Abstract
Sandwich-cultured hepatocytes (SCH) are a powerful in vitro tool that can be utilized to study hepatobiliary drug transport, species differences in drug transport, transport protein regulation, drug-drug interactions, and hepatotoxicity. This review provides an up-to-date summary of the SCH model, including a brief history of, and introduction to, the use of SCH, as well as methodology to evaluate hepatobiliary drug disposition. A summary of the literature that has utilized this model to examine the interplay between drug metabolizing enzymes and transport proteins, drug-drug interactions at the transport level, and hepatotoxicity as a result of altered hepatic transport also is provided.
Keywords: Sandwich-Cultured Hepatocytes, Hepatotoxicity, Drug-Induced Liver Injury, Drug-Drug Interactions, Hepatocellularity, BEI, in vitro Biliary Clearance
Introduction
Hepatocyte cultures are a widely accepted in vitro tool to evaluate mechanisms of drug uptake and metabolism, as well as cytochrome P450 induction potential. However, the rapid loss of many liver-specific functions, redistribution of canalicular membrane proteins, loss of cell polarity and architecture including bile canaliculi, and the deterioration of cell viability within several days under conventional culture conditions precludes the use of this system for long-term studies and measurement of drug excretion (Groothuis et al., 1981, Borlak and Klutcka, 2004, Luttringer et al., 2002). In contrast, when cultured between two layers of gelled collagen (i.e. sandwich-cultured configuration) hepatocytes retain more in vivo-like properties, including formation of intact canalicular networks and polarized excretory function. Dunn et al. first demonstrated enhanced morphology and viability of hepatocytes cultured in a sandwich configuration, including normal levels of secretion of many liver-specific proteins and organic compounds including urea, albumin and bile acids (Dunn et al., 1992, Dunn et al., 1989, Dunn et al., 1991). Subsequent studies have demonstrated that the sandwich configuration facilitates the formation of gap junctions and functional bile canalicular networks over days in culture (LeCluyse et al., 1994, Liu et al., 1998).
Many studies have utilized freshly isolated, suspended hepatocytes to examine efflux of compounds, but this approach is unable to differentiate between sinusoidal efflux and canalicular excretion (Oude Elferink et al., 1990, Studenberg and Brouwer, 1993, Tarao et al., 1982). Therefore, hepatocyte suspensions are appropriately restricted to investigating uptake mechanisms. Canalicular excretion has been studied in hepatocyte couplets, but this approach is limited to the use of fluorescent substrates (Graf and Boyer, 1990, Mills et al., 1999). The challenges associated with isolating highly purified fractions of hepatic basolateral and canalicular liver plasma membrane vesicles with proper orientation has limited the use of liver plasma membranes as a commonly employed approach to assess hepatic drug transport. Transfected systems, which are useful for characterizing transport function, identifying driving forces, and determining substrate specificity and inhibitors, are routinely used in drug development, although the relative in vivo contribution of a given protein to hepatic uptake and excretion is difficult to elucidate using current methodologies. The use of whole organ or in vivo studies may be able to address these fundamental questions, but typically these are more labor- and animal-intensive approaches with much lower throughput and greater compound requirements.
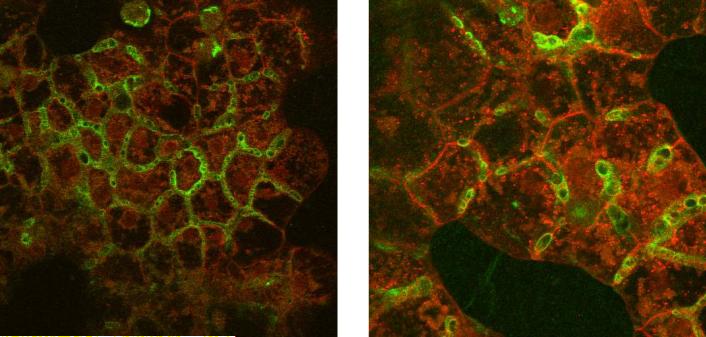
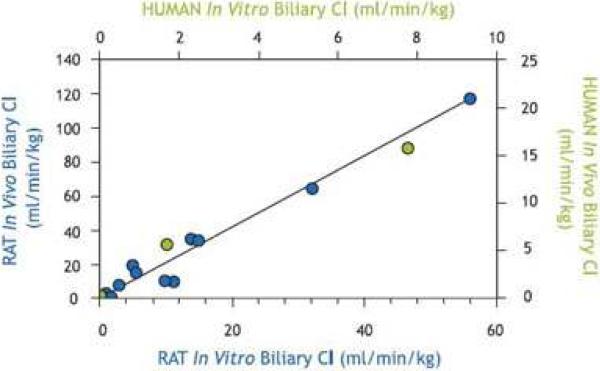
Liu et al. were the first to demonstrate that rat sandwich-cultured hepatocytes (SCH) could be used to investigate the hepatobiliary disposition of substrates (Liu et al., 1999b, Liu et al., 1999c). Initial work to establish the SCH model system involved optimization and characterization. Liu et al. investigated the effects of calcium on tight junction integrity and taurocholate (TC) accumulation in bile canaliculi (Liu et al., 1999c). This work demonstrated that tight junctions were the major diffusional barrier between the canalicular lumen and the extracellular space in SCH; tight junctions could be disrupted rapidly (within 3 min) by depletion of extracellular calcium without altering TC transport. Further work demonstrated that relevant hepatic transport proteins were expressed and properly localized in rat SCH (Turncliff et al., 2004, Hoffmaster et al., 2004, Zhang et al., 2005b), and were induced by well-established modulators such as dexamethasone (DEX) (Turncliff et al., 2004). P-glycoprotein (P-gp) co-localized with the canalicular marker protein dipeptidyl peptidase IV in day 4 and 6 rat and human SCH, respectively (Hoffmaster et al., 2004). Zhang and colleagues demonstrated by confocal microscopy that day 4 rat SCH exhibited extensive canalicular networks and distinct canalicular and basolateral domains, as evidenced by immunofluorescent localization of multidrug resistance-associated proteins 2 (Mrp2) and Mrp3, respectively. As clearly shown in Fig. 1, Mrp2 localized to the tubular structures of the bile canaliculi (green) and did not co-localize with the red staining in the basolateral membrane (Mrp3). Results of studies suggested the involvement of glycosylation in directing the canalicular localization of Mrp2 (Zhang et al., 2005b). A plethora of work has established the function of numerous hepatic uptake and efflux proteins in rat and human SCH. In vitro intrinsic biliary clearance values generated for compounds in SCH, and scaled biliary clearance values, correlate well with in vivo biliary clearance data measured with the same compounds in rats (Abe et al., 2008, Liu et al., 1999a, Fukuda et al., 2008) and humans (Abe et al., 2009, Ghibellini et al., 2007) (Fig. 2).
Figure 1. Confocal microscopy images revealing immunofluorescent localization of Mrp2 (green) on the canalicular domain and Mrp3 (red) on the basolateral domain in day 4 rat SCH.
This figure was published by Zhang et al. (Zhang et al., 2005b), and reprinted with permission from the American Society for Pharmacology and Experimental Therapeutics.
Figure 2. Relationship between in vitro intrinsic Clbiliary values generated for compounds in rat and human SCH with in vivo Clbiliiary data.
Rat in vivo Clbiliary of 11 compounds vs. predicted in vitro Clbiliary data generated in rat SCH [blue; r2=0.99; (Liu et al., 1999a)] and human in vivo Clbiliary of three compounds vs. predicted in vitro Clbiliary data generated in human SCH [green; (Ghibellini et al., 2007)].
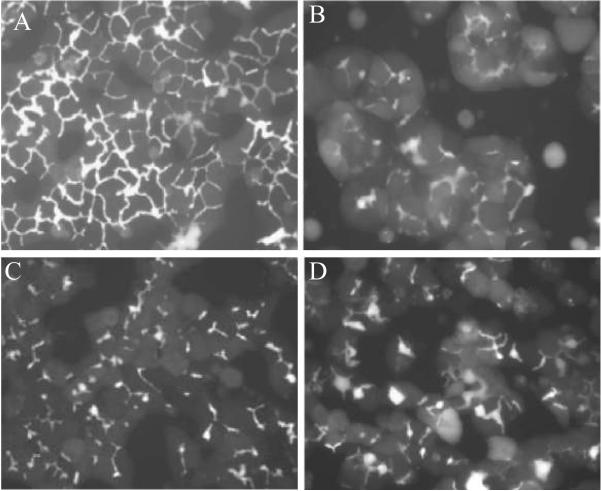
Another useful application of this model is in assessing human hepatobiliary drug disposition and hepatic transporter-based drug-drug interactions (DDI). However, the lack of fresh, healthy human liver tissue suitable for hepatocyte isolation and culture is a significant limitation. Fortunately, the establishment of commercially available, cryopreserved human hepatocytes that retain properties conducive for plating and culturing has expanded the use of this model (Bi et al., 2006). The use of cryopreserved hepatocytes is a significant advantage that allows for greater access to hepatocytes and the ability to conduct routine studies on demand. Applications of this model have been extended to include hepatocytes from other species. The predominant non-rodent species used in preclinical development are dog and monkey. Methods have been established to culture dog and monkey primary hepatocytes in a sandwich configuration (Fig. 3) (Rose et al., 2006, Zhang, 2005), which could be particularly useful in examining species differences in the hepatobiliary disposition of compounds, adverse hepatotoxic events, and/or unexpected pharmacokinetic data. Higher throughput screening systems, such as mouse SCH, may prove to be particularly useful for unraveling the physiological, pharmacological and toxicological roles of hepatic proteins utilizing hepatocytes from gene-disrupted mice, and the phenotypic diversity offered by various mouse strains such as the Collaborative Cross (Threadgill et al., 2002). Towards that end, recent efforts have focused on optimizing conditions for culturing mouse hepatocytes in a sandwich configuration (Fig. 4) (Swift and Brouwer, 2010).
Figure 3. CDF fluorescence microscopy images of rat (A), dog (B), monkey (C), and human (D) SCH.
in six-well plates (maintained in DMEM for 4–7 days). SCH were incubated with 2 μM CDF diacetate for 10 min prior to imaging.
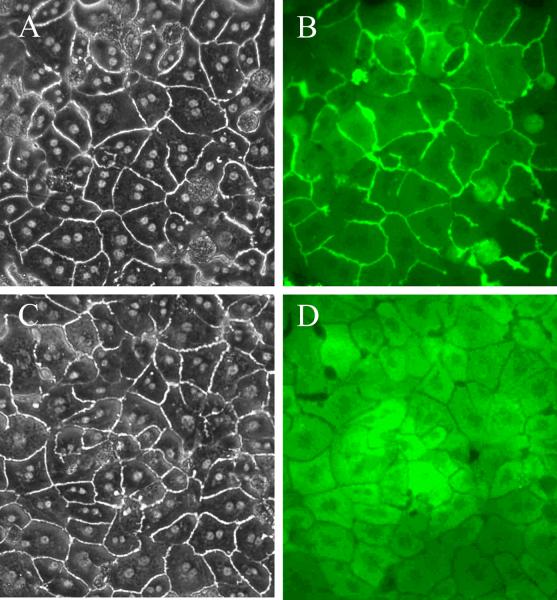
Figure 4. Light (A & C) and CDF fluorescence (B & D) microscopy images of wild-type (A & B) andAbcc2−/− [(Mrp2 knockout); C & D] mouse SCH.
cultured on six-well Biocoat™ plates with rat tail gelled collagen overlay (maintained in DMEM for 4 days). Mouse SCH were incubated with 2 μM CDF diacetate for 10 min prior to imaging. Data generated by Dr. Xianbin Tian.
Methodology
Considerable effort by many investigators has been devoted to describing the influence of numerous culture conditions on hepatocyte viability, morphology, function and growth. A complete discussion of the effects of culture media, supplemental soluble factors, chemical modulators, and extracellular matrix on hepatocytes is beyond the scope of this review [see published reviews (LeCluyse et al., 1996, Ichihara et al., 1982, Rogiers and Vercruysse, 1993)]. Detailed below is a brief review of just a few of the many factors that influence the optimization of canalicular network formation, as well as transport protein expression and function in hepatocytes that are already in a differentiated state, specifically for purposes of studying hepatobiliary disposition.
It is well understood that plating hepatocytes onto inflexible or rigid substrata such as plastic or simple protein coatings, typical of conventional cell culture conditions, is not conducive to normal expression of liver-specific and cytochrome P450 genes (Reid and Jefferson, 1984). Therefore, liver-specific extracellular matrix proteins such as collagen (types I–IV), laminin, fibronectin, and heparin sulfate proteoglycans were evaluated as potential substrata in culture (Stamatoglou and Hughes, 1994, Reid et al., 1992). Results from various studies demonstrated type I and IV collagen were superior to fibronectin and laminin (Sudhakaran et al., 1986, Bissell et al., 1986, Sawada et al., 1987). Further studies focusing on collagen demonstrated that hepatocytes plated between layers of gelled type I collagen maintained a three-dimensional cuboidal shape and distribution of cytoskeletal proteins similar to that observed in vivo whereas hepatocytes grown on ungelled collagen flattened and spread to confluence (Rubin et al., 1981, Lindblad et al., 1991). Other types of substrata also have been used successfully to culture hepatocytes including biomatrix (Rojkind et al., 1980), a complex, partially-purified extract of extracellular matrix material prepared from normal rat liver, and Matrigel™ (Bissell et al., 1987), an acid-urea extract prepared from Engelbreth-Holm-Swarm tumor tissue excised from lathyritic mice (Kleinman et al., 1982, Orkin et al., 1977).
In addition to extracellular matrix, investigators also have focused on determining the critical components in media that are responsible for enhancing hepatocyte function and viability. The effects of various media supplements such as amino acids, hormones, growth factors, vitamins, trace elements and other cofactors on hepatocyte function and viability in primary culture have been described extensively (Berry et al., 1991, Dich and Grunnet, 1989, Guguen-Guillouzo and Guillouzo, 1983). A large variety of commercially available media formulations have been employed for culturing primary hepatocytes including Waymouth's MB-752/1, Ham's F12, RPMI 1640, Dulbecco's modified Eagle's medium, Williams' medium E, Leibovitz' L15 and modified Chee's medium. The use of highly enriched media such as modified Chee's medium and Leibovitz' L15, which contain amino acid concentrations that are 5–10 times higher than most standard media, are superior for maintenance of cell survival, preserving cellular protein levels and liver specific functions (Jauregui et al., 1986, Sawada et al., 1987). Higher levels of amino acids have been suggested to aid in recovery of hepatocytes following collagenase digestion, arrest lysosomal protein degradation (Jauregui et al., 1988), and inhibit the rate of autophagic protein and RNA degradation while stabilizing the activity of some liver specific enzymes (Balavoine et al., 1993, Hutson et al., 1987, Lee et al., 1992, Seglen et al., 1980). Collectively, the results of these studies emphasize the importance of supplementing standard media with various commercially-available amino acid formulations. For example, Brouwer and co-workers supplement Dulbecco's modified Eagle's medium with L-glutamine and MEM non-essential amino acids solution (Invitrogen, Carlsbad, CA).
Culture media also have been supplemented with serum, based on reported findings following early attempts to culture rat hepatocytes in vitro (Bissell et al., 1973, Bonney et al., 1974) demonstrating that serum improves cell attachment, survival and morphology (Williams et al., 1977). However, serum has a dedifferentiating effect on hepatocytes, inhibits the induction effects of phenobarbital, inhibits the re-establishment of bile canaliculi, and favors attachment of non-parenchymal cells (Clayton and Darnell, 1983, Enat et al., 1984, Jefferson et al., 1984, Waxman et al., 1990, Terry and Gallin, 1994). Hepatocytes cultured on an extracellular matrix such as Matrigel™, or in co-culture with other cell types that promote a differentiated state, do not exhibit the same serum effects as those maintained on simple rigid substratum (Dunn et al., 1991, Guguen-Guillouzo et al., 1983, Ichihara, 1991, Vandenberghe et al., 1988, LeCluyse et al., 1994, Chandra et al., 2001). Other important supplements include the addition of hormones such as DEX and insulin. DEX increases secretion of blood clotting factors which aggregate into fibrin meshworks that are resistant to urea, promotes an ordered arrangement of the cytoskeleton, enhances gap junction expression and function, supports cytochrome P450 activity, curtails decreased protein synthesis that is observed during the first 24 h in culture, and enhances the formation of bile canalicular networks (Arterburn et al., 1995, Forster et al., 1986, Kwiatkowski et al., 1994, Ren et al., 1994, Lambiotte et al., 1973, LeCluyse et al., 1994). Insulin is reported to improve survival and attachment, enhance amino acid transport, protein synthesis, glycogenesis and lipogenesis; insulin also inhibits protein degradation and potentiates the inhibition of RNA degradation by amino acids (Balavoine et al., 1993, Ballard et al., 1980, Chapman et al., 1973, Laishes and Williams, 1976, Dahn et al., 1993, Flaim et al., 1985, Guguen-Guillouzo and Guillouzo, 1983, Schwarze et al., 1982).
