Abstract
Vitamin B12 or cobalamin deficiency, a rare clinical entity in pediatric age, is found most exclusively in breastfed infants, whose mothers are strictly vegetarian non-supplemented or with pernicious anaemia. In this article, the authors describe a 10-month-old infant admitted for vomiting, refusal to eat and prostration. The infant was exclusively breastfed and difficulties in introduction of new foods were reported. Failure to thrive since 5 months of age was also noticed. Laboratory evaluation revealed severe normocytic normochromic anaemia and cobalamin deficit. A diagnosis of α-thalassemia trait was also made. Maternal investigation showed autoimmune pernicious anaemia. This case shows the severity of vitamin B12 deficiency and the importance of adopting adequate and precocious measures in order to prevent potentially irreversible neurologic damage.
Background
Human species does not synthesise vitamin B12 (cobalamin), and therefore the exogenous intake occurs through ingestion of animal products (fish, meat and dairy products).1–7 In developed countries, vitamin B12 deficiency is found primarily in exclusively breastfed infants of mothers with deficit of this vitamin1 3 4 6 8–10: strict vegetarian or suffering from undiagnosed or untreated pernicious anaemia,1–4 7–9 11 12 malnutrition or other serious malabsorption syndrome.1 5 7
In Western countries, this disease is rare in infancy,3 4 9 with unknown incidence.3 The cobalamin deficit presents biochemical, haematologic and neurologic manifestations and their reversibility depends on early intervention.10 12
Given the rarity of this disease and the severity of its potential sequelae, we present the case of an infant exclusively breastfed with severe vitamin B12 deficiency, whose mother had undiagnosed pernicious anaemia, alerting to vitamin B12 deficit and the importance of adopting measures that prevent neurologic lesions potentially irreversible.
Case presentation
A 10-month-old Caucasian girl, born after a normal full-term pregnancy and delivery, whose Apgar scores were recorded as 9 and 10 at 1st and 5th min respectively, is described. Intrauterine growth restriction was apparent at birth weight of 2710 g (10–25th percentile), length 44.5 cm (below the 10th percentile) and head circumference 32 cm (10–25th percentile). First child of young parents not consanguineous, apparently healthy, asymptomatic and with no history of haematologic disease or other. Due to syndromic facies (facial dysmorphism with coarse facies, blepharophimosis, convex filter with thin upper lip, micrognathia, ankyloglossia) and intrauterine growth restriction, karyotype, skeletal radiography, cranial ultrasound, ophthalmologic examination and mucopolysaccharides and oligosaccharides study were made which did not show any findings. Since 5 months of age, weight percentile down crossing was noticed (from 50th percentile to below the 5th percentile) and also length and head circumference (from 25 to 50th percentile to below the 5th percentile (figure 1)). She was exclusively breastfed since birth. It was reported that the infant was reluctant to new foods and food diversification began only at 7 months of age and in trace quantities. At 10 months of age, the infant was admitted for intermittent vomiting, poor feeding, drowsiness and prostration with 2 weeks of evolution. Physical examination revealed marked pallor, slightly jaundiced appearance, mild dehydration, generalised axial hypotonia and weak cry to stimulation. She was afebrile with normal blood pressure, shallow breathing, respiratory rate of 30–40 cycles per minute, tachycardia with resting heart rate of 170 beats/min, a II/VI grade systolic murmur at the left sternal border and 3 cm hepatomegaly not crossing the midline and without palpable spleen. No signs of bleeding diathesis.
Figure 1.
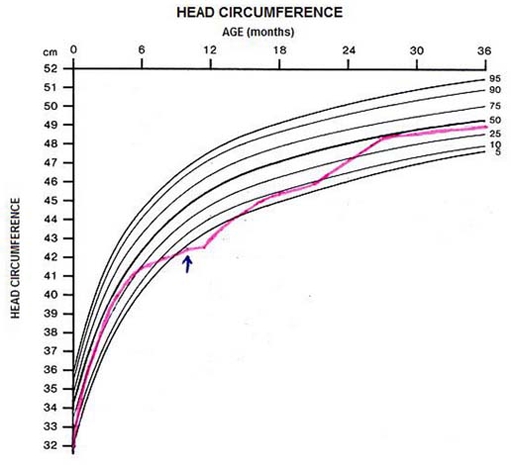
Head circumference evolution of the infant.
