Children treated with prolonged-course antibiotics for ventilator-associated tracheitis have a similar likelihood of progression to nosocomial pneumonia compared to children treated with short-course therapy, but prolonged-course therapy may predispose to higher rates of acquisition of multidrug-resistant organisms.
Abstract
Background. The optimal duration of antibiotic therapy for ventilator-associated tracheitis (VAT) has not been defined, which may result in unnecessarily prolonged courses of antibiotics. The primary objective of this study was to determine whether prolonged-course (≥7 days in duration) therapy for VAT was more protective against progression to hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP), compared with short-course antibiotics (<7 days in duration). The secondary objective was to determine whether prolonged-course therapy was more likely to result in the acquisition of multidrug-resistant organisms (MDROs) compared with short-course therapy.
Methods. We conducted a retrospective cohort study of children ≤18 years of age hospitalized in the intensive care unit and intubated for ≥48 h from January 2007 through December 2009 who received antibiotic therapy for VAT.
Results. Of the 1616 patients intubated for at least 48 h, 150 received antibiotics for clinician-suspected VAT, although only 118 of these patients met VAT criteria. Prolonged-course antibiotics were not protective against subsequent development of HAP or VAP (hazard ratio [HR], 1.08; 95% confidence interval [CI], 0.40–2.91). Factors associated with subsequent MDRO colonization or infection included prolonged-course antibiotic therapy (HR, 5.15; 95% CI, 1.54–7.19), receipt of combination antibiotic therapy (HR, 3.24; 95% CI, 1.54–6.82), and days of hospital exposure prior to completing antibiotic therapy (HR, 1.08; 95% CI, 1.04–1.12).
Conclusions. A prolonged course of antibiotics for VAT does not appear to protect against progression to HAP or VAP compared with short-course therapy. Furthermore, prolonged antibiotic courses were associated with a significantly increased risk of subsequent MDRO acquisition.
With improving survival rates in neonatal and pediatric intensive care units (ICU), the number of children requiring invasive devices who subsequently develop nosocomial infections continues to increase [1]. Among the most common indwelling devices is the endotracheal tube (ETT). Although ETTs provide some obvious benefits, their presence facilitates the movement of bacteria from the oropharyngeal region into the lower respiratory tract.
When the burden of bacterial organisms in the trachea surpasses a critical threshold, ventilator-associated tracheitis (VAT) may result. VAT appears to lie on the continuum between respiratory tract colonization and ventilator-associated pneumonia (VAP). VAP is the most common nosocomial infection in ICUs and is associated with an attributable mortality rate of up to 50% [2, 3]. Optimizing treatment for VAT may prevent subsequent VAP or hospital-acquired pneumonia (HAP).
Two randomized controlled trials conducted in adult populations that compared antibiotic therapy (in the range of 8–14 days duration) with no antibiotic therapy for VAT demonstrated a significantly greater number of mechanical ventilation–free days and decreased subsequent rates of HAP or VAP among those receiving antibiotics [4, 5]. However, the optimal duration of antimicrobial therapy for VAT has not yet been defined, and there have been no studies of VAT in the pediatric population. Data from adult studies indicate that VAP may be successfully treated with antibiotic courses as short as 8 days [6]. Unlike VAT, nosocomial pneumonia results in lung damage, making it reasonable to hypothesize that VAT may be successfully treated with shorter courses of therapy. Additionally, shortened antibiotic courses may decrease the emergence of multidrug-resistant organisms (MDROs), which can result from the ubiquitous use of antibiotics [7–9].
We hypothesized that the judicious use of antibiotics in children with VAT was associated with decreased antimicrobial resistance and did not compromise clinical outcomes. We conducted a retrospective cohort study to determine whether the duration of antibiotic treatment for VAT impacted subsequent development of HAP or VAP and the emergence of MDROs.
PATIENTS AND METHODS
Study Design and Setting
Our retrospective cohort study was conducted at The Johns Hopkins Hospital (JHH) Children's Center. The JHH Children's Center is a >175-bed tertiary care pediatric hospital containing a 26-bed pediatric ICU (PICU) and 45-bed neonatal ICU (NICU) serving Maryland and neighboring states. Children ≤18 years of age who were admitted to the NICU or PICU from 1 January 2007 through 31 December 2009 and required at least 48 h of invasive mechanical ventilation were included. The 48-h limit was based on previous literature [4, 10–13]. Children with a preexisting tracheostomy or a new infiltrate visible on chest imaging, when compared with radiographic findings from the time of intubation, were excluded.
