Whitney test. Second, several sets of data were analyzed together using the Kruskal–Wallis test. In both Wilcoxon–Mann–Whitney and Kruskal–Wallis tests, if the p-value is below 0.05, the conclusion is that there is a difference between/among the data.
RESULTS
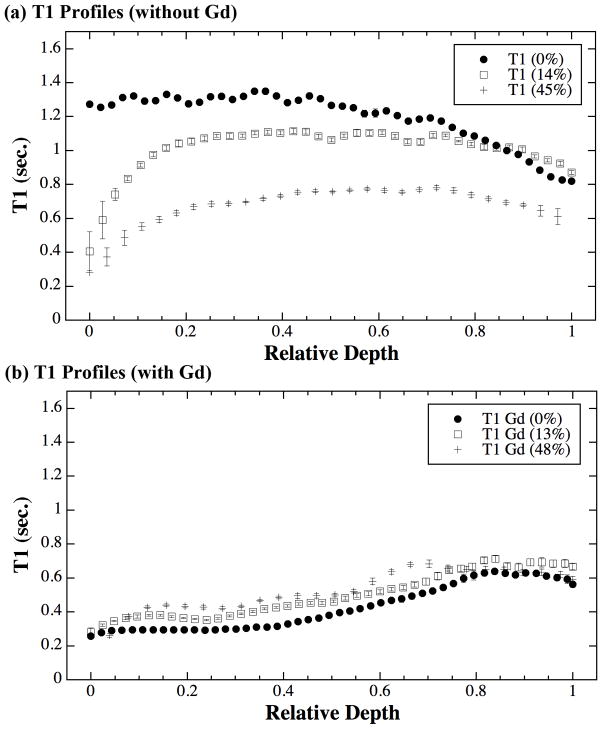
Fig 1 shows the T1 images for both specimens, without (Fig 1a) and in the presence of (Fig 1b) gadolinium immersion. Fig 2 shows the central depth-dependent profiles of all T1 images, plotted on the relative depth scale (0 = articular surface, 1 = cartilage-bone interface). Several features are clearly visible (Fig 2a) in the T1 profiles without the presence of gadolinium ions (T1before profiles). Firstly, the T1 images and T1before profile in the uncompressed tissue are highly consistent with several previous reports from our lab that uses this group of nearly identical dogs for over the last 10 years (13). This result demonstrates that the T1before profile without gadolinium is relatively uniform across its tissue depth. Second, once the tissue is compressed, the T1 values of the tissue reduce significantly, both as the function of tissue depth and the function of mechanical strain. Third, the initial part of the cartilage (the superficial zone) has the strongest reduction in T1 under compression – Even at a modest 14% overall compression, the T1 in the initial part of the tissue can reduce by up to 68%. Finally, when the tissue block was further compressed to about 45%, most of the additional reductions in T1 came from the middle and deep parts of the tissue – the surface T1 remained approximately at the values when it was under the 14% strain.
Fig 1.
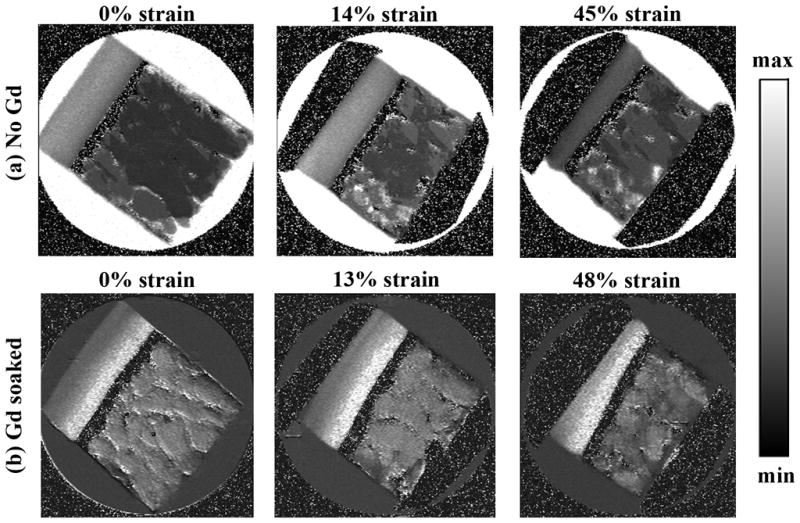
The T1 maps of articular cartilage, without the presence of Gd (a) and in the presence of Gd (b). The image display limits are 0 – 2s for (a) and 0 – 1s for (b).