Investigations
She presented severe anaemia: erythrocytes 1.54×1012/l, haemoglobin 4.6 g/dl, haematocrit 12.8%, reticulocytes 0.4%, 6160/µl (reference value (r.v.) 0.5–2.5%; 0.05–0.1×106/µl); mean corpuscular volume (MCV) 83 fL ( r.v. 77–95 fL), white cell count 4900/μl, neutrophils 1484/ml, 30.3% and platelets 150 000/μl. The peripheral blood smear showed anisomacrocytosis and no blasts. Lactate dehydrogenase (LDH) level was 11198 U/l (r.v. 100–140 U/l), total bilirubin 2.4 mg/dl (r.v. 0.3–1.0 mg/dl), direct bilirubin 0.5 mg/dl (r.v. <0.3 mg/dl), aspartate transaminase 95 U/L (r.v. 22–44 UI/l), alanine aminotransferase 20 U/l (r.v. 12–34 UI/l) and C reactive protein 2.2 mg/dl (r.v. 0.5–1 mg/dl). Anaemia investigation: ferritin 156.5 ng/ml (r.v. 10–82 ng/ml), transferrin 189 mg/dl (r.v. 202–364 mg/dl), haptoglobin <1.94 mg/dl (r.v. 30–200 mg/dl), very decreased serum vitamin B12 level 3.3 pg/ml (r.v. 187–1059 pg/ml) and normal serum folic acid level 11.8 pg/ml (r.v. 3.5–16.1 pg/ml). The myelogram obtained by bone marrow puncture revealed a hypercellular marrow: myelo-erythrocyte ratio reversed, erythroid hyperplasia, left shift and marked dyserythropoiesis, hyperplasia of the granulocytic series, some giant metamyelocytes; megakaryocytic series: normal number and hypersegmented nuclei; some grade I and II rare ringed sideroblasts(figure 2). Vitamin B12 level in breast milk was also reduced – 1.4 ng/ml (r.v. > 3 ng/ml). Later, genetic mutation was found on chromosome 16, gene 3.7 - α-thalassemia (-α/αα). Mother’s laboratory evaluation showed haemoglobin 8.5 g/dl, haematocrit 24%, reticulocytes 1.7%, MCV 107 fL, mean corpuscular haemoglobin 37.9 pg (r.v. 25–33 pg), platelets 181 000/μl, neutrophils 2050/μl, peripheral blood smear with anisomacrocytosis, serum levels of vitamin B12 – 41.6 pg/ml, normal serum folate and positive anti-intrinsic antibody factor. In mother’s serial evaluations during pregnancy there was a progressive increase in MCV. A diagnosis of pernicious anaemia in the mother was established. There was spontaneous disappearance of anti-intrinsic factor antibody in 2–3 years. Father’s blood count was unremarkable. The diagnosis of infant megaloblastic anaemia attributed to severe reduction of vitamin B12 intake.
Figure 2.
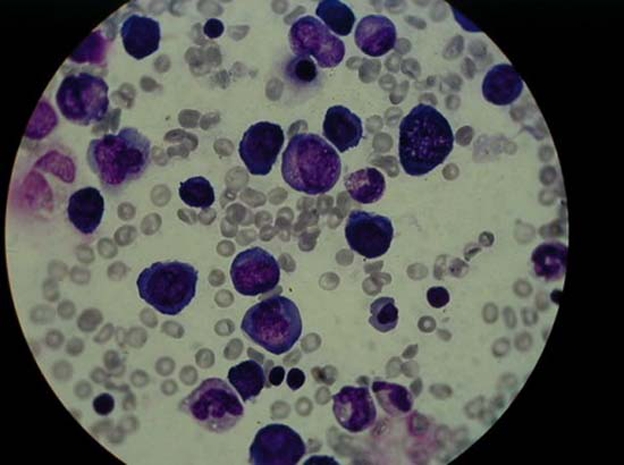
Medullar blood smears obtained by bone marrow puncture showed erythroblastic and granulopoietic hyperplasia, megaloblasts, giant metamyelocytes and hyperlobulated megakaryocytes.
