Abstract
Coenzyme Q10 (CoQ10) is an essential electron carrier in the mitochondrial respiratory chain and an important antioxidant. Deficiency of CoQ10 is a clinically and molecularly heterogeneous syndrome, which, to date, has been found to be autosomal recessive in inheritance and generally responsive to CoQ10 supplementation. CoQ10 deficiency has been associated with five major clinical phenotypes: (1) encephalomyopathy, (2) severe infantile multisystemic disease, (3) cerebellar ataxia, (4) isolated myopathy, and (5) nephrotic syndrome. In a few patients, pathogenic mutations have been identified in genes involved in the biosynthesis of CoQ10 (primary CoQ10 deficiencies) or in genes not directly related to CoQ10 biosynthesis (secondary CoQ10 deficiencies). Respiratory chain defects, ROS production, and apoptosis contribute to the pathogenesis of primary CoQ10 deficiencies. In vitro and in vivo studies are necessary to further understand the pathogenesis of the disease and to develop more effective therapies.
Keywords: coenzyme Q10, respiratory chain activity, ROS, oxidative stress
Coenzyme Q or ubiquinone is a lipophilic molecule present in all tissues and cells that is located mainly in the inner mitochondrial membrane. It is composed of a redox active benzoquinone ring conjugated to an isoprenoid chain. The length of the chain differs among species; in humans, ubiquinone contains predominantly 10 isoprenyl units and is designated CoQ10. CoQ shuttles electrons from complex I and II to complex III of the mitochondrial respiratory chain; it also functions as a lipid-soluble antioxidant, scavenges oxygen reactive species, and is involved in multiple aspects of cellular metabolism [Turunen et al., 2004].
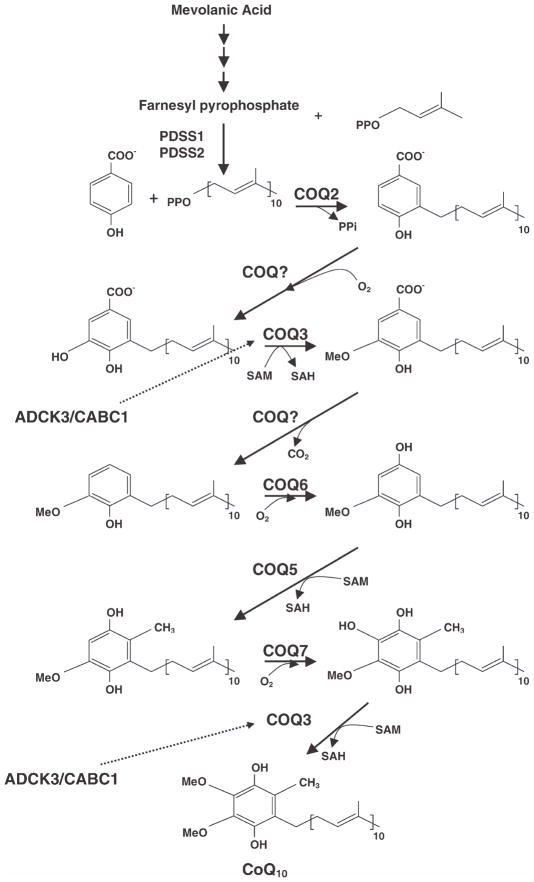
Current knowledge of the CoQ biosynthetic pathway in eukaryotes is mostly derived from characterization of CoQ-deficient mutant strains of Saccharomyces cerevisiae [Kawamukai, 2009]. The benzoquinone ring is derived from tyrosine, whereas the polyisoprenoid tail is assembled by polyprenyldiphosphate synthase. Then, polyprenyl diphosphate:4-HB transferase catalyzes the formation of covalent linkage between the benzoquinone head group and the isoprenoid tail, followed by modifications of the aromatic ring (Fig. 1). Ten complementation groups of CoQ yeast mutants have been identified, and mammalian homologues of the yeast Coq genes (COQ in humans) have been identified via sequence homology [Tran and Clarke, 2007; Kawamukai, 2009].
Fig. 1.
CoQ10 biosynthesis pathway. CoQ10 is composed of a benzoquinone ring and a decaprenyl side chain. ADCK3 (CABC1) is a kinase that modulate CoQ10 synthesis, possibly through phosphorylation of COQ3 [Tauche et al., 2008]. The function of COQ9 is unknown.
CoQ10 deficiency causes clinically heterogeneous diseases. In most cases, family history suggests an autosomal recessive mode of inheritance, because siblings are often affected, whereas parents are typically unaffected and sometimes consanguineous. In 18 patients, pathogenic mutations in genes encoding for proteins involved in the biosynthesis of CoQ10 have been identified (primary CoQ10 deficiencies) [Lopez et al., 2006; Quinzii et al., 2006; Diomedi-Camassei et al., 2007; Mollet et al., 2007, 2008; Lagier-Tourenne et al., 2008; Duncan et al., 2009]. Most patients respond to CoQ10 supplementation [Quinzii et al., 2008a; Duncan et al., 2009].
