Abstract
Objective
To examine the degree to which obesity during infancy, consistent exposure to secondhand smoke, and parenting (positive attention, maternal involvement, and negative control) were related to early development of wheezing in a cohort of African American premature infants at 2, 6, 12, 18, and 24 months corrected age.
Design
Secondary analysis of a subset of variables from a larger nursing support intervention study.
Setting
Two regional perinatal centers in the southeastern United States.
Participants
One hundred sixty-eight African American premature infants (70 boys, 98 girls) who weighed less than 1,750 g or required mechanical ventilation and their mothers.
Methods
The presence of wheezing was obtained from maternal report at 2, 6, 12, 18, and 24 months. Infants were considered to have medically significant wheezing if they were using bronchodilators or pulmonary anti-inflammatory medications.
Results
The percent of infants who had medically significant wheezing increased from 12% at 2 months to 24% at 24 months corrected age. Infants who received more positive attention from their mothers had a slightly higher increase in the probability of developing wheezing over time. Infants of mothers who received public assistance had an increased probability of wheezing. Consistent exposure to secondhand smoke, obesity during infancy, maternal negative control, and maternal involvement were not related to the development of wheezing.
Conclusion
These findings suggest that the likelihood of developing wheezing in African American premature infants is associated with receiving more positive attention from their mothers and having mothers who receive public assistance. Since modifiable risk factors were not highly related to wheezing, intervention efforts need to focus on early identification and treatment of wheezing and asthma-related symptoms.
Premature infants, and especially minorities, are at risk for respiratory illness in infancy due to incomplete lung development (Landau, 1996; Rona, Gulliford, & Chinn, 1993; Sherriff, Peters, Henderson, & Strachan, 2001). As a group, African American premature infants are more likely to experience wheezing and asthma than White premature infants (Grischkan et al., 2004; Holditch-Davis, Merrill, Schwartz, & Scher, 2008). Both illness severity (e.g., birthweight and neonatal illness severity) and demographic factors (e.g., gender, socioeconomic status, marital status, and race) have been found to be associated with wheezing and asthma in prematurely born children (Berz et al., 2007; Greenough et al., 2005; Holditch-Davis et al., 2008). However, most risk factors previously studied are non-modifiable and thus would not be amenable to interventions to reduce the incidence of respiratory illnesses. The purpose of this study, therefore, was to examine modifiable risk factors for the development of wheezing in African American premature infants (obesity during infancy, consistent exposure to secondhand smoke, and parenting) from hospital discharge through 24 months corrected age. The respiratory outcome in this study is wheezing; however, literature on both wheezing and asthma was reviewed because studies varied on their definitions of wheezing and asthma.
Wheezing and asthma symptoms have been found to be related to obesity during infancy and childhood. In a cross-sectional study examining children ages 16 years and younger, Arif and colleagues (2004) found an increased odds of wheezing in overweight children. Similar findings were reported for a younger sample of Israeli children less than 2 years of age; overweight infants were found to suffer more frequently from respiratory illnesses, such as asthma and wheezing (Shibli, Rubin, Akons, & Shaoul, 2008). In prematurely born children, asthma was associated with increased body mass in childhood (Grischkan et al., 2004). Few investigators have examined the effects of obesity in infancy on the development of wheezing, and even fewer have focused on African American premature infants, but previous findings suggested that obesity during infancy resulted in a greater wheezing risk in toddlerhood in a multiethnic group of prematures (Holditch-Davis et al., 2008).
Wheezing and asthma symptoms are also related to tobacco smoke exposure. Using the National Medical Expenditure Survey, Stoddard and Miller (1995) found that children and youth less than 18 years of age of prenatal smokers were more likely than children of nonsmokers to experience wheezing. The greatest association was observed in infants less than 2 years of age. In a British Cohort Study, exposure to prenatal smoking predicted wheezing by 5 years of age (Lewis, Richards, Bynner, Butler, & Britton, 1995). Other researchers have found a relationship between smoke exposure and wheezing primarily during the first year of life. Murray and colleagues (2004) followed infants from birth to 3 years of age and found wheezing in the first year of life to be more common in infants of prenatal smokers. A small number of studies have reported that prenatal smoke exposure resulted in the development of wheezing in very premature infants and low birthweight infants in early infancy and childhood (Darlow, Horwood, & Magridge, 2000; Halterman et al., 2009).
Further, a dose-response relationship has been reported between exposure to secondhand smoke and wheezing in premature infants. Wheezing incidence increased for premature infants whose mothers smoked more cigarettes per day compared to those whose mothers smoked fewer cigarettes per day (Elder et al., 1996). Respiratory morbidity resulting from secondhand smoke exposure was assessed in a racially diverse sample (White, African American, Hispanic, and other) of very low birthweight infants (<1500 grams) through 1 year of age, and infants exposed to secondhand smoke were found to be more likely to require acute care for respiratory problems, such as wheezing, than non exposed infants (Halterman et al., 2009). Although evidence clearly supports the association between prenatal tobacco smoke exposure and development of wheezing in infants and young children, less evidence supports an association between postnatal secondhand smoke exposure and development of wheezing in African American premature infants beyond 1 year of age.
