Abstract
Cocaine use is associated with poorer HIV clinical outcomes and may contribute to neurobiological impairments associated with impulsive decision making. This study examined the effect of cocaine dependence on brain activation during a delay discounting task involving choices between smaller immediate rewards and larger delayed ones. Participants were 39 HIV-positive adults on antiretroviral therapy who had current cocaine dependence (“active,” n=15), past cocaine dependence (“recovered,” n=13), or no lifetime substance dependence (“naïve,” n=11). Based on responses on a traditional delay discounting task, three types of choices were individualized for presentation during fMRI scanning: hard (similarly valued), easy (disparately valued), and no (single option). Active participants had significantly smaller increases in activation than naïve participants during hard versus easy choices bilaterally in the precentral gyrus and anterior cingulate cortex and in the right frontal pole (including dorsolateral, ventrolateral, and orbitofrontal cortex). During hard and easy choices relative to no choices, active participants had smaller increases in activation compared to naïve participants in frontoparietal cortical regions. These deficits in the executive network during delay discounting choices may contribute to impulsive decision making among HIV-positive cocaine users, with implications for risk behaviors associated with disease transmission and progression.
Keywords: cocaine dependence, HIV/AIDS, functional magnetic resonance imaging, delay discounting, decision making
1. Introduction
Drug use disorders are common among adults receiving care for HIV in the United States, with approximately half reporting drug use in the past year and a quarter of drug users screening in for current drug dependence (Bing et al., 2001). Cocaine use, in particular, is associated with increased likelihood of engaging in behaviors, such as risky sex and treatment non-adherence, that are associated with HIV disease transmission and progression (Arnsten et al., 2002; Harzke et al., 2009; Hinkin et al., 2007; Tucker et al., 2004). Cocaine use is also associated with poor HIV clinical outcomes, including higher viral load, CD4 cell count decline, AIDS-related illnesses, and death (Baum et al., 2009; Cook et al., 2008; Webber et al., 1999). Many cocaine users do not receive substance abuse treatment (SAMSHA, 2008); dropout rates are high among those who do (Kampman et al., 2001; Siqueland et al., 2002); and there are no FDA-approved pharmacological treatments for cocaine dependence. Thus, HIV patients with cocaine dependence are at particularly high risk for poor clinical and behavioral outcomes.
The high rate of impulsivity observed in cocaine users may help to explain continued engagement in health risk behaviors. Delay discounting is a measure of cognitive impulsivity that describes the relationship between the delay of a reward and its perceived value (Ainslie, 1975). Greater impulsivity is defined as a preference for smaller immediate rewards over larger delayed rewards (Rachlin and Green, 1972). Decision-making is central to this conceptualization, as immediate reward contingencies and future outcomes influence behavioral choice. Prior research has found that individuals who use drugs, including cocaine, methamphetamine, and heroin, are more cognitively impulsive than healthy controls or former users (Coffey et al., 2003; Heil et al., 2006; Hoffman et al., 2006; Kirby and Petry, 2004).
While numerous studies have examined behavioral aspects of delay discounting, its underlying neurobiology is less well understood. Functional magnetic resonance imaging (fMRI) studies in healthy adults suggest that cortical areas, particularly the lateral prefrontal and posterior parietal cortices, are involved in hard decisions between similarly valued immediate and delayed rewards (McClure et al., 2004; Tanaka et al., 2004). Only two studies have examined neural activity associated with delay discounting in drug users compared to healthy controls, and both focused on methamphetamine. These studies found that hard choices are associated with bilateral activation in the dorsal and ventral lateral prefrontal cortices (D/VLPFC), intraparietal sulcus (IPS), anterior cingulate cortex (ACC), insula, and supplemental motor area (SMA) (Hoffman et al., 2008; Monterosso et al., 2007). Neural activation was generally greater during hard choices than easy choices of disparately valued rewards, but this increase was smaller in drug users compared to healthy controls (Hoffman et al., 2008; Monterosso et al., 2007).
The neural correlates of delay discounting have not been examined in cocaine users specifically. Given the many similarities between cocaine and methamphetamine on neurocognitive and neurobiological functioning, the above studies may inform further research on cocaine dependence. Specifically, both cocaine and methamphetamine dependent individuals demonstrate impairments in cognitive flexibility, working memory, and complex decision-making (Sofuoglu et al., 1999; van der Plas et al., 2009). Furthermore, use of these drugs is associated with brain activation patterns characterized by attenuated activation in the cingulate, frontal and dorsalateral prefrontal cortices, and parietal regions (Aron and Paulus, 2007). Both cocaine and methamphetamine affect dopamine D2 receptors, which are believed to be critical in reward-processing behavior (Aron and Paulus, 2007). Consequently, studies of HIV-infected individuals frequently examine the deleterious health effects of cocaine and/or methamphetamine use, known broadly as “stimulants” (Hinkin et al., 2007; Lyons et al., 2006; Zule et al., 2007). Thus, cocaine users may, like methamphetamine users, also demonstrate less neural efficiency when making delay discounting choices, possibly biasing them towards less complex, immediate options.
Despite the high rate of drug abuse among HIV-infected individuals, delay discounting has not been examined in this population. This is an important area to explore because impulsive decision making may be predictive of health risk behaviors, including continued drug use, risky sex, and treatment non-adherence (Hayaki et al., 2006; Lejuez et al., 2004), thereby contributing to the worse HIV clinical and behavioral outcomes observed in drug users. The prevalence of HIV-associated neurocognitive disorders is high (Cysique and Brew, 2009), and HIV patients who abuse drugs may be particularly vulnerable to impulsive decision making. Studies comparing substance abusers with and without co-occurring HIV infection have found that HIV-infected substance abusers demonstrate greater cognitive impulsivity on the Iowa Gambling Task (Gonzalez et al., 2005; Hardy et al., 2006; Martin et al., 2004). Specifically, they tend to select more cards from the disadvantageous, or “risky,” decks characterized by relatively large payoffs but also large penalties. HIV is believed to cause disruption in frontostriatal circuitry (Heaton et al., 1995) and most prominently affects sustained attention, executive functioning, and memory (Brew, 2004; Chang et al., 2001; Grassi et al., 1999; Heaton et al., 1995; Martin et al., 1995; Woods et al., 2008). The co-occurrence of drug abuse in HIV patients may contribute to neurocognitive impairments mediated by prefrontal-subcortical networks, including deficits in impulsivity and working memory (Farinpour et al., 2000; Martin et al., 2004; Rippeth et al., 2004). Additionally, HIV is an ultimately fatal disease that may alter an individual's time perspective and potentially increase the likelihood of engaging in behaviors that have immediate benefits, despite long-term consequences (Petry et al., 1998). Understanding the additive effects of substance use on cognitive impairment associated with HIV infection may inform the development of interventions aimed at reducing risk behaviors in HIV patients.