Definitions
VAT was defined using the following criteria: (1) fever (temperature, >38°C) or hypothermia (temperature, <36°C), leukocytosis >12,000 leukocytes/mm3 or leukopenia <4000 leukocytes/mm3, plus new onset of purulent endotracheal secretions; (2) Gram stain with moderate-heavy polymorphonuclear cells with moderate-heavy bacterial growth; and (3) no radiographic evidence of a new lung infiltrate [10–12, 14]. VAP (if the patient was intubated) and HAP (if the patient was not intubated) were defined by the presence of a new or progressive infiltrate on chest imaging in a hospitalized patient and at least 3 of the following: fever or hypothermia, leukocytosis or leukopenia, purulent endotracheal aspirate, worsening cough or dyspnea, worsening gas exchange, and changing respiratory examination findings [15].
Acquisition of an MDRO was defined as newly identified colonization or infection by methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus species (VRE), MDR Enterobacteriaceae, MDR Acinetobacter, MDR Pseudomonas, Stenotrophomonas maltophilia, and Burkholderia cepacia within 30 days of initiation of antibiotics for VAT [16]. To be considered an MDR Enterobacteriaceae, MDR Acinetobacter, or MDR Pseudomonas, the organism must have been resistant to >3 classes of antibiotics (aminoglycosides, penicillins, cephalosporins, carbapenems, or fluoroquinolones). If the organism implicated in VAT was isolated again within 30 days and developed a ≥4-fold increase in minimum inhibitory concentration to the antibiotic that was used to treat VAT, this was also considered to be an MDRO.
Combination antibiotic therapy was defined as the use of a β-lactam (either a penicillin, cephalosporin, or carbapenem) plus an aminoglycoside as definitive antibiotic therapy for >24h after bacterial susceptibility data were known. Antibiotic therapy administered for ≥7 days was categorized as prolonged-course therapy, and any duration <7 days was categorized as short-course therapy. The cutoff period of 7 days was decided after identifying the median duration of therapy for all children. If a shorter cutoff period was chosen, the number of children in the short-course group would have been too small for a meaningful analysis.
Data Collection
Medical records for all children who received antibiotic therapy for clinician-suspected VAT were reviewed and demographic, microbiologic, radiographic, and clinical data were collected. Children were categorized as to whether they met the defined criteria for VAT. Those who did not meet criteria were classified as treated for clinician-suspected VAT. The Johns Hopkins University Institutional Review Board approved the study with a waiver of informed consent.
Outcome Measures
The exposed group consisted of children who received ≥7 days of antibiotic therapy for VAT, and the unexposed group consisted of children who were prescribed <7 days of antibiotics for VAT. The primary outcome was diagnosis of HAP or VAP within 10 days of discontinuing antibiotic therapy for VAT [17]. The secondary outcome was acquired colonization or infection with an MDRO within 30 days of initiating antibiotics for VAT. Only children who met the published criteria for VAT were included in the analyses of the primary outcome [10–12, 14]. All children treated for clinician-suspected VAT were included in analyses of the secondary outcome. For both outcomes, children treated with prolonged-course antibiotic therapy were compared with children who were prescribed short-course antibiotic therapy for VAT.
Statistical Analysis
Relationships between factors hypothesized to influence outcomes and days of antibiotic treatment were explored using scatterplots and univariate tests of association. For analysis of the primary outcome, children who did not develop HAP or VAP were censored at the time of hospital discharge or 10 days after completing antibiotic therapy. For the secondary outcome, children who did not acquire MDROs were censored at the time of hospital discharge or 30 days after completing antibiotic therapy. The crude cumulative incidence of HAP or VAP and colonization with an MDRO was calculated using 1 minus the Kaplan-Meier estimate of the survivor function, and a log-rank test was used to evaluate significant differences in outcomes between the short-course and prolonged-course therapy groups.