Optimization of bile canalicular network formation in SCH is critical for studying hepatobiliary disposition and accurately assessing biliary excretion. Chandra et al. refined culture conditions for determining the hepatobiliary disposition of TC in rat SCH by evaluating the time of collagen overlay, the volume of collagen (rat tail type I), media type, and media additives (Chandra et al., 2001). Results demonstrated that Williams' medium E and Dulbecco's modified Eagle's medium produced greater taurocholate accumulation and biliary excretion compared to modified Chee's medium. Other parameters, such as time and volume of collagen overlay, Dulbecco's modified Eagle's medium additives such as 5% fetal bovine serum or DEX (0.1 μM versus 1 μM) did not produce significant differences in taurocholate accumulation or biliary excretion (Chandra et al., 2001). A separate study investigated the effects of culture conditions such as extracellular substratum, culture medium, and cell density on the expression and function of the bile salt export pump (Bsep), Mrp2, and multidrug resistance 1 (Mdr1a/b, P-glycoprotein, P-gp) in rat SCH (Turncliff et al., 2006b). In general, protein expression and function were not influenced by the extracellular matrices examined (rat tail gelled collagen type I vs. Biocoat™). Similar to the findings of Chandra et al., Williams' medium E and Dulbecco's modified Eagle's medium, compared to modified Chee's medium, resulted in greater function of Bsep, Mrp2 and Mdr1a/b based on hepatocellular accumulation and biliary excretion of taurocholate, 5(and 6)-carboxy-2',7'dichlorofluorescein (CDF), and rhodamine 123. Decreasing seeding density from 100% confluency significantly altered Mrp2 and Mdr1a/b expression and/or function (Turncliff et al., 2006b). Work by Zhang and colleagues investigated the influence of 6-, 12- and 24-well plates, three different types of media [Williams' medium E, Dulbecco's modified Eagle's medium, and hepatocyte maintenance medium (Cambrex Corporation, Baltimore, MD)], and two different extracellular matrix substrata [gelled collagen (rat tail type I) under- and overlay (GC/GC) and Biocoat™ (collagen type I, BD Biosciences, Bedford, MA) with Matrigel™ (BD Biosciences, Bedford, MA) overlay (BC/MG)] on transport protein function and expression in fresh, primary human SCH (Zhang et al., 2005a). Various media types and plate formats resulted in no difference in the biliary excretion of the probe substrates taurocholate, digoxin, estradiol-17-β-D-glucuronide and D-penicillamine2,5enkephalin. BC/MG yielded higher attachment and increased levels of P-gp, Bsep and Mrp2 protein compared to GC/GC. The use of Biocoat™ plates with Matrigel™ overlay has the additional advantage of uniformity, due to the lack of interindividual differences in manual coating with gelled collagen. Further work with cryopreserved human (Bi et al., 2006) and freshly isolated mouse hepatocytes (Bi et al., 2006, Swift and Brouwer, 2010) has confirmed the superiority of the BC/MG combination. Interestingly, dog hepatocytes cultured on Biocoat™ plates and overlaid with an alkaline gelled collagen (rat tail type I; pH 9.0) resulted in increased excretion of taurocholate compared to physiologic pH 7.4 (Rose et al., 2006).
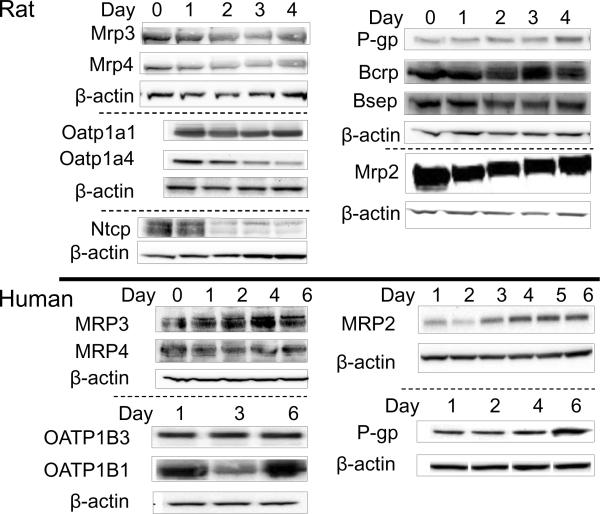
There are many other parameters in addition to media type/components and extracellular matrix to consider when culturing hepatocytes in a sandwich configuration for hepatobiliary transport studies. The extent of bile canalicular network formation and optimal levels of metabolizing enzymes and transport proteins are important factors to consider when determining the appropriate time in culture to conduct functional studies. LeCluyse et al. showed the development of bile canaliculi in rat SCH during the first 24–48 h, followed by a more uniform and homogeneous network through days 3–7; canalicular networks were maintained for at least 2–3 weeks (LeCluyse et al., 1994). The biliary excretion of TC increased and reached a maximum value four days after overlay of rat hepatocytes (Liu et al., 1999b). Likewise, the biliary excretion of [D-penicillamine2,5]enkephalin, a good P-gp substrate, increased up to day 4 after overlay and then started to decrease (Hoffmaster et al., 2004). The levels of many hepatic transport proteins in rat and human SCH over days in culture have been determined (Fig. 5); proper localization of Mrp2 and Mrp3 (Fig. 1), as well as P-gp have been investigated at day 4 after overlay in rat SCH (Hoffmaster et al., 2004, Zhang et al., 2005b). The dynamic process of canalicular network formation may take longer for human SCH, which are typically cultured for up to 6–10 days after overlay. The opposite is true for mouse hepatocytes, in which optimal cell morphology and biliary excretion of TC and CDF occurred on day 3 after overlay (Swift and Brouwer).
Figure 5. Transport protein levels over days in culture in rat and human SCH.
Representative immunoblots of transport proteins in rat and human hepatocytes cultured in six-well Biocoat™ plates with Matrigel™ overlay and maintained with DMEM for 4 or 6 days, respectively. β-actin was used as a loading control. Immunoblots of rat Mrp3 and Mrp4 were provided by Tracy Marion; rat Ntcp immunoblots were provided by Katie Paul; rat Bcrp, Bsep, P-gp, and human MRP3 and MRP4 immunoblots were provided by Dr. Wei Yue. Select data were published previously: rat Mrp2 (Zhang et al., 2005b); rat Oatp1a1, Oatp1a4, P-gp, and for human OATP1B1, OATP1B3, MRP2 and P-gp (Hoffmaster et al., 2004); reproduced, in part, with kind permission from the American Society for Pharmacology and Experimental Therapeutics, and from Springer Science and Business Media, respectively.
Cell density influences the morphology of hepatocytes in culture and is important for cell-cell contact (Hamilton et al., 2001). The confluency of hepatocytes cultured in a sandwich configuration is also crucial to the induction response. Initially, cryopreserved hepatocytes were unsuitable for induction studies because of their low attachment efficiency and poor quality. A great deal of emphasis has been placed on improving cryopreservation techniques to enhance the quality of hepatocytes and increase recovery and attachment. Greuet et al. demonstrated that maintaining primary human hepatocytes at a low density in the presence of epidermal growth factor (promoting proliferation and dedifferentiation) reduced the ability of the cells to respond to the prototypical inducer rifampin (Greuet et al., 1997). Decreased seeding densities of human hepatocytes to yield confluencies of 50, 30 and 25% of normal caused a decrease in the induction of CYP3A4 by 10 μM rifampin, as measured using the probe substrate testosterone; decreased seeding density also changed the phenotype of the hepatocytes resulting in more fibroblast-like (de-differentiated) characteristics (LeCluyse, 2001). Furthermore, Turncliff et al. demonstrated that a decrease in seeding density from 100% confluency resulted in decreased Mrp2 and Mdr1a/1b expression and/or function (Turncliff et al., 2006b). See Table 1 for a summary of recommended seeding densities based on the literature and experience by Brouwer and coworkers.
Table 1.
Sandwich-Cultured Hepatocyte Specifications for Development of Bile Canalicular Networks in Multiple Species, both Freshly Isolated and Cryopreserved
| Species | Plate Format | Extracellular Substratum | Seeding Density (cells/well) | Medium Volume (mL) | Culture Day for Transport Experiments |
|---|---|---|---|---|---|
| Human | 6-well | Biocoat/Matrigel | 1.75 × 106 | 1.5 | 7 |
| 24-well | Biocoat/Matrigel | 0.35 × 106 | 0.5 | 7 | |
|
| |||||
| Cryopreserved Human | 24-well | Biocoat/Matrigel | 0.35 × 106 | 0.5 | 5 |
|
| |||||
| Rat | 6-well | Biocoat/Matrigel | 1.75 × 106 | 1.5 | 4 |
| 24-well | Biocoat/Matrigel | 0.35 × 106 | 0.5 | 4 | |
|
| |||||
| TR− Wistar Rat | 6-well | Biocoat/Matrigel | 1.5 × 106 | 1.5 | 4 |
|
| |||||
| Mouse (C57BL/6) | 6-well | Biocoat/Matrigel | < 1.25 × 106 | 1.5 | 3 |
|
| |||||
| Monkey | 6-well | Biocoat/Gelled Collagen | 1.75 × 106 | 1.5 | 4 |
|
| |||||
| Dog (Beagle) | 6-well | Biocoat/Gelled Collagen (pH 9.0) | 1.75 × 106 | 1.5 | 4 |
Hepatobiliary disposition is evaluated on a specific day (or range of days) in culture based on optimal canalicular network development in each species. Two different protocols using B-CLEAR® technology (U.S. Pat. No. 6,780,580, Pat. No. 7,604,934 and other US and International patents both issued and pending) to quantify hepatic uptake and biliary excretion are discussed below; calcium depletion is utilized to disrupt the tight junctions and subsequently rinse the bile canalicular networks.
Accumulation Studies
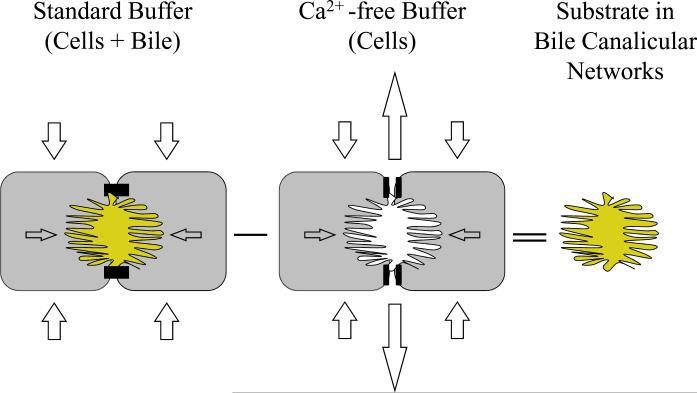
This protocol involves initial incubation of SCH with Hanks' Balanced Salts solution (HBSS) containing Ca2+ (standard HBSS) or Ca2+-free HBSS for 10 min [to maintain tight junction integrity and bile canalicular networks, or disrupt tight junctions and open bile canalicular networks, respectively (Fig. 6)]. Accumulation of taurocholate and CDF in rat SCH was reduced within one to two minutes following incubation with Ca2+-free HBSS (Liu et al., 1999b, Liu et al., 1999c). The bile canalicular space also was reduced based on phase contrast microscopy after a 10-min incubation with Ca2+-free HBSS (Liu et al., 1999c). Following the 10-min preincubation, buffer is removed and all cells are incubated with substrate(s) in standard HBSS for a predetermined period of time based on the substrate (time and concentration should be within the linear range of uptake, preferably within the first 10 to 15 min). This short-term accumulation phase should be conducted in standard HBSS to prevent potential interference due to the absence of Ca2+ on substrate uptake in SCH. Subsequently, SCH are rinsed three times with ice-cold standard buffer, lysed, and lysate is subjected to analysis for determination of substrate accumulation, by scintillation counting, high-performace liquid chromatagraphy (HPLC) or liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) in cells+bile (hepatocytes preincubated in standard HBSS) and cells (hepatocytes preincubated in Ca2+-free HBSS). Liu et al. demonstrated that rat SCH incubated for 10 min in Ca2+-free HBSS followed by incubation in Ca2+-containing HBSS exhibited gradual restoration of bile canalicular accumulation of taurocholate, reaching ~80% of standard HBSS-treated cells after 60 min; canaliculi re-dilated based on phase contrast microscopy (Liu et al., 1999c). Optimal study design should take into account the possibility that measurable substrate may begin to accumulate in bile canaliculi of SCH preincubated in Ca2+-free HBSS within 30 min following exposure to Ca2+-containing HBSS due to re-establishment of tight junction integrity. Furthermore, as discussed below, incubation of rat SCH in Ca2+-free HBSS for more than 30 min causes increased cell death.
Figure 6. Schematic depicting calculation of substrate accumulation in hepatocytes and bile canalicular networks of SCH.
SCH are preincubated in Ca2+-containing HBSS (standard buffer) or Ca2+-free HBSS (Ca2+-free buffer) to disrupt tight junctions sealing the bile canalicular spaces (Liu et al., 1999b, Liu et al., 1999c). Subsequently, cultures are rinsed and substrate uptake in SCH is determined in standard buffer for a designated period of time within the linear range of uptake (typically 10 min). In SCH preincubated with standard buffer, substrate is taken up into SCH and available for excretion into the bile canalicular networks; substrate accumulation (cells+bile) is determined at the end of the uptake period. In SCH preincubated with Ca2+-free buffer to open tight junctions, substrate excreted into the bile canalicular networks is released into the medium; substrate accumulation (cells) is determined at the end of the uptake period. The mass of substrate excreted into the bile canalicular networks during the uptake period is estimated as the difference in accumulation in SCH with intact and disrupted tight junctions [(cells+bile) − (cells)].
The intracellular concentration of a given substrate may be estimated from the accumulated mass and the hepatocellular volume. The average hepatocellular volume of rat SCH in 6-well and 24-well plates was 6.2 × 10−6 μl/hepatocyte, determined using 3-O-methyl-D-glucose, a metabolically stable hexose that is transported across cell membranes by facilitated diffusion [(Uhal and Roehrig, 1982), Jin K. Lee, personal communication, Qualyst, Inc., Durham, NC].
Hepatic uptake clearance of substrates may be quantified based on the total accumulation of substrate in standard HBSS in the linear range of uptake according to the following equation:
| (1) |
where AUC0-T represents the product of the incubation time (T) and the initial concentration in the medium, assuming that the concentration at T is not less than 10% different from the initial concentration. In the absence of sink conditions, the AUC should be calculated based on the log-linear trapezoidal method.
Using B-CLEAR® technology, the biliary excretion index (BEI) of substrates may be quantified based on the fraction of accumulated substrate that resides in the bile canaliculi using the following equation:
| (2) |
and in vitro biliary clearance (Clbiliary) may be quantified based on the total accumulation of substrate in the bile canalicular networks divided by the area under the concentration curve of the dosing medium using the following equation:
| (3) |
In vitro Clbiliary values can be scaled to per kilogram of body weight, depending on the species (see Table 2). In vitro Clbiliary values have been shown to correlate well with in vivo intrinsic Clbiliary values in rats and humans for a number of compounds (Fig. 2) (Yue et al., 2009, Abe et al., 2008, Ghibellini et al., 2007, Liu et al., 1999a, Fukuda et al., 2008). Use of the well-stirred model for scaling Clbiliary, expressed as μL/min/million cells, to in vivo hepatic clearance, expressed as mL/min/kg body weight, has been shown to correlate well in rats (Abe et al., 2008).
Table 2.
In Vitro CLbiliary Scaling Factors
| Species | Protein Concentration per Gram of Liver Tissue (mg/g) | # of Hepatocytes/Gram of Liver Tissue (106cell/g) | Grams of Liver Tissue/kg Body Weight (g/kg) |
|---|---|---|---|
| Human | 90(3), 32(5) | 139(3), 107(4), 99(5), 120(7) | 25.7(1) |
| Rat | 200(2), 112(3) | 117(3), 96(6) 120(7) | 40(1) |
| Mouse | 115(3) | 135(3) | 87.5(1) |
| Monkey | 30(1) | ||
| Dog | 103(3) | 215(3), 165(6), 240(7) | 32(1) |
Inhibitors may be added during the preincubation phase in standard and Ca2+-free HBSS to preload the hepatocytes with inhibitor, and/or added during the substrate uptake phase in standard HBSS. However, depending on the inhibitor, modulation of substrate disposition may be due to inhibition of uptake, and/or inhibition of basolateral and/or canalicular efflux, making the results difficult to interpret. Inducers may be added to the culture medium for specified periods of time prior to conducting uptake studies. In order to avoid direct effects of the inducer or inhibitor on substrate uptake, a modulator-washout phase may be necessary, and modulators also may need to be removed from the standard HBSS buffer during the uptake phase.
Efflux Studies
Compared to the accumulation protocol, basolateral and canalicular efflux studies in SCH are defined by the reversal of substrate incubation and tight junction disruption steps. This efflux approach was first evaluated by Liu (Liu et al., 1999b), and has been utilized by Kostrubsky and colleagues as well as Jemnitz and coworkers in a number of studies (Feng et al., 2009, Kostrubsky et al., 2006, Lengyel et al., 2005, Lengyel et al., 2008). First, the substrate of interest is incubated with SCH in standard HBSS to preload the cells. The loading phase is stopped by removing the buffer and washing hepatocytes three times with warm standard HBSS or Ca2+-free HBSS. Efflux studies are initiated by incubating preloaded hepatocytes with their respective buffers; aliquots are taken at designated time points for no longer than 30 min (Fig. 7). If inhibitor(s) are included in the incubation during the efflux phase, the amount of substrate in the lysed SCH at the end of the efflux phase must be measured in addition to the amount of substrate in the aliquots in order to evaluate potential modulation of the transport of the preloaded substrate. The degree of basolateral and canalicular efflux can be determined based on the following equations:
| (4) |
| (5) |
In both cases, if multiple samples of HBSS buffer are taken over a period of time, the change in volume needs to be accounted for based on the number and volume of aliquots taken. Both basolateral and canalicular efflux values can be normalized to the total amount of pre-loaded substrate using the sum of the total mass in the efflux medium and hepatocyte lysate at the end of the efflux phase.