The present study examined cognitive impulsivity and brain functioning during a delay discounting task in HIV-positive adults with current, remitted, and no cocaine dependence. We investigated the effects of cocaine dependence because cocaine remains the most frequently abused illicit drug other than marijuana in HIV-positive adults (Cook et al., 2007; Korthuis et al., 2008; Kuo et al., 2004) and is associated with high levels of impulsivity (Coffey et al., 2003; Lejuez et al., 2005; Verdejo-Garcia et al., 2007). We hypothesized that active cocaine users would: (1) demonstrate greater delay discounting compared to recovered and naïve cocaine users; and (2) exhibit smaller increases in brain activation in prefrontal, anterior cingulate, and posterior parietal cortices during difficult choices relative to easy choices.
2. Methods
2.1. Participants and procedures
HIV-positive adults were recruited via advertisements in two local newspapers and on craigslist.com, and flyers distributed in community-based organizations serving HIV-positive adults. Eligibility criteria were 18-59 years of age, diagnosis of HIV infection, history of CD4 cell count <500/mL, and verified prescription for antiretroviral medications. Additionally, participants had to meet criteria for one of three cocaine groups: “active”, “recovered”, or “naïve”. The active group met diagnostic criteria for current cocaine dependence and had used cocaine in the past month. They could have other substance use disorders, but cocaine was the principal diagnosis and drug of choice. The recovered group met criteria for past cocaine dependence (as principal diagnosis and drug of choice) in sustained full remission and had not used cocaine, other illicit drugs, or alcohol to intoxication in ≥1 year. The naïve group had no lifetime substance use disorders. The Structured Clinical Interview for DSM-IV (First et al., 1996) and the Addiction Severity Index (McLellan et al., 1992) were used to diagnose substance use disorders and assess current and lifetime substance use. At both visits, urine toxicology screens were used to corroborate self-report of recent cocaine and other drug use.
Exclusion criteria were lifetime psychotic or bipolar disorder, acute psychiatric distress, loss of consciousness for ≥30 minutes and/or requiring medical treatment, major cognitive impairment, MRI contraindications, and illiteracy or lack of English fluency. The Mini International Neuropsychiatric Interview was used to confirm the absence of disqualifying psychiatric disorders and conditions (Sheehan et al., 1998). The Mini Mental State Exam was administered to screen and rule out individuals with gross cognitive impairment (score <24) (Folstein et al., 1975); all participants scored above this cut-off and were retained.
After providing written informed consent, participants completed clinical interviews and questionnaires and provided urine and blood samples. Eligible participants returned for a second visit that included additional clinical assessments and an MRI brain scan. Participants were compensated $50 for the first visit, regardless of eligibility, and $100 for the second visit. Of 42 eligible participants, three had unusable MRI data (one fell asleep, one was uncooperative, and one had dental work that caused an artifact in scan images), leaving a final sample of 39.
2.2. Measures
The Monetary Choice Questionnaire (MCQ) is a delay discounting task that measures cognitive impulsivity by examining the relationship between the value of a reward (in this case money) and the time to its delivery. Greater impulsivity is characterized by a preference for smaller immediate rewards over larger delayed rewards. The MCQ allows one to compute an individual's point of indifference where the perceived value of a smaller immediate reward is equivalent to a larger delayed reward (Kirby et al., 1999). Previous research has found that the relationship between the perceived value of a reward and the length of the delay to its receipt can be described using the following hyperbolic function: Vimmediate= Vdelayed/(1 + kD), in which V is value in dollars, D is delay in days, and k is a free parameter that determines the discount rate (Mazur and Coe, 1987). Individuals with higher k-values discount the future more steeply and are considered more impulsive (Hernstein, 1981). The MCQ consists of a fixed set of 27 choices between smaller immediate rewards ($11-78) and larger delayed rewards ($25-85, 7-186 days) (Kirby et al., 1999). Administration of the MCQ was computerized, and the order of choices was random across participants. Using the procedure described by Kirby, Petry, and Bickel (1999), values of the hyperbolic discount rate (i.e., the k-value) were inferred based on participant's choices between smaller immediate and large delayed rewards.
The Intertemporal Choice Task (ICT) is another delay discounting task that employs the same logic as the MCQ. However, the task was designed specifically for fMRI experiments (Monterosso et al., 2007). It consists of three types of choices: “hard,” “easy,” and “no.” Hard and easy trials involve choices between smaller immediate rewards and larger delayed rewards. The later options ranged in value ($20-50 for small rewards and $70-100 for large rewards) and in delay (7-90 days). A computer program was used to randomly generate the sequence and value of the later options once before any participants were scanned. The same sequence was then used for all participants. The corresponding immediate options were calculated for each individual based on their k-value from screening using the above discount function equation. For hard choices, the k-value remained constant, resulting in two options of approximately equivalent. For easy choices, the same procedure was used, except that the k-value was multiplied by 10 or 0.1, resulting in options of very different value. As a result, participants' choices for easy choices could be predicted and were expected to be similar across participants. For “no” choices, either an immediate or delayed option was presented with an alternative of “nothing”; this served as the baseline.