Cox proportional hazards models were used to obtain unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CI). Covariates with P values <.20 in the unadjusted model were included in the adjusted model. Additionally, any variable specified as a potential confounder a priori based on previous literature or biological plausibility was also included in the model. Days of antibiotic therapy dichotomized at 7 days, patient's pediatric risk of mortality (PRISM) score at time of VAT diagnosis, hospital unit of admission, ETT removal during antibiotic therapy, use of additional antibiotics for >48 h within 10 days or 30 days of VAT diagnosis (depending on outcome being assessed), use of combination antibiotic therapy, and recovery of Gram-negative organisms from sputum cultures were included in the multivariable model as independent variables. In addition to these variables, days of hospitalization prior to identification of an MDRO was added to the model of MDRO colonization or infection. The addition of variables not included in the final models changed the point estimate for the effect of prolonged antibiotic use by <10%. The proportional hazards assumption was tested by visually inspecting scaled Schoenfeld residuals versus ranked time and by including interaction terms with functions of time in the Cox models.
After obtaining results from multivariable models, we repeated our results to determine whether they would change significantly using propensity scores. Propensity scores for days of antibiotic therapy were incorporated into the models. The propensity score was generated on the basis of age, sex, reason for hospital admission, unit of admission, length of stay prior to VAT diagnosis, PRISM score at the time of VAT diagnosis, presence of a Gram-negative organism in a sputum sample, and >48 h of additional antibiotic therapy. Incorporating propensity scores did not significantly change our estimates, and thus they were not included in the final model. For all statistical tests, 2-sided P values of <.05 were considered to be statistically significant. Statistical analyses were performed using Stata software, version 10.0 (Stata Corp).
RESULTS
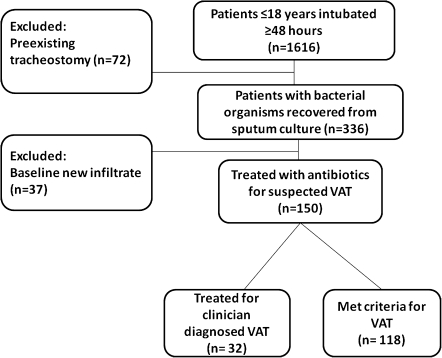
There were 1616 children ≤ 18 years of age intubated for at least 48 h between 1 January 2007 and 31 December 2009. The medical records of all of these children were reviewed to determine whether they met inclusion criteria. Seventy-two children were excluded because of preexisting tracheostomies (Figure 1). Of the 336 children with endotracheal tubes who had bacteria cultured from their sputum samples, 37 children were excluded because of a new infiltrate on chest imaging. One-hundred fifty of the remaining children received antibiotics for clinician-suspected VAT, and 118 of these children met the defined criteria for VAT. The incidence of VAT was 7.3%, similar to the incidence described in published studies from adult ICUs [11, 18–20]. The median duration from time of intubation to development of VAT was 6 days.
Figure 1.
Study design of ventilator-associated tracheitis (VAT) in the neonatal and pediatric intensive care units between 1 January 2007 and 31 December 2009.
Baseline Characteristics and Development of HAP or VAP Among Children Meeting Criteria for VAT
Demographic and clinical characteristics for children meeting criteria for VAT are displayed in Table 1. Eighty-two (69%) of the children received a prolonged course of antibiotics. The median duration of therapy for these children was 9.8 days. In the short-course therapy group, the median duration of antibiotic therapy was 5.9 days. The short-course and prolonged-course therapy groups were similar with regards to age, PRISM score at time of diagnosis of VAT, unit of admission, sex, presence of Gram-negative VAT, and preexisting medical conditions.
Table 1.
Baseline Characteristics of Children Who Received Antibiotics for Ventilator-Associated Tracheitis (VAT)
| 118 patients meeting VAT criteriaa |
150 patients with clinician-suspected VATb |
|||||
| Variable | ≤6 days of antibiotics (n = 36) | ≥7 days of antibiotics (n = 82) | P | ≤6 days of antibiotics (n = 50) | ≥7 days of antibiotics (n = 100) | P |
| Age, median years (IQR) | 1.0 (0.9–12) | 0.9 (0.6–14) | 0.8 (0.5–12) | 0.8 (0.5–14) | ||
| PRISMc score, median value (IQR) | 12 (8–20) | 12 (8–21) | .60 | 12 (9–20) | 12 (10–26) | |
| Unit | .24 | .56 | ||||
| NICU | 28 (78) | 55 (67) | 18 (36) | 41 (41) | ||
| PICU | 8 (22) | 27 (33) | 32 (64) | 59 (59) | ||
| Male sex | 24 (67) | 54 (66) | .93 | 35 (70) | 63 (63) | .40 |
| Gram-negative organisms from sputum | 26 (72) | 68 (83) | .18 | 30 (60) | 78 (78) | .02 |
| Preexisting conditions | .22 | .47 | ||||
| Prematurity | 6 (17) | 22 (27) | 15 (30) | 33 (33) | ||
| Neuromuscular | 9 (25) | 10 (12) | 9 (18) | 10 (10) | ||
| Cardiovascular | 3 (8) | 13 (16) | 5 (10) | 16 (16) | ||
| Genetic/metabolic | 2 (6) | 4 (5) | 2 (4) | 6 (6) | ||
| Respiratory | 3 (8) | 12 (15) | 3 (6) | 13 (13) | ||
| Trauma | 7 (20) | 10 (12) | 8 (16) | 10 (10) | ||
| Gastrointestinal | 4 (11) | 2 (2) | 4 (8) | 2 (2) | ||
| Immunocompromised | 0 (0) | 1 (1) | 0 (0) | 2 (2) | ||
NOTE. Data are numbers (%) of patients, unless otherwise indicated. IQR, interquartile range; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Based on previously published definition of VAT [10–12, 14].