Differential diagnosis
Folate deficiency and other causes of vitamin B12 deficiency were excluded. Also, other haemoglobinopathies and glucose-6-phosphate dehydrogenase deficiency were excluded.
Treatment
Red blood cells transfusion, cyanocobalamin (50 μg subcutaneously, five doses; 100 μg, oral, per day for 1 month), folic acid (5 mg/day), and food diversification with therapist training were provided to the infant.
Outcome and follow-up
Biochemical and haematologic remission were achieved after 1 week and 1 month, respectively (table 1). Infant development was normalised at 24 months (including head circumference) (figure 1). Psychomotor development at 2 and 3 years was normal as also brain MRI. She remains clinically well with excellent school performance at 12 years of age.
Table 1.
Haematologic evolution of the infant
| 3rd month | Hospital stay (days) | 11th month | 23rd month | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 8 | 30 | ||||
| Haemoglobin (g/dl) (11.5–15.5) | 10.4 | 4.6 | 7 | 6.4 | 7.9 | 8.6 | 9.3 | 11.7 | 13.6 |
| Haematocrit (%) (40–54%) | 30.4 | 12.8 | 19.6 | 18.0 | 21.3 | 24.4 | 27.7 | 34.2 | 39.2 |
| Erythrocytes (×1012/l) (4.6–6.2) | 4.04 | 1.54 | 2.33 | 2.16 | 2.55 | 2.81 | 3.16 | 4.09 | 5.46 |
| White blood cells (×103/µl) (4.5–13.5) | 11.8 | 4.9 | 2.9 | 5.4 | 3.1 | 2.7 | 4.4 | 7.5 | 9.3 |
| Platelets (×103/µl) (150–400) | 418 | 150 | 107 | 59 | 58 | 112 | 294 | 477 | 362 |
Discussion
The level of vitamin B12 in infants whose mothers lack this vitamin, is very precarious,1 3–5 9 especially in those exclusively breastfed.1 3–5 In our case, exclusive breastfeeding associated with low levels of maternal cobalamin has conditioned a deficit state of this vitamin. The normal newborn has enough vitamin B12 for 6–8 months,3 8 even in the presence of a restricted diet or inadequate absorption, so the signs and symptoms of disease occur after a normal developmental period,1 2 4 6 characteristically between the 4th and 8th months of age,1–3 8 as in this case.
Clinical manifestations are predominantly neurologic and haematologic,9 but are often unspecific.1 3 4 8 9 The earliest signs include progressive lethargy, apathy, irritability1 5 6 8 9 13 14 and developmental delay with gross motor dysfunction which contributes to feeding difficulties.4–6 9 14 There occurs refusal to wean and refusal to solid food, vomiting and failure to thrive,1 5 8 9 14 as in the case presented. There may be weakness, anorexia and weak cry,6 8 9 and also hyporreflexia, hypotonia and choreathetotic movements.5 6 8 9 14 As the disease progresses, it may appear hypothermia, encephalopathy and coma.8 9 Lemon pale skin with slight jaundice due to intramedullary haemolysis and erythematous tongue,8 13 mild hepatosplenomegaly, diarrhea and palmar hyperpigmentation9 are also common findings. The case described presented lethargy, apathy, poor weight gain, vomiting, solid food refusal, pallor, mild jaundice and hepatomegaly. The evolution to encephalopathy was interrupted by the timely diagnosis.