Fig 2.
The T1 profiles of articular cartilage, plotted as the relative tissue depth (0 = articular surface, 1 = cartilage-bone interface).
With the immersion of gadolinium ions, the relationship of the T1 changes as the function of strain (Fig 2b). Firstly, the uncompressed T1Gd profile is consistent with several previous reports (7,13), which shows T1 increases from the articular surface to the cartilage-bone interface (the deep tissue), reflecting the GAG concentration profiles in cartilage. Secondly, when the tissue was compressed at both 13% and 48%, the T1 profiles remained approximately the same shapes; however, its values increased slightly but significantly when compared with the uncompressed T1.
Since the resolution of most MRI experiments cannot reach tens of microns, the trends of T1 as the functions of both tissue depth and mechanical strain were averaged over its sub-tissue histological zones on a relative depth scale, shown in Table 1. The division of the histological zones adopted a set of criteria that was developed and validated by both μMRI and polarized light microscopy on a group of nearly identical cartilage (28), which yielded the percentage thicknesses of the three zones in humeral cartilage to be approximately 8% for the superficial zone, 16% for the transitional zone, and 76% for the radial zone.
Table 1.
The averaged T1 (RMS ± SD in sec.) without and with Gd as the function of strains (%)
| Averaging Region | T1 without Gd (sec.) | T1 with Gd (sec.) | ||||
|---|---|---|---|---|---|---|
| 0 % | 14% | 45% | 0% | 13% | 48% | |
| Full thickness | 1.20±0.15 | 1.02±0.15 | 0.67±0.15 | 0.45±0.131 | 0.52±0.131 | 0.54±0.111 |
| SZ (0–0.08) | 1.28±0.02 | 0.60±0.17 | 0.33±0.06 | 0.28±0.022 | 0.32±0.032 | 0.32±0.082 |
| TZ (0.08–0.24) | 1.30±0.02 | 0.94±0.08 | 0.55±0.05 | 0.29±0.01 | 0.37±0.01 | 0.42±0.03 |
| RZ (0.24–1) | 1.17±0.16 | 1.06±0.06 | 0.72±0.05 | 0.48±0.13 | 0.56±0.12 | 0.58±0.09 |
Note 1: Three sets of the original T1 data were statistically different (see the text for discussion).
Note 2: Three sets of the original T1 data in SZ were statistically identical (see the text for discussion).
Three sets of statistical analysis were carried out. In the first analysis, the Wilcoxon–Mann–Whitney test was performed to compare the difference between the T1s averaged over the full thickness under three different strains (the first row data in Table 1). The p-values for the two separate analyses were both less than 0.0001, demonstrating their significant differences. The two other statistical analyses concern the zonal averaged T1 data, which is also plotted in Fig 3. In one zonal analysis (the Wilcoxon–Mann–Whitney test), which concerns the difference between the T1 in the native SZ at 45% strain and the T1 in the Gd-immersed SZ, the p-values showed that there was no significant difference between the T1 in any Gd-immersed SZ and the native SZ at 45% strain. In the second zonal analysis (the Kruskal–Wallis test), the p-value was 0.305 for the three sets of T1 values in the Gd-immersed SZ, demonstrating that there was no statistical difference in SZ-averaged T1Gd, no matter what were the mechanical strains (from 0% to 48%).
Fig 3.
