Abstract
Almost half the patients with colorectal cancer will develop liver metastasis at some stage during their disease. Potentially curative surgical resection is possible in some of these patients. In those patients unsuitable for surgery, treatment with systemic chemotherapy and external radiation therapy is relatively ineffective. Many studies have described the successful use of selective internal radiation therapy (SIRT) with 90Y-SIR-Spheres microspheres in patients with inoperable liver metastasis. The authors report on a patient who has been in complete remission for 1 year after treatment with SIRT.
Background
Colorectal cancer is the third most common cancer in men and the second most common cancer in women in the UK. Over half of patients with colorectal cancer will develop metastatic disease, with a quarter having distant metastatic lesions at diagnosis,1 often in the liver. Surgical resection of colorectal liver metastases is a potentially curative option, with reported 5-year survival of 28–39%.2 However, about 80% of patients with colorectal liver metastases have unresectable disease at presentation,3 and long-term survival is low. For those patients treatment aims to palliate and prolong survival. The treatment options include systemic chemotherapy, thermal ablation techniques, trans-arterial chemo embolisation and external beam radiotherapy. In the last years the SIRT (micro-brachytherapy or ‘radio-embolisation’) for the treatment of diffuse non-resectable colorectal hepatic metastases has emerged. Here we report on patient with liver metastases from a colon cancer, who has achieved a long-term remission after therapy with selective internal radiation therapy (SIRT).
Case presentation
Our patient is a 73-year-old woman presented with weight loss and increasing right upper quadrant abdominal pain. Six months prior to this she had a thoracotomy for spontaneous right pneumothorax having failed conventional management.
Investigations
CT examination showed a large caecal mass and two liver metastases in segments IV and V. She proceeded to an emergency right hemicolectomy which confirmed a pT4b, N1 (3/27), poorly differentiated mucinous adenocarcinoma of the ascending colon with extramural vascular invasion. All margins were free of cancer. Postoperative 18F-FDG PET CT study performed to assess resectability of liver metastasis demonstrated two large foci of markedly increased uptake in segments IV and V of the liver. In the chest there was further uptake in the subcarinal lymph node and in the right cardiophrenic regions.
Treatment
Because of the possible extra-hepatic disease surgical resection was considered inappropriate and the patient was referred for palliative chemotherapy. Six days after the first cycle of 5-FU, Oxaliplatin and Folinic acid (FOLFOX) she was admitted for 11 days for grade III/IV diarrhoea and vomiting. As a result, the patient refused further chemotherapy. SIRT was explained and agreed by the patient.
Before we carried out the SIRT a preliminary hepatic angiography was performed to assess the arterial anatomy and to embolise aberrant vessels to prevent non-target radio-embolisation. In our case the gastroduedenal artery was embolised. There was variant anatomy with the right hepatic artery arising from the superior mesenteric artery. The proximal left hepatic artery was therefore embolised to allow for redistribution of left hepatic arterial supply from the variant right hepatic artery so that radio-embolisation could be performed via the right hepatic artery alone (figure 1). Following embolisation, a ‘break-through’ lung scintigram using 99mTc-macroaggregates of albumin (99mTc-MAA) was undertaken mainly to determine potential shunt of microspheres into the lungs and to assess distribution in the liver. The radiotherapy dose was calculated based on percent of tumour involvement measured on the CT scan. A week later patient was treated with 90Y-SIR-Spheres microspheres to a total radiation dose of 1.46 GBq. No significant treatment-related toxicity was detected during or after the procedure.
Figure 1.
