Abstract
Objective
Hypoxia has been established as a key factor influencing the pathophysiology of malignant growth. Hypoxia-induced changes in gene expression are coordinated primarily by hypoxia inducible factor-1 alpha (HIF-1α) and HIF-2α. The purpose of this study was to determine whether or not HIF-2α expression is associated with survival and response to radiation in patients with cervical cancer.
Methods
After reviewing the medical records of 119 patients treated in our institution by primary therapy for stage IIB-IVA cervical cancer, we performed a case-control study. Cases (n=12) were selected from patients with local recurrence or radiation failure after primary radiation therapy with or without concurrent chemoradiation. For each case, we selected two controls from patients who had no evidence of local recurrence. Using pre-treatment paraffin-embedded tissues, we evaluated the expression of HIF-2α by immunohistochemistry. Staining was scored based on intensity (intensity score [IS], 0-3) and proportion (proportion score [PS], 0-100). The results were analyzed by the Student t-test, Mann-Whitney U test, Fisher's exact test, and Cox proportional hazards regression model.
Results
Cytoplasmic expression of HIF-2α, representing the degree of hypoxia, had a relationship with poor response to radiotherapy. The hazard ratio of recurrence was 1.71 for the HIF-2α IS (p=0.110) and 1.04 for the HIF-2α PS (p<0.001), indicating that the HIF-2α staining area correlates weakly with the risk for recurrence.
Conclusion
The HIF-2α expression area may have an important role in radioresistance in patients with locally advanced cervical cancer. We conclude that a wider area of hypoxia predicts an increased probability of radioresistance.
Keywords: Cervical neoplasm, Radiation, HIF-2α, Survival, Hypoxia
INTRODUCTION
Radiation therapy with or without cisplatin-containing chemotherapy is the most commonly used treatment modality in patients with advanced cervical cancer [1]. However, recurrence after radiotherapy remains a problem in the treatment of locally advanced cervical cancer. Therefore, a study focusing on the molecules affecting radioresistance is important to determine the mechanism underlying radioresistance.
Hypoxia has been established as a key factor influencing the pathophysiology of malignant growth. Most measurable solid tumors, which consume a large amount of oxygen, have regions with inefficient vascular supplies. Such low-oxygen regions have a poorer prognosis and worse response to treatment than better oxygenated tumors. Tumor hypoxia is known to be an important factor associated with chemoresistance and radioresistance [2-4].
Hypoxia is associated with radiation therapy because depletion of oxygen disturbs radiolysis of H2O and reduces the production of reactive and cytotoxic species, and because radiation-induced DNA damage is fixed and irreparable under normoxia [5,6]. Members of the hypoxia inducible factor (HIF) family of transcription factors regulate the cellular response to hypoxia. There have been several studies regarding HIF-1α and radioresistance [7,8]. On the contrary, there have been few studies regarding HIF-2α. Moreover, the results are conflicting.
HIF-1α and HIF-2α have contrasting roles, as evidenced by the opposing regulation of c-Myc [9]. Moeller et al. [10] reported that HIF-1α stimulates p53 phosphorylation and p53-dependent cell death in cancer cells. Conversely, Bertout et al. [11] demonstrated that HIF-2α inhibition promotes p53 activity.
A clinical study of radiation treatment for head and neck cancers reported that the levels of both HIF-1α and HIF-2α correlate with radiation resistance [8]. However, in experiments by Palayoor et al. [12], transfection of von Hippel-Lindau (VHL) into renal cell carcinoma lacking this gene did not alter radiation sensitivity, despite leading to an increase in protein levels for HIF-1α and HIF-2α. A recent study conducted by Franovic et al. [13] showed that HIF-2α has a greater role in tumorigenesis than HIF-1α via deactivation of select receptor tyrosine kinases, including epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor (IGF1R), as well as downstream extracellular-regulated kinase (ERK)/Akt signaling.
It has been reported that overexpression of HIF-1α in uterine cervical cancer cells prior to treatment serves as a predictive marker for poor prognosis following treatment by radiotherapy [7,14-16]. Recently, the same results regarding HIF-2α have been released showing that HIF-2α expression is associated with prognosis in patients with cervical cancer undergoing radiotherapy [17].