While lying in the scanner, participants viewed the choices on a computer screen and indicated their preference by pressing the corresponding button on a handheld fiber-optic response pad. Participants had been told that one of the choices would be randomly selected and honored. The scanning session included four runs of 36 trials each, separated by 2-6 min rest periods. Each trial had a fixed length of 9 s; the choice was presented for 8 s, followed by a 1 s blank screen. All participants received the same sequences of trials (differing only in the value of the immediate option). The runs were counterbalanced such that each contained 12 trials of the three choice types. All responses were checked for quality and consistency. Six participants chose the same now-later response for all easy and hard choices in a particular run. Three of these participants had only one such run; this run was deemed aberrant and was therefore discarded. The other three participants had three or four such runs, and so all runs were retained.
2.3. Imaging protocol
Scans were performed on a 3T Siemens Trio MR imaging system at the McLean Hospital Brain Imaging Center. T2 functional data were acquired during four scans of 5.5min each using single-shot gradient-echo echoplanar imaging (TR/TE= 3000/30 ms, matrix= 64*64, FOV= 224*224 mm, α= 90°). A head coil was used to obtain 41 3.5 mm interleaved axial slices spanning the whole brain acquired parallel to the AC-PC plane. This blood oxygenation-level dependent (BOLD) sequence yielded 108 images of isotropic 3.5 mm voxels for each scan. Automatic second-order shimming was performed over the fMRI imaging volume prior to acquisition. Other scans were inserted between the functional scans and served as inter-trial breaks. This included a conventional T1-weighted scan performed on the same functional prescription with identical susceptibility distortion (“matched-warped;” identical geometry to the fMRI scans except matrix= 256*256) and a T1-weighted MP-RAGE3D scan (FOV= 256*256*170 mm, voxels= 256*256*128).
2.4. Data analysis
Chi-square and ANOVA tests were used to compare the active, recovered, and naïve groups on socio-demographic and HIV disease characteristics and behavioral performance on the MCQ and ICT. The fMRI data were processed using FSL 4.1.2 (FMRIB Analysis Group, www.fmrib.ox.ac.uk/fsl). Functional images were de-spiked using an in-house program, corrected for slice timing and motion, spatially smoothed (5 mm FWHM Gaussian kernel), global 4D normalized, and high-pass temporal filtered. Functional images were first aligned to the high-resolution T1-weighted “matched-warped” scan, then to the MP-RAGE3D scan, and then to standard stereotaxic Montreal Neurological Institute coordinates. Data from individual runs for each participant were subjected to a general linear model. Regressors were derived from choice type (hard, easy, no), size of monetary reward (small, large), selection (now, later), and six motion estimates. A principal component analysis was performed on the 5,000 voxels in the residuals having the largest variance. The first eight components were used as additional nuisance regressors in a new general linear model, using the same pre-processed functional data (Madsen and Lund, 2006). From the resulting parameter estimates, three contrasts were examined: easy-no, hard-no, and hard-easy.
For each participant, data from individual runs were combined using a fixed-effects analysis. These results were then combined across all participants using random-effects models for each contrast. All results were thresholded to P< 0.01 uncorrected, and then cluster thresholded to P< 0.05 corrected over the entire brain using Gaussian random field theory (Worsley et al, 1992). For regions that exhibited significant F-values, all six group comparisons were examined using post-hoc t-tests, voxelwise thresholded to P< 0.01. Locations were assisted by the Harvard-Oxford cortical and subcortical structural atlases available in FSL.
Finally, a functional regions-of-interest (ROI) approach was employed to further illuminate the direction and magnitude of group differences in brain activation. ROIs were obtained using the hard-easy contrast averaged across all participants and the method delineated above. Activation, expressed as a percent change from the mean BOLD signal, was averaged across each ROI for each participant and contrast. These reduced data were compared across the three groups (active, recovered, and naïve) using ANOVA, with post-hoc t-tests employed as indicated and Cohen's d computed to estimate effect sizes.
3. Results
3.1. Participant characteristics
Table 1 describes the sample. The three groups did not differ significantly on any of these socio-demographic or HIV disease characteristics. Participants were 69% male, aged 27-58 years (48.1 ± 7.4, M ± SD), ethnically diverse (41% African-American, 35% Caucasian, and 23% Hispanic), and socioeconomically disadvantaged (41% living under the Federal poverty threshold). They had been diagnosed with HIV for 3-26 years (15.2 ± 6.0), with a mean CD4 cell count of 617.41 (SD= 354.05), and 64% had an AIDS diagnosis. The active and recovered groups had used cocaine regularly for an average of 20.3 ± 8.2 and 14.4 ± 5.6 years, respectively. Active participants had used cocaine on an average of 8.1 ± 7.5 days in the past month, and recovered participants had been in sustained full remission for an average of 10.9 ± 6.4 years. Co-occurring substance dependence was rare; only two participants in the active group had current alcohol dependence, and none had other drug dependencies.
Table 1. Participant characteristics by cocaine group.