All blood cells lineage are affected.9 13 Red blood cells vary in size and shape and are frequently larger than normal.13 Anaemia is macrocytic (>100 fL)9 11 13 14 and there is reticulocytopenia.6 9 13 In some situations, megaloblastic anaemia morphology does not show the usual variations15 16 and macrocytosis may be absent in individuals with microcytic anaemia such as iron deficiency anaemia, α or β-thalassemia or chronic inflammatory disease11 13 15–17 as in this case. Granulocyte precursors are also hypersegmented6 9 13 and megakaryocytes show similar changes.9 13 Megaloblastic anaemia is associated with ineffective erythropoiesis and haemolysis, and consequently increased erythrocyte precursors and plasma iron, LDH and bilirubin levels,8 13 findings found in this case. Pancytopenia may also be found.4 9 Bone marrow smears shows a cellular bone marrow with megaloblastic changes, especially in the erythroid series.13
In childhood, megaloblastic changes of cell lines may be due to deficiency of folate, vitamin B12 or, less often, to inborn errors of metabolism of vitamin B12, folate, purine or pyrimidine.1 6 13 Vitamin B12 deficiency should always be considered in children with neurologic symptoms associated with megaloblastosis and failure to thrive8 and must be included in the differential diagnosis of failure to thrive, regression of psychomotor development and neurologic, psychiatric and haematologic manifestations.3 10 The confirmation of diagnosis is made by elevated urinary or serum methylmalonic acid and serum homocysteine levels and decreased vitamin B12 serum levels.2 5 7 13 Methylmalonic acid, except when caused by inborn errors of metabolism,1 6 13 18 is the most sensitive and specific markers of preclinical vitamin B12 deficiency2 3 5 6 8 13 18 and is a useful tool for differentiating from folate deficiency.18 Unfortunately, in this case, we have no data on urine methylmalonic acid before the beginning of the treatment.
In this case, the thalassaemia trait masked macrocytosis and the absence of known mother’s anaemia delayed the diagnosis. Neutrophil hypersegmentation visible on peripheral blood smear, pancytopenia, abnormal findings in blood and bone marrow smears suggested the deficit of cobalamin. Frequently, vitamin B12 and folate deficiency coexist, so they must be investigated together. It is the discovery of vitamin B12 deficiency in a breastfed infant that leads to maternal diagnosis, usually mild and asymptomatic.12 14 Mothers with subclinical pernicious anaemia are not usually anaemic4 5 8 12 and serum levels of vitamin B12 frequently are at the lower limit of normal.8
Vitamin B12 treatment is not well established.8 Characteristically, treatment of infants with haematologic and neurologic manifestations includes 1 mg intramuscular vitamin B12, for 4 days,1 followed sometimes by large oral doses to replete stores.1 There have been described intramuscular or subcutaneous hydroxicobalamin administration (1000 µg in adults), six doses, during several weeks, several times a week7 18 or daily during 2 weeks, weekly until the haematocrit is normal and then monthly for life13 or for 6 months in the presence of neurologic manifestations.13 Red blood cells transfusion is also recommended for children with severe anaemia.8 13 18 In our case, we chose initial subcutaneous cobalamin, followed by daily vitamin doses associated with folate. We also promoted the food diversification and training as a source of cobalamin.
After therapy, there are clinical improvement and normalisation of haematologic and neurologic parameters.1 4 9 13 In 12 h, bone marrow becomes normoblastic with complete recovery in 2–3 days.13 After 1 week, there is increased reticulocyte count and 1 month later complete blood count returns to normal.9 13 In this case, after cobalamin administration and food diversification, there was significant clinical improvement with regression of neurologic and haematologic findings.
Precocious treatment offers a dramatic improvement.9 14 17 Cerebral atrophy and desmielinisation may reverse in several months1 as well as growth velocity normalisation,1 which occurred in the case described, including microcephaly reversibility. When not properly treated, these infants show poor weight gain, haematologic changes and irreversible neurologic consequences.2 14 The extent and degree of disability depends on the deficiency severity and duration.1 3 4 6 7 10 14
There are several cases of exclusively breastfed infants suffering from megaloblastic anaemia born to mothers with undiagnosed pernicious anaemia. However, this case had an exuberant clinical presentation with neurologic reversibility, which is rare in those described in the literature. It is also referred that the coexistence of vitamin B12 deficiency and thalassemia could have worsened the prognosis by delaying the diagnosis which did not happen in this case.
Learning points.
-
▶
It is very important to keep high suspicion for the diagnosis of vitamin B12 deficiency in an exclusively breastfed infant with clinical and laboratory manifestations, even in the absence of macrocytosis and/or positive maternal anamnesis.
-
▶
Anaemia and megaloblastosis may be late consequences of vitamin B12 deficiency and its absence in children whose mothers have vitamin B12 deficiency history should not exclude the diagnosis.
-
▶
In the presence of reduced levels of cobalamin in an infant, maternal causes of vitamin B12 must be investigated and if the mother has a varied diet, other aetiologies must be investigated, such as pernicious anaemia.
-
▶
In some cases like this one, the appreciation of the progressive increase in MCV during pregnancy could have allowed primary prevention measures in the infant.