CLINICAL AND MOLECULAR HETEROGENEITY OF CoQ10 DEFICIENCY
Encephalomyopathy
In 1989, Ogasahara and colleagues reported the first patients with CoQ10 deficiency. Two sisters presented with mitochondrial myopathy, elevated serum creatine kinase (CK), recurrent myoglobinuria, lactic acidosis, and central nervous system signs (seizures and mental retardation) that was associated with decreased activities of complexes I + III and II + III and markedly reduced CoQ10 in muscle [Ogasahara et al., 1989]. Since then, several patients with encephalomyopathy manifesting the same clinical triad of mitochondrial myopathy, recurrent myoglobinuria, and encephalopathy have been described [Sobreira et al., 1997; Boitier et al., 1998; Di Giovanni et al., 2001; Aure et al., 2004]. Among this group of patients, molecular defects have been found in only one patient who carries mutations in the ADCK3/CABC1 gene [Aure et al., 2004; Mollet et al., 2008], the human ortholog of the yeast Coq8 gene. ADCK3 encodes a putative mitochondrial kinase thought to be involved in the regulation of the biosynthesis of CoQ, possibly by phosphorylating Coq3 protein (Coq3p) [Iiizumi et al., 2002; Tauche et al., 2008].
Childhood-Onset Cerebellar Ataxia and Atrophy
In 2001, Musumeci and colleagues described the most common phenotype associated with CoQ10 deficiency, which is characterized by childhood-onset cerebellar ataxia and atrophy; other variably associated manifestations include: neuropathy, seizures, mental retardation, muscle weakness, and hypogonadism [Musumeci et al., 2001; Lamperti et al., 2003; Gironi et al., 2004; Artuch et al., 2006; Lagier-Tourenne et al., 2008; Mollet et al., 2008]. Partial CoQ10 deficiency was documented in muscle and in most fibroblasts (9/11) and lymphoblasts (2/3). Although muscle biopsies did not show abnormal mitochondrial proliferation or lipid storage in the first reports [Musumeci et al., 2001; Lamperti et al., 2003], skeletal muscle in one of the patients reported later revealed mitochondrial accumulation and lipid droplets in 10–20% of the fibers [Mollet et al., 2008]. A subgroup of patients with juvenile-onset cerebellar ataxia has primary CoQ10 deficiency due to ADCK3/CABC1 mutations [Lagier-Tourenne et al., 2008; Mollet et al., 2008]. Secondary CoQ10 deficiency has been found to result from a stop codon mutation in the APTX gene encoding aprataxin [Quinzii et al., 2005; Le Ber et al., 2007], which is a protein involved in double-stranded DNA repair [Reynolds et al., 2009] and is known to cause ataxia-oculomotor-apraxia 1 (AOA1) [Date et al., 2001; Moreira et al., 2001]. In these patients, CoQ10 deficiency was not correlated with disease duration, severity, or progression or with biological measures, indicating that CoQ10 deficiency is not the primary or the only cause of neurological decline in AOA1. Nevertheless, three patients improved considerately after CoQ10 supplementation [Quinzii et al., 2005].
Multisystemic Infantile Form
In contrast to the ataxic form, most patients with the multisystemic infantile form have had genetically confirmed primary CoQ10 deficiency. The infantile-onset disorder was described initially in 2000 by Rötig et al. in three siblings who presented soon after birth with neurological symptoms, including nystagmus, optic atrophy, sensorineural hearing loss, ataxia, dystonia, weakness, and rapidly progressive nephropathy [Rötig et al., 2000]; however, the causative mutation in this family has not been reported. In 2006, we reported the first mutation causing primary CoQ10 deficiency in a proband with infantile-onset multisystemic disease and his younger sister, who shared a homozygous missense mutation in the COQ2 gene encoding parahydroxybenzoate-polyprenyl transferase [Quinzii et al., 2006]. The elder sibling was a 33-month-old boy who was noted to have nystagmus at 2 months. At 12 months, he was hospitalized because of a severe nephrotic syndrome and neurological examination showed hypotonia and mild psychomotor delay. At 18 months, he developed frequent vomiting, psychomotor regression, tremor, weakness, hypotonia, and status epilepticus. Brain MRI showed cerebral and cerebellar atrophy and stroke-like lesions. He received a successful renal transplant at 3 years of age. The sister developed nephrotic syndrome at 12 months of age without any clinical signs of neurological involvement [Salviati et al., 2005; Diomedi-Camassei et al., 2007]. Both siblings improved with CoQ10 supplementation [Diomedi-Camassei et al., 2007; Montini et al., 2008].
Rötig and colleagues also reported a girl with neonatal neurological distress, nephrotic syndrome, hepatopathy, pancytopenia, diabetes, seizures, and lactic acidosis progressing to fatal multiorgan failure at age 12 days [Mollet et al., 2007]. The older brother also had anemia, liver failure, and renal insufficiency and died at the age of 1 day. Both siblings harbored a homozygous base-pair deletion in exon 7 of the COQ2 gene.