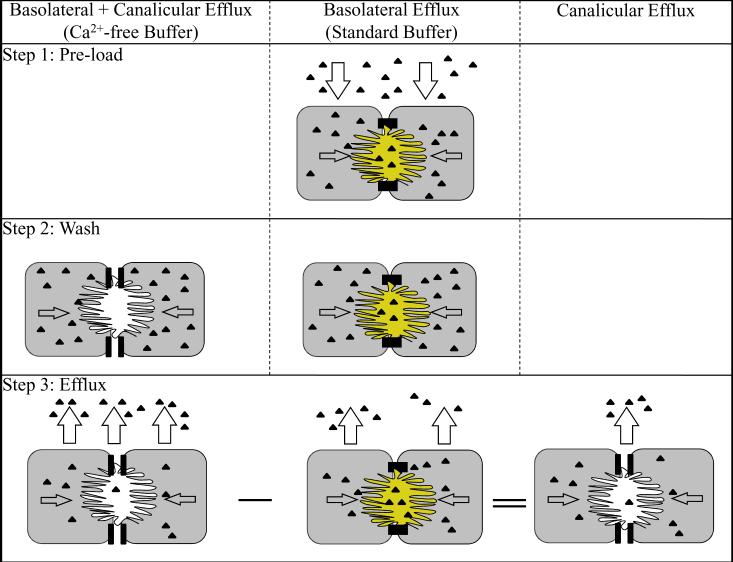
Figure 7. Schematic depicting calculation of canalicular and basolateral efflux of substrate in SCH.
Step 1: SCH are preincubated with substrate (▲) in Ca2+-containing HBSS (standard buffer) to preload the cells; substrate is taken up into SCH and excreted into the bile canalicular networks. Step 2: Cultures are rinsed in standard buffer, or Ca2+-free HBSS (Ca2+-free buffer) to disrupt tight junctions sealing the bile canalicular space. Step 3: SCH are incubated in their respective buffers, and aliquots are taken at designated time points for no longer than 30 min. In SCH rinsed and incubated with standard buffer, substrate efflux occurs across the basolateral membrane only. In SCH rinsed and incubated with Ca2+-free buffer to open tight junctions, substrate efflux occurs across the basolateral and canalicular membrane. The canalicular efflux is estimated as the difference in efflux between SCH with disrupted and intact tight junctions [(basolateral + canalicular efflux) − (basolateral efflux)].
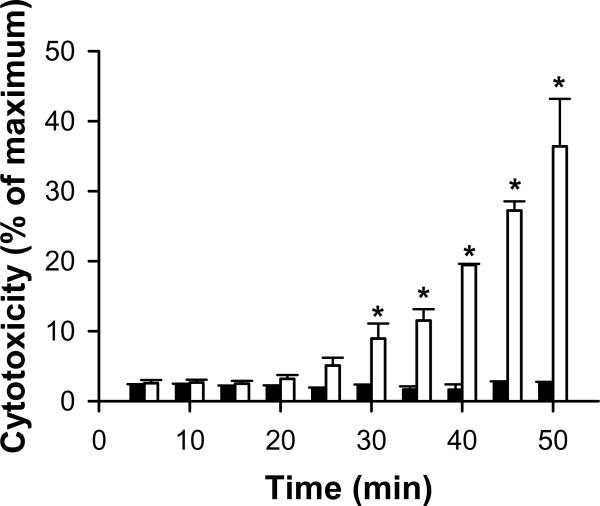
There are several caveats that must be considered when designing efflux experiments and interpreting the results. First, it is imperative that the incubation with Ca2+-free HBSS is for a limited period of time (e.g., not greater than 30 min). Omission of extracellular Ca2+ from cells incubated with a buffered salt solution is known to result in cell death (Reed et al., 1990); rat SCH incubated in Ca2+-free HBSS exhibited a significant increase in lactate dehydrogenase (LDH) leakage within 30 min (Fig. 8). Another limitation to this method is the unknown degree of substrate accumulation in the bile canaliculi during the preloading period, which is not washed away when transport is stopped and dosing solutions are removed. This may result in an overestimate of canalicular efflux. If chemical inhibitors of active transport processes are added during the substrate preloading phase, as discussed above, decreased efflux may be due to impaired uptake and/or efflux; evaluation of the sum of the total mass in the efflux medium and hepatocyte lysate in the absence and presence of inhibitor(s) would be required to distinguish between these two possibilities. Alternatively, inhibitor(s) could be added after the preloading step to avoid potential interference with the uptake process when concomitantly incubated. However, the time required for the inhibitor(s) to reach the site of transport inhibition must be considered in this study design. As mentioned with the accumulation protocol, perturbations in substrate disposition observed during the efflux period may be due to inhibition of basolateral or canalicular efflux, as well as to inhibition of substrate re-uptake. Another consideration is that medium concentrations may be influenced significantly by basolateral efflux of substrate during the efflux phase due to the reversal of the concentration gradient. In contrast, basolateral efflux of substrate would be expected to have minimal effects on medium concentrations in the accumulation protocol due to sink conditions.
Figure 8. Effect of incubation time in standard HBSS (dark bars) and Ca2+-free HBSS (white bars) on viability of rat SCH.
Day 4 SCH from wild-type Wistar rats in six-well Biocoat™ plates with Matrigel™ overlay were incubated with 1.5 mL/well of warm standard HBSS for 20 min, followed by two rinses with 2 mL/well warm standard HBSS or Ca2+-free HBSS, and incubation with 1.5 mL/well of the same buffer. Cytotoxicity was determined by measuring lactate dehydrogenase (LDH) release into the respective HBSS buffers after designated times using a cytotoxicity kit according to the manufacturer's instruction (Roche, Indianapolis, IN; Each bar represents the mean ± SEM; n=3 livers in triplicate; p<0.05).
The use of chemical inhibition to investigate the role of specific transport proteins in the disposition of substrates is widely used and accepted. It is not the most precise method, however, due to incomplete knowledge about the specificity of inhibitors for individual transport proteins. Naturally occurring genetically-deficient rodents lacking a specific transport protein such as the Mrp2-deficient Wistar (TR−) and Eisai hyperbilirubinemic Sprague-Dawley rats (EHBR), and the Mdr1a-deficient CF-1 mice, have been useful tools for elucidating the role of these transport proteins in the disposition of endogenous compounds and xenobiotics; incorporation of hepatocytes from these mutants in the sandwich-cultured model has been of great utility. Abe and colleagues confirmed in vivo reports that olmesartan, valsartan, pravastatin and rosuvastatin were Mrp2 substrates by demonstrating that SCH from TR− rats exhibited decreased BEI and in vitro Clbiliary relative to SCH from transport-competent Wistar controls (Abe et al., 2008). Interestingly, pitavastatin BEI was reduced despite previous data demonstrating that the in vivo biliary excretion of pitavastatin was similar in wild-type and EHBR rats (Hirano et al., 2005). Further investigation revealed a novel finding: breast cancer resistance protein (Bcrp) levels were considerably lower in SCH from TR− rats compared to Wistar control rats based on immunoblot analysis (Abe et al., 2008). Many transport proteins have been knocked out alone or in combination in mice using various gene disruption techniques. However, mouse SCH have not been investigated as extensively as rat or human SCH, and mouse hepatocytes are known to be more difficult to maintain in culture.
RNA interference (RNAi) leads to post-transcriptional, sequence-specific gene silencing and is a powerful tool to study the effect of loss-of-function of genes. The application of this technology to suppress specific transport proteins in SCH (U.S. Pat. No. 6,780,580, Pat. No. 7,601,494 and other US and International patents both issued and pending) has provided another tool, in addition to knockout mouse models, to gain insight into the function of specific transport proteins. Brouwer and coworkers successfully applied this approach using small interfering RNA (siRNA) targeting Mrp2 and Mrp3; protein levels were decreased by ~50% in rat SCH, and Mrp2 and Mrp3 function also was decreased based on the hepatobiliary disposition of CDF, a probe substrate for both transport proteins (Tian et al., 2004). More recently, Brouwer and coworkers have used adenoviral vector-mediated delivery of short hairpin (sh) RNA to knock down Bcrp in rat and human SCH; decreased expression and function have been confirmed using the probe drug, nitrofurantoin (Yue et al., 2009).
Another unique tool that has been utilized with the SCH model system is incubation with fluorescent probes to study transport protein expression, localization and function. CDF and cholyl-lysyl-fluorescein (CLF), a fluorescent bile acid, have been used to visualize the development of bile canalicular networks over days in culture (Kostrubsky et al., 2003, Liu et al., 1999b, LeCluyse et al., 1994, Zhang et al., 2005b, Bi et al., 2006). CDF accumulated in the canalicular networks of SCH from C57BL/6 wild-type but not Abcc2−/− (Mrp2 knockout) mice (Fig. 6), demonstrating the important role of Mrp2 in the biliary excretion of CDF. Ruthenium red staining also has been used to demonstrate that the bile canaliculi are sealed until Ca2+ depletion disrupts the tight junctions (Liu et al., 1999c). An important advantage of fluorescent probe substrates is the straightforward quantification by fluorescence spectroscopy. CDF diacetate (2 μM) has been used to phenotype the function of Mrp2 and Mrp3 after siRNA knockdown in rat SCH, as mentioned above (Tian et al., 2004). Rhodamine 123, a model P-gp substrate, has been used in rat SCH to assess P-gp-mediated biliary excretion, and to examine drug interactions affecting P-gp function (Annaert and Brouwer, 2005, Annaert et al., 2001). Cholyl-glycylamido-fluoroscein (CGamF) is a fluorescent bile acid that preferentially undergoes organic anion transporting polypeptide (Oatp)-mediated hepatic uptake, as opposed to taurocholate, which is predominantly Sodium taurocholate cotransporting polypeptide (Ntcp)-mediated. CGamF was used recently to assess drug interactions in hepatic uptake with the HIV protease inhibitors ritonavir, saquinavir, atazanavir, darunavir, and amprenavir in rat SCH (Ye et al., 2008). To date, application of fluorescent probes has focused primarily on characterizing hepatobiliary transport function and transporter-based DDIs. Fluorescent probe substrates, or fluorescence-tagged xenobiotics, may be useful in the future to elucidate mechanisms of intracellular trafficking of xenobiotics. For example, the innate fluorescence of the cation, daunorubicin, was utilized to demonstrate the intracellular sequestration of daunorubicin in vesicles in the pericanalicular region of rat isolated perfused livers (IPLs) and isolated rat hepatocyte couplets (Hayes et al., 1999). Other work has shown the expression of functional P-gp when localized to the Golgi and mitochondria of doxorubicin-resistant K562 cells (Munteanu et al., 2006). This supports the hypothesis that other transport proteins may reside at intracellular sites and play a role in xenobiotic distribution within the hepatocyte. This could be investigated with the use of the sandwich-cultured model system.
Use of Sandwich-Cultured Hepatocytes to Investigate the Interplay Between Drug Metabolism and Transport
The liver plays a major role in the biotransformation of many endogenous and exogenous compounds, and also represents an important target organ for toxicity. Hepatic cell lines, precision-cut liver slices, and primary hepatocyte cultures from various species represent predominant and well-established in vitro systems for conducting these studies. Advantages of intact hepatocytes include the presence of: (1) interacting enzyme systems, intracellular machinery and physiological concentrations of cofactors allowing for coupled phase I and phase II reactions to take place; (2) the entire array of hepatic transport proteins involved in hepatic uptake and excretion, some of which may limit access to the site of metabolism; and (3) nuclear receptors, which allow regulation and induction of enzymes and transport proteins. The sandwich-cultured configuration is important for maintaining the differentiated morphology of hepatocytes and longevity in culture (Tuschl and Mueller, 2006). The major limitation to using hepatocyte cultures to study drug metabolism is the decline in cytochrome P450 enzyme activity (Hoen et al., 2000, Boess et al., 2003), although medium additives can help maintain function, as detailed below. Despite this, sandwich-cultured primary hepatocytes remain a well-established model for induction studies due to the lack of phenotypic gene expression in nearly all immortalized cell lines and the short-term viability of liver slices (LeCluyse, 2001).
In order to reverse the decline of cytochrome P450 enzymes, well known inducers such as phenobarbital and DEX have been added as medium supplements, separately or in combination (LeCluyse et al., 1996, Miyazaki et al., 1998, Pichard-Garcia et al., 2002). The addition of phenobarbital, DEX and β-naphthoflavone to the culture medium of rat SCH preserves phase I and II enzyme levels almost equivalent to those in liver in vivo, as determined by gene expression and enzyme activity analysis (Kienhuis et al., 2007). A discussion of the expression and function of phase I and phase II biotransformation enzymes in SCH is beyond the scope of this paper, but has been reviewed by many others (Hewitt et al., 2007a, Hewitt et al., 2007b, LeCluyse, 2001, Rogiers and Vercruysse, 1993, Maurel, 1996, Ferrini et al., 1997). The current review will focus on the interplay between drug metabolism and disposition of the parent and preformed or generated metabolites in the sandwich-cultured model system.
It is not surprising that interplay exists between transport proteins and metabolizing enzymes due to the common regulatory pathways mediated by the nuclear receptors, and the substrate overlap of the parent compound and/or generated metabolites for the transport proteins. The most well-studied example of this interplay is the common regulation and substrate/inhibitor overlap between P-gp and CYP3A4 (Wacher et al., 1995, Yasuda et al., 2002). This coordinated clearance pathway has been examined in rat SCH using the drug/metabolite pair terfenadine, a known CYP3A4 substrate (Yun et al., 1993, Jurima-Romet et al., 1994), and fexofenadine, both known P-gp substrates (Hait et al., 1993, Cvetkovic et al., 1999). Turncliff et al. determined the metabolism of terfenadine as well as the basolateral efflux and biliary excretion of terfenadine and fexofenadine (both preformed and generated) in control and DEX-treated rat SCH (Turncliff et al., 2006a). Treatment with 100 μM DEX increased the formation rates of the terfenadine metabolites, azacyclonol and fexofenadine, approximately 20- and 2-fold, respectively. Pharmacokinetic modeling of the data indicated that the rate constant for hepatocyte uptake was faster for terfenadine compared with preformed fexofenadine (2.5 vs. 0.08 h−1, respectively), whereas the biliary excretion rate constant for preformed fexofenadine exceeded that of terfenadine (0.44 vs. 0.039 h−1, respectively). The rate constants for basolateral excretion of terfenadine and fexofenadine were comparable (3.2 vs. 1.9 h−1, respectively) (Turncliff et al., 2006a). This study emphasizes the utility of the SCH model as an in vitro system capable of assessing, in an integrated fashion, metabolism, biliary excretion, and basolateral transport processes.
Rat SCH have been used to compare the hepatobiliary disposition of two orally active pentamidine analogues, and pharmacokinetic modeling has been utilized to examine the rates of hepatic uptake, metabolism, biliary excretion and basolateral efflux (Yan et al., 2008). Conversion of pafuramidine to the active metabolite, furamidine, is catalyzed by cytochrome b5/NADH-cytochrome b5 and many CYP450 enzymes, notably CYP4F (Wang et al., 2006, Saulter et al., 2005). The purpose of these studies was to determine the mechanism(s) responsible for the improved efficacy of DB868, an analog of the prodrug pafuramidine, in a late-stage mouse model of African trypanosomiasis. DB829, the active metabolite of DB868, has a higher systemic exposure, due either to increased metabolism and/or increased hepatic basolateral efflux of the active metabolite. The extent of formation of the active metabolite DB829 was consistently higher than that of furamidine over time in rat SCH. In addition, the net hepatic basolateral efflux was greater for DB829 compared to furamidine, consistent with pharmacokinetic modeling in which the rate constant representing basolateral efflux was increased 6-fold for DB829 compared to furamidine (Yan et al., 2008).
The examples given above involve phase I oxidation reactions, but additional examples have focused on the formation and hepatobiliary disposition of phase II conjugates. Wolf et al. characterized the accumulation, glucuronidation and excretion of morphine in rat SCH (Wolf et al., 2008). In vitro data were in good agreement with in vivo data indicating that morphine 3-glucuronide (M3G) was the primary metabolite formed (Wolf et al., 2008). In rat SCH, M3G was eliminated across the basolateral membrane; ~99% of the drug-related mass was recovered in medium during the 120-min study as M3G. M3G reuptake in rat SCH appeared to be negligible based on pharmacokinetic modeling, consistent with findings in the rat IPL when preformed M3G was administered (Doherty et al., 2006, Ouellet and Pollack, 1995). Another example of glucuronidation in rat SCH is with the opioid antagonist naloxone (Ansede and Brouwer, 2008). Following incubation of 10 μM naloxone for 80 min, less than 1% of naloxone was recovered in the hepatocytes or bile canaliculi; 89.3% of the dose was recovered as naloxone 3-glucuronide in medium (Ansede and Brouwer, 2008). This is consistent with in vivo rat studies demonstrating that naloxone glucuronide is the major metabolite, with 15.4 and 1.2% of naloxone 3-glucuronide recovered in urine and feces, respectively, after 96 hr (Misra et al., 1976). SCH from both humans and rats also are capable of forming other conjugates based on data generated utilizing troglitazone (Lee et al., 2010). Troglitazone sulfate was the predominant metabolite in medium and hepatocytes, followed by troglitazone glucuronide and troglitazone quinone, over a 120-min incubation period with 10 μM troglitazone in rat and human SCH. Similar to in vivo, the biliary excretion of trogitazone sulfate was greater than troglitazone glucuronide, while troglitazone and troglitazone quinone underwent negligible biliary excretion in rat and human SCH (Loi et al., 1999a, Loi et al., 1999b). The impact of modulating the biliary excretion of troglitazone sulfate and troglitazone glucuronide on hepatic accumulation was assessed utilizing a Monte Carlo simulation approach. Interestingly, changes in hepatocyte concentrations were more sensitive to changes in biliary excretion than medium concentrations, suggesting that plasma concentrations may not reflect increased liver accumulation and the potential for hepatotoxicity in vivo (Lee et al., 2010).