-
▶
A precocious diagnosis of vitamin B12 deficiency and appropriate measures can prevent neurologic lesions potentially irreversible.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Dror DK, Allen LH. Effect of vitamin B12 deficiency on neurodevelopment in infants: current knowledge and possible mechanisms. Nutr Rev 2008;66:250–5 [DOI] [PubMed] [Google Scholar]
- 2.Marble M, Copeland S, Khanfar N, et al. Neonatal vitamin B12 deficiency secondary to maternal subclinical pernicious anemia: identification by expanded newborn screening. J Pediatr 2008;152:731–3 [DOI] [PubMed] [Google Scholar]
- 3.From the Centers for Disease Control and Prevention. Neurologic impairment in children associated with maternal dietary deficiency of cobalamin–Georgia, 2001. JAMA 2003;289:979–80 [PubMed] [Google Scholar]
- 4.Yenicesu I. Pancytopenia due to vitamin B12 deficiency in a breast-fed infant. Pediatr Hematol Oncol 2008;25:365–7 [DOI] [PubMed] [Google Scholar]
- 5.Stabler SP, Allen RH. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr 2004;24:299–326 [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Fogelman Y, Bennett M. Severe vitamin B12 deficiency in an infant associated with a maternal deficiency and a strict vegetarian diet. J Pediatr Hematol Oncol 2004;26:270–1 [DOI] [PubMed] [Google Scholar]
- 7.Oral or intramuscular vitamin B12? Drug Ther Bull 2009;47:19–21 [DOI] [PubMed] [Google Scholar]
- 8.Zengin E, Sarper N, Caki Kiliç S. Clinical manifestations of infants with nutritional vitamin B deficiency due to maternal dietary deficiency. Acta Paediatr 2009;98:98–102 [DOI] [PubMed] [Google Scholar]
- 9.Doyle JJ, Langevin AM, Zipursky A. Nutritional vitamin B12 deficiency in infancy: three case reports and a review of the literature. Pediatr Hematol Oncol 1989;6:161–72 [DOI] [PubMed] [Google Scholar]
- 10.Banka S, Roberts R, Plews D, et al. Early diagnosis and treatment of cobalamin deficiency of infancy owing to occult maternal pernicious anemia. J Pediatr Hematol Oncol 2010;32:319–22 [DOI] [PubMed] [Google Scholar]
- 11.Goldberg MA, Schwartz SO. Mediterranean anemia in a Negro complicated by pernicious anemia of pregnancy: report of a case. Blood 1954;9:648–54 [PubMed] [Google Scholar]
- 12.Erdeve O, Arsan S, Atasay B, et al. A breast-fed newborn with megaloblastic anemia-treated with the vitamin B12 supplementation of the mother. J Pediatr Hematol Oncol 2009;31:763–5 [DOI] [PubMed] [Google Scholar]
- 13.Lichtman MA, Beutler E, Kipps TJ, et al. Williams Hematology. Seventh Edition New York, NY: McGraw-Hill Medical; 2006 [Google Scholar]
- 14.Honzik T, Adamovicova M, Smolka V, et al. Clinical presentation and metabolic consequences in 40 breastfed infants with nutritional vitamin B12 deficiency–what have we learned? Eur J Paediatr Neurol 2010;14:488–95 [DOI] [PubMed] [Google Scholar]
- 15.Tavil B, Sipahi T. Masked deficit of vitamin B12 in a Turkish girl with thalassemia. Pediatr Hematol Oncol 2004;21:363–5 [DOI] [PubMed] [Google Scholar]
- 16.Green R, Kuhl W, Jacobson R, et al. Masking of macrocytosis by alpha-thalassemia in blacks with pernicious anemia. N Engl J Med 1982;307:1322–5 [DOI] [PubMed] [Google Scholar]
- 17.Bilic E, Zagar M, Juric S. Masked deficit of vitamin B12 in the patient with heterozygous beta-thalassemia and spastic paraparesis. Acta Neurol Belg 2004;104:173–5 [PubMed] [Google Scholar]
- 18.Arceci RJ, Hann I, Smith OP. Pediatric Hematology. Third Edition Boston, MA: Blackwell Publishing; 2006 [Google Scholar]