In this study, we evaluated HIF-2α expression in patients with locally advanced cervical cancer using immunohistochemistry, and correlated HIF-2α staining with local recurrence or failure to respond to radiation treatment.
MATERIALS AND METHODS
1. Patient selection
According to the availability of cancer tissue, we identified 119 patients with locally advanced stage IIB-IVA cervical cancer who completed primary radiotherapy in the Department of Radiation Oncology at Samsung Medical Center of Sungkyunkwan University School of Medicine between 1994 and 2004. We reviewed charts according to Institutional Review Board guidelines and performed a case-control study. Cases (n=12) were selected from patients with local recurrence or radiation failure after primary radiation therapy, and constituted the radioresistant group. Age-matched control patients were selected who had no local recurrence for at least 3 years after radiation therapy, which constituted the radiosensitive group (n=24). Slides were prepared from stored pre-treatment paraffin-embedded tissue blocks from the 36 patients.
External beam radiotherapy (EBRT) and high-dose rate (HDR) intracavitary brachytherapy [18] were completed according to schedule. The whole pelvis total dose was 50.40 gray (Gy) with 1.8 Gy daily fractions administered 5 times a week. HDR brachytherapy was started 4-5 weeks after initiation of EBRT. The dose of HDR brachytherapy was 24 Gy at point A, with 4 Gy per fraction twice a week for 3 weeks. Progression-free survival (PFS) was defined from the end of treatment to the day of recurrence by imaging studies or biopsies.
2. Immunohistochemical staining
Sections (6-µm thick) were deparaffinized with xylene, rehydrated, and incubated with fresh 0.3% hydrogen peroxide in methanol for 30 minutes at room temperature. Specimens were rehydrated through a graded ethanol series and washed in phosphate-buffered saline (PBS). After a blocking treatment, the specimens were incubated with anti-HIF-2α antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at a dilution of 1:200 in PBS containing 1% bovine serum albumin (BSA) at 4℃ overnight. Sections were then washed with PBS and incubated in secondary antibody for 30 minutes at room temperature. The tissue sections were lightly counterstained with hematoxylin, then examined by light microscopy. There was no detectable staining in negative controls, which were prepared by omitting the primary antibody [19].
3. Evaluation
Two examiners without knowledge of the clinical outcome evaluated the staining. One gynecologic pathologist (COS) and one gynecologic oncologist (TJK) reviewed the blinded slides and evaluated the immunohistochemical data independently.
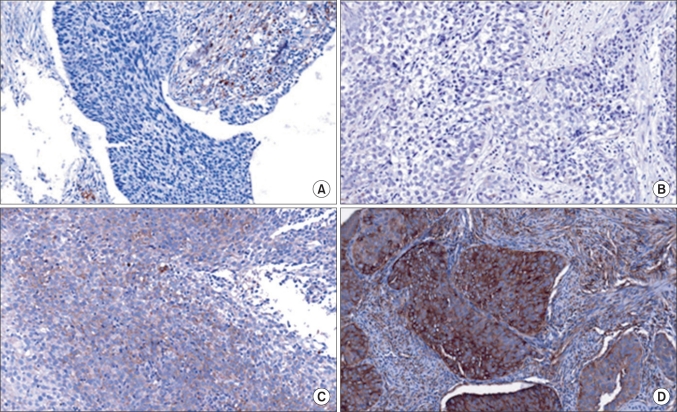
Staining intensity was scored from 0-3, as follows: 0, no appreciable staining in the tumor cells; 1, barely detectable staining in the cytoplasm, nucleus, or membrane compared with stromal elements; 2, readily detectable brown staining distinctly marking the tumor cell cytoplasm, nucleus, or membrane; and 3, dark brown staining in tumor cells completely obscuring the cytoplasm, nucleus, or membrane [20]. The staining was scored based on intensity (intensity score [IS], 0-3) and proportion (proportion score [PS], 0-100) (Fig. 1).
Fig. 1.
Proportion score and intensity score examples of hypoxia inducible factor-2α expression (×200). A: 0 (0), B: 1 (1), C: 30 (2), D: 90 (3).