Evaluation of other substrate/metabolite pairs undergoing biotransformation by phase I and/or II enzymes, and further characterization of the biliary elimination of unchanged compounds and generated metabolites, will enhance understanding of the utility of this model system. Mass balance data detailing the parent compound and generated metabolites in medium, cells and bile canaliculi, coupled with pharmacokinetic modeling, can provide a robust evaluation of the hepatobiliary disposition of compounds, including relative rates for the hepatic uptake, metabolism, basolateral efflux and biliary excretion of the parent compound and generated metabolites. The SCH model offers the ability to quantify the impact of modulation of both hepatic transport systems and metabolism of drugs on overall disposition in a single in vitro system, which has significant advantages over existing methodologies.
Sandwich-Cultured Hepatoctyes as a Tool to Predict Drug Interactions in Hepatobiliary Transport
Inhibition of Bile Acid Transport by Drugs
Vectorial transport of bile acids from the blood into bile is a multistep process starting with uptake into hepatocytes, mediated predominantly by NTCP (SLC10A1), with a minor component mediated by OATP (SLCO) isoforms. Bile acids are effluxed from hepatocytes into bile, in conjugated or unconjugated form, by canalicular ATP-binding cassette (ABC) transport proteins, primarily ABCB11 (BSEP), but also by ABCC2 (MRP2) and others (Marion et al., 2007). Basolateral excretion of bile acids from hepatocytes into blood is mediated by MRP3 (ABCC3) and MRP4 (ABCC4), providing a compensatory pathway under certain conditions, such as cholestasis or inhibition of canalicular excretion. The organic solute transporter (OSTα/β) recently was identified as another basolateral transport protein that functions to excrete bile acids and other compounds, similar to MRP3 and MRP4 (Boyer et al., 2006, Alrefai and Gill, 2007).
The association of elevated serum bile acid concentrations with drugs known to cause hepatotoxicity led to the hypothesis that altered hepatic bile acid transport may be one mechanism of drug-induced liver injury [DILI, (Funk et al., 2001b, Fattinger et al., 2001, Funk et al., 2001a, Stieger et al., 2000, Kostrubsky et al., 2001, Roman et al., 1990, Preininger et al., 1999)]. A number of hepatotoxic drugs, including bosentan, troglitazone, and cyclosporine, inhibit BSEP-mediated bile acid transport in isolated in vitro systems (Stieger et al., 2000, Funk et al., 2001b, Fattinger et al., 2001, Funk et al., 2001a, Byrne et al., 2002).
TC, a model bile acid, has been used to evaluate the potential for drugs to inhibit bile acid transport in the SCH system. Kostrubsky et al. demonstrated inhibition of TC transport in human SCH with a set of structurally diverse compounds that were eliminated preferentially via bile and known to cause liver toxicity in humans (cyclosporine A, bosentan, glyburide, troleandomycin and CI-1034) (Kostrubsky et al., 2003). Kostrubsky and colleagues also established that potent inhibition of TC efflux in SCH correlated with clinical reports of liver toxicity, and elevated serum bile acids in rats (see below: Direct Assessment of Cytotoxicity in SCH from Relevant Species). In a later study, Kostrubsky and colleagues again confirmed that potent inhibition of TC efflux was predictive of the hepatotoxic potential of nefazodone compared to a pair of non-toxic structural analogues, trazodone and buspirone (Kostrubsky et al., 2006). Nefazodone was a potent inhibitor of TC transport in Sf9-derived BSEP-expressing membrane vesicles; nefazodone also inhibited biliary excretion of TC in rat SCH, and elevated serum bile acids when administered to rats in vivo. Nefazodone-associated decreases in protein synthesis as a measure of cellular toxicity, and the role of phase I and phase II metabolism in the hepatotoxicity of nefazodone also were evaluated, capitalizing on the ability of SCH to maintain a complex array of liver-specific functions [see below: Hepatocyte Accumulation of Toxic Species, (Kostrubsky et al., 2006)].
The role of impaired bile acid transport in troglitazone- and bosentan-associated hepatotoxicity initially focused on BSEP-mediated canalicular efflux as the primary mechanism leading to elevated serum bile acid concentrations (Funk et al., 2001b, Kostrubsky et al., 2001, Fattinger et al., 2001, Funk et al., 2001a, Byrne et al., 2002). More recently, Kemp et al. observed that while troglitazone and bosentan both decreased the BEI of TC in SCH, consistent with Bsep inhibition, cellular accumulation of TC did not increase, as might be expected (Kemp et al., 2005). In freshly isolated rat hepatocytes, 10 and 100 μM troglitazone and bosentan each significantly decreased the uptake rate and accumulation of the probe substrates TC (1 μM) and estradiol-17β-D-glucuronide (1 μM), an Oatp substrate, consistent with inhibition of both Ntcp- and Oatp-mediated uptake mechanisms. Taken together, these results suggest that drugs known to inhibit biliary excretion of bile acids also may inhibit hepatic uptake, thus attenuating elevations of intracellular bile acid concentrations and potential toxicity that may result. This phenomenon is analogous to down-regulation of basolateral uptake mechanisms in cholestatic conditions, which effectively minimizes accumulation of toxic bile acids inside hepatocytes (Zollner et al., 2003, Lee et al., 2000, Lee and Boyer, 2000, Gartung et al., 1996, Gartung et al., 1997). Preferential inhibition of basolateral uptake, as opposed to canalicular efflux, by drugs would similarly reduce hepatocyte exposure to bile acids, while increasing bile acid concentrations in the systemic circulation. This effect was explored by Leslie et al. as an explanation for the species difference in bosentan toxicity between humans and rats [see below: Direct Assessment of Cytotoxicity in SCH from Relevant Species, (Leslie et al., 2007)].
Inhibition of bile acid transport was identified as a potential mechanism of antiretroviral-associated hepatotoxicity using multiple model systems, including human and rat SCH (McRae et al., 2006). BSEP-mediated biliary excretion, and NTCP- and OATP-mediated uptake, of TC were inhibited differentially by the antiretroviral agents ritonavir, saquinavir, and efavirenz, but not nevirapine. Potential hepatocyte accumulation of TC due to BSEP inhibition was attenuated by concomitant inhibition of NTCP- and/or OATP-mediated uptake of TC. The hepatotoxic potential of the antiretroviral agents could not be predicted by rank order of TC inhibition in isolated uptake or efflux systems or SCH, suggesting that “hepatotoxicity associated with antiretroviral therapy is likely complex and multifactorial, such that inhibition of bile acid transport may be only one of several contributing factors (McRae et al., 2006).”
An important application of the SCH model is in classifying compounds based on their relative effect on bile acid uptake and/or efflux, which may provide useful information concerning compounds that alter bile acid concentrations in vivo. For example, cyclosporine A and glyburide decreased total accumulation (cells+bile), increased cellular accumulation, and decreased BEI and Clbiliary of deuterium-labeled taurocholate (d8-TC), suggesting that bile acid efflux was affected primarily (Ansede et al., 2009 in press). In contrast, erythromycin-estolate, troglitazone and bosentan decreased d8-TC accumulation (both cells+bile and cells), BEI and Clbiliary of d8-TC, suggesting that bile acid uptake was the predominant pathway affected. Recently, the utility of a cassette dosing approach to identify compounds that inhibit hepatic uptake and/or excretion of bile acids was demonstrated in rat and human SCH (Wolf et al., 2009).
Given the requisite overlap in substrate specificity of basolateral and canalicular hepatic transport proteins necessary to mediate vectorial transport of bile acids, it is not surprising that xenobiotics may modulate multiple hepatic bile acid transport mechanisms. The relative effect of xenobiotics on both processes should be considered in order to predict the potential consequences of modulating bile acid transport, disposition and homeostasis. SCH have been used successfully to study hepatobiliary transport kinetics and mechanisms of both hepatic uptake and biliary excretion of bile acids, simultaneously.
Drug-Drug Interactions in Hepatobiliary Transport
Hepatic clearance is an important pathway of elimination for many, if not most, drugs. Many drugs and/or generated metabolites possess limited diffusion potential and rely on transport proteins to mediate uptake into hepatocytes and/or excretion into bile. Thus, DDIs at the level of hepatic uptake or efflux processes may be responsible for significant changes in plasma or hepatocellular drug concentration-time profiles, with potential consequences for therapeutic or toxic effects. Furthermore, identifying the extent and relevance of transporter-mediated DDIs in human liver can be challenging, due to inaccessibility of the liver and bile compartments. SCH combined with probe substrates exhibiting known transport protein specificity offer the ability to identify transport proteins involved in the disposition of new chemical agents, as well as the potential for new agents to be “victims” or “perpetrators” of DDIs, in a physiologically-relevant model system.
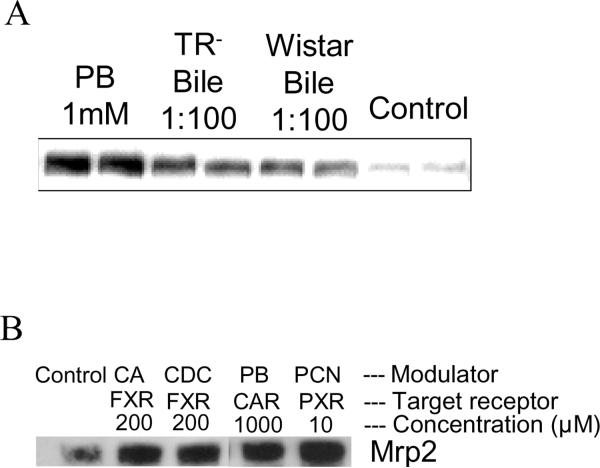
Early work was performed in the Brouwer laboratory to optimize and validate the rat SCH model for the study of P-gp-mediated biliary excretion using substrates such as rhodamine 123 and digoxin (Annaert et al., 2001). Addition of the P-gp (Mdr1) inhibitor, GF120918, resulted in a 75% reduction in the BEI of rhodamine 123 and digoxin. Prediction of hepatic P-pg-mediated drug interactions was later established in rat SCH using the high-affinity probe substrate rhodamine 123 and various P-gp modulators (Annaert et al., 2001, Annaert and Brouwer, 2005). The classical P-gp inhibitors verapamil and progesterone significantly reduced the BEI and in vitro CLbiliary ofrhodamine 123, while inhibition by 100 μM quinidine did not reach statistical significance. Quercetin, a P-gp activator, significantly increased in vitro CLbiliary but not the BEI of rhodamine 123, reflecting the fact that cellular accumulation was enhanced to a greater extent than excretion into bile. Treatment of SCH with verapamil, rifampin and DEX was generally inconclusive in terms of P-gp induction. However, results from DEX treatment implicated the involvement of an inducible element to the basolateral uptake of rhodamine 123, which was supported further by inhibition of rhodamine 123 uptake by digoxin, verapamil and quinidine, all documented substrates/inhibitors of Oatp1a4. Indeed, Turncliff, et al. demonstrated that 48 h DEX treatment induced protein expression and function of CYP3A1/2, Oatp1a4 and Mrp2, but not Mdr1 [see below: Drug-Hepatic Transport Protein Interactions (Turncliff et al., 2004)]. In rat SCH, Mrp3 protein levels, determined by immunoblot analysis, were increased following incubation with 1 mM phenobarbital for 72 hr; diluted bile from Wistar wild-type and TR- rats also induced Mrp3, suggesting that one or more bile constitutents, other than glucuronide or glutathione conjugates, were responsible for Mrp3 induction (Fig. 9a). Mrp2 protein was increased after incubation of rat SCH for 72 hr with selected nuclear receptor agonists including cholic acid, chenodeoxycholic acid, phenobarbital and pregnenolone 16α-carbonitrile (Fig. 9b).
Figure 9. Immunoblot analysis of the influence of modulators on (a) Mrp3 and (b) Mrp2 protein in rat SCH.
Rat SCH cultured for 72 hr with (a) phenobarbital (1 mM) or diluted bile (1:100) from TR− and Wistar control rats exhibited increased Mrp3 protein. Data generated by Dr. Peijin Zhang; (b) Mrp2 protein was increased after incubation of rat SCH for 72 hr with selected nuclear receptor agonists including cholic acid (CA; 200 μM), chenodeoxycholic acid (CDC; 200 μM), phenobarbital (PB; 1000 μM) and pregnenolone 16α-carbonitrile (PCN; 10 μM). Data generated by Dr. Yong Hae Han.
Transport of the fluorescent probe CDF in rat liver was characterized using a number of model systems (Zamek-Gliszczynski et al., 2003). Involvement of Mrp2 and Mrp3 was confirmed in Mrp-expressing Sf9 membrane vesicles and IPLs from wild-type (WT) and Mrp2-deficient TR− rats. Saturable CDF uptake was observed in WT rat SCH and probed using nonspecific Oatp inhibitors, bromosulfophthalein (BSP) and probenecid, and specific inhibitors digoxin (Oatp2), 100 μM para-aminohippurate (PAH) (Oat2), and 1 mM PAH (Oat3). Only BSP and probenecid inhibited uptake, suggesting CDF is taken up by an Oatp isoform(s) other than Oatp2.
Annaert and colleagues characterized the kinetics of the fluorescent bile acid analogue CGamF in rat SCH and assessed its utility as a probe for studying interactions with hepatic uptake of endogenous or exogenous compounds using seven HIV protease inhibitors [PI, (Ye et al., 2008)]. The known Oatp inhibitor, rifampin, reduced uptake of 1 μM CGamF by 70% (vs. 25% for 1 μM TC); removal of sodium produced no change in CGamF while completely abolishing TC uptake. These observations confirm the well-described routes of TC uptake (80% NTCP/20% OATPs) and suggested a complimentary role for CGamF as a more sensitive probe for Oatp-mediated inhibition (Meier et al., 1997, Kouzuki et al., 2000). Comparing the inhibitory potential of HIV PIs on the uptake of TC and CGamF in standard and sodium-free conditions, a preferential interference with sodium-independent processes (likely Oatps) emerged. Another notable observation was the striking potency of ritonavir (IC50 = 0.25 μM) as an inhibitor of CGamF uptake compared to other PIs, indicating that ritonavir may be a potent Oatp inhibitor, which is not detectable using TC in standard (+ sodium) conditions. The opportunity remains to elucidate the extent and possible mechanisms of CGamF biliary excretion and potential for use as a probe of overall hepatobiliary disposition.
Treijtel et al. utilized rat SCH to explore multiple processes responsible for the clearance of the frequently co-administered HIV PIs saquinavir and ritonavir (Treijtel et al., 2009). Saquinavir metabolism was significantly reduced by 5 μM ketoconazole, a known Cyp3a inhibitor, and was even more sensitive to 1 μM ritonavir, confirming the role of metabolism in the saquinavir-boosting effect of ritonavir coadministration. Biliary excretion of ritonavir was investigated in rat SCH in the presence and absence of the P-gp inhibitors verapamil, cyclosporine A and PSC833. Verapamil had little to no effect, but cyclosporine A and PSC833 decreased ritonavir cellular accumulation ~50% due to decreased biliary excretion and subsequently increased metabolism (Treijtel et al., 2009).
In a recent publication from Brouwer and coworkers, fexofenadine was evaluated as a probe for studying hepatobiliary transport using a combination of pharmacokinetic modeling/simulation of clinical and preclinical data from human SCH and rat isolated perfused livers (IPLs) (Swift et al., 2009). Simulation studies indicated that systemic exposure to fexofenadine in humans was sensitive to changes in hepatic uptake but not biliary efflux rates. Inhibition of P-gp-mediated fexofenadine efflux in human SCH using GF120918 had no effect on fexofenadine BEI or in vitro CLbiliary, further supporting simulation results. However, fexofenadine is a known substrate of additional canalicular efflux proteins, MRP2 and BSEP, which cannot be selectively modulated with known chemical probes, but presumably could compensate for P-gp inhibition. Of note, pharmacokinetic simulations also indicated that inhibition of fexofenadine biliary excretion may result in a significant (~20–60%) increase in hepatic exposure that is undetectable based on monitoring plasma concentrations. These studies demonstrate the utility of SCH in combination with pharmacokinetic modeling and simulation to explore and confirm mechanisms of DDIs.
The maintenance of cell polarity in vitro in the SCH model allows the unique ability to simultaneously evaluate hepatic uptake and efflux processes at the basolateral membrane, and biliary excretion processes at the canalicular membrane. A number of chemical probes with known transport protein specificity and modulating effects (inhibition, activation, induction) further facilitate the prediction of DDIs at the level of hepatic transport proteins. Despite previous work to establish conditions for evaluating interactions with individual or limited combinations of hepatic transport proteins, “a sufficiently diverse panel of model substrates (and inhibitors) needs to be identified and profiled with respect to transporter affinity and interaction,” to enable “rapid and reliable screening for drug interaction and cellular toxicity potential (Ye et al., 2008).”