Finally, wheezing and asthma symptoms have been related to parenting. A number of studies have demonstrated that less positive parenting precedes the onset of wheezing, suggesting that problematic parenting plays a role in the development of childhood asthma (Holditch-Davis et al., 2008; Klinnert, Kaugars, Strand, & Silveira, 2008; Klinnert et al., 2001). Mantymaa and colleagues (2003) found a relationship between less positive parenting at 2 months of age and chronic health problems (e.g., asthma) at 2 years of age in fullterm infants. In a sample of children who were genetically at risk for developing asthma, less positive parenting in early infancy was related to asthma onset between 6 and 8 years of age (Klinnert et al., 2001). In a follow-up study of fullterm infants, less positive parenting predicted asthma onset by 4 years of age (Klinnert et al., 2008). Infants in these two studies were racially diverse. In a study that examined wheezing as an outcome in multi-ethnic sample of premature infants, development of wheezing was related to less positive parenting at 6 and 18 months (Holditch-Davis et al., 2008). Collectively, these findings suggest that interactions between a mother and her infant may result in negative infant health outcomes such as wheezing and asthma (Mantymaa et al., 2003).
Most studies examining wheezing and asthma have been conducted in White fullterm infants and older children. Studies of wheezing in premature infants have focused primarily on non-modifiable risk factors, such as medical history, race, and socioeconomic status. Modifiable risk factors such as obesity in infancy, exposure to tobacco smoke, and parenting may be more amenable to nursing interventions than the more frequently studied risk factors. Identifying modifiable factors related to wheezing risk in African American premature infants could lead to interventions to improve their respiratory outcomes. Thus, the purpose of this secondary analysis was to examine modifiable risk factors related to the development of wheezing in African American premature infants over the first 24 months of age corrected for prematurity. Specifically, we examined the degree to which obesity during infancy, consistent exposure to secondhand smoke, and parenting were related to early development of wheezing in a sample of African American premature infants at 2, 6, 12, 18, and 24 months corrected age. In these analyses, we controlled for other variables known to affect wheezing development, including birthweight, neonatal illness severity, gender, socioeconomic status (as evidenced by receipt of public assistance), and marital status (Greenough et al., 2005; Halterman et al., 2009; Holditch-Davis et al., 2008).
Methods
This study was a secondary analysis from a larger nursing support intervention study (n = 197) conducted at two regional perinatal centers in the southeastern United States from 2001 to 2007 (Holditch-Davis et al., 2009; Miles, Holditch-Davis, Thoyre, & Beeber, 2005). The outcome variable, wheezing, was not affected by the intervention; thus, infants from both the intervention and control groups were included in this study.
Participants
Infants from either singleton and twin births were eligible for the study if they were of African American race, born less than 35 weeks gestational age, and considered high risk for developmental and health problems because they either weighed less than 1,750 grams at birth or required mechanical ventilation. Infants were excluded if they had congenital neurological problems (such as Down Syndrome, congenital hydrocephalus, or microcephaly), were symptomatic from substance exposure, were hospitalized longer than 1 month post-term, were part of a higher order multiple set, or were not in the custody of the biological mother. Infants also were excluded if follow-up for 24 months was unlikely (such as out-of-state visitors as mothers) or if the family situation made asking for consent intrusive or affected the mother’s ability to respond to the intervention (such as maternal HIV, maternal age less than 15, current diagnosis of major depression, or non-English speaking mothers).
The present study focused on the 168 infants from the original sample who had data on wheezing and at least 6 months of data beyond term to provide sufficient time for infants to develop wheezing not resulting from neonatal respiratory problems such as chronic lung disease. Table 1 shows the demographics and birth characteristics of these infants. A power analysis using the specialized procedures for power analysis with repeated measures data (Jung, and Ahn, 2003) showed that with 168 participants we would be able to detect odds ratios between 1.45 and 2.1 (80% power, alpha = .05, 2 tailed) depending upon the metric and distribution of different predictor variables.
Table 1.
Demographic Characteristics of the 168 African American Premature Infants and Their Mothers
| Mean(SD) | Percent | |
|---|---|---|
| Gestational Age (weeks) | 28.4 (2.92) | |
| Birth Weight (grams) | 1121 (393.4) | |
| Small for Gestational Age | 20.9 | |
| Sex of Child: | ||
| Male | 42.8 | |
| Female | 57.2 | |
| Mechanical Ventilation (days) | 15 (25.8) | |
| Maternal Age (years) | 25.9 (6.4) | |
| Married Mothers | 27.4 | |
| Maternal Education (years) | 12.6 (1.8) | |
| Public Assistance | 51.8 | |
| Weight for Length2 (kg/cm2): | ||
| Early Agesa | 1.7 (0.2) | |
| Later Agesb | 1.6 (0.2) | |
| Exposure to tobacco smoke*: | ||
| Early Agesa | 0.2 (0.3) | |
| Later Agesb | 0.2 (0.4) | |
| Parenting Quality: | ||
| Negative Control | −0.02 (0.80) | |
| Positive Attention | 0.04 (0.67) | |
| Involvement | −0.01 (0.66) | |
2, 6, and 12 months;
18 and 24 months.