Mutations in PDSS2, which encodes one of two subunits of polyprenyl diphosphate synthase, the first enzyme of the CoQ10 biosynthetic pathway, have been reported in a male infant with nephrotic syndrome and Leigh syndrome [Lopez et al., 2006]. The boy presented with neonatal pneumonia and hypotonia. At 3 months of age, he developed seizures and subsequently became progressively floppy, had difficulty feeding, severe episodic vomiting, and lactic acidosis and died at age 8 months due to severe refractory focal status epilepticus. In a consanguineous family, two siblings had CoQ10 deficiency due to a homozygous PDSS1 mutation manifesting as a multisystem disease with early-onset deafness, encephaloneuropathy, obesity, livedo reticularis, and cardiac valvulopathy [Mollet et al., 2007].
Last year, Duncan and colleagues reported mutations in another gene, COQ9, required for the biosynthesis of CoQ10 in a boy who presented at 6 hr of age with poor feeding, hypothermia, and “shaking of both arms” [Rahman et al., 2001; Duncan et al., 2009]. He had generalized limb hypertonia with reduced truncal tone, lactic acidosis, renal tubulopathy, and cardiomyopathy. Brain MRI revealed cerebral and cerebellar atrophy. He developed severe seizures and dystonia and died at 2 years of age.
In all of the infantile multi-systemic syndromes, levels of CoQ10 were decreased in muscle and fibroblasts. Noteworthily, all patients with PDSS1, PDSS2, COQ2, and COQ9 mutations had renal disease [Lopez et al., 2006; Quinzii et al., 2006; Diomedi-Camassei et al., 2007; Mollet et al., 2007; Duncan et al., 2009] as did Pdss2 mutant mice that show glomerulonephritis as the sole clinical manifestation [Peng et al., 2008].
Glomerulopathy
Diomedi-Cassadei and colleagues reported two patients with early-onset glomerulopathy due to mutations in the COQ2 gene [Diomedi-Camassei et al., 2007]. The first patient presented with steroid-resistant nephrotic syndrome at age 18 months as a result of collapsing glomerulopathy, without extrarenal manifestations. The second patient presented at 5 days of life with oliguria, had severe extracapillary proliferation on renal biopsy, rapidly developed end-stage renal disease, and died at the age of 6 months after a course complicated by progressive epileptic encephalopathy. Combined complex II + III activity and CoQ10 level were decreased in renal cortex as well as in skeletal muscle [Diomedi-Camassei et al., 2007].
Myopathy
Finally, two groups have described a pure myopathic form, with lipid storage myopathy and respiratory chain dysfunction [Lalani et al., 2005; Horvath et al., 2006]. In 2007, Gempel and colleagues [Gempel et al., 2007] found in the patients reported by Horvath and colleagues mutations in the ETFDH gene encoding electron-transferring-flavoprotein dehydrogenase, which previously had been associated with glutaric aciduria type II (multiple acyl-CoA dehydrogenase deficiency [MADD]). In that report, all seven patients from five families presented with exercise intolerance, fatigue, proximal myopathy, and high-serum CK. Muscle histology showed lipid storage and subtle signs of mitochondrial myopathy. In contrast, other studies reported patients with MADD and ETFDH mutations who had normal CoQ10 levels in muscle [Liang et al., 2009; Ohkuma et al., 2009].
Other Clinical Presentations
In addition to the main phenotypes described above, CoQ10 deficiency has been reported in two adults sisters with childhood-onset Leigh syndrome encephalopathy, growth retardation, infantilism, ataxia, deafness, and lactic acidosis [Van Maldergem et al., 2002]. CoQ10 deficiency has also been reported in a patient with cardiofaciocutaneous syndrome due to a BRAF mutation [Aeby et al., 2007]. Leshinsky-Silver and colleagues reported a patient who presented with neonatal liver disease, pancreatic insufficiency, tyrosinemia, hyperammonemia, subsequent sensorineural hearing loss, and Leigh syndrome [Leshinsky-Silver et al. 2003]. Liver biopsy revealed markedly reduced complexes I + III and II + III activities that were restored by addition of CoQ10 to the liver homogenate indicating ubiquinone deficiency.
Moreover, CoQ10 deficiency has been reported in patients with a variety of mitochondrial diseases including mitochondrial DNA depletion [Matsuoka et al., 1991; Montero et al., 2005; Miles et al., 2008; Sacconi et al., 2010].