Drug-Hepatic Transport Protein Interactions
Xeno- and endobiotics modulate transport proteins in a number of ways besides direct, competitive interactions at the active site(s) responsible for binding and translocation of substrates. Alterations in transport protein regulation at the level of transcription, translation or post-translational modifications do not require direct interactions with the transport protein. Both transcriptional and post-transcriptional regulation involves complex networks of co-activators and co-repressors that provide multiple checkpoints and ensure balanced activity of the pathways under normal conditions. The time scale of changes in transport protein expression and activity are different, however. Transcriptional regulation produces long-term changes in the total amount of transport protein that is available, while short-term changes can only result from a re-arrangement of preexisting protein, which is mobilized as a result of post-translational modifications.
A number of ligand-activated transcription factors have been associated with altered gene expression of hepatic transport proteins in various in vitro and in vivo models, including the pregnane X receptor (PXR/NR1I2), constitutive androstane receptor (CAR/NR1I3), farnesoid X-activated receptor (FXR/NR1H4), retinoid X receptor alpha (RXRα/NR2B1), aryl hydrocarbon receptor (AHR), peroxisome proliferator-activated receptors (PPARα,γ/NR1C1,3), glucocorticoid receptor (GR), small heterodimer partner (SHP), liver X receptor (LXR), nuclear factor E2-related factor 2 (Nrf2) and hepatic nuclear factor 1 alpha [HNF1α, (Kast et al., 2002, Jigorel et al., 2006, Klaassen and Slitt, 2005, Aleksunes et al., 2009, Staudinger et al., 2003, Kullak-Ublick and Becker, 2003). Similar to the transport proteins themselves, nuclear receptors (NRs) are bound by an overlapping spectrum of endogenous (hormones, bile acids) and exogenous (drugs, toxins) ligands (Parks et al., 1999, Moore et al., 2000). Thus, drug-induced NR activation may result from direct drug interactions with a given NR(s), or by altered intracellular disposition of endogenous agents, such as bile acids, that are themselves NR ligands. Examples of drugs known to directly activate transcription factors include: PXR - DEX, spironolactone; PPAR - fibrates; Nrf2 – ursodeoxycholic acid (UDCA) (Maher et al., 2005, Cheng et al., 2005, Okada et al., 2008). Other drugs, such as phenobarbital and rifampin, can alter transport protein function by both direct (competitive) and indirect (up- or down-regulation) mechanisms (Patel et al., 2003, Lam et al., 2006). Such complex and potentially confounding mechanisms present a serious challenge to differentiate between transport protein induction and inhibition.
Although mechanistic work to investigate transcriptional regulation using SCH has been minimal, routine sandwich-culture methodology takes advantage of maintained NR activity in primary hepatocytes. DEX has long been used as a media supplement due to its modulating effects on drug disposition mechanisms. DEX treatment is able to attenuate or reverse changes in gene expression observed in cultured hepatocytes and, together with a sandwich-cultured configuration, maintains expression of most relevant functions at near-physiological levels. DEX treatment of rat SCH restores physiologic expression of Mdr2 and Oatp1a4 mRNA by up-regulation, while maintaining Mdr1b and Mrp3 at baseline levels by preventing their up-regulation (Luttringer et al., 2002). However, Mrp2 mRNA is up-regulated by DEX beyond physiologic levels. The effects of DEX on protein expression and function in rat SCH have been evaluated (Turncliff et al., 2004). Forty-eight hr DEX pre-treatment increased expression of CYP3A1/2, Oatp1a4 and Mrp2, decreased expression of Ntcp, and had no effect on Oatp1a1, Mrp3, Mdr1a/b and Bsep. Modest changes in the transport of probe substrates were consistent with changes in protein expression, while patterns of protein expression generally agreed with earlier gene expression data by Luttringer et al., with the exception of Oatp1a4. Despite this example, mRNA and protein expression levels do not always correlate, and must be evaluated on a case-by-case basis for transport proteins (Rodriguez-Antona et al., 2001, Ho et al., 2006).
Short-term regulation of hepatic transport protein trafficking to and from the plasma membrane is mediated by post-translational modifications and second messenger signaling pathways (Kipp et al., 2001, Anwer, 2004). “Signal” regions in the protein structure are necessary for proper vesicle sorting and trafficking of newly-synthesized membrane-bound proteins from the endoplasmic reticulum and Golgi network to final membrane locations. These signals can take the form of specific peptide sequences or post-translational modifications, such as glycosylation. The role of glycosylation in Mrp2 expression, localization and function was investigated in rat SCH (Zhang et al., 2005b). Although the amount of cellular Mrp2 protein did not change, a gradual increase (~10kDa by western blot) in molecular weight over days in culture correlated with increased staining at the canalicular membrane by confocal microscopy, on day 4, relative to diffuse intracellular and ER co-localization at day 0 or day 1 in culture. Molecular weight and canalicular localization were decreased by treatment with tunicamycin, an inhibitor of glycosylation.
Cellular trafficking also is mediated by other modification “signals,” such as phosphate or ubiquitin residues, on transport proteins themselves, or on the chaperone proteins that move them around within the cell (Glavy et al., 2000). The cellular machinery responsible for modifying and mobilizing proteins is under the control of a complex series of second messenger pathways. A number of second messengers have been associated with changes in transport protein expression, localization and function, including cyclic AMP (cAMP), cGMP, intracellular Ca2+, phosphoinositol-3-kinase (PI3K), protein kinases A, B and C (PKA, PKB, PKC), and p38 mitogen activated protein kinase [MAPK, (Anwer, 2004, Roelofsen et al., 1998, Misra et al., 1998, Kubitz et al., 2004)]. The effect of cAMP and PKC activators, glucagon and phorbol 12-myristate 13-acetate (PMA), on Mrp3/MRP3 regulation was investigated in rat and human SCH and rat IPLs (Chandra et al., 2005). Glucagon increased CDF excretion and Mrp3/MRP3 localization at the basolateral membrane in both rat and human SCH, while PMA had no effect. Glucagon also increased CDF efflux in rat IPLs, suggesting that cAMP effects are recapitulated in the SCH model and similar across species. PMA decreased CDF efflux in rat IPLs, consistent with known down-regulation mediated by PKC activation, though it is unclear why PMA had no effect in SCH. These trafficking pathways can play a significant role in transport protein function, and may be modulated by xenobiotics, potentially including the recently developed family of kinase inhibitors (Katayama et al., 2007). SCH clearly demonstrate functional post-translational and second messenger pathways, although further characterization is needed.
Use of Sandwich-Cultured Hepatocytes to Predict Drug-Induced Liver Injury and Examine Mechanisms of Hepatotoxicity
DILI is historically one of the most common reasons that drugs fail in development or are removed from the market after being approved (Lee, 2003). Therefore, experimental models to better predict DILI are still needed. Furthermore, when DILI is predicted, it would be beneficial to understand the mechanism(s) of hepatotoxicity in order to assist in the design and testing of alternate compounds to circumvent toxic potential. In theory, a useful in vitro model would recapitulate relevant liver functions and mechanisms of hepatotoxicity, and resemble the in vivo situation as closely as possible. As detailed above, SCH are a unique in vitro system that maintains specific hepatic cytomorphology and function relevant to drug metabolism, disposition, and toxicity, and thus, closely resembles the in vivo setting.
Direct Assessment of Cytotoxicity in SCH from Relevant Species
It is an unfortunate fact that many hepatotoxic compounds go unrecognized in preclinical toxicology studies using animal models or screening for cytotoxicity. Species differences in metabolism or transport pathways can lead to marked differences in tolerance to toxic substances. In addition, in vitro models may not recapitulate the complex processes that often lead to gross toxicity. Therefore, the choice of preclinical toxicity models and endpoints can influence predictability of adverse events in humans.
SCH have contributed a great deal to our understanding of hepatotoxicity caused by inhibition of bile acid transport that may go unrecognized in animal studies. Kostrubsky and colleagues assessed the effects of numerous hepatotoxicants on human SCH [see above: Inhibition of Bile Acid Transport by Drugs (Kostrubsky et al., 2003, Kostrubsky et al., 2006)]. They established, for a series of macrolide antibiotics, that potent inhibition of TC efflux in human SCH correlated with clinical reports of liver toxicity, suggesting that rank order of in vitro TC inhibition may be predictive of hepatotoxic potential in vivo. Although preclinical rodent toxicity studies were previously negative, Kostrubsky and coworkers demonstrated that two inhibitors of TC transport in human SCH (CI-1034 and glyburide) caused a transient, 2.4-fold increase in serum bile acids when dosed individually in vivo in rats; coadministration resulted in a synergistic (6.8-fold) increase. Later, the effect of nefazodone was compared to its nontoxic structural analogues, buspirone and trazodone, in human SCH and intact rats (Kostrubsky et al., 2006). Cellular toxicity was assessed in SCH by measuring a decrease in protein synthesis after drug treatment and pulse labeling with 14C-leucine. Toxicity was associated exclusively with nefazodone, and correlated with accumulation of parent drug (see below: Hepatocyte Accumulation of Toxic Species). Nefazodone, but not buspirone, elevated serum bile acids in rats 1 hr after dosing, which was resolved by 24 hr. These studies provide several preclinical alternatives with the potential to identify hepatotoxic compounds that act by different mechanisms and demonstrate species differences.
Mechanistic understanding of species differences in hepatotoxicity has the potential to identify new opportunities for predicting toxic events that previously went undetected until reaching the clinic. The hepatotoxic potential of HIV PIs was compared in human and rat SCH, with similar patterns of TC inhibition, although PIs appeared to be more potent inhibitors of rat than human TC biliary efflux (McRae et al., 2006). Rank order of inhibition of TC efflux was not able to predict PI hepatotoxicity, as it did for macrolide antibiotics (Kostrubsky et al., 2003). However, it was shown that potential accumulation of bile acids due to PI-mediated BSEP inhibition was attenuated by concomitant inhibition of TC uptake in human SCH, similar to the findings of Kemp et al. in rat SCH (Kemp et al., 2005). In another publication from Brouwer and coworkers, this effect was explored as an explanation for the species difference in bosentan toxicity between humans and rats (Leslie et al., 2007). Although bosentan inhibited uptake as well as efflux in human SCH, resulting in decreased TC accumulation, inhibition of bile acid uptake is not expected at relevant concentrations in human. However, bosentan-mediated inhibition of Na+-dependent TC uptake was approximately 6− and 30-fold more potent in rat vs. human suspended hepatocytes and NTCP-/Ntcp-transfected HEK cells, respectively. Thus, preferential inhibition of rat Ntcp remains a plausible explanation for the observed resistance of rats to bosentan-induced hepatotoxicity observed clinically in humans.
Similarly, differential effects on TC disposition were observed in the seminal publication establishing sandwich-cultured conditions for dog and monkey hepatocytes (Rose et al., 2006). Glyburide abolished TC efflux in dog SCH, with no effect on efflux in monkey SCH. Conversely, glyburide inhibited TC uptake in monkey but not dog SCH, while cyclosporin A inhibited efflux but not uptake similarly in both dog and monkey SCH. These marked differences in TC inhibition underscore potential differences in observed hepatotoxic potential across preclinical species.
SCH recently were evaluated for the potential to assess hepatobiliary disposition of copper (Ansede et al., 2009). Copper (Cu) is an essential nutrient necessary for the activity of various enzymes, but also can accumulate to toxic levels when Cu elimination is disrupted by drugs or genetic disorders, such as in Wilson's Disease, which is characterized by massive Cu accumulation in lysosomes and lack of excretion in bile, the major route (85%) of Cu elimination. Hepatic Cu disposition is fairly well characterized: basolateral uptake is mediated by Cu transporter 1 (CTR1), while the ATP-dependent ATP7B transports Cu into trans-Golgi Network (TGN) vesicles for incorporation into ceruloplasmin and, apical membrane vesicles for biliary excretion (via exocytosis) of excess Cu. Cu transport was evaluated in rat, dog and human hepatocytes. CTR1/Ctr1 and ATP7B/Atp7b expression was confirmed in hepatocytes from all species by western blot analysis, with slight changes in expression observed in some species following exposure to Cu-supplemented media. CTR1/Ctr1 expression was decreased slightly in human and rat SCH, while Atp7b was elevated slightly in rat SCH; all changes were consistent with a protective role of reducing hepatic exposure in the presence of excess Cu. Relative differences in endogenous Cu levels in SCH from rat, dog and human (17.2, 490 and 43.5 ng/well, respectively) were consistent with published values for Cu content in livers of intact animals from corresponding species (3.56–5.56, 57.32–173.3, 6.2 μg/g in rat, dog and human, respectively). Increased uptake and efflux were observed with increases in Cu concentration and incubation time, confirming functional Cu transport and the potential for SCH to serve as a useful model to study the role of transport in Cu homeostasis.
Various assays have been used to assess viability in cytotoxicity studies as a direct measure of toxic effects. The utility of five such assays was compared in rat SCH to measure the toxic effects of increased bile acid accumulation resulting from inhibition of bile acid efflux by troglitazone, bosentan and glibenclamide (Kemp and Brouwer, 2004). The neutral red assay was not amenable for use in SCH due to crystal formation, while the LDH, MTT, propidium iodine and alamar blue assays were useful for viability assessment, having low variability and correlating well with morphologic changes observed by light microscopy. The LDH and propidium iodide assays are the methods of choice because they are amenable to multiple time point measurements and high-throughput analysis.
Toxicogenomics is another approach to measure compound-induced changes in multiple genes and signaling pathways, with the potential to extrapolate in vitro toxicology experiments to the in vivo situation. Comparison of gene expression and traditional toxicology assays, both in vitro and in vivo, reveals that genes are affected earlier and at lower dose levels than needed to detect toxicity by traditional measures (Heinloth et al., 2004, Kienhuis et al., 2006). Thus, gene expression changes may be more sensitive indicators of potential adverse effects. The primary limitation of most hepatic in vitro assays used in toxicogenomics studies is the loss of liver-specific functions. Among in vitro systems, SCH exclusively maintain polarized morphology and the panoply of liver-specific functions reflective of the in vivo situation. This includes the ability to induce enzyme systems, which can be used to study induction as a mechanism of DDIs or toxicity, as well as enhance enzyme activity to study compounds for which toxicity depends on activation by P450s or other enzyme systems. In one example, SCH were maintained in standard medium or medium modified with known P450 inducers phenobarbital, DEX and β-naphthoflavone to study coumarin-induced cytotoxicity and gene expression profiles (Kienhuis et al., 2006). Coumarin metabolism and resulting cytotoxicity correlated with in vivo toxicity studies only in hepatocytes maintained in modified culture medium, while gene expression changes in the modified system were also much more reflective of in vivo studies. This demonstrates that even when standard conditions for sandwich-culture are not entirely reflective of the desired endpoint(s), maintenance of liver-specific functions, such as induction, allows the model to be modified to recapitulate the desired effect observed in vivo.
Hepatocyte Accumulation of Toxic Species
Accumulation of toxic compounds within hepatocytes can occur by a number of mechanisms, including efficient uptake, inefficient or compromised excretion, and sequestration into subcellular compartments. Accumulation of bile acids to toxic levels by inhibition of canalicular efflux, including the potential attenuation of accumulation by concomitant inhibition of hepatic uptake, has been discussed extensively above.
Returning again to the study by Kostrubsky and colleagues in 2006, accumulation of parent nefazodone correlated with toxicity in human SCH (Kostrubsky et al., 2006). Total protein synthesis, determined by pulse-labeling with 14C-leucine, was reported as a measure of toxicity. Nefazodone, but not buspirone or trazodone, resulted in decreased protein synthesis after treatment for 6 hr with 10, 50 and 100 μM of the antidepressants. The time course of nefazodone toxicity was evaluated by treating SCH with nefazodone (10, 50 and 100 μM) for 1, 6 and 24 hr. Decreased protein synthesis was detected at 1 hr for all doses of nefazodone. At 24 hr, protein synthesis returned to normal for 10 μM but not 50 or 100 μM nefazodone. Further analysis by LC/MS/MS for nefazodone and metabolites in media revealed a 90% decrease in parent nefazodone at 24 hr after treatment with 10 μM; no decrease in nefazodone following incubation with 50 or 100 μM suggested that metabolism was compromised or insufficient for higher doses, resulting in prolonged exposure to parent compound, a sustained decrease in protein synthesis, and morphological cell death by microscopy. This effect was confirmed by treating cells with 10 μM nefazodone + 1 mM 1-aminobenzotriazole (ABT, a P450 inhibitor), resulting in accumulation of parent nefazodone, decreased formation of conjugated metabolites, and lack of recovery in protein synthesis at 24 hr.
Trebectadin cytotoxicity and attenuation by DEX pretreatment were successfully recapitulated in rat SCH, allowing investigation of the mechanism(s) by which DEX attenuates hepatotoxicity in rats and humans (Lee et al., 2008). DEX-mediated induction of Cyp3A and Mrp2 were both postulated mechanisms of hepatoprotection. Although a 75% reduction in biliary excretion in Mrp2-deficient TR− versus wild-type rat IPLs confirmed the involvement of Mrp2 in the biliary excretion of trebectadin, TR− rat SCH were surprisingly more resistant to trebectadin-induced cytotoxicity. Increased expression and activity of P450 enzymes, glutathione (GSH) and basolateral Mrp3 in TR− rats were proposed as possible explanations. Pre-treatment with 500 μM buthionine sulfoximine (BSO), which depletes cellular GSH, resulted in an unexpected decrease in cytotoxicity. Western blot analysis of cell lysates from BSO-treated and control SCH revealed that Mrp2, Mrp3 and Mrp4 protein were increased by 1.5±0.7-, 1.9±0.4- and 3.1±0.7-fold, respectively. Results of this study suggest that trebectadin-mediated cytotoxicity can be attenuated by numerous mechanisms that reduce cellular accumulation of intact trebectadin, whether by DEX-mediated increases in CYP3A metabolism and Mrp2 excretion, or markedly increased basolateral excretion due to up-regulation of Mrp3 and/or Mrp4 in the presence of BSO. Rat SCH were used successfully to reproduce cytotoxicity of trebectadin observed in intact animals and explore the role of transport proteins in attenuation of cytotoxicity.