Measures
Presence of wheezing
The presence of wheezing was assessed using data collected from an infant health history completed by the mother at 2, 6, 12, 18, and 24 months of age corrected for prematurity. Although a description of wheezing was not given prior to data collection, mothers completed the infant health history in the presence of a research assistant and were provided the opportunity to ask questions during the assessment. At each age, infants reported by mothers (a) to experience wheezing or asthma in the period since the last contact and (b) to be using pulmonary anti-inflammatory medications or bronchodilators (in a separate question) were classified as having medically significant wheezing. Infants reported to have experienced wheezing or asthma since the last contact without the need for pulmonary anti-inflammatory medications or bronchodilators were classified as having mild wheezing. Infants whose mothers reported no incidence of wheezing or asthma since the last contact were classified as having no wheezing.
Obesity during infancy
Obesity during infancy was assessed using infant height and weight measurements recorded at 2 months of corrected age by maternal report or medical record review when available. Additional height and weight measurements were collected by a research assistant during home visits at 6 and 18 months and by a nurse clinician during clinic visits at 12 and 24 months corrected age: weight was reported in kilograms using a calibrated scale and length was reported in centigrams using a height measuring board. Weight for length was calculated by dividing weight in kilograms by length (cm2). The mean weight for length values at early ages (mean of 2, 6, and 12 months corrected age) and at later ages (mean of 18 and 24 months corrected age) were used in analyses.
Consistent exposure to secondhand smoke
Infant exposure to secondhand smoke was assessed from an infant health history provided by the mother at each follow-up contact (2, 6, 12, 18, and 24 months of corrected age). Infants whose mothers reported smoke exposure at every age were classified as exposed (1) and all other infants were scored as 0.
Parenting
Parenting was measured by three parenting dimensions: positive attention, maternal involvement, and negative control (Holditch-Davis et al., 2007). These dimensions were scored from 45-minute videotapes of mother-infant interactions and the Home Observation for Measurement of the Environment (HOME; Caldwell & Bradley, 1980), both scored at 6 and 18 months. Two groupings of the mother behaviors from the videotapes were each coded by two scorers, who were initially trained to adequate inter-rater reliability (more than 85% exact agreement and kappas greater than .70). Inter-rater reliability was verified every 2 months by having each pair of scorers code the same videotape, and kappas for the variables used in this report ranged from .72 to .94 with a mean of .83. The overall HOME score had an internal consistency alpha of .81 at 6 months and .86 at 28 months.
Positive attention - the amount of holding, affectionate behaviors, and tactile behaviors that the mother displays toward the infant - was created by summing the Z-scores of three mother behaviors (positive, touch, and hold; Holditch-Davis, Schwartz, Black, & Scher, 2007; Lee, Holditch-Davis, & Miles, 2007). Z-scores are a way of standardizing scores so that 0 equals the mean, 1 equals one standard deviation above the mean, and -1 equals one standard deviation below the mean. Maternal involvement - the amount of involvement, interaction, and play that the mother displays toward the infant - was created by summing the Z-scores of three mother behaviors from videotaped observation (amount of interaction, uninvolved with infant [reversed], and play with infant) and the maternal involvement subscale from the HOME (Holditch-Davis et al., 2007; Lee, Holditch-Davis, & Miles, 2007). Negative control - the mother’s use of psychological and physical control behaviors toward the infant - was calculated by summing the Z-scores of the reverse acceptance of the infant’s behavior subscale of the HOME Inventory minus two items not directly related to negative control (presence of a pet, number of books in the home; Holditch-Davis et al., 2007). Each variable was converted to a Z-score and then the scores of each variable in the dimension were summed, separately for 6 and 18 months (Holditch-Davis et al., 2007).
Internal consistencies for these parenting dimensions ranging from .56 to .71 and significant correlations over ages have been reported in samples of premature infants and toddlers with HIV-infected mothers (Holditch-Davis et al., 2007). Internal consistencies for the parenting dimensions in this sample were 0.74 for positive attention, 0.76 for maternal involvement, and .57 for negative control. In this study, parenting dimensions were assessed at 6 and 18 months corrected age and the scores were averaged to produce a mean score for each parenting dimension.
Covariates
Demographic information was collected at enrollment (Holditch-Davis et al., 2007) and was updated at 2, 6, 12, 18, and 24 months corrected age. Maternal age, use of public assistance (a proxy for socioeconomic status), and marital status were included as covariates. Other covariates were data about infant characteristics (birthweight, gender, and size for gestational age) and neonatal illness severity (length of mechanical ventilation in days) collected from the infant’s medical record.