Children receiving antibiotic courses for clinician-suspected VAT (inclusive of the 118 patients who met criteria for VAT).
Pediatric risk of mortality score from time of VAT diagnosis.
The causative bacterial microorganisms of VAT are described in Table 2. In the NICU, VAT was generally associated with Enterobacteriaceae and Pseudomonas aeruginosa. Although S. aureus VAT was observed in the NICU, it was more predominant in the PICU. Not surprisingly, in patients who were older than neonates, Streptococcus pneumoniae and Haemophilus influenzae were implicated as frequent causes of VAT.
Table 2.
Bacterial Organisms Cultured from Sputum of 118 Children With Ventilator-Associated Tracheitis (VAT) in the Neonatal and Pediatric Intensive Care Units
| No. (%) of patients |
||
| Organism | NICU | PICU |
| Pseudomonas aeruginosa | 11 (31) | 20 (24) |
| Klebsiella species | 10 (29) | 4 (5) |
| Escherichia coli | 5 (14) | 4 (5) |
| Enterobacter cloacae | 3 (8) | 6 (7) |
| Serratia marcescens | 3 (9) | 3 (3) |
| Staphylococcus aureusa | 2 (6) | 15 (18) |
| Citrobacter species | 1 (3) | … |
| Stenotrophomonas maltophilia | … | 1 (1) |
| Proteus mirabilis | … | 1 (1) |
| Streptococcus pneumoniae | … | 7 (8) |
| Haemophilus influenzae | … | 21 (25) |
NOTE. NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Of the 17 Staphylococcus aureus strains associated with VAT, 7 were methicillin resistant.
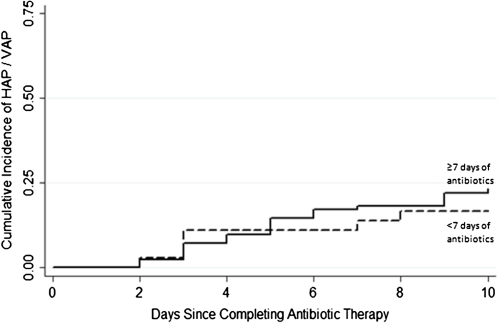
The median time to develop VAP from discontinuation of antibiotics for VAT was 4.5 days. Nineteen (23%) of the 82 children who received prolonged-course therapy and 6 (20%) of the 36 children who received short-course therapy developed HAP or VAP within 10 days of completing antibiotics for VAT. (Table 3) After adjusting for ETT removal, PRISM score at time of VAT diagnosis, presence of Gram-negative VAT, use of additional antibiotics for >48 h, use of combination antibiotic therapy, and unit of admission, receiving prolonged-course antibiotics was not protective against the subsequent development of HAP or VAP (HR, 1.08; 95% CI, 0.40–2.91). An estimate of the cumulative incidence of HAP or VAP versus days after antibiotic therapy is depicted in Figure 2 using a log-rank test to compare the prolonged-course and short-course treatment groups (P = .50). The risk of developing HAP or VAP was ∼4 times greater among those children whose ETTs were not removed during antibiotic therapy (HR, 4.16; 95% CI, 1.39–12.45).
Table 3.