4. Statistical analysis
We used SAS ver. 9.1.3 (SAS Institute Inc., Cary, NC, USA) for statistical calculations. For continuous variables, we used the t-test for variables that were normally distributed and the Mann-Whitney U test for variables that were not normally distributed. Fisher's exact test was performed for categorical variables. To evaluate the relationship of HIF-2α staining to PFS, we used a Cox proportional hazards regression model.
RESULTS
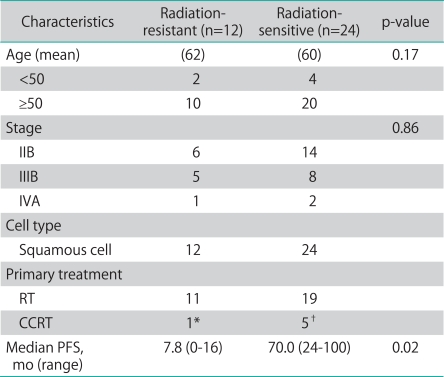
The patient characteristics are described in Table 1. The median follow-up for the patients studied was 49 months. At the time of analysis, all case patients had succumbed from causes related to cervical cancer, and all control patients were alive with no evidence of recurrence.
Table 1.
Patient characteristics
RT: radiation therapy, CCRT: concurrent chemoradiation therapy, PFS: progression free survival, FU: fluorouracil.
*Cisplatin+5FU. †Cisplatin+5FU (n=4) and weekly Cisplatin (n=1).
There were no statistical differences in age, cell type, and International Federation of Gynecology and Obstetrics (FIGO) stages between the two groups. Fig. 1 shows the examples of cellular distribution of HIF-2α staining. The results of immunohistochemical expression of HIF-2α in 36 cervical squamous cell carcinomas (12 radioresistant and 24 radiosensitive) are summarized in Tables 2-4. The expression of HIF-2α was significantly more frequent between one and two stainings in both groups (Table 2). But we showed that the IS (p=0.133), unlike the PS, did not have a close relationship with survival in the case group (Table 3).
Table 2.
Immunohistochemical analysis of HIF-2α expression in radiation-sensitive and radiation-resistant cervical cancer tissues
HIF: hypoxia inducible factor, IS: intensity score.
Table 4.
Relationship between HIF-2α PS and hazard ratio of recurrence
HIF: hypoxia inducible factor, PS: proportion score, HR: hazard ratio, CI: confidence interval.
Table 3.
Distribution of HIF-2α staining in two groups
HIF: hypoxia inducible factor, IS: intensity score, PS: proportion score.
Intensity shows degree or severity of staining, and proportion means area or boundary of staining. Therefore PS more accurately represented the hypoxic area in tumors regarding risk of recurrence (Table 4).
Average PS were 20±40 (radiation-resistant group) and 10±17 (radiation-sensitive group) (p<0.05).
The PFS of all patients was analyzed based upon HIF-2α expression. The median PFS was 9 months for patients in the radioresistant group and 73 months for the radiosensitive group. The hazard ratio of recurrence was 1.525 for HIF-2α PS (p<0.05) (Table 4), indicating that HIF-2α PS correlates weakly with recurrence risk and is a pathologic prognostic factor.
DISCUSSION
In the current study, HIF-2α expression was evaluated in patients with locally advanced cervical cancer prior to treatment with primary radiotherapy, with a positive association between HIF-2α expression in terms of PS and local recurrence or radiation failure. In addition, the correlation between the area of staining with the risk of recurrence correlated with prognosis in patients with cervical cancer. With 10 unit increase of PS, risk of recurrence increases 1.525 times more than lower unit. Based on the data shown in Tables 3 and 4, we conclude that a wider area of hypoxia increase the probability of radioresistance. With regard to PS, it appears that the stained area represents a more hypoxic than other area. Although we did not know the exact mechanism of radioresistance, it may provide some hint with regard to the relationship with oxygen and radiation.
According to a previous study regarding HIF-1α with radiation, it promotes tumor vasculature radioresistance through the release of proangiogenic cytokines such as VEGF [21]. HIF-2α is the second HIF family member to be identified and shares approximately 48% amino-acid sequence homology with HIF-1α. So it shares some similarity.
Kawanaka et al. [17] suggested the ratio of the HIF-2α positive cell in tumor infiltrative macrophages may be a new predictive indicator for prognosis. Our study also solidified that HIF-2α staining may be a key in radioresistance in cervical cancer.