Data Analysis
The data analysis for this paper was generated using SAS® software, version 9.1 (SAS Institute Inc., Cary, NC). Generalized linear mixed models calculated from Proc Glimmix were used to estimate the models of the relationship between potential predictors and wheezing severity over time (2, 6, 12, 18, and 24 months). Potential predictors included obesity in infancy, consistent exposure to secondhand smoke, and parenting (positive attention, maternal involvement, and negative control). Time (2, 6, 12, 18, and 24 months corrected age) was modeled as a continuous explanatory variable. The outcome variable used in the generalized linear mixed models was wheezing severity at each time point, categorized as 2 (medically significant wheezing), 1 (mild wheezing), and 0 (no wheezing) and treated as a three category ordinal measure. The generalized linear mixed model (Breslow & Clayton, 1993) extends the generalized linear model (McCullagh & Nelder, 1989) by incorporating normally distributed random effects and allows for participant-specific (conditional) and population-averaged (marginal) inference. Repeated measures within a participant were treated as correlated.
Generalized linear mixed modeling was performed in two steps. Potential predictors and their interactions with one another and with time were assessed individually and retained if p = 0.15. Backward selection was then used to eliminate non-significant predictors and interaction terms that were no longer significant at the 0.05 level when analyzed together in the model, resulting in a final model. Results are presented as odds ratios (OR) and 95% confidence intervals (95% CI).
Results
Table 2 presents the prevalence of wheezing at 2, 6, 12, 18, and 24 months corrected age. The percentage of infants who were reported to have medically significant wheezing increased from 12% at 2 months to 24% by 24 months corrected age. The percentage of infants who were reported to have no wheezing decreased from 76% to 51% by 24 months corrected age.
Table 2.
Percent of African American Premature Infants Reported to Have Each Wheezing Category at Each Age
| Age (months) | Na | No Wheezing (%) | Mild Wheezing (%) | Medically Significant Wheezing (%) |
|---|---|---|---|---|
| 2 months | 154 | 76.6 | 11.0 | 12.3 |
| 6 months | 141 | 61.7 | 17.0 | 21.3 |
| 12 months | 157 | 52.2 | 26.1 | 21.7 |
| 18 months | 126 | 54.8 | 19.0 | 26.2 |
| 24 months | 139 | 51.1 | 25.2 | 23.7 |
The N varies over time because of missing contacts, study withdraws, and incomplete questionnaires.
Table 3 shows the results from the preliminary models and the final reduced model. In the preliminary model, public assistance and the interaction between maternal positive attention and time were associated with wheezing with p < .05. Obesity, maternal involvement, negative control, maternal age, marital status, and their interactions with time had p levels > .15 so they were not included in the final model.
Table 3.
Initial and Final Model of Wheezing by Explanatory Variables, Covariates, and Interaction Terms
| Initial Model | Final Model | |||
|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Odds Ratios Estimate (CLs) | ||
| Wheezing | Intercept1a | −0.89 (1.78) | −1.78** (0.33) | |
| Intercept2b | −2.44 (1.78) | −3.29** (0.34) | ||
| Time | −0.19 (0.29) | 0.21** (0.04) | 1.23(1.133 – 1.342) | |
| Time2 | 0.01 (0.01) | −0.01**( 0.00) | 0.99(0.989 – 0.996) | |
| Obesity | 0.02 (0.01) | |||
| Smoke Exposure | 0.04 (0.44) | 0.13 (0.41) | 0.87(0.388 – 1.967) | |
| Parenting Quality | Positive Attention | −0.06 (0.44) | −0.07 (0.33) | 0.94(0.486 – 1.805) |
| Involvement | −0.18 (0.38) | |||
| Negative Control | 0.07 (0.23) | |||
| Covariates | Public Assistance | 1.13 (0.54)* | 0.69* (0.34) | 1.99(1.014 – 3.910) |
| Mechanical Ventilation | 0.01 (0.01) | 0.01 (0.01) | 1.01(0.995 – 1.027) | |
| Maternal Age | −0.01 (0.04) | |||
| Marital Status | −0.59 (0.48) | |||
| Interactions | Public Assistance × Time | −0.14 (0.09) | ||
| Mechanical Ventilation × Time | 0.00 (0.00) | |||
| Maternal Age × Time | 0.01 (0.01) | |||
| Marital Status × Time | 0.11 (0.08) | |||
| Positive Attention × Time | 0.06 (0.02)** | 0.05** (0.02) | 1.05(1.015 – 1.094) | |
| Public Assistance × Time2 | 0.01 (0.00) | |||
| Mechanical Ventilation × Time2 | −0.00 (0.00) | |||
| Maternal Age × Time2 | −0.00 (0.00) | |||
| Marital Status × Time2 | −0.00 (0.00) | |||
Intercept for the odds of mild wheezing versus no wheezing.