| Active n = 15 | Recovered n = 13 | Naïve n = 11 | Statistic | p-value | |
|---|---|---|---|---|---|
| Age in years, M (SD) | 50.0 (6.96) | 49.8 (4.3) | 43.6 (9.3) | F(2, 36) = 3.15 | 0.055 |
| Male (%) | 73.3% | 53.8% | 81.8% | χ2(2) = 2.38 | 0.304 |
| Race/ethnicity (%) | χ2(6) = 7.52 | 0.139 | |||
| African-American | 60.0% | 46.2% | 9.1% | ||
| Caucasian | 20.0% | 38.5% | 54.4% | ||
| Hispanic | 20.0% | 15.4% | 36.4% | ||
| Years of education, M (SD) | 13.9 (2.2) | 13.4 (2.3) | 14.7 (2.8) | F(2, 36) = 0.94 | 0.400 |
| Currently single (%) | 86.7% | 92.3% | 100% | χ2(2) = 1.59 | 0.452 |
| Under Federal poverty threshold (%) | 46.7% | 46.2% | 27.2% | χ2(2) = 1.20 | 0.549 |
| Years since HIV diagnosis, M (SD) | 15.3 (6.4) | 17.1 (3.0) | 12.9 (7.8) | F(2, 36) = 1.45 | 0.248 |
| Diagnosis of AIDS | 66.7% | 53.8% | 72.7% | χ2(2) = 0.99 | 0.609 |
| Current CD4 count, M (SD) | 558.1 (351.6) | 764.9 (371.4) | 524.0 (307.5) | F(2, 36) = 1.79 | 0.181 |
3.2. Behavioral performance on delay discounting tasks
The mean discount rates on the MCQ for the active, recovered, and naïve groups were 0.059 ± 0.071, 0.025 ± 0.031, and 0.017 ± 0.027, respectively. To illustrate this, an active cocaine user with a discount rate of 0.059 was approximately indifferent in choosing between $31 immediately and $85 in 30 days. In contrast, a drug naïve participant with a discount rate of 0.017 was approximately indifferent in choosing between $56 immediately and $85 in 30 days. As in prior studies (Heil et al., 2006; Monterosso et al., 2007), the MCQ discount rates were skewed, so the data were normalized using a natural log transformation. The group difference in discount rates was not statistically significant [F(2,36)= 2.27, P= 0.118], but the effect sizes were strong (d = 0.620 for active versus recovered and d = 0.782 for active versus naïve).
As expected, the three groups performed similarly on the ICT administered during fMRI scanning. Because participants' choices were individualized based on their MCQ discount rates, we were able to predict their responses for the no and easy choice trials. For no trials, 98.2%, 97.1%, and 98.7% of active, recovered, and naïve participants, respectively, chose responses in the predicted direction [F(2,36)= 0.324, P= 0.725]. For easy trials, 80.9%, 87.4%, and 89.8% of active, recovered, and naïve participants chose responses in the predicted direction [F(2,36)= 1.509, P= 0.235]. For hard choices, the now and later choices were of equivalent value, so it was not possible to predict individuals' choices. All of these behavioral data were rerun controlling for age, gender, and HIV disease characteristics (years since HIV diagnosis, current CD4 count, and AIDS diagnosis), and results were unaffected.
3.3. Brain activation during ICT
Initially, data from all groups were combined to examine task-related activation during the ICT. As expected, there was increased activation during easy versus no choice trials (easy-no), and even greater increases during hard versus no choice trials (hard-no). Table 2 shows the five clusters of activation during easy-no trials. Activation was observed bilaterally in the prefrontal cortices, primary motor cortex (M1), ACC, SMA, IPS and parietal lobules, lateral occipital cortex, primary visual cortex, and cerebellum. During hard-no trials, activation was observed in similar regions captured as a single large cluster of 426.51 ml, with peak activation within the occipital pole (32, -96, 0; z-max= 7.93, cluster P< 0.00001). As shown in Table 2, there were significantly greater increases in activation during hard versus easy choice trials (hard-easy) bilaterally in the prefrontal cortices, ACC and SMA, IPS and parietal lobules, thalamus, and cerebellum.
Table 2.
Task-related activation during delay discounting choices (all participants combined).
| Contrast | Cluster size in ml | Max Z-score | MNI coordinates (x, y, z) at peak | Anatomical region at peak | Other regions in cluster |
|---|---|---|---|---|---|
| Easy-no | 5.39 | 5.61 | -30, 52, -18 | L frontal pole | L OFC, VLPFC, MFC |
| 6.21 | 4.37 | -6, 20, 44 | L/R ACC | L/R SMA | |
| 6.93 | 5.01 | -42, 2, 26 | L M1 | L/R VLPFC, DLPFC | |
| 19.46 | 5.76 | 50, 36, 24 | R frontal pole | R VLPFC, DLPFC, precentral gyrus | |
| 248.04 | 7.45 | 18, -96, -8 | R/L occipital pole | R/L IPS, parietal lobules, fusiform gyrus, visual cortex, cerebellum | |
| Hard-easy | 2.84 | 3.83 | 46, -60, -40 | R cerebellum | -- |
| 3.61 | 3.81 | -10, -16, 8 | R/L thalamus | R/L putamen | |
| 4.74 | 4.23 | -36, -66, -30 | L cerebellum | -- | |
| 5.20 | 3.66 | 50, 22, 32 | R DLPFC | R VLPFC, M1 | |
| 5.40 | 3.72 | 30, 52, 20 | R frontal pole | R DLPFC | |
| 5.78 | 4.39 | 36, -66, 34 | R IPS | R parietal lobules | |
| 6.30 | 4.01 | -38, -50, 42 | L IPS | L parietal lobules | |
| 8.80 | 4.58 | 0, 10, 64 | L superior frontal gyrus | L SMA, ACC | |
| 11.94 | 4.69 | -44, 38, 18 | L frontal pole | L DLPFC, VLPFC |
ACC = anterior cingulate cortex, DLPFC = dorsolateral prefrontal cortex, IPS = intraparietal sulcus, M1 = primary motor cortex, MFC = middle frontal cortex, OFC = orbitalfrontal cortex, VLPFC = ventrolateral prefrontal cortex, SMA = supplemental motor area.
Next, we compared the groups on the three contrasts. In clusters with significant group differences, active cocaine users consistently had smaller increases in brain activation relative to drug naïve participants (Table 3). In the easy-no contrast, the active group had smaller increases bilaterally in the IPS, parietal lobules, precuneus, ACC, and postcentral gyrus. In the hard-no contrast, they had smaller increases bilaterally in the VLPFC, IPS, parietal lobules, in the right DLPFC, thalamus, striatum, and insula, and in the left M1 and cuneal cortices. In the hard-easy contrast, they had smaller increases bilaterally in the M1 and ACC and in the right frontal pole (see Fig. 1).
Table 3.