In our study, we did not demonstrate a strong relationship between IS and radioresistance. However, staining may change subjectively, thus a more accurate in vitro test and more samples are needed to confirm the relationship.
Although HIF-2α PS was related with PFS, the hazard ratio of IS and PS did not meet the criteria of the previous study.
Footnotes
No potential conflict of interest relevant to this article was reported.
This abstract was adopted as a poster presentation in 2010 AACR (American Association for Cancer Research) annual meeting.
References
- 1.Petignat P, Roy M. Diagnosis and management of cervical cancer. BMJ. 2007;335:765–768. doi: 10.1136/bmj.39337.615197.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hockel M, Vorndran B, Schlenger K, Baussmann E, Knapstein PG. Tumor oxygenation: a new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol Oncol. 1993;51:141–149. doi: 10.1006/gyno.1993.1262. [DOI] [PubMed] [Google Scholar]
- 3.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and radiation response in human tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 4.Sutherland RM, Ausserer WA, Murphy BJ, Laderoute KR. Tumor hypoxia and heterogeneity: challenges and opportunities for the future. Semin Radiat Oncol. 1996;6:59–70. doi: 10.1053/SRAO0060059. [DOI] [PubMed] [Google Scholar]
- 5.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 6.Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955;9:539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa H, Sakurai H, Hasegawa M, Mitsuhashi N, Takahashi M, Masuda N, et al. Expression of hypoxic-inducible factor 1alpha predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;60:513–521. doi: 10.1016/j.ijrobp.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos C, Turley H, Talks K, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192–1202. doi: 10.1016/s0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]
- 9.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Bertout JA, Majmundar AJ, Gordan JD, Lam JC, Ditsworth D, Keith B, et al. HIF2alpha inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci U S A. 2009;106:14391–14396. doi: 10.1073/pnas.0907357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palayoor ST, Burgos MA, Shoaibi A, Tofilon PJ, Coleman CN. Effect of radiation and ibuprofen on normoxic renal carcinoma cells overexpressing hypoxia-inducible factors by loss of von Hippel-Lindau tumor suppressor gene function. Clin Cancer Res. 2004;10:4158–4164. doi: 10.1158/1078-0432.CCR-04-0005. [DOI] [PubMed] [Google Scholar]
- 13.Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2alpha oncogenic axis. Proc Natl Acad Sci U S A. 2009;106:21306–21311. doi: 10.1073/pnas.0906432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchison GJ, Valentine HR, Loncaster JA, Davidson SE, Hunter RD, Roberts SA, et al. Hypoxia-inducible factor 1alpha expression as an intrinsic marker of hypoxia: correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clin Cancer Res. 2004;10:8405–8412. doi: 10.1158/1078-0432.CCR-03-0135. [DOI] [PubMed] [Google Scholar]
- 15.Bachtiary B, Schindl M, Potter R, Dreier B, Knocke TH, Hainfellner JA, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234–2240. [PubMed] [Google Scholar]
- 16.Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:494–501. doi: 10.1016/s0360-3016(02)04579-0. [DOI] [PubMed] [Google Scholar]
- 17.Kawanaka T, Kubo A, Ikushima H, Sano T, Takegawa Y, Nishitani H. Prognostic significance of HIF-2alpha expression on tumor infiltrating macrophages in patients with uterine cervical cancer undergoing radiotherapy. J Med Invest. 2008;55:78–86. doi: 10.2152/jmi.55.78. [DOI] [PubMed] [Google Scholar]
- 18.Nakano T, Kato S, Ohno T, Tsujii H, Sato S, Fukuhisa K, et al. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer. 2005;103:92–101. doi: 10.1002/cncr.20734. [DOI] [PubMed] [Google Scholar]
- 19.Lee SY, Chung SM. Neovastat (AE-941) inhibits the airway inflammation via VEGF and HIF-2 alpha suppression. Vascul Pharmacol. 2007;47:313–318. doi: 10.1016/j.vph.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Freitas S, Moore DH, Michael H, Kelley MR. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: correlations with tumor progression and platinum resistance. Clin Cancer Res. 2003;9:4689–4694. [PubMed] [Google Scholar]
- 21.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]