Human SCH were employed to determine the role of nucleoside transporters in the hepatobiliary disposition of nucleosides and nucleoside drugs in the liver (Govindarajan et al., 2008). Protein expression and localization of equilibrative and concentrative nucleoside transporters (ENTs and CNTs) in human SCH were determined, and mRNA expression was compared to human liver samples. Expression levels of ENT2 and CNT2 mRNA in SCH format were similar to intact liver, but 2-D cultures (no overlay) resulted in significantly lower levels of ENT2 and CNT2 mRNA. Uptake into hepatocytes and elimination, by metabolism and/or vectorial transport into bile canaliculi, were demonstrated for 5 nucleosides and nucleoside analogues [guanidine, thymidine, ribavirin, formycin B and fialuridine (FIAU)]. Conditions were established to allow deconvolution of the relative contribution of individual transporters to the overall uptake of each substrate, which was varied and substrate-dependent. However, common patterns were observed, such as the significant (if not predominant) role of ENT1 for all substrates, and an inverse relationship between the extent of metabolism and excretion into bile. An example of the applicability of this system was the high extent of FIAU accumulation that, coupled with previous data detailing its mitochondrial transport and resulting cytotoxicity, may explain the significant hepatotoxicity of this drug (Lai et al., 2004). A more recent study extended the scope of ENT expression data to include ENT3, which displayed a higher level of mRNA expression than CNT1 and ENT1 in human liver samples and SCH; based on immunostaining, ENT3 was localized exclusively in the cytoplasm, co-localized with markers of both mitochondrial and non-mitochondrial compartments (Govindarajan et al., 2009).
Rat and human SCH recently were reported as a model to determine the formation of acyl glucuronides and covalent binding to cellular proteins (Dong and Smith, 2009). Glucuronidation and protein adduct formation were monitored following exposure of rat SCH to benoxaprofen, a known hepatotoxicant, and two related NSAIDs, flunoxaprofen and ibuprofen. Glucuronide and protein adducts were detected in the order: benoxaprofen > flunoxaprofen > ibuprofen. Despite this correlation, modulation of glucuronidation and oxidative metabolism pathways suggested that covalent binding to proteins was mediated only partly by reactive glucuronide formation and not at all by oxidative metabolites, leaving an unknown component of protein adduct formation. Although glucuronidation rates varied between donors of human SCH, as might be expected, glucuronide and protein adduct formation were greatest for benoxaprofen. These studies demonstrate the utility of SCH for determining relative risk of forming protein adducts from reactive acyl glucuronides.
Hepatic Transport Protein Knockdown and Knockout Models
RNA interference (RNAi) of single or multiple transport proteins is a powerful tool to explore potential rate-limiting steps in hepatic transport and consequences (“worst-case scenario”) of altered hepatic transport protein function caused by disease states, DDIs or polymorphisms. This approach is particularly useful to evaluate transport proteins for which no “specific” inhibitor has been identified. Synthetic small interfering RNA (siRNA) was transfected successfully into rat SCH to specifically knock down the efflux transporters Mrp2 and Mrp3 (Tian et al., 2004). The extent of protein modulation (~50% knockdown) agreed well with changes in activity, as determined by CDF disposition. More recently, adenoviral vectors expressing short hairpin (sh)RNA targeting Bcrp were delivered successfully into rat SCH to specifically, efficiently and sustainably suppress Bcrp protein expression and activity (Yue et al., 2009). Bcrp knockdown decreased nitrofurantoin BEI and CLbiliary to 11 and 14% of control, respectively. Digoxin, a P-gp substrate, was not affected by Bcrp knockdown; however, GF120918 significantly reduced the biliary excretion of both digoxin and nitrofurantoin, consistent with known inhibition of both P-gp and Bcrp. These studies demonstrated that knockdown of specific transport proteins in SCH is possible, and mimics the effect of inhibitors on substrate disposition.
Hepatocytes from animals with transport protein-deficient genotypes, such as Mrp2-deficient TR− rats may be cultured in a sandwich configuration (Abe et al., 2008, Lee et al., 2008). Another promising source of hepatocytes for SCH studies is from genetically-modified mouse strains such as Mrp2/Abcc2−/− knockout mice. However, care must be taken to fully characterize these models, since loss of expression or function of one transport protein may change the route and/or extent of excretion due to compensatory changes in drug metabolizing enzymes or transport proteins. For example, Nezasa et al. demonstrated that hepatic Mrp3/Abcc3 protein levels were approximately 60% higher in Mrp2/Abcc2−/− mice compared to wild-type mice, corresponding to a 4-fold increase in the rate of basolateral CDF excretion (Nezasa et al., 2006). In addition, it was established recently that Mrp2-deficient TR− rats also partially lack full Bcrp expression and activity (Abe et al., 2008). SCH, coupled with knockdown or knockout technology, represent a useful in vitro model to determine the contribution of specific canalicular or basolateral transport proteins to drug/metabolite disposition, and to examine the impact of loss-of-function of transport proteins on drug/metabolite disposition.
Future Challenges
Culturing hepatocytes in a sandwich configuration to maintain cell viability, polarized architecture and liver-specific function has led to significant advances in our understanding of hepatic transport processes and related interactions leading to pharmacokinetic and pharmacodynamic changes in endo- and xenobiotic disposition and hepatotoxicity. Despite these advances, a number of opportunities remain to further develop and characterize the SCH model. The limited availability of fresh hepatocytes from a variety of species, particularly human, has stimulated interest in the use of cryopreserved SCH. Unfortunately, not all batches of cryopreserved hepatocytes are suitable for culturing in a sandwich configuration, due to limitations in cell attachment and loss of transport protein expression, localization and/or function. Further research is needed to improve the cryopreservation process to yield hepatocytes amenable for SCH studies. Modifications of the culture system to incorporate sinusoidal flow and exteriorized bile flow may improve hepatocyte morphology and help retain liver-specific properties. Optimization of culture medium to maintain in vivo-like cytochrome 450 activity in addition to transport function would enhance utility of the model. Further use of this model as a screening tool in drug development to evaluate metabolite formation and routes of excretion, predict in vivo biliary clearance, and evaluate DDIs in hepatobiliary transport will undoubtedly yield a wealth of new information about species differences in hepatic transport, mechanisms of DILI, and xenobiotic-transport interactions.
Acknowledgements
The authors would like to thank the following individuals: Dr. Xianbin Tian, for providing the fluorescent images of CDF accumulation in wild-type and Abcc2−/− mouse SCH; Dr. Wei Yue, Tracy Marion and Katie Paul for providing immunoblots of transport proteins in rat and human SCH; Drs. Peijin Zhang and Yong Hae Han for providing immunoblots of modulator effects on Mrp2 and Mrp3, respectively; Dr. Jin Lee for calculation of the hepatocyte volume in SCH; and Dr. Kenneth Brouwer for insightful suggestions during the preparation and review of this manuscript. This research was supported by a grant from the National Institutes of Health (R01 GM41935). Brandon Swift is supported by an Eli Lilly and Company predoctoral fellowship. Nathan Pfeifer is supported by the UNC Royster Society of Fellows.
This research was supported by a grant from the National Institutes of Health (R01GM41935). Brandon Swift is supported by an Eli Lilly and Company predoctoral fellowship. Nathan Pfeifer is supported by the UNC Royster Society of Fellows.
Abbreviations Used
- SCH
Sandwich-cultured hepatocytes
- Mrp2
Multidrug Resistance-associated protein 2
- Mrp3
Multidrug resistance-associated protein 3
- Bsep
Bile salt export pump
- Mdr1
Multidrug resistance p-glycoprotein
- CDF
5(and 6)-carboxy-2',7'dichlorofluorescein
- NTCP
Sodium taurocholate cotransporting polypeptide
- HBSS
Hanks' balanced salt solution
- LDH
Lactate dehydrogenase
- EHBR
Eisai hyperbilirubinemic Sprague-Dawley rats
- Bcrp
breast cancer resistance protein
- RNAi
RNA interference
- siRNA
small interfering RNA
- shRNA
short hairpin RNA
- CLF
cholyl-lysyl-fluorescein
- CGamF
Cholylglycylamido-fluoroscein
- Oatp
Organic anion transporting polypeptide
- DDI
Drug-drug interaction
- IPL
Isolated perfused liver
- OSTα/β
Organic Solute Transporter
- DILI
Drug-induced liver injury
- TC
Taurocholate
- DEX
Dexamethasone
- BSP
bromosulfophthalein
- PAH
para-aminohippurate
- PI
Protease Inhibitor
- PXR/NR1I2
pregnane X receptor
- CAR/NR1I3
constitutive and rostane receptor
- FXR/NR1H4
farnesoid X-activated receptor
- RXRα/NR2B1
retinoid X receptor alpha
- AHR
aryl hydrocarbon receptor
- PPARα,γ/NR1C1,3
peroxisome proliferator-activated receptors
- GR
glucocorticoid receptor
- SHP
small heterodimer partner
- LXR
liver X receptor
- Nrf2
nuclear factor E2-related factor 2
- HNF1α
hepatic nuclear factor 1 alpha
- NRs
nuclear receptors
- UDCA
ursodeoxycholic acid
- PI3K
phosphoinositol-3-kinase
- PKA, PKB, PKC
protein kinases A, B and C
- MAPK
p38 mitogen-activated protein kinase
- PMA
phorbol 12-myristate 13-acetate
- GSH
glutathione
- DMEM
buthionine sulfoximine, BSO Dulbecco's modified Eagle's medium
- BC/MG
biocoat/matrigel
- GC/GC
gelled collagen/gelled collagen
- M3G
morphine 3-glucuronide
- IPL
isolated perfused liver
- ER
endoplasmic reticulum
Footnotes
Conflict of Interest: Dr. Kim Brouwer is a co-founder and Chair of the Scientific Advisory Board for Qualyst, Inc., which has exclusively licensed the sandwich-cultured hepatocyte technology for quantification of biliary excretion (B-CLEAR®).
Declaration of Interest KLRB is a cofounder and chair of the Scientific Advisory Board for Qualyst, Inc., which has exclusively licensed the sandwich-cultured hepatocyte technology for the quantification of biliary excretion (B-CLEAR®).
References
- Abe K, Bridges AS, Brouwer KL. Use of sandwich-cultured human hepatocytes to predict biliary clearance of angiotensin II receptor blockers and HMG-CoA reductase inhibitors. Drug Metab Dispos. 2009;37:447–52. doi: 10.1124/dmd.108.023465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe K, Bridges AS, Yue W, Brouwer KL. In vitro biliary clearance of angiotensin II receptor blockers and 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in sandwich-cultured rat hepatocytes: comparison with in vivo biliary clearance. J Pharmacol Exp Ther. 2008;326:983–90. doi: 10.1124/jpet.108.138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Yeager RL, Klaassen CD. Application of multivariate statistical procedures to identify transcription factors that correlate with MRP2, 3, and 4 mRNA in adult human livers. Xenobiotica. 2009;39:514–22. doi: 10.1080/00498250902952514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24:1803–23. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- Annaert PP, Brouwer KL. Assessment of drug interactions in hepatobiliary transport using rhodamine 123 in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2005;33:388–94. doi: 10.1124/dmd.104.001669. [DOI] [PubMed] [Google Scholar]
- Annaert PP, Turncliff RZ, Booth CL, Thakker DR, Brouwer KL. P-glycoprotein-mediated in vitro biliary excretion in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2001;29:1277–83. [PubMed] [Google Scholar]
- Ansede JH, Brouwer KR. Transport of Naloxone Glucuronide in B-Clear (Sandwich-Cultured Rat Hepatocytes) and its Application as an In Vitro Model for Determining the Directional Transport of Metabolites. 10th European ISSX Meeting; Vienna, Austria. 2008. [Google Scholar]
- Ansede JH, Wright MR, St Claire RL, Hart RW, Gefroh HA, Brouwer KR. Characterization of Sandwich-Cultured Hepatocytes as an In Vitro Model to Assess the Hepatobiliary Disposition of Copper. Drug Metab Dispos. 2009 doi: 10.1124/dmd.108.024638. [DOI] [PubMed] [Google Scholar]
- Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–90. doi: 10.1002/hep.20090. [DOI] [PubMed] [Google Scholar]
- Arterburn LM, Zurlo J, Yager JD, Overton RM, Heifetz AH. A morphological study of differentiated hepatocytes in vitro. Hepatology. 1995;22:175–87. [PubMed] [Google Scholar]
- Balavoine S, Feldmann G, Lardeux B. Regulation of RNA degradation in cultured rat hepatocytes: effects of specific amino acids and insulin. J Cell Physiol. 1993;156:56–62. doi: 10.1002/jcp.1041560109. [DOI] [PubMed] [Google Scholar]
- Ballard FJ, Wong SS, Knowles SE, Partridge NC, Martin TJ, Wood CM, Gunn JM. Insulin inhibition of protein degradation in cell monolayers. J Cell Physiol. 1980;105:335–46. doi: 10.1002/jcp.1041050216. [DOI] [PubMed] [Google Scholar]
- Barter ZE, Bayliss MK, Beaune PH, Boobis AR, Carlile DJ, Edwards RJ, Houston JB, Lake BG, Lipscomb JC, Pelkonen OR, Tucker GT, Rostami-Hodjegan A. Scaling factors for the extrapolation of in vivo metabolic drug clearance from in vitro data: reaching a consensus on values of human microsomal protein and hepatocellularity per gram of liver. Curr Drug Metab. 2007;8:33–45. doi: 10.2174/138920007779315053. [DOI] [PubMed] [Google Scholar]
- Bayliss MK, Bell JA, Jenner WN, Park GR, Wilson K. Utility of hepatocytes to model species differences in the metabolism of loxtidine and to predict pharmacokinetic parameters in rat, dog and man. Xenobiotica. 1999;29:253–68. doi: 10.1080/004982599238650. [DOI] [PubMed] [Google Scholar]
- Berry MN, Edwards AM, Barritt GJ. Isolated Hepatocytes: Preparation, Properties and Applications. Elsevier Science Publishing Co.; New York: 1991. [Google Scholar]
- Bi YA, Kazolias D, Duignan DB. Use of cryopreserved human hepatocytes in sandwich culture to measure hepatobiliary transport. Drug Metab Dispos. 2006;34:1658–65. doi: 10.1124/dmd.105.009118. [DOI] [PubMed] [Google Scholar]
- Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79:801–12. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell DM, Hammaker LE, Meyer UA. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. I. Functional studies. J Cell Biol. 1973;59:722–34. doi: 10.1083/jcb.59.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell DM, Stamatoglou SC, Nermut MV, Hughes RC. Interactions of rat hepatocytes with type IV collagen, fibronectin and laminin matrices. Distinct matrix-controlled modes of attachment and spreading. Eur J Cell Biol. 1986;40:72–8. [PubMed] [Google Scholar]
- Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci. 2003;73:386–402. doi: 10.1093/toxsci/kfg064. [DOI] [PubMed] [Google Scholar]
- Bonney RJ, Becker JE, Walker PR, Potter VR. Primary monolayer cultures of adult rat liver parenchymal cells suitable for study of the regulation of enzyme synthesis. In Vitro. 1974;9:399–413. doi: 10.1007/BF02615992. [DOI] [PubMed] [Google Scholar]
- Borlak J, Klutcka T. Expression of basolateral and canalicular transporters in rat liver and cultures of primary hepatocytes. Xenobiotica. 2004;34:935–47. doi: 10.1080/00498250400008363. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–30. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123:1649–58. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- Chandra P, Lecluyse EL, Brouwer KL. Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. In Vitro Cell Dev Biol Anim. 2001;37:380–5. doi: 10.1007/BF02577575. [DOI] [PubMed] [Google Scholar]
- Chandra P, Zhang P, Brouwer KL. Short-term regulation of multidrug resistance-associated protein 3 in rat and human hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1252–8. doi: 10.1152/ajpgi.00362.2004. [DOI] [PubMed] [Google Scholar]
- Chapman GS, Jones AL, Meyer UA, Bissell DM. Parenchymal cells from adult rat liver in nonproliferating monolayer culture. II. Ultrastructural studies. J Cell Biol. 1973;59:735–47. doi: 10.1083/jcb.59.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33:1276–82. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- Clayton DF, Darnell JE., Jr. Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983;3:1552–61. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–71. [PubMed] [Google Scholar]