DIAGNOSIS AND TREATMENT
The content of CoQin organs and membranes depends on functional requirements. It exhibits great variations not only in different tissues but also within the same organ and among membranes of individuals. Moreover, tissue levels of CoQ10 depend mainly on de novo synthesis [Dallner and Sindelar, 2000]. In contrast, plasma concentrations of CoQ10 are significantly influenced by dietary uptake. Because plasma concentration may not adequately represent cellular concentrations, blood cells and platelets may be suitable for assessing intracellular CoQ10 concentration for ubiquinone deficiency in treatment-naïve patients [Niklowitz et al., 2004, 2007; Miles et al., 2008]. Deficiency of CoQ10 in skin fibroblasts can be an important confirmation of a primary defect of CoQ10 biosynthesis in patients with multisystemic disease; however, normal levels of CoQ10 in fibroblasts do not exclude deficiency of CoQ10 in muscle [Lagier-Tourenne et al., 2008; Montero et al., 2008]. Therefore, direct measurement of CoQ10 levels in muscle is the most reliable test for diagnosis. Reduced biochemical activities of complexes I + III (NADH:cytochrome c oxidoreductase) and II + III (succinate:cytochrome c oxidoreductase) in the presence of normal activities of isolated complex I and III suggest severe CoQ10 deficiency, although activities of these enzymes may be normal with partial ubiquinone deficiency.
Although early supplementation in patients with COQ2 mutations appears to alleviate the nephropathy and may prevent development of neurological signs and symptoms [Montini et al., 2008], patients with PDSS2 and COQ9 mutations described by Lopez et al. and Rahman et al. died despite CoQ10 replacement. CoQ10 treatment was accompanied by mild, subjective, clinical improvement in patients with cerebellar ataxia associated with mutations in ADCK3/CABC1 [Rahman et al., 2001; Lopez et al., 2006; Lagier-Tourenne et al., 2008; Mollet et al., 2008; Duncan et al., 2009]. All the patients with pure myopathy showed dramatic improvements after CoQ10 supplementation [Gempel et al., 2007]. Interestingly, in patients with the encephalomyopathic form treated with CoQ10 supplementation, all showed improvements of muscle symptoms, while only some had nervous system improvements. The lack of improvement of cerebellar symptoms may be due to poor transfer of CoQ10 across the blood-brain barrier, irreversible structural alterations in the cerebellum, or both [Bentinger et al., 2003].
In general, disparate responses to CoQ10 supplementation suggest differences in the pathophysiologic mechanisms of these disorders and/or in the pharmacokinetics of CoQ10 supplementation.
CoQ10 is a lipophilic molecule, which accumulates in membranes reaching saturated concentrations. Thus, only a small proportion reaches the mitochondria. In addition, much of the CoQ10 in mitochondria is likely to be trapped in the outer membrane and, consequently, not available to the respiratory chain, which is located in the mitochondrial inner membrane [Geromel et al., 2002].
We have evaluated the in vitro efficacy of CoQ10 supplementation in normalizing the bioenergetic status and oxidative balance in fibroblasts of CoQ10 deficient patients compared with the efficacy of short-tail ubiquinone analogs (idebenone and CoQ2), which are less lipophilic, as well as vitamin C, a hydrophilic antioxidant [López et al., in press]. We observed that after 24 hr of 5 μM CoQ10 supplementation (the approximate concentration reached in the plasma of patients supplemented with high-doses of oral CoQ10), cellular CoQ10 levels increased dramatically and all four compounds significantly reduced superoxide anion levels and cell death in mutant fibroblasts with increased oxidative stress. However, after 24 hr of CoQ10 treatment, none of the cell lines showed significant improvement in ATP levels or in ATP/ADP ratios, which are markers of respiratory chain function. In marked contrast to treatment for 24 hr of CoQ10, incubation of ubiquinone-deficient fibroblasts for 1 week with 5 μM CoQ10 increased ATP levels and ATP/ADP ratios significantly, indicating normalization of the bioenergetic status.
Similarly, yeast coq mutants showed inefficient uptake of exogenous CoQ6 to the mitochondrial inner membrane, which was reflected in a low-succinate cytochrome c reductase activity after 2–15 μM CoQ6 supplementation for 48 hr. Complete rescue of growth of the yeast coq mutants’ supplemented with 15 μM CoQ6 was possible after 6–8 days [Do et al., 2001; Jonassen et al., 2002; Santos-Ocaña et al., 2002]. These results suggest that the pharmacokinetic constraints of CoQ10 in reaching the mitochondrial respiratory chain are key limiting factors in determining its efficacy in CoQ10 deficient patients. Furthermore, an in vivo study of PDSS2 mutant mice treated for 4 months with 100 mg/kg body weight (b.w.)/day or 200 mg/kg b.w./day of water-soluble CoQ10 showed that only the highest dose of CoQ10 improved the renal function in these CoQ deficient animals [Saiki et al., 2008]. Thus, the dose of CoQ10 is another important factor influencing the effectiveness of CoQ10 supplementation in ubiquinone deficient patients.
In contrast to CoQ10, short-tail ubiquinone analogs and vitamin C failed to increase ATP levels and ATP/ADP ratio in patients’ cells after both 24 hr and 1 week of treatment. These results are in agreement with previous studies showing that hydrophilic ubiquinone analogs (CoQ2 and idebenone) or mitochondrial-targeted ubiquinone (MitoQ) are less efficient than hydrophobic ubiquinone analogs in enhancing energy production by the mitochondrial respiratory chain [Degli Esposti et al., 1996a,b; King et al., 2009].