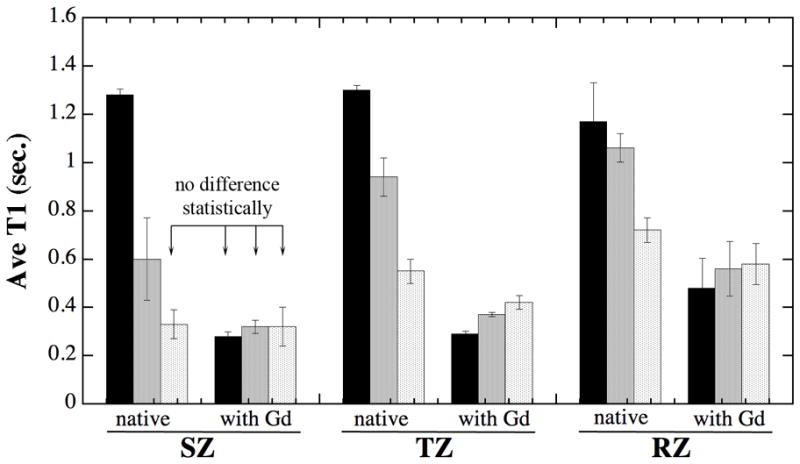
The trends of T1 changes under strains in all sub-tissue zones, based on the data in Table 1. The black columns are no loading; the grey columns are modest loading (13–14%), and the light columns are heavy loading (45–48%). The divisions of three tissue zones are based on the high resolution imaging data shown in Table 1.
DISCUSSION
Articular cartilage is known to have a depth-dependent GAG concentration (6,7), which governs its depth-dependent mechanical properties (29). The strain-dependent T1 profiles found in this report is due precisely to this depth-dependent mechanical property in articular cartilage.
For the native tissue (without gadolinium), a modest compression (e.g., 14% in Fig 2a) will inevitably cause the softest part of cartilage (i.e., the surface tissue) to be compressed the most, while most of the deep tissue remains largely uncompressed (29). Consequently, water in the surface portion of the tissue will be lost the most, resulting in a significantly reduced T1 in the surface portion of the tissue. When the tissue is further compressed (e.g., 45% in Fig 2a), its surface, which is already being compressed the most and hence becomes much harder due to an increased charge density from its more compacted GAG molecules, will resist any further compression. Consequently, any further compression will occur at the deeper part of the tissue. The three T1 profiles in the native tissue shown in Fig 2a reflect precisely these complex sequences of tissue’s adaptation to the external loading. Since T1 relaxation in native tissue is known to be isotropic to the tissue orientation (5,13), which implies that T1 values in cartilage are independent of the orientation of the collagen fibrils in the magnetic field. Consequently, the T1 changes in Fig 2a are not caused by the orientational changes of the collagen fibrils in compressed tissue, but by the reduction of the mobile water and the increase of the solid components in a compressed cartilage.
For the Gd-immersed tissue, its T1 values under no compression are inversely proportional to the amount of gadolinium ions in cartilage (7). When a Gd-immersed tissue is compressed, the tissue will also lose water – and together with the gadolinium ions – presumably in the same manner as in the native tissue. There must still be a sufficient quantity of gadolinium ions remaining in the compressed tissue, which ensures the T1 profile in the tissue to have essentially the same character as the fully-doped and fully-relaxed tissue (i.e., at 0% strain). However, there must be a loss of some gadolinium ions from the tissue, which causes a small but significant increase of the T1 in the compressed and Gd-immersed tissue.
In the dGEMRIC procedure, the full equation that calculates the GAG concentration in cartilage contains an expression (1/T1Gd – 1/T1before) (30). To save the patient time, it is common in clinics to use the T1Gd as a “dGEMRIC index” (31) without the measurement of T1before. This is a valid approximation as long as the profile of T1before is relatively uniform and 1/T1Gd is much larger than 1/T1before. However, when T1before is reduced significantly, close to the same level of T1Gd (e.g., the SZ tissue in Fig 3), the full expression (1/T1Gd – 1/T1before) must be adapted. This strain- and depth-dependencies of T1 in articular cartilage could become important in clinical MRI, since mechanical loading is an inevitable consequence of human daily activities, and since lesioned cartilage is much softer than healthy cartilage, hence, easier to be compressed. In fact, a recent clinical MRI report has documented the in vivo effects of static compression on T1Gd in healthy human knee, which was compressed to 50% of body weight (32). Although the image resolution was limited in clinical MRI, their observation agrees with the results in this project.
In conclusion, this study demonstrated that the T1 profile in articular cartilage was strongly strain-dependent. This strain dependency of T1 profile also depended upon whether the tissue was immersed in gadolinium ions or not. The implication of this finding in the clinical application of gadolinium contrast agent in MRI of cartilage (the dGEMRIC procedure) was discussed. The zonal averaged T1 in this project can be used as the references in the clinical MRI experiments that have lower image resolution than tens of microns in this project.