Intercept for the odds of medically significant wheezing versus no wheezing.
p < .05
p <.01
In the final model, one dimension of parenting quality was related to wheezing. The probability of having wheezing rate increased slightly more rapidly over time for African American premature infants who received more positive attention from their mothers [OR = 1.05, CL (1.015 – 1.094)]. Figure 1 shows the probability of the development of wheezing for African American premature infants receiving varying amounts of positive attention from their mothers.
Figure 1.
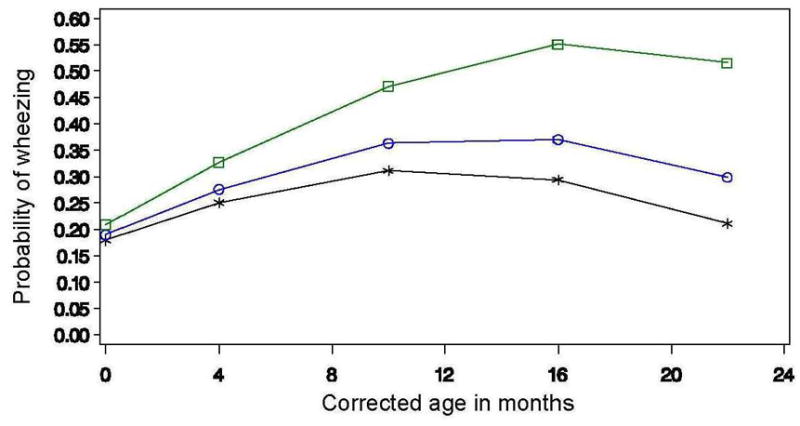
Predicted means of wheezing by positive attention and age. The line with the squares shows the predicted probability of wheezing for infants who received the most positive attention, the line with the circles shows the predicted probability of wheezing for infants who received a moderate amount of positive attention, and the line with the stars shows the predicted probability of wheezing for infants who received the least positive attention.
Results also indicated that the probability of wheezing was higher among infants whose mothers received public assistance. Infants whose mothers received public assistance had 1.99 times the odds of wheezing as infants whose mothers did not receive public assistance. Secondhand smoke exposure, obesity in infancy, maternal involvement, and maternal negative control had no effect on wheezing in this sample.
Discussion
In this secondary analysis of a study of African American premature infants, we found the rate of medically significant wheezing increased from 12% at 2 months to 24% by 24 months corrected age; whereas 51% of infants were reported to have no wheezing at 24 months corrected age. These percentages are similar to those of previous studies examining wheezing in multi-ethnic samples of premature infants (Greenough et al., 2005; Holditch-Davis et al., 2008).
One dimension of parenting was related to the development of wheezing. African American premature infants who received more positive attention from their mothers had a slightly greater increase over time in their rate of wheezing than infants who received less positive attention. In other studies, wheezing and other respiratory problems were associated with greater maternal punitiveness (Holditch-Davis et al., 2008; Klinnert et al., 2001; Mantymaa et al., 2003), but positive attention has not previously been associated with wheezing development. Most likely, our finding is due to infants who were wheezing receiving more attention from their mothers. Other studies have found that mothers provide more positive attention to sick premature infants to compensate them for stressful experiences related to the NICU environment and immature infant behavior (Holditch-Davis, Cox, Miles, & Belyea, 2003; Holditch-Davis et al., 2007; Lee, Holditch-Davis, & Miles, 2007). In addition, positive attention may be a proxy for other factors related to wheezing, such as better maternal awareness of wheezing or more frequent visits to primary care.
We also found that the probability of wheezing was higher in African American premature infants whose mothers received public assistance. Similarly, Margolis and colleagues (1992) reported an increased risk for respiratory problems in infants of low socioeconomic status compared to those of middle or high socioeconomic status. Further, being African American was related to both low socioeconomic status and persistent respiratory symptoms in the first year of life (Margolis et al, 2002). Several researchers found that African American children were more likely to receive Medicaid than White children and were more likely to utilize urgent care more frequently for asthma symptoms (Akinbami & Schoendorf, 2002; Jones, Lin, Munsie, Radigan, & Hwang, 2008; Stingone & Claudio, 2006). The relationship between low socioeconomic status and risk for the development of wheezing and asthma may be partially explained by environmental factors. For example, Leaderer and colleagues (2002) found an association between low income in African American families and the presence of elevated cockroach allergens in the home. Thus, African American premature infants whose mothers utilize public assistance may be at risk for exposure to certain environmental allergens that may trigger wheezing and asthma.
Although other investigators (Elder et al., 1996; Halterman et al., 2009) reported increased risk of wheezing with exposure to secondhand smoke, we did not find a significant effect of consistent secondhand smoke exposure in our sample of African American premature infants. Like researchers who reported an increase in wheezing risk for children with the number of parents currently smoking, the number of cigarettes smoked per day in the home, and maternal smoking during pregnancy with subsequent postnatal exposure (Johansson, Ludvigsson, & Hermansson, 2008; Jurado, Munoz, Luna, & Munoz-Hoyos, 2005), we expected that consistent secondhand smoke exposure would have the greatest effect on the development of wheezing. However, because we used previously collected data, we were limited in the type of information that was available, such as the frequency and timing of smoke exposure. We also did not have data on prenatal smoking, which has been implicated in other studies (Halterman et al., 2009). Further, the limited infant exposure to prenatal smoking in the third trimester and to early postnatal smoking as a result of premature birth and NICU admission may have affected our ability to find an association between secondhand smoke and wheezing in our sample.