Thus, initial clinical experience and in vitro studies suggest that in CoQ10 deficient patients, early treatment based on early diagnosis is critical to maximize the efficacy of ubiquinone supplementation. In addition, administration of antioxidants with high bioavailability may be helpful; however, short-tail ubiquinone analogs are not suitable substitutes for CoQ10. An important determinant of efficacy is absorption/bioavailability of CoQ10 in the various available formulations. Therefore, dosage and monitoring of plasma levels and clinical response are important [Bhagavan and Chopra, 2006; Miles, 2007].
PATHOGENESIS OF PRIMARY CoQ10 DEFICIENCY: IN VITRO AND IN VIVO STUDIES
In addition to shuttling electrons in the mitochondrial respiratory chain, CoQ10 serves several additional cellular functions including transfer of electrons in plasma membranes [Sun et al., 1992] and lysosomes [Gille and Nohl, 2000], modulation of apoptosis [Fontaine et al., 1998; Walter et al., 2002], proton transport of uncoupling proteins [Echtay et al., 2000], and antioxidant activity including inhibition of lipid peroxidation [Villalba et al., 2001] although under certain conditions CoQ may serve as a pro-oxidant [Bentinger et al., 2007]. Consequently, deficiency of CoQ10 may disrupt several vital cellular functions. Studies of skeletal muscle biopsies from patients with all forms of CoQ10 deficiency have shown variable defects of respiratory chain enzyme activities (coupled complexes I + III and II + III) [Ogasahara et al., 1989; Musumeci et al., 2001; Lamperti et al., 2003; Lalani et al., 2005; Horvath et al., 2006]. In addition, muscle from patients with myopathic CoQ10-deficiencies has revealed increased markers of apoptosis including increased DNA fragmentation by terminal deoxynucleotidyl transferase mediated dUTP nick end-labeling (TUNEL), increased expression of proapoptotic FAS proteins, and activation of capase 3 [Di Giovanni et al., 2001; Horvath et al., 2006]. Lopez-Martin and colleagues showed that COQ2 mutant fibroblasts require uridine to maintain growth and proposed that deficiency of CoQ10 caused a defect of de novo pyrimidine biosynthesis because of the dependence of dihydro-orotate de-hydrogenase on ubiquinol [Lopez-Martin et al., 2007].
Initial studies of cultured fibroblasts from two siblings with infantile-onset CoQ10-deficiency showed mild respiratory chain defects but no evidence of increased superoxide anions, lipid peroxidation, or apoptosis-mediated cell death [Geromel et al., 2001]. To explore these putative pathogenic mechanisms, we have investigated the consequence of severe CoQ10 deficiency on bioenergetics, oxidative stress, and antioxidant defenses in cultured skin fibroblasts harboring COQ2 and PDSS2 mutations. These studies suggest that defects in the first two committed steps of the CoQ10 biosynthetic pathway produce different biochemical alterations. PDSS2 mutant fibroblasts have 12% CoQ10 content and 28% residual CII + III activity relative to control cells with markedly reduced ATP synthesis, but do not show increased ROS production, signs of oxidative stress, or increased antioxidant defense markers. In contrast, COQ2 mutant fibroblasts have 30% CoQ10 content and 48% residual CII + III activity with mild defects of ATP synthesis and show significantly increased ROS production as well as oxidation of lipids and proteins [Quinzii et al., 2008b]. To better understand the pathogenesis of CoQ10 deficiency, we have now characterized the effects of varying severity of CoQ10 deficiency on ROS production and mitochondrial bioenergetics in additional cells harboring genetic defects of CoQ10 biosynthesis. We have observed a correlation between levels of CoQ10 and ROS production: 10–15% or >60% residual CoQ10 content are not associated with significant ROS production, whereas 30–50% residual CoQ10 content is associated with maximal increases in ROS production and cell death. These studies confirm that varying degrees of CoQ10 variably impair ATP synthesis and induce oxidative stress [Quinzii et al., in press].
Although studies of both muscle biopsies and cultured fibroblasts indicate that CoQ10 deficiency impairs oxidative phosphorylation, activates apoptosis, and induces oxidative stress, pathogenic effects may be cell-specific and the relative contributions of each pathway to cell death are unknown. A role of tissue-specificity in CoQ10 deficiency is supported by studies in the kd/kd mice with a spontaneous missense mutation (called kidney disease) in the Pdss2 gene [Lyon and Hulse, 1971; Peng et al., 2008]. Homozygotes for the kd allele appear healthy for the first 8 weeks of life but then develop a lethal kidney disease [Lyon and Hulse, 1971; Peng et al., 2004; Madaio et al., 2005]. Extensive phenotyphic evaluation of mice older than 120 days failed to detect any overt extrarenal disease manifestations, suggesting that early mortality from kidney failure precluded additional manifestations of CoQ10 deficiency that might have otherwise developed over time [Peng et al., 2008]. Saiki and colleagues noted evidence suggesting a primary role of oxidative stress in the pathogenesis of the disease in the kd/kd mice [Saiki et al., 2008].