Risk of Hospital-Acquired Pneumonia (HAP) or Ventilator-Associated Pneumonia (VAP) Among the 118 Children Meeting Criteria for Ventilator-Associated Tracheitis (VAT)
| Variable | Unadjusted HR (95% CI) | P | Adjusted HRa (95% CI) | P |
| ≥7 days of antibiotics for VAT | 1.41 (0.56–3.52) | .47 | 1.08 (0.40–2.91) | .88 |
| ETT remainsb | 4.85 (1.67–14.16) | <.01 | 4.16 (1.39–12.45) | <.01 |
| PRISM scorec | 1.04 (0.95–1.15) | .37 | 1.03 (0.94–1.13) | .50 |
| Gram-negative organism from sputum | 3.24 (0.76–13.73) | .11 | 2.18 (0.50–9.61) | .30 |
| Combination antibioticsd | 1.81 (0.83–3.97) | .14 | 1.31 (0.49–3.47) | .59 |
| Additional antibiotic therapy for >48 h within 10 days of VAT diagnosis | 1.20 (0.40–2.11) | .43 | 1.43 (0.59–3.58) | .36 |
| NICUe | 0.49 (0.22–1.08) | .08 | 0.93 (0.36–2.39) | .88 |
NOTE. CI, confidence interval; ETT, endotracheal tube; HR, hazard ratio; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; PRISM, pediatric risk of mortality score.
HR adjusted for variables in table from diagnosis of VAT until development of HAP or VAP or 10 days after antibiotic completion.
ETT remained in place for duration of antibiotic course for VAT.
Per 1 unit increase.
Combination therapy defined as the use of a β-lactam plus aminoglycoside as definitive antibiotic therapy for >24h after bacterial susceptibility data known.
Infant admitted to NICU compared with admission to PICU.
Figure 2.
Cumulative distribution function of hospital-acquired pneumonia (HAP), shown for 118 children who met the definition of ventilator-associated tracheitis (VAT). P = .46, by log-rank test.
Baseline Characteristics and Acquisition of MDROs Among Children Receiving Antibiotics for Clinician-Suspected VAT
Of the 150 children who received antibiotics for clinician-suspected VAT, 100 children received a prolonged course of antibiotics (Table 1). Baseline characteristics were similar between the 2 groups; however, children who received a diagnosis of Gram-negative VAT were significantly more likely to receive >7 days of antibiotic therapy, compared with children with Gram-positive VAT (78 vs 22; P = .02).
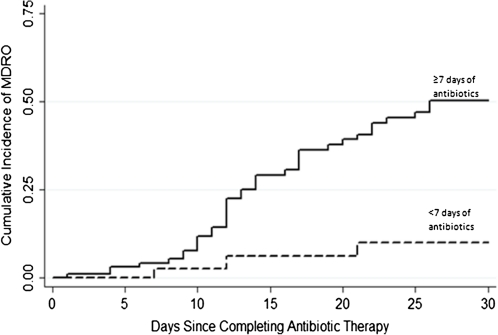
Thirty-eight (38%) of the 100 children who received prolonged-course therapy for clinician-suspected VAT and 4 (8%) of the 50 children who received short-course therapy acquired a new MDRO within 30 days of completing antibiotic therapy for VAT (Figure 3). The unadjusted hazard of colonization or infection with an MDRO was >4 times greater among children who received prolonged-course therapy, and this association was only mildly attenuated after adjusting for other variables, including days from initial hospital admission until acquisition of MDRO (adjusted HR, 5.15; 95% CI, 1.54 to–7.19) (Table 4). Importantly, the use of combination therapy was also strongly associated with the emergence of MDROs in both univariate and multivariable models (HR, 3.24; 95% CI, 1.54–6.82). Median follow-up time from completion of antibiotic course for VAT was 24.3 days. Not surprisingly, days of hospital exposure prior to completing antibiotic therapy was an independent risk factor for subsequent colonization or infection with an MDRO (HR, 1.08; 95% CI, 1.04–1.12).
Figure 3.
Cumulative distribution function of colonization or infection with a multidrug-resistant organism (MDRO), shown for 150 children receiving antibiotic therapy for ventilator-associated tracheitis (VAT). P ≤ .01, by log-rank test.
Table 4.