Unlike others (Holditch-Davis et al., 2008), we did not find a relationship between obesity in infancy and the development of wheezing in African American premature infants. Because African Americans are at risk for obesity (Anderson & Whitaker, 2009; Ogden, Carroll, & Flegal, 2008) and rapid weight gain during infancy has been associated with obesity in childhood and young adulthood (Stettler, Kumanyika, Katz, Zemel, & Stallings, 2003), African American premature infants with rapid weight gain during infancy may be at increased risk for obesity in childhood and young adulthood and thus, more likely to experience asthma and other respiratory problems (Flaherman & Rutherford, 2006; Grischkan et al., 2004). However, some investigators have found an inverse relationship between obesity in infancy and development of wheezing and asthma at later ages (Mai, Gaddlin, Nilsson, & Leijon, 2005; Turner et al., 2008). Differences in findings may be attributed to variations in methods used in measurement, ages of the infants at assessment, and the race of the samples. Also, infants in this study had a lower mean birthweight and lower mean values of weight for age than the infants in the study finding obesity effects on wheezing in premature infants (Holditch-Davis et al., 2008).
There are some limitations to this analysis. First, the generalizability of this study may be affected by the use of maternal report as the method to collect information pertaining to the respiratory symptoms experienced by the infant. Previous studies indicated that there is less than 50% agreement between parents’ and clinicians’ reports of asthma (Cane, Ranganathan, & McKenzie, 2000). Diagnoses of asthma were not determined by medical report for the purposes of this study. Rather, maternal report of infant wheezing and the use bronchodilators or pulmonary anti-inflammatory medications was used for classification of wheezing status, and the mothers may have differed in their interpretation of which symptoms met the criteria of wheezing. Future studies focused on respiratory health problems of African American premature infants may need to consider the use of both maternal and clinician reports for determining medical diagnoses to ensure that the findings are generalizable. Likewise, maternal report about smoking may be biased because mothers are generally told at discharge that the infant should not be exposed to secondhand smoke. Several mothers in the intervention group dropped from the study when the nurse gently focused on the issue of parental smoking.
Future Research
Future studies examining the effects of socioeconomic status and presence of environmental allergens on the development of wheezing and asthma symptoms in African American premature infants are warranted. Additional efforts to understand the effects of obesity in infancy and to further evaluate modifiable factors associated on the development of wheezing, particularly among African American premature infants, are also recommended due to the lack of research specifically focusing on this population. Also, because the effects of secondhand smoke exposure may take more than 2 years to be apparent, additional prospective longitudinal research beyond 2 years of life is needed to examine the frequency and timing of smoke exposure on the development of wheezing in African American premature infants.
Implications for Practice
Because African American premature infants are at substantially greater risk for the development of wheezing and asthma in childhood than White premature infants (Grischkan et al., 2004; Holditch-Davis et al., 2008), it is imperative that clinicians recognize and address the factors contributing to wheezing early in infancy. The modifiable risk factors examined in this study did not appear to have a major impact on the development of wheezing in the first 2 years of life in African American premature infants; thus, clinicians might be better served by early identification of and treatment of infants with wheezing and by anticipatory guidance of mothers in high risk groups as identified in this study (i.e., poverty). Clinicians can adopt a preventative approach in reducing wheezing and other respiratory morbidities experienced by African American premature infants by assessing for factors known to be associated with wheezing, including illness severity (e.g., birthweight and neonatal illness severity), demographic factors (e.g., gender, socioeconomic status, marital status, and race), and environmental factors (e.g, prenatal smoking; Berz et al., 2007; Greenough et al., 2005; Holditch-Davis et al., 2008; Kurukulaaratchy et al., 2005). Assessment and treatment of wheezing in early infancy and toddlerhood may lead to a reduction in wheezing incidence and severity in this at-risk group of infants.
Acknowledgments
Supported by Grant R01 NR035962 from the National Institute for Nursing Research, NIH to the second author.
Footnotes
Disclosure: The author reports no conflict of interest or relevant financial relationships.
Contributor Information
Jada L. Brooks, School of Nursing, Duke University, Durham, NC.
Diane Holditch-Davis, School of Nursing, Duke University, Durham, NC.
Lawrence R. Landerman, School of Medicine, Duke University, Durham, NC.
Margaret Shandor Miles, School of Nursing, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Stephen C. Engelke, Brody School of Medicine, East Carolina University and health system pediatric neonatologist at the Pitt County Memorial Hospital, Greenville, NC.