Autophagy may also play a role in the pathogenesis of CoQ10 deficiency. Rodriguez-Hernandez and colleagues studied four cell lines from patients with CoQ10 deficiency, two carrying COQ2 mutations, and two with unknown molecular defects. They observed increased levels of lysososomal markers as well as enhanced expression of transcriptional and translational levels of autophagic genes [Rodríguez-Hernández et al., 2009]. Because inhibition of autophagy resulted in apoptotic cell death, the authors suggested that autophagy is a protective mechanism involved in the degradation of dysfunctional mitochondria. Interestingly, findings of mitophagy and upregulation of autophagy were also noted in liver-conditional Pdss2 knockout mice [Peng et al., 2004, 2008].
CONCLUSIONS
CoQ10 deficiencies are clinically and genetically heterogeneous diseases that are potentially treatable and therefore important to diagnose early. In vitro studies from our and other groups have revealed that CoQ10 deficiency leads to diverse biochemical consequences that play different roles in the demise of mutant fibroblasts. Further studies on the pathogenesis of CoQ10 deficiency in patients with different molecular defects and in animal models will lead to improved therapies in patients.
Acknowledgments
Grant sponsor: NIH; Grant numbers: HD32062, NS11786; Grant sponsors: Muscular Dystrophy Association (MDA), Marriott Mitochondrial Disorder Clinical Research Fund (MMDCRF).
References
- Aeby A, Sznajer Y, Cave H, et al. Cardiofaciocutaneous (CFC) syndrome associated with muscular coenzyme Q10 deficiency. J Inherit Metab Dis. 2007;30:827. doi: 10.1007/s10545-007-0612-0. [DOI] [PubMed] [Google Scholar]
- Artuch R, Brea-Calvo G, Briones P, et al. Cerebellar ataxia with coenzyme Q(10) deficiency: diagnosis and follow-up after coenzyme Q(10) supplementation. J Neurol Sci. 2006;246:153–158. doi: 10.1016/j.jns.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Aure K, Benoist JF, Ogier de Baulny H, et al. Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology. 2004;63:727–729. doi: 10.1212/01.wnl.0000134607.76780.b2. [DOI] [PubMed] [Google Scholar]
- Bentinger M, Brismar K, Dallner G. The antioxidant role of coenzyme Q. Mitochondrion. 2007;7 (Suppl):S41–S50. doi: 10.1016/j.mito.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bentinger M, Dallner G, Chojnacki T, et al. Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic Biol Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- Boitier E, Degoul F, Desguerre I, et al. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci. 1998;156:41–46. doi: 10.1016/s0022-510x(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Dallner G, Sindelar PJ. Regulation of ubiquinone metabolism. Free Radic Biol Med. 2000;29:285–294. doi: 10.1016/s0891-5849(00)00307-5. [DOI] [PubMed] [Google Scholar]
- Date H, Onodera O, Tanaka H, et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat Genet. 2001;29:184–188. doi: 10.1038/ng1001-184. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M, Ngo A, Ghelli A, et al. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch Biochem Biophys. 1996a;330:395–400. doi: 10.1006/abbi.1996.0267. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M, Ngo A, McMullen GL, et al. The specificity of mitochondrial complex I for ubiquinones. Biochem J. 1996b;313 (Pt 1):327–334. doi: 10.1042/bj3130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni S, Mirabella M, Spinazzola A, et al. Coenzyme Q10 reverses pathological phenotype and reduces apoptosis in familial CoQ10 deficiency. Neurology. 2001;57:515–518. doi: 10.1212/wnl.57.3.515. [DOI] [PubMed] [Google Scholar]
- Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- Do TQ, Hsu AY, Jonassen T, et al. A defect in coenzyme Q biosynthesis is responsible for the respiratory deficiency in Saccharomyces cerevisiae abc1 mutants. J Biol Chem. 2001;276:18161–18168. doi: 10.1074/jbc.M100952200. [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Bitner-Glindzicz M, Meunier B, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Winkler E, Klingenberg M. Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature. 2000;408:609–613. doi: 10.1038/35046114. [DOI] [PubMed] [Google Scholar]
- Fontaine E, Ichas F, Bernardi P. A ubiquinone-binding site regulates the mitochondrial permeability transition pore. J Biol Chem. 1998;273:25734–25740. doi: 10.1074/jbc.273.40.25734. [DOI] [PubMed] [Google Scholar]
- Gempel K, Topaloglu H, Talim B, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain. 2007;130(Pt 8):2037–2044. doi: 10.1093/brain/awm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geromel V, Darin N, Chretien D, et al. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- Geromel V, Kadhom N, Ceballos-Picot I, et al. Human cultured skin fibroblasts survive profound inherited ubiquinone depletion. Free Radic Res. 2001;35:11–21. doi: 10.1080/10715760100300551. [DOI] [PubMed] [Google Scholar]
- Gille L, Nohl H. The existence of a lysosomal redox chain and the role of ubiquinone. Arch Biochem Biophys. 2000;375:347–354. doi: 10.1006/abbi.1999.1649. [DOI] [PubMed] [Google Scholar]