Clusters showing smaller increases in activation during delay discounting choices among active cocaine versus drug naïve participants.
| Contrast | Cluster size in ml | Max Z-score | MNI coordinates (x, y, z) at peak | Anatomical region at peak | Other regions in cluster |
|---|---|---|---|---|---|
| Easy-no | 19.96 | 4.97 | 30, -68, 28 | R IPS | R/L parietal lobules, precuneus, ACC, postcentral gyus, L IPS |
| Hard-no | 4.45 | 4.55 | -52, 10, 2 | L VLPFC | L M1 |
| 5.46 | 5.79 | -24, -72, 44 | L superior parietal lobule | L IPS, inferior parietal lobules, cuneal cortices | |
| 6.06 | 5.4 | 46, 50, 12 | R frontal pole | R DLPFC, VLPFC | |
| 6.08 | 4.98 | 16, -16, 14 | R thalamus | R striatum (putamen, caudate), insula | |
| 8.44 | 4.45 | 32, -66, 32 | R IPS | R parietal lobules | |
| Hard-easy | 2.73 | 4.47 | -48, 2, 28 | L M1 | -- |
| 2.87 | 4.2 | 42, -2, 32 | R M1 | -- | |
| 4.46 | 4.24 | -6, 24, 36 | L/R ACC | L/R superior frontal gyrus | |
| 10.99 | 4.02 | 40, 54, 8 | R frontal pole | R OFC, VLPFC, DLPFC |
In no regions did active cocaine participants have larger increases in activation relative to drug naïve participants. ACC = anterior cingulate cortex, DLPFC = dorsolateral prefrontal cortex, IPS = intraparietal sulcus, M1 = primary motor cortex, MFC = middle frontal cortex, OFC = orbitalfrontal cortex, VLPFC = ventrolateral prefrontal cortex, SMA = supplemental motor area.
Fig. 1.
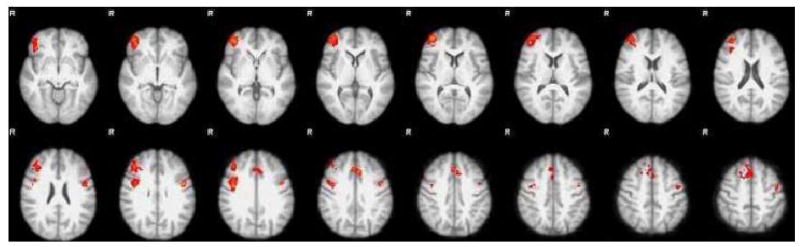
Differences in brain activation between naïve and active groups during hard versus easy choice trials. Colored regions represent clusters in which the naïve group had significantly greater increases in activation compared to the active group (see Table 4 for detailed information about the clusters). Images were thresholded at P< 0.01 uncorrected and then cluster thresholded to P< 0.05 corrected over the entire brain using Gaussian random field theory. Colors represent Z scores, and range from 2.3 (red) to 4.5 (yellow).
The pattern of activation was more complex for the recovered group (Table 4). In the easy-no contrast, recovered users had greater increases relative to active users bilaterally in the lateral occipital cortex and in the left frontal pole, and they had similar increases (i.e., no differences) relative to drug naïve participants. In the hard-no contrast, recovered users had larger increases relative to active users in the left inferior parietal lobule and IPS, the right cerebellum, and the brain stem, but they had smaller increases relative to drug naïve participants in the left inferior parietal lobule and bilaterally in the prefrontal cortices, postcentral gyrus, IPS, precuneus, insula, among other regions. Conversely, in the hard-easy contrast, recovered users had similar increases in brain activation relative to active cocaine users, and they had smaller increases relative to drug naïve participants bilaterally in the frontal pole, including prefrontal cortices. These results suggest that recovered cocaine users have similar patterns of activation relative to drug naïve participants during easy choices, but demonstrate impairments during hard choices.
Table 4.
Clusters showing smaller or larger increases in activation during delay discounting choices among recovered versus active and naïve participants.
| Contrast | Cluster size in ml | Max Z-score | MNI coordinates (x, y, z) at peak | Anatomical region at peak | Other regions in cluster |
|---|---|---|---|---|---|
| Easy-no | |||||
| Recovered > Active | 5.41 | 4.44 | -40, 46, 0 | L frontal pole | L OFC, VLPFC |
| 17.79 | 4.09 | 42, -80, 42 | R lateral occipital cortex (inferior parietal lobule) | R/L parietal lobules, precuneous | |
| Hard-no | |||||
| Recovered > Active | 3.35 | 4.03 | 38, -46, -38 | R cerebellum | R fusiform cortex, inferior temporal gyrus |
| 4.25 | 4.52 | -2, -38, -38 | R/L brain stem | ||
| 8.04 | 4.28 | 52, -50, 60 | L inferior parietal lobule | L IPS, lateral occipital cortex | |
| Recovered < Naïve | 4.03 | 4.06 | -58, -30, 38 | L inferior parietal lobule | L/R postcentral gyrus, IPS, precuneus, insula, parietal operculum, DLPFC, VLPFC; L frontal operculum, M1; R thalamus, striatum, pallidum, frontal pole |
| Hard-easy | |||||
| Recovered < Naïve | 2.80 | 3.55 | 44, 54, 10 | L frontal pole | L OFC, VLPFC |
| 3.26 | 4.18 | 44, 34, 28 | L DLPFC | L frontal pole, VLPFC | |
| 3.44 | 3.74 | -42, 36, 12 | R frontal pole | R VLPFC, DLPFC |
DLPFC = dorsolateral prefrontal cortex, IPS = intraparietal sulcus, OFC = orbitalfrontal cortex, VLPFC = ventrolateral prefrontal cortex.