References
- Akinbami LJ, Rhodes JC, Lara M. Racial and ethnic differences in asthma diagnosis among children who wheeze. Pediatrics. 2005;115(5):1254–1260. doi: 10.1542/peds.2004-0897. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Whitaker RC. Prevalence of obesity among US preschool children in different racial and ethnic groups. Archives of Pediatrics and Adolescent Medicine. 2009;163(4):344–348. doi: 10.1001/archpediatrics.2009.18. [DOI] [PubMed] [Google Scholar]
- Arif AA, Borders TF, Patterson PJ, Rohrer JE, Xu KT. Prevalence and correlates of paediatric asthma and wheezing in a largely rural USA population. Journal of Paediatrics and Child Health. 2004;40(4):189–194. doi: 10.1111/j.1440-1754.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- Berz JB, Carter AS, Wagmiller RL, Horwitz SM, Murdock KK, Briggs-Gowan M. Prevalence and correlates of early onset asthma and wheezing in a healthy birth cohort of 2- to 3-year olds. Journal of Pediatric Psychology. 2007;32(2):154–166. doi: 10.1093/jpepsy/jsj123. [DOI] [PubMed] [Google Scholar]
- Breslow NE, Clayton DG. Approximate Inference in Generalized Linear Mixed Models. Journal of the American Statistical Association. 1993;88(421):9–25. [Google Scholar]
- Caldwell B, Bradley R. Home Observation for Measurement of the Environment. Little Rock: University of Arkansas at Little Rock; 1980. [Google Scholar]
- Cane RS, Ranganathan SC, McKenzie SA. What do parents of wheezy children understand by “wheeze”? Archives of Disease in Childhood. 2000;82(4):327–332. doi: 10.1136/adc.82.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlow BA, Horwood LJ, Mogridge N. Very low birthweight and asthma by age seven years in a national cohort. Pediatric Pulmonology. 2000;30:291–296. doi: 10.1002/1099-0496(200010)30:4<291::aid-ppul3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Elder DE, Hagan R, Evans SF, Benninger HR, French NP. Recurrent wheezing in very preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition. 1996;74(3):F165–171. doi: 10.1136/fn.74.3.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Archives of Disease in Childhood. 2006;91:334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough A, Limb E, Marston L, Marlow N, Calvert S, Peacock J. Risk factors for respiratory morbidity in infancy after very premature birth. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2005;90(4):F320–323. doi: 10.1136/adc.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grischkan J, Storfer-Isser A, Rosen CL, Larkin EK, Kirchner HL, South A, et al. Variation in childhood asthma among former preterm infants. The Journal of Pediatrics. 2004;144(3):321–326. doi: 10.1016/j.jpeds.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Halterman JS, Lynch KA, Conn KM, Hernandez TE, Perry TT, Stevens TP. Environmental exposures and respiratory morbidity among very low birth weight infants at 1 year of life. Archives of Disease in Childhood. 2009;94(1):28–32. doi: 10.1136/adc.2008.137349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch-Davis D, Cox MF, Miles MS, Belyea M. Mother-infant interactions of medically fragile and non-chronically ill premature infants. Research in Nursing & Health. 2003;26:300–311. doi: 10.1002/nur.10095. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Merrill P, Schwartz T, Scher M. Predictors of wheezing in prematurely born children. Journal of Obstetrics, Gynecologic and Neonatal Nursing. 2008;37(3):262–273. doi: 10.1111/j.1552-6909.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch-Davis D, Miles MS, Burchinal M, O’Donnell K, McKinney R, Lim W. Parental caregiving and developmental outcomes of infants of mothers with HIV. Nursing Research. 2001;50(1):5–14. doi: 10.1097/00006199-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Holditch-Davis D, Miles MS, Weaver MA, Black BP, Beeber LS, Thoyre S, Engelke S. Patterns of distress in African-American mothers of preterm infants. Journal of Behavioral and Developmental Pediatrics. 2009;30(3):193–205. doi: 10.1097/DBP.0b013e3181a7ee53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holditch-Davis D, Schwartz T, Black B, Scher M. Correlates of mother-premature infant interactions. Research in Nursing & Health. 2007;30(3):333–346. doi: 10.1002/nur.20190. [DOI] [PubMed] [Google Scholar]
- Johansson A, Ludvigsson J, Hermansson G. Adverse health effects related to tobacco smoke exposure in a cohort of three-year olds. Acta Paediatrica. 2008;97(3):354–357. doi: 10.1111/j.1651-2227.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- Jones R, Lin S, Munsie JP, Radigan M, Hwang S. Racial/ethnic differences in asthma-related emergency department visits and hospitalizations among children with wheeze in Buffalo, New York. Journal of Asthma. 2008;45:916–922. doi: 10.1080/02770900802395488. [DOI] [PubMed] [Google Scholar]
- Jung SH, Ahn C. Sample size estimation for Gee method for comparing slopes in repeated measurements data. Statistics in Medicine. 2003;22(8):1305–1315. doi: 10.1002/sim.1384. [DOI] [PubMed] [Google Scholar]