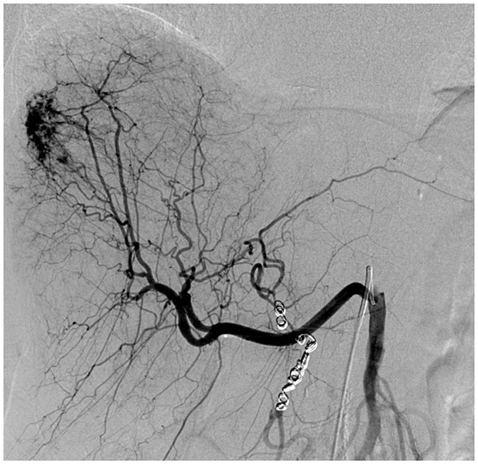
Selective catheterisation of the variant right hepatic artery from SMA shows tumour vascularity in the right lobe. Note the redistribution of left hepatic arterial supply following embolisation of proximal left hepatic artery. Coils in left hepatic artery and GDA.
Outcome and follow-up
Clinical follow-up and CT scans at 3 and 9 months showed complete remission with a performance status of zero. Twelve months follow-up PET CT scan showed resolution of the high tracer uptake in the liver. The increased uptake in the subcarinal lymph node and in the right cardiophrenic region was still visible but had not increased in size (figure 2). An endoscopic ultrasound was carried out to sample the subcarinal lymph node and the fine needle aspiration cytology showed no evidence of adenocarcinoma. The increased uptake in the thoracic region was attributed to the previous thoracotomy. Following this finding a subsegmental liver resection of segments IV and V was undertaken. This showed two discrete areas of fibrosis with a small amount of extracellular mucin, but no epithelial component confirming complete pathological resolution. The biospheres were clearly seen both within these regressed areas and within portal vein tributaries in the background parenchyma (figure 3).
Figure 2.
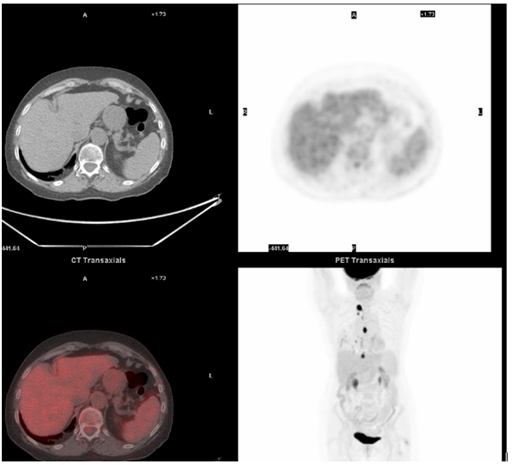
Increased uptake in the subcarinal lymph node and in the right cardiophrenic region is still visible but stable in size. No uptake within the liver.
Figure 3.
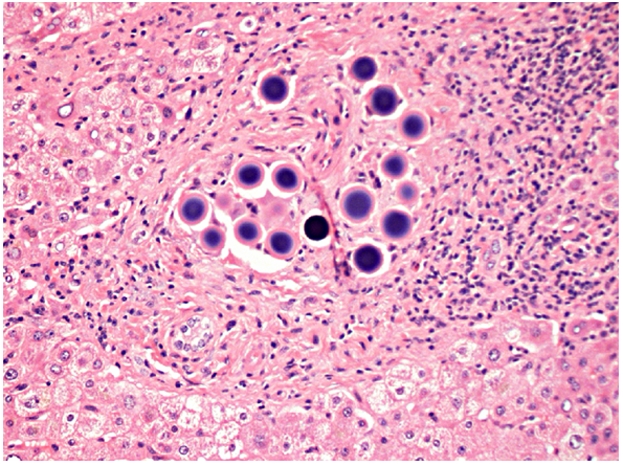
The biospheres are clearly seen trapped within the background parenchyma.