3.4. ROI analyses
Fig. 2 shows the percent BOLD signal change during easy-no (2A) and hard-no (2B) contrasts in the nine ROIs (listed in Table 2 as clusters activated in hard-easy contrast). Both types of choices were associated with increased activation relative to baseline in the plotted ROIs, with hard choices requiring substantially more activation than easy choices. In general, active cocaine users had smaller increases in activation during both easy and hard choices. In the easy-no contrast, the active group had minimal increases in BOLD signal change relative to the recovered and naïve groups, with significant differences in the left frontal pole [t(37)= -2.27, P= 0.029, d= -0.717], left IPS [t(37)= -3.11, P= 0.004, d= -0.956), and right IPS [t(37)= -3.01, P= 0.005, d= -0.920). Active users had greater increases in activation in the hard-no contrast than the easy-no contrast. For example, the mean change in the right IFG cluster was 0.21% for hard choices versus 0.03% for easy choices. However, once again, active users had smaller increases relative to naïve participants, with significant differences in the right frontal pole [t(24)= 2.60, P= 0.016, d= -1.037], left ACC/SMA [t(24)= 2.58, P= 0.016, d= -0.717], right DLPFC [t(24)= -2.40, P= 0.025, d= -0.985], and right IPS [t(24)= 2.40, P= 0.025, d= -0.980]. In the hard-no contrast, recovered users had increases that were generally intermediate relative to active and naïve users; they differed significantly only relative to active cocaine users in the right IPS [t(26)= -2.05, P= 0.050, d= -0.785].
Fig. 2.
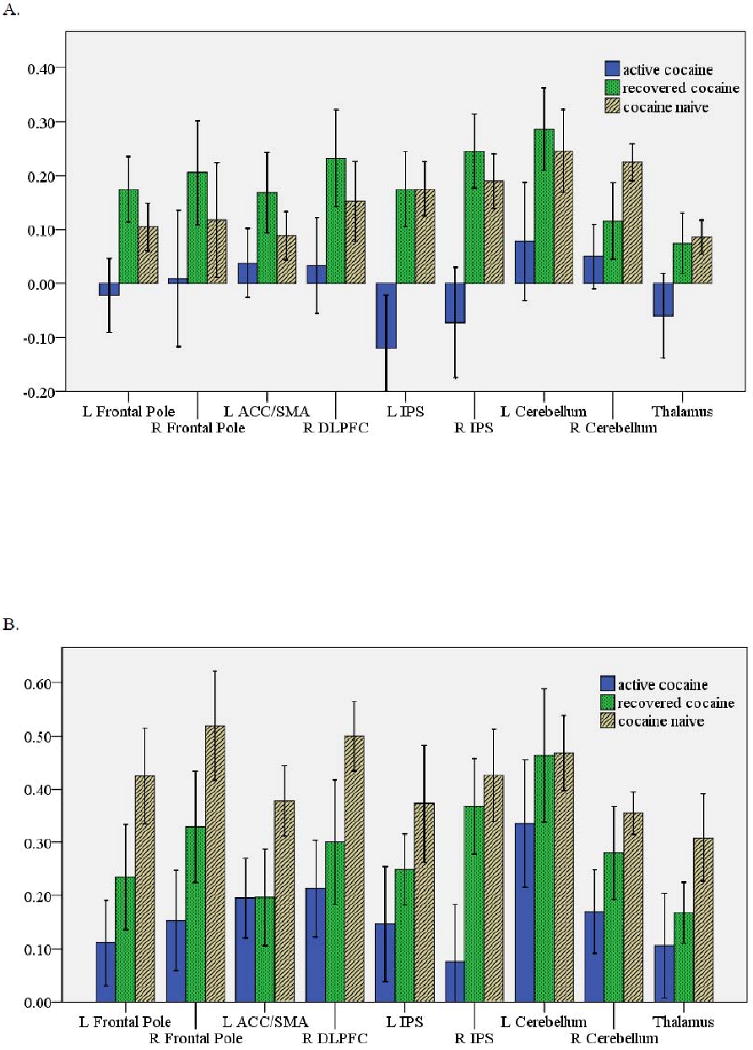
Percent BOLD signal change (mean ± standard error) for each group for: (A) easy relative to no choice trials, and (B) hard relative to no choice trials. In the easy-no contrast, the active group had significantly reduced increases in activation compared to the recovered and naïve groups in the L frontal pole, L IPS, and R IPS. In the hard-no contrast, the active group had significantly less increases in activation compared to the naïve group in the L frontal pole, R frontal pole, R DLPFC, and R IPS.
4. Discussion
Despite the poor clinical and behavioral outcomes associated with cocaine use, many HIV patients continue to use cocaine (Cook et al., 2007; Korthuis et al., 2008; Kuo et al., 2004). This study is the first to examine the effects of cocaine dependence on brain functioning associated with delay discounting choices among HIV patients, and to tease apart effects due to current versus past cocaine dependence. We found that participants with current cocaine dependence had smaller increases in activation in executive regions of the brain when making both easy and hard choices, with deficits primarily in the frontoparietal system. These impairments may help to explain why so many HIV-infected individuals who currently abuse cocaine, relative to those who do not abuse drugs, engage in behaviors associated with HIV disease progression and transmission, including continued cocaine use, risky sex, and medication non-adherence.
The executive network that exerts cognitive control over reward-related decision making requires neural recruitment in the frontoparietal network (Cole and Schneider, 2007). Our results support the involvement of the executive system, characterized by frontoparietal activation, during delay discounting choices. Consistent with previous studies (Hoffman et al., 2008; McClure et al., 2004; Monterosso et al., 2007; Tanaka et al., 2004), the delay discounting task in this study evoked bilateral activation in the prefrontal cortices, ACC, SMA, IPS, parietal lobules, and visual cortex, with greater increases in activation during hard versus easy choices. This broad pattern of activity likely reflects the complex nature of the task, which requires evaluation of immediate and delayed options, comparison of the options, selection of the preferred option, and a motor response to indicate the final choice. Deficits in the executive decision making network among active cocaine users were evident during easy choices in which the discounted values of the immediate and delayed values differed dramatically, with smaller increases in activation broadly in the lateral occipital cortex, including the IPS and parietal lobules. These differences were evident despite the fact that active users were equally likely to choose immediate versus delayed options, suggesting that the effects were associated with underlying differences in the neural processes associated with decision making rather than different response patterns. Deficits persisted during hard choices in which the discounted values of the immediate and delayed options were approximately equivalent. During the hard-easy contrast, smaller increases in activation were evident bilaterally in the ACC and primary motor cortex and the right prefrontal cortices.