- Jurado D, Munoz C, Luna JD, Munoz-Hoyos A. Is maternal smoking more determinant than paternal smoking on the respiratory symptoms of young children? Respiratory Medicine. 2005;99(9):1138–1144. doi: 10.1016/j.rmed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Kaugars AS, Strand M, Silveira L. Family psychological factors in relation to children’s asthma status and behavioral adjustment at age 4. Family Process. 2008;47(1):41–61. doi: 10.1111/j.1545-5300.2008.00238.x. [DOI] [PubMed] [Google Scholar]
- Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108(4):E69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- Kurukulaaratchy RJ, Waterhouse L, Matthews SM, Arshad SH. Are influences during pregnancy associated with wheezing phenotypes during the first decade of life? Acta Paediatrica. 2005;94(5):553–558. doi: 10.1111/j.1651-2227.2005.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Landau LI. Risks of developing asthma. Pediatric Pulmonology. 1996;22(5):314–318. doi: 10.1002/(SICI)1099-0496(199611)22:5<314::AID-PPUL4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, McSharry J, Platts-Mills TAE, Chapman MD, Bracken MB. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: Impact of socioeconomic factors and population density. Environmental Health Perspectives. 2002;110(4):419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TY, Holditch-Davis D, Miles MS. The influence of maternal and child characteristics and paternal support on interactions of mothers and their medically fragile infants. Research in Nursing & Health. 2007;30(1):17–30. doi: 10.1002/nur.20184. [DOI] [PubMed] [Google Scholar]
- Lewis S, Richards D, Bynner J, Butler N, Britton J. Prospective study of risk factors for early and persistent wheezing in childhood. The European Respiratory Journal. 1995;8(3):349–356. doi: 10.1183/09031936.95.08030349. [DOI] [PubMed] [Google Scholar]
- Mai XM, Gaddlin PO, Nilsson L, Leijon I. Early rapid weight gain and current overweight in relation to asthma in adolescents born with very low birth weight. Pediatric Allergy and Immunology. 2005;16(5):380–385. doi: 10.1111/j.1399-3038.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Mantymaa M, Puura K, Luoma I, Salmelin R, Davis H, Tsiantis J, Ispanovic-Radojkovic V, Paradisiotou A, Tamminen T. Infant-mother interaction as a predictor of child’s chronic health problems. Child Care Health and Development. 2003;29(3):181–191. doi: 10.1046/j.1365-2214.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- Margolis PA, Greenberg RA, Keyes LL, LaVange LM, Chapman RS, Denny FW, Bauman KE, Boat BW. Lower respiratory illness in infants and low socioeconomic status. American Journal of Public Health. 1992;82(8):1119–1126. doi: 10.2105/ajph.82.8.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2. London: Chapman and Hall; 1989. [Google Scholar]
- Miles MS, Holditch-Davis D, Thoyre S, Beeber L. Rural African American mothers parenting prematurely-born infants: An ecological systems perspective. Newborn and Infant Nursing Reviews. 2005;5(3):142–148. [Google Scholar]
- Murray CS, Woodcock A, Smillie FI, Cain G, Kissen P, Custovic A. Tobacco smoke exposure, wheeze, and atopy. Pediatric Pulmonology. 2004;37(6):492–498. doi: 10.1002/ppul.20019. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. The Journal of the American Medical Association. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Rona RJ, Gulliford MC, Chinn S. Effects of prematurity and intrauterine growth on respiratory health and lung function in childhood. British Medical Journal. 1993;306(6881):817–820. doi: 10.1136/bmj.306.6881.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherriff A, Peters TJ, Henderson J, Strachan D. Risk factor associations with wheezing patterns in children followed longitudinally from birth to 3(1/2) years. International Journal of Epidemiology. 2001;30(6):1473–1484. doi: 10.1093/ije/30.6.1473. [DOI] [PubMed] [Google Scholar]
- Shibli R, Rubin L, Akons H, Shaoul R. Morbidity of overweight ( 85th percentile) in the first 2 years of life. Pediatrics. 2008;122(2):267–272. doi: 10.1542/peds.2007-2867. [DOI] [PubMed] [Google Scholar]
- Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. The American Journal of Clinical Nutrition. 2003;77:1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- Stingone JA, Claudio L. Disparities in the use of urgent health care services among asthmatic children. Annals of Allergy and Immunology. 2006;97(2):244–250. doi: 10.1016/S1081-1206(10)60021-X. [DOI] [PubMed] [Google Scholar]
- Stoddard JJ, Miller T. Impact of parental smoking on the prevalence of wheezing respiratory illness in children. American Journal of Epidemiology. 1995;141(2):96–102. doi: 10.1093/oxfordjournals.aje.a117418. [DOI] [PubMed] [Google Scholar]
- Turner S, Zhang G, Young S, Cox M, Goldblatt J, Landau L, et al. Associations between postnatal weight gain, change in postnatal pulmonary function, formula feeding and early asthma. Thorax. 2008;63(3):234–239. doi: 10.1136/thx.2006.064642. [DOI] [PubMed] [Google Scholar]