Discussion
SIRT is performed under local anaesthesia where microscopic radioactive spheres are delivered through femoral artery catheter and injected through the hepatic artery to the site of the tumour. The radiotherapy dose delivered depends on the percentage of the liver involvement with metastasis. Liver tumours receive the majority of their blood supply from the hepatic artery while healthy liver tissue receives most of its blood supply via the portal vein. As a result, catheterisation of the hepatic artery permits the targeting of therapeutic material to liver cancer. The microscopic spheres, each approximately 35 μm, are labelled with 90Yttrium (90Y), a pure β emitter with a physical half-life of about two-and-a-half days. The microspheres are preferably trapped in the tumour’s vascular bed, where they destroy the tumour by reducing its blood supply and through local radiation damage to the cancer cells’ DNA. The radiation is wholly contained within the patient’s body, and is continually delivered over approximately 2 weeks, at which point the microspheres are no longer radioactive. Ideal patients have no significant extra-hepatic disease, have sufficient remaining healthy liver and are well enough to tolerate the treatment. Greater than 20% lung shunting or significant reflux of hepatic arterial blood to the stomach, pancreas or bowel is contraindication to SIRT. Possible side effects are nausea, transient fever and liver capsular pain. Theoretical adverse events may include radiation-induced liver damage or hepatitis, pneumonitis, gastrointestinal haemorrhage or ulceration, cholecystitis, biliary strictures, pancreatitis and radiation dermatitis.
SIRT has now been tested in three randomised controlled trials (RCT).4–6 Gray et al4 compared hepatic artery chemotherapy alone with hepatic artery chemotherapy plus SIRT. In this trial, there was significant improvement in complete and partial response but no significant increase in median survival was demonstrated. In the RCT conducted by Van Hazel et al,5 systemic chemotherapy alone was compared to SIRT plus systemic chemotherapy. The median survival was significantly longer in the SIRT plus systemic chemotherapy group (29.4 months) than in the systemic chemotherapy alone group (12.8 months), HR 0.33 (95% CI 0.12 to 0.91) (p=0.025). The third RCT6 compared continuous 5FU infusion versus the same plus SIR-Spheres on day 1, cycle 1. At progression, patients in the 5FU control arm could receive SIR-Spheres if they were still suitable. Although this design blunts the ability of the trial to show a difference in the survival of patients, it was considered by the investigators to be an ethical necessity. The study demonstrated significantly prolonged time to liver progression in patients receiving SIR-Spheres + 5FU compared to 5FU alone: 5.5 versus 2.1 months (p=0.003). The HR was low at 0.38 (95% CI 0.20 to 0.72). Currently SIRT is being tested in a randomised phase II-III trial of FOLFOX ± interventional radio-embolisation with 90-Yttrium- SIR-Spheres in metastatic colorectal cancer funded by Cancer Research, UK.7
Learning points.
-
▶
In our patient, SIRT using 90Y-SIR-Spheres microspheres achieved complete resolution of the two metastatic liver lesions.
-
▶
After 1 year, the patient remains in complete pathological remission. This study supports the use of SIRT in carefully selected patients with colorectal liver metastasis.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 2004;96:1420–5 [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309–18; discussion 318–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711–20 [DOI] [PubMed] [Google Scholar]
- 5.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78–85 [DOI] [PubMed] [Google Scholar]
- 6.Hendlisz A, Van den Eynde M, Peeters M, et al. Phase III trial comparing protracted intravenous fluorouracil infusion alone or with yttrium-90 resin microspheres radioembolization for liver-limited metastatic colorectal cancer refractory to standard chemotherapy. J Clin Oncol 2010;28:3687–94 [DOI] [PubMed] [Google Scholar]
- 7.Sharma R. CRUK/07/030: FOXFIRE: Randomised Phase II-III trial of 5-Fluorouracil, Oxaliplatin, Folinic acid +/- Interventional Radio-Embolisation with Yttrium-90 SIR-Spheres in Metastatic Colorectal Cancer (Supported by the Bobby Moore Fund). http://science.cancerresearchuk.org/research/who-and-what-we-fund/browse-by-location/oxford/university-of-oxford/grants/ricky-sharma-8971-cruk-07-030-foxfire-randomised-phase-ii-iii (accessed 4 April 2011).