- Gironi M, Lamperti C, Nemni R, et al. Late-onset cerebellar ataxia with hypogonadism and muscle coenzyme Q10 deficiency. Neurology. 2004;62:818–820. doi: 10.1212/01.wnl.0000113719.67643.b7. [DOI] [PubMed] [Google Scholar]
- Horvath R, Schneiderat P, Schoser BG, et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66:253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- Iiizumi M, Arakawa H, Mori T, et al. Isolation of a novel gene, CABC1, encoding a mitochondrial protein that is highly homologous to yeast activity of bc1 complex. Cancer Res. 2002;62:1246–1250. [PubMed] [Google Scholar]
- Jonassen T, Marbois BN, Faull KF, et al. Development and fertility in Caenorhabditis elegans clk-1 mutants depend upon transport of dietary coenzyme Q8 to mitochondria. J Biol Chem. 2002;277:45020–45027. doi: 10.1074/jbc.M204758200. [DOI] [PubMed] [Google Scholar]
- Kawamukai M. Biosynthesis and bioproduction of coenzyme Q10 by yeasts and other organisms. Biotechnol Appl Biochem. 2009;53(Pt 4):217–226. doi: 10.1042/BA20090035. [DOI] [PubMed] [Google Scholar]
- King MS, Sharpley MS, Hirst J. Reduction of hydrophilic ubiquinones by the flavin in mitochondrial NADH:ubiquinone oxidoreductase (Complex I) and production of reactive oxygen species. Biochemistry. 2009;48:2053–2062. doi: 10.1021/bi802282h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Tazir M, Lopez LC, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani SR, Vladutiu GD, Plunkett K, et al. Isolated mitochondrial myopathy associated with muscle coenzyme Q10 deficiency. Arch Neurol. 2005;62:317–320. doi: 10.1001/archneur.62.2.317. [DOI] [PubMed] [Google Scholar]
- Lamperti C, Naini A, Hirano M, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60:1206–1208. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Dubourg O, Benoist JF, et al. Muscle coenzyme Q10 deficiencies in ataxia with oculomotor apraxia 1. Neurology. 2007;68:295–297. doi: 10.1212/01.wnl.0000252366.10731.43. [DOI] [PubMed] [Google Scholar]
- Leshinsky-Silver E, Levine A, Nissenkorn A, et al. Neonatal liver failure and Leigh syndrome possibly due to CoQ-responsive OXPHOS deficiency. Mol Genet Metab. 2003;79:288–293. doi: 10.1016/s1096-7192(03)00097-0. [DOI] [PubMed] [Google Scholar]
- Liang WC, Ohkuma A, Hayashi YK, et al. ETFDH mutations. CoQ10 levels, and respiratory chain activities in patients with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency. Neuromuscul Disord. 2009;19:212–216. doi: 10.1016/j.nmd.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López LC, Quinzii C, Area E, et al. Treatment of CoQ10 deficient fibroblasts with ubiquinone, analogs, and vitamin C: time- and compound-dependent effects on bioenergetic and oxidative stress status. PLoS One. doi: 10.1371/journal.pone.0011897. (in press) [DOI] [PMC free article] [PubMed]
- Lopez LC, Schuelke M, Quinzii CM, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martin JM, Salviati L, Trevisson E, et al. Missense mutation of the COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum Mol Genet. 2007;16:1091–1097. doi: 10.1093/hmg/ddm058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, Hulse EV. An inherited kidney disease of mice resembling human nephronophthisis. J Med Genet. 1971;8:41–48. doi: 10.1136/jmg.8.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaio MP, Ahima RS, Meade R, et al. Glomerular and tubular epithelial defects in kd/kd mice lead to progressive renal failure. Am J Nephrol. 2005;25:604–610. doi: 10.1159/000089709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Maeda H, Goto Y, et al. Muscle coenzyme Q10 in mitochondrial encephalomyopathies. Neuromuscul Disord. 1991;1:443–447. doi: 10.1016/0960-8966(91)90007-f. [DOI] [PubMed] [Google Scholar]
- Miles MV. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7 (Suppl):S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Miles MV, Miles L, Tang PH, et al. Systematic evaluation of muscle coenzyme Q10 content in children with mitochondrial respiratory chain enzyme deficiencies. Mitochondrion. 2008;8:170–180. doi: 10.1016/j.mito.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Mollet J, Delahodde A, Serre V, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet J, Giurgea I, Schlemmer D, et al. Pre-nyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117:765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero R, Artuch R, Briones P, et al. Muscle coenzyme Q10 concentrations in patients with probable and definite diagnosis of respiratory chain disorders. Biofactors. 2005;25:109–115. doi: 10.1002/biof.5520250112. [DOI] [PubMed] [Google Scholar]
- Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849–2850. doi: 10.1056/NEJMc0800582. [DOI] [PubMed] [Google Scholar]
- Moreira MC, Barbot C, Tachi N, et al. The gene mutated in ataxiaocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet. 2001;29:189–193. doi: 10.1038/ng1001-189. [DOI] [PubMed] [Google Scholar]