Acknowledgments
Yang Xia is grateful to the National Institutes of Health for the R01 grants (AR 045172 and AR 052353) and an instrument endorsement from R.B. and J.N. Bennett. The authors are indebted to Drs. Cliff Les and Hani Sabbah (Henry Ford Hospital, Detroit) for providing the canine specimens, Ms Janelle Spann (Michigan Resonance Imaging, Rochester Hills, Michigan) for providing the contrast agent, and Ms Carol Searight (Dept of Physics, Oakland University) for editorial comments.
Support Grants: NIH R01 Grants (AR045172, AR052353)
References
- 1.Xia Y. Magic Angle Effect in MRI of Articular Cartilage - A Review. Invest Radiol. 2000;35:602–621. doi: 10.1097/00004424-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Gray ML, Burstein D, Xia Y. Biochemical (and Functional) Imaging of Articular Cartilage. Semin Musculoskelet Radiol. 2001;5:329–344. doi: 10.1055/s-2001-19043. [DOI] [PubMed] [Google Scholar]
- 3.Gold GE, Han E, Stainsby J, Wright G, Brittain J, Beaulieu C. Musculoskeletal MRI at 3.0 T: relaxation times and image contrast. Am J Roentgenol. 2004;183:343–351. doi: 10.2214/ajr.183.2.1830343. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Mankin H. Articular cartilage I. Tissue design and chondrocyte matrix interactions. J Bone Joint Surgery. 1997;79A:600–611. [PubMed] [Google Scholar]
- 5.Xia Y. Relaxation Anisotropy in Cartilage by NMR Microscopy (μMRI) at 14 μm Resolution. Magn Reson Med. 1998;39:941–949. doi: 10.1002/mrm.1910390612. [DOI] [PubMed] [Google Scholar]
- 6.Maroudas A, Bayliss MT, Venn M. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis. 1980;39:514–534. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Y, Zheng S, Bidthanapally A. Depth-dependent Profiles of Glycosaminoglycans in Articular Cartilage by μMRI and Histochemistry. J Magn Reson Imaging. 2008;28:151–157. doi: 10.1002/jmri.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein D, Gray ML. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis? Osteoarthritis Cartilage. 2006;14:1087–1090. doi: 10.1016/j.joca.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y. Resolution ‘scaling law’ in MRI of articular cartilage. Osteoarthritis Cartilage. 2007;15:363–365. doi: 10.1016/j.joca.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhadlaq H, Xia Y, Moody JB, Matyas J. Detecting Structural Changes in Early Experimental Osteoarthritis of Tibial Cartilage by Microscopic MRI and Polarized Light Microscopy. Ann Rheum Dis. 2004;63:709–717. doi: 10.1136/ard.2003.011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng S, Xia Y. Multi-components of T2 relaxation in ex vivo cartilage and tendon. J Magn Reson. 2009;198:188–196. doi: 10.1016/j.jmr.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne JS, Kraft KA, Shields KJ, Yin C, Owen JR, Disler DG. MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology. 2003;228:493–499. doi: 10.1148/radiol.2282012012. [DOI] [PubMed] [Google Scholar]
- 13.Zheng S, Xia Y, Bidthanapally A, Badar F, Ilsar I, Duvoisin N. Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging. 2009;27:648–655. doi: 10.1016/j.mri.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML. Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Gray ML, Burstein D, Kim YJ, Maroudas A. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26:281–291. doi: 10.1002/jor.20482. [DOI] [PubMed] [Google Scholar]
- 16.Lüsse S, Knauss R, Werner A, Gründer W, Arnold K. Action of Compression and Cations on the Proton and Deuterium Relaxation in Cartilage. Magn Reson Med. 1995;33:483–489. doi: 10.1002/mrm.1910330405. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein JD, Kim JK, Henkelman RM. Effects of compression and recovery on bovine articular cartilage: appearance on MR images. Radiology. 1996;201:843–850. doi: 10.1148/radiology.201.3.8939241. [DOI] [PubMed] [Google Scholar]