Risk of Colonization or Infection with Multidrug-Resistant Organisms (MDROs) Among 150 Patients Treated with Antibiotics for Clinician-Suspected Ventilator-Associated Tracheitis (VAT)
| Variable | Unadjusted HR (95% CI) | P | Adjusted HRa (95% CI) | P |
| ≥ 7 days of antibiotics for VAT | 6.26 (1.93–8.31) | <.01 | 5.15 (1.54–7.19) | <.01 |
| Combination therapyb | 3.01 (1.58–5.56) | <.01 | 3.24 (1.54–6.82) | <.01 |
| PRISM scorec | 0.95 (0.87–1.03) | .20 | 0.96 (0.89–1.03) | .29 |
| Length of stay in hospital prior to completing antibiotic therapyd | 1.07 (1.04–1.11) | <.01 | 1.08 (1.04–1.12) | <.01 |
| Gram-negative organism in sputum | 1.06 (0.53–2.12) | .87 | 0.49 (0.22–1.10) | .08 |
| Additional antibiotic therapy for >48 h within 30 days of VAT diagnosis | 1.30 (0.60–2.81) | .51 | 1.53 (0.59–3.98) | .38 |
| ETT remainse | 0.91 (0.46–1.78) | .77 | 0.97 (0.46–1.91) | .86 |
| NICUf | 0.83 (0.44–1.51) | .55 | 0.62 (0.32–1.34) | .25 |
NOTE. CI, confidence interval; ETT, endotracheal tube; HR, hazard ratio; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; PRISM, pediatric risk of mortality score.
HR adjusted for variables in table as well as receipt of any additional antibiotics from diagnosis of VAT until development of colonization or infection with MDRO or 30 days after antibiotic therapy initiation.
Combination therapy defined as the use of a β-lactam plus aminoglycoside as definitive antibiotic therapy for >24h after bacterial susceptibility data known.
Per 1 unit increase.
Length of stay prior to completing antibiotic therapy (1 day increase).
ETT remained in place for duration of antibiotic course for VAT.
Infant admitted to NICU compared with admission to PICU.
The median time for development of a new MDRO from completion of VAT therapy was 12.5 days. Sixty-three percent of MDROs were Gram-negative organisms, including 1 MDR Acinetobacter in a patient who required prolonged parenteral colistin sulfate therapy. There were 7 children who had the organism implicated in VAT isolated on a subsequent culture within 30 days, and in each case, the MIC of the antibiotic previously prescribed for the treatment of VAT had increased ≥4-fold. Although this data was not included in the analysis, 3 children, 2 of whom received antibiotics for >14 days, developed Clostridium difficile infection within 5 days of antibiotic therapy completion.
DISCUSSION
Our study demonstrates that children treated with prolonged-course antibiotic therapy for VAT have a similar likelihood of progression to HAP or VAP, compared with children treated with short-course therapy, but prolonged-course therapy may predispose to higher rates of acquisition of MDROs. Our results suggest that antibiotic therapy administered for 6 days is adequate for treating VAT and preventing subsequent HAP or VAP in pediatric patients. Antibiotic therapy as short as 3 days has been documented to sterilize the lungs of patients with nosocomial pneumonia [21]. Because tracheitis is a more localized infection than pneumonia, prospective studies are needed to determine whether the treatment duration for VAT can be even shorter.
Respiratory tract infections account for ∼50% of all antibiotics prescribed in the ICU; however only 63% of them are prescribed for proven bacterial respiratory tract infections [22]. Indeed, in the current study, 21% of children received some duration of antibiotic therapy for clinician-suspected VAT when recovery of bacteria from sputum cultures appeared to signify bacterial colonization and not infection. When taking into consideration the number of patients treated for VAT without meeting criteria and the additional days of antibiotics that may have been unnecessary, the possible costs, toxicities, and emergence of resistance may be substantial.
The only significant predictor of progression to HAP or VAP in our cohort was continued intubation. Our results indicate the adjusted hazard of developing HAP or VAP was 4 times greater among children with VAT who retained their ETT, compared with the adjusted hazard among those with ETT removal. Similar to Foley catheters and central lines, daily evaluation of the continued need for ETTs in patients receiving mechanical ventilation should be a routine part of ICU care, because indwelling catheters clearly pose an infection risk to patients [23–27]. In one institution, a daily checklist that reviewed the continued need for intubation led to an 82% decrease in the incidence of VAP [26].
In our cohort, subsequent development of colonization or infection with MDROs was documented in 38% of children who received prolonged-course antibiotics, compared with 8% of children who received short-course antibiotics for VAT. Colonization or infection with an MDRO was also strongly associated with previous prescription of combination antibiotics. Our results were similar to those of a study conducted by Singh et al [7], in which patients with suspected nosocomial pneumonia with low-risk clinical pulmonary infection scores were randomized to receive 3 days of monotherapy or standard care, which consisted of antibiotics administered for 10–21 days, frequently as combination regimens. The cohort that received short-course therapy had a significantly lower incidence of subsequent infection by MDROs, and clinical outcomes were not adversely affected. Antibiotic therapy has great ecological impact, and in the age of an increasing number of antimicrobial-resistant organisms, the benefits of curtailing antibiotic usage are clear [7–9, 28–30].