When examining the pattern of brain activation as a function of choice difficulty, we found that active cocaine users had minimal neural recruitment in the prefrontal cortex and IPS during easy choices, while both recovered and naïve cocaine users evidenced substantial increases in neural recruitment in these regions. This was in contrast to two prior studies that found minimal change in activation among controls and greater increases among methamphetamine users during easy choices (Hoffman et al., 2008; Monterosso et al., 2007). The different patterns of activation in our study could relate to the fact that our participants also had HIV infection. The HIV virus infiltrates the central nervous system shortly after infection and becomes localized in various brain regions, causing direct and indirect damage to brain structure and functioning (Anthony and Bell, 2008; Hult et al., 2008). Executive dysfunction associated with HIV disease is believed to be associated specifically with disruptions in fronto-striatal circuitry. Concentrations of HIV viral RNA are highest in the striatum and lowest in the frontal cortex (Kumar et al., 2007). Volume loss in the striatum (Kieburtz et al., 1996) and in the premotor and prefrontal cortices (Thompson et al., 2005) has been found to be associated with greater cognitive impairment. Prior fMRI studies have reported that HIV-positive individuals demonstrate hypoactivity in the striatum and prefrontal cortices relative to HIV-negative individuals (Melrose et al., 2008), and that HIV patients with even mild or undetectable neurocognitive impairment require increased neural recruitment in prefrontal and posterior parietal regions during working memory and attentional tasks, with greater declines in neural efficiency relative to controls over time (Chang et al., 2001; Chang et al., 2004; Ernst et al., 2002; Ernst et al., 2009). Thus, it is possible that our drug naïve group may have required greater neural recruitment than would an HIV-negative healthy comparison group during easy choices due to mild or undetectable neuronal injury caused by HIV infection.
Recovered cocaine users demonstrated a more complex pattern of brain activation during the delay discounting task. For easy choices, they performed similarly to drug naïve participants, with greater increases in activation compared to active cocaine users. In contrast, for hard choices, they evidenced deficits similar to those observed in active cocaine users. It is possible that sustained remission from cocaine use may reverse some of the impairments associated with active cocaine dependence. Specifically, recovered users may regain executive functions that allow them to perform efficiently during relatively easy choices (Ersche et al., 2005). However, deficits associated with prior cocaine dependence may become evident when the brain is taxed during hard choices. Prospective studies that follow cocaine users through the recovery process will be necessary to address these hypotheses.
In sum, results suggest that chronic cocaine dependence among HIV patients is associated with impaired neuronal functioning during delay discounting choices. The smaller increases in activation in the executive network observed among active cocaine users relative to recovered and non users during both hard and easy choices may reflect less efficient cognitive control in the executive decision making network. An inefficient network may make the comparison of alternative choices more difficult, and simpler choices may become more appealing, even if not adaptive in the long-term (Hoffman et al., 2008).
This study was designed as a proof-of-concept and has limitations. First, due to the cross-section design, it is not possible to determine the cause of the differences observed between active, recovered, and naïve groups. Second, the study was underpowered to detect group differences on the MCQ, but the effect sizes were strong and consistent with previous research. Third, we did not include an HIV-negative control group to identify the unique effects of HIV infection on impulsivity and brain functioning. Future studies might consider adding such a control group to test for potential additive and/or interactive effects of HIV infection and cocaine dependence on neurocognitive functioning. Nevertheless, this study is one of the few to examine the effects of cocaine dependence on brain functioning in HIV patients. Given that methamphetamine is also a popular drug of abuse among HIV-infected individuals and has deleterious effects on neurocognitive functioning, future studies might use the delay discounting paradigm to examine its effect on brain functioning in HIV patients and to identify potential differences associated with cocaine and methamphetamine. Additional strengths of this study include the recruitment of non-treatment seeking cocaine users and a comparison group of recovered cocaine users to control for potential socioeconomic and personality differences in individuals with and without histories of drug dependence.
In conclusion, we found that HIV patients who actively use cocaine had deficits in the executive decision making system compared to those who do not use drugs, with smaller increases in activity in the frontoparietal regions when making easy and hard delay discounting choices. These deficits may contribute to impulsive decision making characterized by steeper discounting of future rewards. This may help to explain why HIV patients with cocaine dependence are so vulnerable to engagement in health risk behaviors, such as continued drug use, poor treatment adherence, and sexual risk behavior, that have short-term gains (e.g., pleasure) but long-term consequences (e.g., disease progression). Further research should explore whether the effects of chronic cocaine use on neurobiological functioning may be further exacerbated by the deleterious effects of HIV on the central nervous system.