- 18.Herberhold C, Faber S, Stammberger T, Steinlechner M, Putz R, Englmeier K, Reiser M, Eckstein F. In situ measurement of articular cartilage deformation in intact femoropatellar joints under static loading. J Biomech. 1999;32:1287–1295. doi: 10.1016/s0021-9290(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 19.Regatte RR, Kaufman JH, Noyszewski EA, Reddy R. Sodium and proton MR properties of cartilage during compression. J Magn Reson Imaging. 1999;10:961–967. doi: 10.1002/(sici)1522-2586(199912)10:6<961::aid-jmri8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Gründer W, Kanowski M, Wagner M, Werner A. Visualization of pressure distribution within loaded joint cartilage by application of angle-sensitive NMR microscopy. Magn Reson Med. 2000;43:884–891. doi: 10.1002/1522-2594(200006)43:6<884::aid-mrm15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Neu CP, Hull ML. Toward an MRI-based method to measure non-uniform cartilage deformation: an MRI-cyclic loading apparatus system and steady-state cyclic displacement of articular cartilage under compressive loading. J Biomech Eng. 2003;125:180–188. doi: 10.1115/1.1560141. [DOI] [PubMed] [Google Scholar]
- 22.Alhadlaq H, Xia Y. The Structural Adaptations in Compressed Articular Cartilage by Microscopic MRI (μMRI) T2 Anisotropy. Osteoarthritis Cartilage. 2004;12:887–894. doi: 10.1016/j.joca.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Nag D, Liney GP, Gillespie P, Sherman KP. Quantification of T(2) relaxation changes in articular cartilage with in situ mechanical loading of the knee. J Magn Reson Imaging. 2004;19:317–322. doi: 10.1002/jmri.20000. [DOI] [PubMed] [Google Scholar]
- 24.Alhadlaq HA, Xia Y. Modifications of orientational dependence of microscopic magnetic resonance imaging T2 anisotropy in compressed articular cartilage. J Magn Reson Imaging. 2005;22:665–673. doi: 10.1002/jmri.20418. [DOI] [PubMed] [Google Scholar]
- 25.Baldassarri M, Goodwin JS, Farley ML, Bierbaum BE, Goldring SR, Goldring MB, Burstein D, Gray ML. Relationship between cartilage stiffness and dGEMRIC index: Correlation and prediction. J Orthop Res. 2007;25:904–912. doi: 10.1002/jor.20378. [DOI] [PubMed] [Google Scholar]
- 26.de Visser SK, Crawford RW, Pope JM. Structural adaptations in compressed articular cartilage measured by diffusion tensor imaging. Osteoarthritis Cartilage. 2008;16:83–89. doi: 10.1016/j.joca.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Juras V, Bittsansky M, Majdisova Z, Szomolanyi P, Sulzbacher I, Gabler S, Stampfl J, Schuller G, Trattnig S. In vitro determination of biomechanical properties of human articular cartilage in osteoarthritis using multi-parametric MRI. J Magn Reson. 2009;197:40–47. doi: 10.1016/j.jmr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Xia Y, Moody J, Burton-Wurster N, Lust G. Quantitative In Situ Correlation Between Microscopic MRI and Polarized Light Microscopy Studies of Articular Cartilage. Osteoarthritis Cartilage. 2001;9:393–406. doi: 10.1053/joca.2000.0405. [DOI] [PubMed] [Google Scholar]
- 29.Chen SS, Falcovitz YH, Schneiderman R, Maroudas A, Sah RL. Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis Cartilage. 2001;9:561–569. doi: 10.1053/joca.2001.0424. [DOI] [PubMed] [Google Scholar]
- 30.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 31.Williams A, Mikulis B, Krishnan N, Gray M, McKenzie C, Burstein D. Suitability of T(1Gd) as the dGEMRIC index at 1.5T and 3.0T. Magn Reson Med. 2007;58:830–834. doi: 10.1002/mrm.21376. [DOI] [PubMed] [Google Scholar]
- 32.Mayerhoefer ME, Welsch GH, Mamisch TC, Kainberger F, Weber M, Nemec S, Friedrich KM, Dirisamer A, Trattnig S. The in vivo effects of unloading and compression on T1-Gd (dGEMRIC) relaxation times in healthy articular knee cartilage at 3.0 Tesla. Eur Radiol. 2010;20:443–449. doi: 10.1007/s00330-009-1559-3. [DOI] [PubMed] [Google Scholar]