There are some limitations to consider when interpreting our results. In our institution, surveillance for colonization with Gram-positive MDROs is routinely performed for patients hospitalized in the ICU, but not for patients in the pediatric wards. As a result, the probability of detecting colonization with MDROs generally decreases when a patient is transferred out of the ICU. However, we did not find differences in the proportions of surveillance cultures performed between children who did and children who did not develop colonization or infection due to MRDOs (data not shown), which suggests that surveillance bias did not significantly influence study findings.
Second, our results are not generalizable to children with tracheostomies, because these children were excluded from this study. Children with tracheostomies have risk factors that are likely to be very different from those of children with ETTs, and we believed this heterogeneity would make it difficult to analyze these 2 groups together. As tracheostomies put children at risk for frequent episodes of VAT, this is a population that would benefit from additional studies to determine the optimal management of VAT.
Third, we combined data from the NICU and PICU. In our institution, neonates with congenital cardiac disease, complicated surgical issues, and those requiring extra corporeal membrane oxygenation are frequently admitted to the PICU rather than to the NICU. Thus, not surprisingly, exploratory data analysis indicated that the groups were similar with regards to age, baseline characteristics, and severity of illness scores. This may not be the case in other institutions, however, and this may limit the generalizability of our findings to other PICUs.
Finally, evaluating treatment effects from observational data can be problematic. Prognostic factors may influence treatment decisions, producing a type of bias referred to as confounding by indication [31]. In our study, there is the possibility that children who appeared to be more ill were treated with longer courses of antibiotics, and these children may also be at higher risk for developing MDROs. Using exploratory data analysis, we were unable to find differences in baseline severity of illness (eg, PRISM score, immunocompromised status, prematurity, number of preexisting medical conditions, and need for pressors) between those treated with short-course versus prolonged-course antibiotic therapy. Furthermore, our results were similar and did not change significantly when propensity score techniques were used. We concluded that the difference in treatment duration was more likely to be related to physician preference than to severity of illness.
CONCLUSIONS
Nosocomial lower respiratory tract infections are a frequent cause of morbidity and mortality in patients hospitalized in the ICU who require invasive mechanical ventilation. Although there are numerous studies investigating therapeutic options for nosocomial pneumonia, few have focused on the role of therapy for VAT, and to our knowledge, no studies have evaluated VAT in children. This is the first study to use adult-adapted criteria for the diagnosis of VAT in children.
Lack of adherence to strict diagnostic criteria and lack of guidance regarding the optimal duration of antibiotic therapy may lead to inappropriate antibiotic prescriptions for VAT. Our results suggest that judicious use of antibiotics in children with VAT may decrease subsequent emergence of MDROs without compromising clinical outcomes.
Acknowledgments
We thank Alice Jenh Hsu for her assistance with preparation of this manuscript.
Financial support. National Institutes of Health training grant (T32 AI052071-07 to P. D. T.).
Potential conflicts of interest. A. M. M. has received grant support through his institution from Sage Products and BioMérieux. C. U. L. has received travel reimbursement for board membership from American Medical Informatics Association, has received payment for lectures from South Carolina Medicaid Association, and has received textbook royalties from Springer Verlag. All other authors: no conflicts.