Acknowledgments
Funding for this study was provided by grants from amfAR, The Foundation for AIDS Research (106884-42-RFBR), the National Institute on Drug Abuse (T32-DA01536, K25-DA016612, K05-DA00343), and the Harvard University Center for AIDS Research (P30-AI60354). The authors thank Dr. Steven Safren for his mentorship and Jessica Eldridge, Nina Conn, and Tiffany Chu for their assistance collecting and entering data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Bell JE. The neuropathology of HIV/AIDS. International Review of Psychiatry. 2008;20:15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegran H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102 1:33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. Journal of Acquired Immune Deficiency Syndrome. 2009;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry. 2001;58:721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Brew BJ. Evidence for a change in AIDS dementia complex in the era of highly active antiretroviral therapy and the possibility of new forms of AIDS dementia complex. AIDS. 2004;18 1:75–78. [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neuronal correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, Ernst T. Adaptation of the attention network in human immunodeficiency virus brain injury. Annals of Neurology. 2004;56:259–272. doi: 10.1002/ana.20190. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Levine AM, Grey DD. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS. 2008;22:1355–1363. doi: 10.1097/QAD.0b013e32830507f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA, Grey DD, Burke-Miller JK, Cohen MH, Vlahov D, Kapadia F, Wilson TE, Cook R, Schwartz RM, Golub ET, Anastos K, Ponath C, Goparaju L, Levine AM. Illicit drug use, depression and their association with highly active antiretroviral therapy in HIV-positive women. Drug and Alcohol Dependence. 2007;89:74–81. doi: 10.1016/j.drugalcdep.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychology Review. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang LJ, James N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, Ricardo-Dukelow ML, Chang L. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Annals of Neurology. 2009;65:316–325. doi: 10.1002/ana.21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology. 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinpour R, Martin EM, Seidenberg M, Pitrak DL, Pursell KJ, Mullane KM, Novak RM, Harrow M. Verbal working memory in HIV-seropositive drug users. Journal of the International Neuropsychological Society. 2000;6:548–555. doi: 10.1017/s1355617700655042. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient/Non-patient Edition. Biometrics Research, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Nunnally G, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Grassi B, Garghentini G, Campana A, Grassi E, Bertelli S, Cinque P, Epifani M, Lazzarin A, Scarone S. Spatial working memory in asymptomatic HIV-infected subjects. Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:387–391. doi: 10.1176/jnp.11.3.387. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzke AJ, Williams ML, Bowen AM. Binge use of crack cocaine and sexual risk behaviors among African-American, HIV-positive users. AIDS and Behavior. 2009;13:1106–1118. doi: 10.1007/s10461-008-9450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Anderson B, Stein M. Sexual risk behaviors among substance users: Relationship to impulsivity. Psychology of Addictive Behaviors. 2006;20:328–332. doi: 10.1037/0893-164X.20.3.328. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addictive Behaviors. 2006;31:1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hernstein RJ. Self control as response strength. In: Bradshaw CM, Szabadi E, Lowe CF, editors. Quantification of Steady State Operant Behavior. Elsevier/North Holland Biomedical Press; Amsterdam: 1981. pp. 3–20. [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS and Behavior. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology. 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, Mitchell SH. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology. 2008;201:183–193. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. International Review of Psychiatry. 2008;20:3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O'Brien CP. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychology of Addictive Behaviors. 2001;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, Hickey C, Feigin A, Caine ED. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology. 1996;53:155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Korthuis PT, Zephyrin LC, Fleishman JA, Saha S, Josephs JS, McGrath MM, Hellinger J, Gebo KA. Health-related quality of life in HIV-infected patients: the role of substance use. AIDS Patient Care and STDs. 2008;22:859–867. doi: 10.1089/apc.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. Journal of Neurovirology. 2007;13:210–224. doi: 10.1080/13550280701327038. [DOI] [PubMed] [Google Scholar]
- Kuo WH, Wilson TE, Weber KM, Madhava V, Richardson J, Delapenha R, Des Jarlais D. Initiation of regular marijuana use among a cohort of women infected with or at risk for HIV in the Women's Interagency HIV Study (WIHS) AIDS Patient Care and STDs. 2004;18:702–713. doi: 10.1089/apc.2004.18.702. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Bornovalova MA, Daughers SB, Curtin JJ. Differences in impulsivity and sexual risk behavior among inner-city crack/cocaine users and heroin users. Drug and Alcohol Dependence. 2005;77:169–175. doi: 10.1016/j.drugalcdep.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Simmons BL, Aklin WM, Daughters SB, Dvir S. Risk-taking propensity and risky sexual behavior of individuals in residential substance use treatment. Addictive Behaviors. 2004;29:1643–1647. doi: 10.1016/j.addbeh.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Lyons T, Chandra G, Goldstein J. Stimulant use and HIV risk behavior: the influence of peer support group participation. AIDS Education and Prevention. 2006;18:461–473. doi: 10.1521/aeap.2006.18.5.461. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Pursell KJ, Mullane KM, Novak RM. Delayed recognition memory span in HIV-1 infection. Journal of the International Neuropsychological Society. 1995;1:575–580. doi: 10.1017/s1355617700000710. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Mazur JE, Coe D. Tests of transitivity in choices between fixed and variable reinforcer delays. Journal of the Experimental Analysis of Behavior. 1987;47:287–297. doi: 10.1901/jeab.1987.47-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behavioural Brain Research. 2008;188:337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry N, Bickel W, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- SAMSHA . Results from the 2007 National Survey on Drug Use and Health: National Findings. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: 2008. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Siqueland L, Crits-Christoph P, Gallop R, Barber JP, Griffin ML, Thase ME, Daley D, Frank A, Gastfriend DR, Blaine J, Connolly MB, Gladis M. Retention in psychosocial treatment of cocaine dependence: predictors and impact on outcome. The American Journal on Addictions. 2002;11:24–40. doi: 10.1080/10550490252801611. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel P, Hatsukami D. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and Clinical Psychopharmacology. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neuroscience. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker JS, Orlando M, Burnam MA, Sherbourne C, Kung FY, Gifford AL. Psychosocial mediators of antiretroviral nonadherence in HIV-positive adults with substance use and mental health problems. Health Psychology. 2004;23:363–370. doi: 10.1037/0278-6133.23.4.363. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. Journal of Clinical and Experimental Neuropsychology. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive Behaviors. 2007;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Klein RS. A prospective study of HIV disease progression in female and male drug users. AIDS. 1999;13:257–262. doi: 10.1097/00002030-199902040-00014. [DOI] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22:110–117. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zule WA, Costenbader E, Coomes CM, Meyer WJ, Jr, Riehman K, Poehlman J, Wechsberg WM. Stimulant use and sexual risk behaviors for HIV in rural North Carolina. Journal of Rural Health. 2007;23 Suppl:73–78. doi: 10.1111/j.1748-0361.2007.00127.x. [DOI] [PubMed] [Google Scholar]