- Musumeci O, Naini A, Slonim AE, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–855. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- Niklowitz P, Menke T, Andler W, et al. Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: comparison of the antioxidant level in blood cells and their environment in healthy children and after oral supplementation in adults. Clin Chim Acta. 2004;342:219–226. doi: 10.1016/j.cccn.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Niklowitz P, Sonnenschein A, Janetzky B, et al. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. Int J Biol Sci. 2007;3:257–262. doi: 10.7150/ijbs.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasahara S, Engel AG, Frens D, et al. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyopathy. Proc Natl Acad Sci USA. 1989;86:2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma A, Noguchi S, Sugie H, et al. Clinical and genetic analysis of lipid storage myopathies. Muscle Nerve. 2009;39:333–342. doi: 10.1002/mus.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Falk MJ, Haase VH, et al. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:e1000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Jarett L, Meade R, et al. Mutant prenyltransferase-like mitochondrial protein (PLMP) and mitochondrial abnormalities in kd/kd mice. Kidney Int. 2004;66:20–28. doi: 10.1111/j.1523-1755.2004.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii C, Naini A, Salviati L, et al. A Mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet. 2006;78:345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, López LC, Naini A, et al. Human CoQ10 deficiencies. Biofactors. 2008a;32:113–118. doi: 10.1002/biof.5520320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, Kattah AG, Naini A, et al. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology. 2005;64:539–541. doi: 10.1212/01.WNL.0000150588.75281.58. [DOI] [PubMed] [Google Scholar]
- Quinzii CM, López LC, Gilkerson RW, et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. doi: 10.1096/fj.09-152728. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, Lopez LC, Von-Moltke J, et al. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J. 2008b;22:1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Hargreaves I, Clayton P, et al. Neonatal presentation of coenzyme Q10 deficiency. J Pediatr. 2001;139:456–458. doi: 10.1067/mpd.2001.117575. [DOI] [PubMed] [Google Scholar]
- Reynolds JJ, El-Khamisy SF, Caldecott KW. Short-patch single-strand break repair in ataxia oculomotor apraxia-1. Biochem Soc Trans. 2009;37(Pt 3):577–581. doi: 10.1042/BST0370577. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Hernández A, Cordero MD, Salviati L, et al. Coenzyme Q deficiency triggers mitochondria degradation by mitophagy. Autophagy. 2009;5:19–32. doi: 10.4161/auto.5.1.7174. [DOI] [PubMed] [Google Scholar]
- Rötig A, Appelkvist E-L, Geromel V, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- Sacconi S, Trevisson E, Salviati L, et al. Coenzyme Q10 is frequently reduced in muscle of patients with mitochondrial myopathy. Neuromuscul Disord. 2010;20:44–48. doi: 10.1016/j.nmd.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Saiki R, Lunceford AL, Shi Y, et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati L, Sacconi S, Murer L, et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology. 2005;65:606–608. doi: 10.1212/01.wnl.0000172859.55579.a7. [DOI] [PubMed] [Google Scholar]
- Santos-Ocaña C, Do TQ, Padilla S, et al. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002;277:10973–10981. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- Sobreira C, Hirano M, Shanske S, et al. Mitochondrial encephalomyopathy with coenzyme Q10 deficiency. Neurology. 1997;48:1238–1243. doi: 10.1212/wnl.48.5.1238. [DOI] [PubMed] [Google Scholar]
- Sun IL, Sun EE, Crane FL, et al. Requirement for coenzyme Q in plasma membrane electron transport. Proc Natl Acad Sci USA. 1992;89:11126–11130. doi: 10.1073/pnas.89.23.11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauche A, Krause-Buchholz U, Rodel G. Ubiquinone biosynthesis in Saccharomyces cerevisiae: the molecular organization of O-methylase Coq3p depends on Abc1p/Coq8p. FEMS Yeast Res. 2008;8:1263–1275. doi: 10.1111/j.1567-1364.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- Tran UC, Clarke CF. Endogenous synthesis of coenzyme Q in eukaryotes. Mitochondrion. 2007;7 (Suppl):S62–S71. doi: 10.1016/j.mito.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochim Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Van Maldergem L, Trijbels F, DiMauro S, et al. Coenzyme Q-responsive Leigh’s encephalopathy in two sisters. Ann Neurol. 2002;52:750–754. doi: 10.1002/ana.10371. [DOI] [PubMed] [Google Scholar]
- Villalba JM, López-Lluch G, Santos-Ocaña C, et al. Extramitochondrial functions of coenzyme Q. In: Kagan VE, Quinn PJ, editors. Coenzyme Q: molecular mechanisms in health and disease. Boca Raton, FL: CRC Press; 2001. pp. 83–98. [Google Scholar]
- Walter L, Miyoshi H, Leverve X, et al. Regulation of the mitochondrial permeability transition pore by ubiquinone analogs. A progress report. Free Radic Res. 2002;36:405–412. doi: 10.1080/10715760290021252. [DOI] [PubMed] [Google Scholar]