References
- 1.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 2.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 3.Niederman M, Craven D. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 4.Nseir S, Favory R, Jozefowicz E, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care. 2008;12:R62. doi: 10.1186/cc6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer LB, Smaldone GC, Chen JJ, et al. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med. 2008;36:2008–2013. doi: 10.1097/CCM.0b013e31817c0f9e. [DOI] [PubMed] [Google Scholar]
- 6.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 7.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(Pt 1):505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH, Silver P, Murphy DM, Trovillion E. The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest. 1995;108:1655–1662. doi: 10.1378/chest.108.6.1655. [DOI] [PubMed] [Google Scholar]
- 9.Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–539. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 10.Niederman MS. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis. 2010;51(Suppl. 1):S12–S17. doi: 10.1086/653035. [DOI] [PubMed] [Google Scholar]
- 11.Dallas J, Skrupky L, Abebe N, Boyle WA, 3rd, Kollef MH. Ventilator-associated tracheobronchitis (Vat) in a mixed surgical and medical ICU population. Chest. 2010;139:513–518. doi: 10.1378/chest.10-1336. [DOI] [PubMed] [Google Scholar]
- 12.Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl. 1):S59–S66. doi: 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- 13.Nseir S, Ader F, Marquette CH. Nosocomial tracheobronchitis. Curr Opin Infect Dis. 2009;22:148–153. doi: 10.1097/QCO.0b013e3283229fdb. [DOI] [PubMed] [Google Scholar]
- 14.Craven DE, Chroneou A, Zias N, Hjalmarson KI. Ventilator-associated tracheobronchitis: the impact of targeted antibiotic therapy on patient outcomes. Chest. 2009;135:521–528. doi: 10.1378/chest.08-1617. [DOI] [PubMed] [Google Scholar]
- 15.Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health-care associated infection and criteria for specific types of infections in the acute care setting Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Siegel J, Rhinehart E, Jackson M, Chiarello L. Management of multidrug-resistant organisms in healthcare settings, 2006 Am J Infect Control. 2006;35(Suppl. 2):S165–S193. doi: 10.1016/j.ajic.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009;123:1108–1115. doi: 10.1542/peds.2008-1211. [DOI] [PubMed] [Google Scholar]
- 18.Nseir S, Di Pompeo C, Pronnier P, et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J. 2002;20:1483–1489. doi: 10.1183/09031936.02.00012902. [DOI] [PubMed] [Google Scholar]
- 19.Kampf G, Wischnewski N, Schulgen G, Schumacher M, Daschner F. Prevalence and risk factors for nosocomial lower respiratory tract infections in German hospitals. J Clin Epidemiol. 1998;51:495–502. doi: 10.1016/s0895-4356(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 20.Rello J, Ausina V, Castella J, Net A, Prats G. Nosocomial respiratory tract infections in multiple trauma patients. Influence of level of consciousness with implications for therapy. Chest. 1992;102:525–529. doi: 10.1378/chest.102.2.525. [DOI] [PubMed] [Google Scholar]
- 21.Montravers P, Fagon JY, Chastre J, et al. Follow-up protected specimen brushes to assess treatment in nosocomial pneumonia. Am Rev Respir Dis. 1993;147:38–44. doi: 10.1164/ajrccm/147.1.38. [DOI] [PubMed] [Google Scholar]
- 22.Bergmans DC, Bonten MJ, Gaillard CA, et al. Indications for antibiotic use in ICU patients: a one-year prospective surveillance. J Antimicrob Chemother. 1997;39:527–535. doi: 10.1093/jac/39.4.527. [DOI] [PubMed] [Google Scholar]
- 23.Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35:589–593. doi: 10.1016/j.ajic.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Gardam MA, Amihod B, Orenstein P, Consolacion N, Miller MA. Overutilization of indwelling urinary catheters and the development of nosocomial urinary tract infections. Clin Perform Qual Health Care. 1998;6:99–102. [PubMed] [Google Scholar]
- 25.Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43:1009–1017. doi: 10.1097/01.mlr.0000178199.07789.32. [DOI] [PubMed] [Google Scholar]
- 26.Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17:163–166. doi: 10.1097/JTN.0b013e3181fb38a6. [DOI] [PubMed] [Google Scholar]
- 27.DuBose JJ, Inaba K, Shiflett A, et al. Measurable outcomes of quality improvement in the trauma intensive care unit: the impact of a daily quality rounding checklist. J Trauma. 2008;64:22–27. doi: 10.1097/TA.0b013e318161b0c8. discussion 27–29. [DOI] [PubMed] [Google Scholar]
- 28.Milstone AM, Bryant KA, Huskins WC, Zerr DM. The past, present, and future of healthcare-associated infection prevention in pediatrics: multidrug-resistant organisms. Infect Control Hosp Epidemiol. 2010;31(Suppl. 1):S18–S21. doi: 10.1086/656001. [DOI] [PubMed] [Google Scholar]
- 29.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6:751–763. doi: 10.1586/14787210.6.5.751. [DOI] [PubMed] [Google Scholar]
- 30.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(Suppl. 2):S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 31.Johnston SC. Identifying confounding by indication through blinded prospective review. Am J Epidemiol. 2001;154:276–284. doi: 10.1093/aje/154.3.276. [DOI] [PubMed] [Google Scholar]