- Dahn MS, Hsu CJ, Lange MP, Kimball SR, Jefferson LS. Factors affecting secretory protein production in primary cultures of rat hepatocytes. Proc Soc Exp Biol Med. 1993;203:38–44. doi: 10.3181/00379727-203-43570. [DOI] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- Dich J, Grunnet N. Primary cultures of rat hepatocytes Methods in Molecular Biology: Animal Cell Culture. Humana Press; Clifton, New Jersey: 1989. [DOI] [PubMed] [Google Scholar]
- Doherty MM, Poon K, Tsang C, Pang KS. Transport is not rate-limiting in morphine glucuronidation in the single-pass perfused rat liver preparation. J Pharmacol Exp Ther. 2006;317:890–900. doi: 10.1124/jpet.105.100446. [DOI] [PubMed] [Google Scholar]
- Dong JQ, Smith PC. Glucuronidation and Covalent Protein Binding of Benoxaprofen and Flunoxaprofen in Sandwich-Cultured Rat and Human Hepatocytes. Drug Metab Dispos. 2009 doi: 10.1124/dmd.109.028944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7:237–45. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;116:1043–53. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. Faseb J. 1989;3:174–7. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- Enat R, Jefferson DM, Ruiz-Opazo N, Gatmaitan Z, Leinwand LA, Reid LM. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984;81:1411–5. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, Meier PJ. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–31. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- Feng B, Xu JJ, Bi YA, Mireles R, Davidson R, Duignan DB, Campbell S, Kostrubsky VE, Dunn MC, Smith AR, Wang HF. Role of hepatic transporters in the disposition and hepatotoxicity of a HER2 tyrosine kinase inhibitor CP-724,714. Toxicol Sci. 2009;108:492–500. doi: 10.1093/toxsci/kfp033. [DOI] [PubMed] [Google Scholar]
- Ferrini JB, Pichard L, Domergue J, Maurel P. Long-term primary cultures of adult human hepatocytes. Chem Biol Interact. 1997;107:31–45. doi: 10.1016/s0009-2797(97)00072-0. [DOI] [PubMed] [Google Scholar]
- Flaim KE, Hutson SM, Lloyd CE, Taylor JM, Shiman R, Jefferson LS. Direct effect of insulin on albumin gene expression in primary cultures of rat hepatocytes. Am J Physiol. 1985;249:E447–53. doi: 10.1152/ajpendo.1985.249.5.E447. [DOI] [PubMed] [Google Scholar]
- Forster U, Luippold G, Schwarz LR. Induction of monooxygenase and UDP-glucuronosyltransferase activities in primary cultures of rat hepatocytes. Drug Metab Dispos. 1986;14:353–60. [PubMed] [Google Scholar]
- Fukuda H, Ohashi R, Tsuda-Tsukimoto M, Tamai I. Effect of plasma protein binding on in vitro-in vivo correlation of biliary excretion of drugs evaluated by sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2008;36:1275–82. doi: 10.1124/dmd.107.019026. [DOI] [PubMed] [Google Scholar]
- Funk C, Pantze M, Jehle L, Ponelle C, Scheuermann G, Lazendic M, Gasser R. Troglitazone-induced intrahepatic cholestasis by an interference with the hepatobiliary export of bile acids in male and female rats. Correlation with the gender difference in troglitazone sulfate formation and the inhibition of the canalicular bile salt export pump (Bsep) by troglitazone and troglitazone sulfate. Toxicology. 2001a;167:83–98. doi: 10.1016/s0300-483x(01)00460-7. [DOI] [PubMed] [Google Scholar]
- Funk C, Ponelle C, Scheuermann G, Pantze M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol. 2001b;59:627–35. [PubMed] [Google Scholar]
- Gartung C, Ananthanarayanan M, Rahman MA, Schuele S, Nundy S, Soroka CJ, Stolz A, Suchy FJ, Boyer JL. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- Gartung C, Schuele S, Schlosser SF, Boyer JL. Expression of the rat liver Na+/taurocholate cotransporter is regulated in vivo by retention of biliary constituents but not their depletion. Hepatology. 1997;25:284–90. doi: 10.1002/hep.510250205. [DOI] [PubMed] [Google Scholar]
- Ghibellini G, Vasist LS, Leslie EM, Heizer WD, Kowalsky RJ, Calvo BF, Brouwer KL. In vitro-in vivo correlation of hepatobiliary drug clearance in humans. Clin Pharmacol Ther. 2007;81:406–13. doi: 10.1038/sj.clpt.6100059. [DOI] [PubMed] [Google Scholar]
- Glavy JS, Wu SM, Wang PJ, Orr GA, Wolkoff AW. Down-regulation by extracellular ATP of rat hepatocyte organic anion transport is mediated by serine phosphorylation of oatp1. J Biol Chem. 2000;275:1479–84. doi: 10.1074/jbc.275.2.1479. [DOI] [PubMed] [Google Scholar]
- Govindarajan R, Endres CJ, Whittington D, Lecluyse E, Pastor-Anglada M, Tse CM, Unadkat JD. Expression and hepatobiliary transport characteristics of the concentrative and equilibrative nucleoside transporters in sandwich-cultured human hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295:G570–80. doi: 10.1152/ajpgi.00542.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan R, Leung GP, Zhou M, Tse CM, Wang J, Unadkat JD. Facilitated mitochondrial import of antiviral and anticancer nucleoside drugs by human equilibrative nucleoside transporter-3. Am J Physiol Gastrointest Liver Physiol. 2009;296:G910–22. doi: 10.1152/ajpgi.90672.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Boyer JL. The use of isolated rat hepatocyte couplets in hepatobiliary physiology. J Hepatol. 1990;10:387–94. doi: 10.1016/0168-8278(90)90152-h. [DOI] [PubMed] [Google Scholar]
- Greuet J, Pichard L, Ourlin JC, Bonfils C, Domergue J, Le Treut P, Maurel P. Effect of cell density and epidermal growth factor on the inducible expression of CYP3A and CYP1A genes in human hepatocytes in primary culture. Hepatology. 1997;25:1166–75. doi: 10.1002/hep.510250520. [DOI] [PubMed] [Google Scholar]
- Groothuis GM, Hulstaert CE, Kalicharan D, Hardonk MJ. Plasma membrane specialization and intracellular polarity of freshly isolated rat hepatocytes. Eur J Cell Biol. 1981;26:43–51. [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Clement B, Baffet G, Beaumont C, Morel-Chany E, Glaise D, Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983;143:47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Guillouzo A. Modulation of functional activities in cultured rat hepatocytes. Mol Cell Biochem. 1983:53–54. 35–56. doi: 10.1007/BF00225245. [DOI] [PubMed] [Google Scholar]
- Hait WN, Gesmonde JF, Murren JR, Yang JM, Chen HX, Reiss M. Terfenadine (Seldane): a new drug for restoring sensitivity to multidrug resistant cancer cells. Biochem Pharmacol. 1993;45:401–6. doi: 10.1016/0006-2952(93)90076-9. [DOI] [PubMed] [Google Scholar]
- Hamilton GA, Jolley SL, Gilbert D, Coon DJ, Barros S, Lecluyse EL. Regulation of cell morphology and cytochrome P450 expression in human hepatocytes by extracellular matrix and cell-cell interactions. Cell Tissue Res. 2001;306:85–99. doi: 10.1007/s004410100429. [DOI] [PubMed] [Google Scholar]
- Hayes JH, Soroka CJ, Rios-Velez L, Boyer JL. Hepatic sequestration and modulation of the canalicular transport of the organic cation, daunorubicin, in the Rat. Hepatology. 1999;29:483–93. doi: 10.1002/hep.510290216. [DOI] [PubMed] [Google Scholar]
- Heinloth AN, Irwin RD, Boorman GA, Nettesheim P, Fannin RD, Sieber SO, Snell ML, Tucker CJ, Li L, Travlos GS, Vansant G, Blackshear PE, Tennant RW, Cunningham ML, Paules RS. Gene expression profiling of rat livers reveals indicators of potential adverse effects. Toxicol Sci. 2004;80:193–202. doi: 10.1093/toxsci/kfh145. [DOI] [PubMed] [Google Scholar]
- Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl G, Li AP, Lecluyse E, Groothuis GM, Hengstler JG. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007a;39:159–234. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- Hewitt NJ, Lecluyse EL, Ferguson SS. Induction of hepatic cytochrome P450 enzymes: methods, mechanisms, recommendations, and in vitro-in vivo correlations. Xenobiotica. 2007b;37:1196–224. doi: 10.1080/00498250701534893. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–7. doi: 10.1124/mol.105.014019. [DOI] [PubMed] [Google Scholar]
- Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Hoen PA, Commandeur JN, Vermeulen NP, Van Berkel TJ, Bijsterbosch MK. Selective induction of cytochrome P450 3A1 by dexamethasone in cultured rat hepatocytes: analysis with a novel reverse transcriptase-polymerase chain reaction assay section sign. Biochem Pharmacol. 2000;60:1509–18. doi: 10.1016/s0006-2952(00)00454-8. [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Turncliff RZ, Lecluyse EL, Kim RB, Meier PJ, Brouwer KL. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Pharm Res. 2004;21:1294–302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Stinson-Fisher C, Shiman R, Jefferson LS. Regulation of albumin synthesis by hormones and amino acids in primary cultures of rat hepatocytes. Am J Physiol. 1987;252:E291–8. doi: 10.1152/ajpendo.1987.252.3.E291. [DOI] [PubMed] [Google Scholar]
- Ichihara A. Mechanisms controlling growth of hepatocytes in primary culture. Dig Dis Sci. 1991;36:489–93. doi: 10.1007/BF01298881. [DOI] [PubMed] [Google Scholar]
- Ichihara A, Nakamura T, Tanaka K. Use of hepatocytes in primary culture for biochemical studies on liver functions. Mol Cell Biochem. 1982;43:145–60. doi: 10.1007/BF00223006. [DOI] [PubMed] [Google Scholar]
- Jauregui HO, Mcmillan PN, Driscoll J, Naik S. Attachment and long term survival of adult rat hepatocytes in primary monolayer cultures: comparison of different substrata and tissue culture media formulations. In Vitro Cell Dev Biol. 1986;22:13–22. doi: 10.1007/BF02623436. [DOI] [PubMed] [Google Scholar]
- Jauregui HO, Mcmillan PN, Hevey K, Naik S. A quantitative analysis of lectin binding to adult rat hepatocyte cell surfaces. In Vitro Cell Dev Biol. 1988;24:401–12. doi: 10.1007/BF02628491. [DOI] [PubMed] [Google Scholar]
- Jefferson DM, Clayton DF, Darnell JE, Jr., Reid LM. Posttranscriptional modulation of gene expression in cultured rat hepatocytes. Mol Cell Biol. 1984;4:1929–34. doi: 10.1128/mcb.4.9.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigorel E, Le Vee M, Boursier-Neyret C, Parmentier Y, Fardel O. Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug-sensing receptors in primary human hepatocytes. Drug Metab Dispos. 2006;34:1756–63. doi: 10.1124/dmd.106.010033. [DOI] [PubMed] [Google Scholar]
- Jurima-Romet M, Crawford K, Cyr T, Inaba T. Terfenadine metabolism in human liver. In vitro inhibition by macrolide antibiotics and azole antifungals. Drug Metab Dispos. 1994;22:849–57. [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther. 2007;6:2092–102. doi: 10.1158/1535-7163.MCT-07-0148. [DOI] [PubMed] [Google Scholar]
- Kemp DC, Brouwer KL. Viability assessment in sandwich-cultured rat hepatocytes after xenobiotic exposure. Toxicol In Vitro. 2004;18:869–77. doi: 10.1016/j.tiv.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Kemp DC, Zamek-Gliszczynski MJ, Brouwer KL. Xenobiotics inhibit hepatic uptake and biliary excretion of taurocholate in rat hepatocytes. Toxicol Sci. 2005;83:207–14. doi: 10.1093/toxsci/kfi020. [DOI] [PubMed] [Google Scholar]
- Kienhuis AS, Wortelboer HM, Hoflack JC, Moonen EJ, Kleinjans JC, Van Ommen B, Van Delft JH, Stierum RH. Comparison of coumarin-induced toxicity between sandwich-cultured primary rat hepatocytes and rats in vivo: a toxicogenomics approach. Drug Metab Dispos. 2006;34:2083–90. doi: 10.1124/dmd.106.011262. [DOI] [PubMed] [Google Scholar]
- Kienhuis AS, Wortelboer HM, Maas WJ, Van Herwijnen M, Kleinjans JC, Van Delft JH, Stierum RH. A sandwich-cultured rat hepatocyte system with increased metabolic competence evaluated by gene expression profiling. Toxicol In Vitro. 2007;21:892–901. doi: 10.1016/j.tiv.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–24. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Slitt AL. Regulation of hepatic transporters by xenobiotic receptors. Curr Drug Metab. 2005;6:309–28. doi: 10.2174/1389200054633826. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Mcgarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–93. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Kostrubsky SE, Strom SC, Kalgutkar AS, Kulkarni S, Atherton J, Mireles R, Feng B, Kubik R, Hanson J, Urda E, Mutlib AE. Inhibition of hepatobiliary transport as a predictive method for clinical hepatotoxicity of nefazodone. Toxicol Sci. 2006;90:451–9. doi: 10.1093/toxsci/kfj095. [DOI] [PubMed] [Google Scholar]
- Kostrubsky VE, Strom SC, Hanson J, Urda E, Rose K, Burliegh J, Zocharski P, Cai H, Sinclair JF, Sahi J. Evaluation of hepatotoxic potential of drugs by inhibition of bile-acid transport in cultured primary human hepatocytes and intact rats. Toxicol Sci. 2003;76:220–8. doi: 10.1093/toxsci/kfg217. [DOI] [PubMed] [Google Scholar]
- Kostrubsky VE, Vore M, Kindt E, Burliegh J, Rogers K, Peter G, Altrogge D, Sinz MW. The effect of troglitazone biliary excretion on metabolite distribution and cholestasis in transporter-deficient rats. Drug Metab Dispos. 2001;29:1561–6. [PubMed] [Google Scholar]
- Kouzuki H, Suzuki H, Stieger B, Meier PJ, Sugiyama Y. Characterization of the transport properties of organic anion transporting polypeptide 1 (oatp1) and Na(+)/taurocholate cotransporting polypeptide (Ntcp): comparative studies on the inhibitory effect of their possible substrates in hepatocytes and cDNA-transfected COS-7 cells. J Pharmacol Exp Ther. 2000;292:505–11. [PubMed] [Google Scholar]
- Kubitz R, Sutfels G, Kuhlkamp T, Kolling R, Haussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology. 2004;126:541–53. doi: 10.1053/j.gastro.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Becker MB. Regulation of drug and bile salt transporters in liver and intestine. Drug Metab Rev. 2003;35:305–17. doi: 10.1081/dmr-120026398. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski AP, Baker TK, Klaunig JE. Comparison of glucocorticoid-mediated changes in the expression and function of rat hepatocyte gap junctional proteins. Carcinogenesis. 1994;15:1753–7. doi: 10.1093/carcin/15.8.1753. [DOI] [PubMed] [Google Scholar]
- Lai Y, Tse CM, Unadkat JD. Mitochondrial expression of the human equilibrative nucleoside transporter 1 (hENT1) results in enhanced mitochondrial toxicity of antiviral drugs. J Biol Chem. 2004;279:4490–7. doi: 10.1074/jbc.M307938200. [DOI] [PubMed] [Google Scholar]
- Laishes BA, Williams GM. Conditions affecting primary cell cultures of functional adult rat hepatocytes. II. Dexamethasone enhanced longevity and maintenance of morphology. In Vitro. 1976;12:821–32. doi: 10.1007/BF02796367. [DOI] [PubMed] [Google Scholar]
- Lam JL, Shugarts SB, Okochi H, Benet LZ. Elucidating the effect of final-day dosing of rifampin in induction studies on hepatic drug disposition and metabolism. J Pharmacol Exp Ther. 2006;319:864–70. doi: 10.1124/jpet.106.108282. [DOI] [PubMed] [Google Scholar]
- Lambiotte M, Vorbrodt A, Benedetti EL. Expression of differentiation of rat foetal hepatocytes in cellular culture under the action of glucocorticoids: appearance of bile canaliculi. Cell Differ. 1973;2:43–53. doi: 10.1016/0045-6039(73)90005-5. [DOI] [PubMed] [Google Scholar]
- Lecluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–68. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- Lecluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266:C1764–74. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- Lecluyse EL, Bullock PL, Parkinson A, Hochman JH. Cultured rat hepatocytes. Pharm Biotechnol. 1996;8:121–59. doi: 10.1007/978-1-4899-1863-5_9. [DOI] [PubMed] [Google Scholar]
- Lee J, Boyer JL. Molecular alterations in hepatocyte transport mechanisms in acquired cholestatic liver disorders. Semin Liver Dis. 2000;20:373–84. doi: 10.1055/s-2000-9390. [DOI] [PubMed] [Google Scholar]
- Lee J, Morgan JR, Tompkins RG, Yarmush ML. The importance of proline on long-term hepatocyte function in a collagen gel sandwich configuration: Regulation of protein secretion. Biotechnol Bioeng. 1992;40:298–305. doi: 10.1002/bit.260400214. [DOI] [PubMed] [Google Scholar]
- Lee JK, Leslie EM, Zamek-Gliszczynski MJ, Brouwer KL. Modulation of trabectedin (ET-743) hepatobiliary disposition by multidrug resistance-associated proteins (Mrps) may prevent hepatotoxicity. Toxicol Appl Pharmacol. 2008;228:17–23. doi: 10.1016/j.taap.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Marion TL, Abe K, Lim C, Pollock GM, Brouwer KL. Hepatobiliary disposition of troglitazone and metabolites in rat and human sandwich-cultured hepatocytes: use of Monte Carlo simulations to assess the impact of changes in biliary excretion on troglitazone sulfate accumulation. J Pharmacol Exp Ther. 2010;33:26–34. doi: 10.1124/jpet.109.156653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Trauner M, Soroka CJ, Stieger B, Meier PJ, Boyer JL. Expression of the bile salt export pump is maintained after chronic cholestasis in the rat. Gastroenterology. 2000;118:163–72. doi: 10.1016/s0016-5085(00)70425-2. [DOI] [PubMed] [Google Scholar]
- Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 2003;349:474–85. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- Lengyel G, Veres Z, Szabo P, Vereczkey L, Jemnitz K. Canalicular and sinusoidal disposition of bilirubin mono- and diglucuronides in sandwich-cultured human and rat primary hepatocytes. Drug Metab Dispos. 2005;33:1355–60. doi: 10.1124/dmd.105.004481. [DOI] [PubMed] [Google Scholar]
- Lengyel G, Veres Z, Tugyi R, Vereczkey L, Molnar T, Glavinas H, Krajcsi P, Jemnitz K. Modulation of sinusoidal and canalicular elimination of bilirubin-glucuronides by rifampicin and other cholestatic drugs in a sandwich culture of rat hepatocytes. Hepatol Res. 2008;38:300–9. doi: 10.1111/j.1872-034X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Watkins PB, Kim RB, Brouwer KL. Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1)by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther. 2007;321:1170–8. doi: 10.1124/jpet.106.119073. [DOI] [PubMed] [Google Scholar]
- Lindblad WJ, Schuetz EG, Redford KS, Guzelian PS. Hepatocellular phenotype in vitro is influenced by biophysical features of the collagenous substratum. Hepatology. 1991;13:282–8. [PubMed] [Google Scholar]
- Liu X, Brouwer KL, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, Lecluyse EL. Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res. 1998;15:1533–9. doi: 10.1023/a:1011994831139. [DOI] [PubMed] [Google Scholar]
- Liu X, Chism JP, Lecluyse EL, Brouwer KR, Brouwer KL. Correlation of biliary excretion in sandwich-cultured rat hepatocytes and in vivo in rats. Drug Metab Dispos. 1999a;27:637–44. [PubMed] [Google Scholar]
- Liu X, Lecluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KL. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999b;277:G12–21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- Liu X, Lecluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KL. Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 1999c;289:1592–9. [PubMed] [Google Scholar]
- Loi CM, Alvey CW, Vassos AB, Randinitis EJ, Sedman AJ, Koup JR. Steady-state pharmacokinetics and dose proportionality of troglitazone and its metabolites. J Clin Pharmacol. 1999a;39:920–6. doi: 10.1177/00912709922008533. [DOI] [PubMed] [Google Scholar]
- Loi CM, Young M, Randinitis E, Vassos A, Koup JR. Clinical pharmacokinetics of troglitazone. Clin Pharmacokinet. 1999b;37:91–104. doi: 10.2165/00003088-199937020-00001. [DOI] [PubMed] [Google Scholar]
- Luttringer O, Theil FP, Lave T, Wernli-Kuratli K, Guentert TW, De Saizieu A. Influence of isolation procedure, extracellular matrix and dexamethasone on the regulation of membrane transporters gene expression in rat hepatocytes. Biochem Pharmacol. 2002;64:1637–50. doi: 10.1016/s0006-2952(02)01382-5. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Slitt AL, Dieter MZ, Klaassen CD. Induction of the multidrug resistance-associated protein family of transporters by chemical activators of receptor-mediated pathways in mouse liver. Drug Metab Dispos. 2005;33:956–62. doi: 10.1124/dmd.105.003798. [DOI] [PubMed] [Google Scholar]
- Marion TL, Leslie EM, Brouwer KL. Use of sandwich-cultured hepatocytes to evaluate impaired bile acid transport as a mechanism of drug-induced hepatotoxicity. Mol Pharm. 2007;4:911–8. doi: 10.1021/mp0700357. [DOI] [PubMed] [Google Scholar]
- Maurel P. The use of adult human hepatocytes in primary culture and other in vitro systems to investigate drug metabolism in man. Advanced Drug Delivery Reviews. 1996;22:105–132. [Google Scholar]
- Mcrae MP, Lowe CM, Tian X, Bourdet DL, Ho RH, Leake BF, Kim RB, Brouwer KL, Kashuba AD. Ritonavir, saquinavir, and efavirenz, but not nevirapine, inhibit bile acid transport in human and rat hepatocytes. J Pharmacol Exp Ther. 2006;318:1068–75. doi: 10.1124/jpet.106.102657. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26:1667–77. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- Mills CO, Milkiewicz P, Muller M, Roma MG, Havinga R, Coleman R, Kuipers F, Jansen PL, Elias E. Different pathways of canalicular secretion of sulfated and non-sulfated fluorescent bile acids: a study in isolated hepatocyte couplets and TR- rats. J Hepatol. 1999;31:678–84. doi: 10.1016/s0168-8278(99)80348-1. [DOI] [PubMed] [Google Scholar]
- Misra AL, Pontani RB, Vadlamani NL, Mule SJ. Physiological disposition and biotransformation of [allyl-1', 3' - 14C naloxone in the rat and some comparative observations on nalorphine. J Pharmacol Exp Ther. 1976;196:257–68. [PubMed] [Google Scholar]
- Misra S, Ujhazy P, Gatmaitan Z, Varticovski L, Arias IM. The role of phosphoinositide 3-kinase in taurocholate-induced trafficking of ATP-dependent canalicular transporters in rat liver. J Biol Chem. 1998;273:26638–44. doi: 10.1074/jbc.273.41.26638. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Mars WM, Michalopoulos GK, Namba M. Dose-dependent biphasic effects of phenobarbital on growth and differentiation of primary culture rat hepatocytes. J Gastroenterol Hepatol. 1998;13(Suppl):S78–82. doi: 10.1111/jgh.1998.13.s1.78. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–7. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Munteanu E, Verdier M, Grandjean-Forestier F, Stenger C, Jayat-Vignoles C, Huet S, Robert J, Ratinaud MH. Mitochondrial localization and activity of P-glycoprotein in doxorubicin-resistant K562 cells. Biochem Pharmacol. 2006;71:1162–74. doi: 10.1016/j.bcp.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Nezasa K, Tian X, Zamek-Gliszczynski MJ, Patel NJ, Raub TJ, Brouwer KL. Altered hepatobiliary disposition of 5 (and 6)-carboxy-2',7'-dichlorofluorescein in Abcg2 (Bcrp1) and Abcc2 (Mrp2) knockout mice. Drug Metab Dispos. 2006;34:718–23. doi: 10.1124/dmd.105.007922. [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, Oda K, Warabi E, Ishii T, Osaka K, Hyodo I, Yamamoto M. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–47. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- Orkin RW, Gehron P, Mcgoodwin EB, Martin GR, Valentine T, Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977;145:204–20. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Elferink RP, Ottenhoff R, Liefting WG, Schoemaker B, Groen AK, Jansen PL. ATP-dependent efflux of GSSG and GS-conjugate from isolated rat hepatocytes. Am J Physiol. 1990;258:G699–706. doi: 10.1152/ajpgi.1990.258.5.G699. [DOI] [PubMed] [Google Scholar]
- Ouellet DM, Pollack GM. Biliary excretion and enterohepatic recirculation of morphine-3-glucuronide in rats. Drug Metab Dispos. 1995;23:478–84. [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- Patel NJ, Zamek-Gliszczynski MJ, Zhang P, Han YH, Jansen PL, Meier PJ, Stieger B, Brouwer KL. Phenobarbital alters hepatic Mrp2 function by direct and indirect interactions. Mol Pharmacol. 2003;64:154–9. doi: 10.1124/mol.64.1.154. [DOI] [PubMed] [Google Scholar]
- Pichard-Garcia L, Gerbal-Chaloin S, Ferrini JB, Fabre JM, Maurel P. Use of long-term cultures of human hepatocytes to study cytochrome P450 gene expression. Methods Enzymol. 2002;357:311–21. doi: 10.1016/s0076-6879(02)57689-8. [DOI] [PubMed] [Google Scholar]
- Preininger K, Stingl H, Englisch R, Furnsinn C, Graf J, Waldhausl W, Roden M. Acute troglitazone action in isolated perfused rat liver. Br J Pharmacol. 1999;126:372–8. doi: 10.1038/sj.bjp.0702318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DJ, Pascoe GA, Thomas CE. Extracellular calcium effects on cell viability and thiol homeostasis. Environ Health Perspect. 1990;84:113–20. doi: 10.1289/ehp.9084113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LM, Fiorino AS, Sigal SH, Brill S, Holst PA. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology. 1992;15:1198–203. doi: 10.1002/hep.1840150635. [DOI] [PubMed] [Google Scholar]
- Reid LM, Jefferson DM. Culturing hepatocytes and other differentiated cells. Hepatology. 1984;4:548–59. doi: 10.1002/hep.1840040332. [DOI] [PubMed] [Google Scholar]
- Ren P, De Feijter AW, Paul DL, Ruch RJ. Enhancement of liver cell gap junction protein expression by glucocorticoids. Carcinogenesis. 1994;15:1807–13. doi: 10.1093/carcin/15.9.1807. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Antona C, Donato MT, Pareja E, Gomez-Lechon MJ, Castell JV. Cytochrome P-450 mRNA expression in human liver and its relationship with enzyme activity. Arch Biochem Biophys. 2001;393:308–15. doi: 10.1006/abbi.2001.2499. [DOI] [PubMed] [Google Scholar]
- Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci. 1998;111(Pt 8):1137–45. doi: 10.1242/jcs.111.8.1137. [DOI] [PubMed] [Google Scholar]
- Rogiers V, Vercruysse A. Rat hepatocyte cultures and co-cultures in biotransformation studies of xenobiotics. Toxicology. 1993;82:193–208. doi: 10.1016/0300-483x(93)02611-j. [DOI] [PubMed] [Google Scholar]
- Rojkind M, Gatmaitan Z, Mackensen S, Giambrone MA, Ponce P, Reid LM. Connective tissue biomatrix: its isolation and utilization for long-term cultures of normal rat hepatocytes. J Cell Biol. 1980;87:255–63. doi: 10.1083/jcb.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman ID, Monte MJ, Gonzalez-Buitrago JM, Esteller A, Jimenez R. Inhibition of hepatocytary vesicular transport by cyclosporin A in the rat: relationship with cholestasis and hyperbilirubinemia. Hepatology. 1990;12:83–91. doi: 10.1002/hep.1840120114. [DOI] [PubMed] [Google Scholar]
- Rose KA, Kostrubsky V, Sahi J. Hepatobiliary disposition in primary cultures of dog and monkey hepatocytes. Mol Pharm. 2006;3:266–74. doi: 10.1021/mp0501022. [DOI] [PubMed] [Google Scholar]
- Rubin K, Hook M, Obrink B, Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981;24:463–70. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Saulter JY, Kurian JR, Trepanier LA, Tidwell RR, Bridges AS, Boykin DW, Stephens CE, Anbazhagan M, Hall JE. Unusual dehydroxylation of antimicrobial amidoxime prodrugs by cytochrome b5 and NADH cytochrome b5 reductase. Drug Metab Dispos. 2005;33:1886–93. doi: 10.1124/dmd.105.005017. [DOI] [PubMed] [Google Scholar]
- Sawada N, Tomomura A, Sattler CA, Sattler GL, Kleinman HK, Pitot HC. Effects of extracellular matrix components on the growth and differentiation of cultured rat hepatocytes. In Vitro Cell Dev Biol. 1987;23:267–73. doi: 10.1007/BF02623709. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Solheim AE, Seglen PO. Amino acid and energy requirements for rat hepatocytes in primary culture. In Vitro. 1982;18:43–54. doi: 10.1007/BF02796384. [DOI] [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB, Poli A. Amino acid inhibition of the autophagic/lysosomal pathway of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980;630:103–18. doi: 10.1016/0304-4165(80)90141-5. [DOI] [PubMed] [Google Scholar]
- Smith R, Jones RD, Ballard PG, Griffiths HH. Determination of microsome and hepatocyte scaling factors for in vitro/in vivo extrapolation in the rat and dog. Xenobiotica. 2008;38:1386–98. doi: 10.1080/00498250802491662. [DOI] [PubMed] [Google Scholar]
- Sohlenius-Sternbeck AK. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol In Vitro. 2006;20:1582–6. doi: 10.1016/j.tiv.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Stamatoglou SC, Hughes RC. Cell adhesion molecules in liver function and pattern formation. Faseb J. 1994;8:420–7. doi: 10.1096/fasebj.8.6.8168692. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos. 2003;31:523–7. doi: 10.1124/dmd.31.5.523. [DOI] [PubMed] [Google Scholar]
- Stieger B, Fattinger K, Madon J, Kullak-Ublick GA, Meier PJ. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–30. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- Studenberg SD, Brouwer KL. Effect of phenobarbital and phydroxyphenobarbital glucuronide on acetaminophen metabolites in isolated rat hepatocytes: use of a kinetic model to examine the rates of formation and egress. J Pharmacokinet Biopharm. 1993;21:175–94. doi: 10.1007/BF01059769. [DOI] [PubMed] [Google Scholar]
- Sudhakaran PR, Stamatoglou SC, Hughes RC. Modulation of protein synthesis and secretion by substratum in primary cultures of rat hepatocytes. Exp Cell Res. 1986;167:505–16. doi: 10.1016/0014-4827(86)90190-4. [DOI] [PubMed] [Google Scholar]
- Swift B, Brouwer KL. Influence of seeding density and extracellular matrix on bile Acid transport and mrp4 expression in sandwich-cultured mouse hepatocytes. Mol Pharm. 2010;7:491–500. doi: 10.1021/mp900227a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift B, Tian X, Brouwer KL. Integration of preclinical and clinical data with pharmacokinetic modeling and simulation to evaluate fexofenadine as a probe for hepatobiliary transport function. Pharm Res. 2009;26:1942–51. doi: 10.1007/s11095-009-9909-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarao K, Olinger EJ, Ostrow JD, Balistreri WF. Impaired bile acid efflux from hepatocytes isolated from the liver of rats with cholestasis. Am J Physiol. 1982;243:G253–8. doi: 10.1152/ajpgi.1982.243.4.G253. [DOI] [PubMed] [Google Scholar]
- Terry TL, Gallin WJ. Effects of fetal calf serum and disruption of cadherin function on the formation of bile canaliculi between hepatocytes. Exp Cell Res. 1994;214:642–53. doi: 10.1006/excr.1994.1302. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Hunter KW, Williams RW. Genetic dissection of complex and quantitative traits: from fantasy to reality via a community effort. Mamm Genome. 2002;13:175–8. doi: 10.1007/s00335-001-4001-Y. [DOI] [PubMed] [Google Scholar]
- Tian X, Zamek-Gliszczynski MJ, Zhang P, Brouwer KL. Modulation of multidrug resistance-associated protein 2 (Mrp2) and Mrp3 expression and function with small interfering RNA in sandwich-cultured rat hepatocytes. Mol Pharmacol. 2004;66:1004–10. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- Treijtel N, Van Eijkeren JC, Nijmeijer S, De Greef-Van Der Sandt IC, Freidig AP. Clearance and clearance inhibition of the HIV-1 protease inhibitors ritonavir and saquinavir in sandwich-cultured rat hepatocytes and rat microsomes. Toxicol In Vitro. 2009;23:185–93. doi: 10.1016/j.tiv.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Hoffmaster KA, Kalvass JC, Pollack GM, Brouwer KL. Hepatobiliary disposition of a drug/metabolite pair: Comprehensive pharmacokinetic modeling in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther. 2006a;318:881–9. doi: 10.1124/jpet.106.102616. [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Meier PJ, Brouwer KL. Effect of dexamethasone treatment on the expression and function of transport proteins in sandwich-cultured rat hepatocytes. Drug Metab Dispos. 2004;32:834–9. doi: 10.1124/dmd.32.8.834. [DOI] [PubMed] [Google Scholar]
- Turncliff RZ, Tian X, Brouwer KL. Effect of culture conditions on the expression and function of Bsep, Mrp2, and Mdr1a/b in sandwich-cultured rat hepatocytes. Biochem Pharmacol. 2006b;71:1520–9. doi: 10.1016/j.bcp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Tuschl G, Mueller SO. Effects of cell culture conditions on primary rat hepatocytes-cell morphology and differential gene expression. Toxicology. 2006;218:205–15. doi: 10.1016/j.tox.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Uhal BD, Roehrig KL. Effect of dietary state on hepatocyte size. Biosci Rep. 1982;2:1003–7. doi: 10.1007/BF01122168. [DOI] [PubMed] [Google Scholar]
- Vandenberghe Y, Ratanasavanh D, Glaise D, Guillouzo A. Influence of medium composition and culture conditions on glutathione S-transferase activity in adult rat hepatocytes during culture. In Vitro Cell Dev Biol. 1988;24:281–8. doi: 10.1007/BF02628828. [DOI] [PubMed] [Google Scholar]
- Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–34. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- Wang MZ, Saulter JY, Usuki E, Cheung YL, Hall M, Bridges AS, Loewen G, Parkinson OT, Stephens CE, Allen JL, Zeldin DC, Boykin DW, Tidwell RR, Parkinson A, Paine MF, Hall JE. CYP4F enzymes are the major enzymes in human liver microsomes that catalyze the O-demethylation of the antiparasitic prodrug DB289 [2,5-bis(4-amidinophenyl)furanbis-O-methylamidoxime] Drug Metab Dispos. 2006;34:1985–94. doi: 10.1124/dmd.106.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Morrissey JJ, Naik S, Jauregui HO. Phenobarbital induction of cytochromes P-450. High-level long-term responsiveness of primary rat hepatocyte cultures to drug induction, and glucocorticoid dependence of the phenobarbital response. Biochem J. 1990;271:113–9. doi: 10.1042/bj2710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GM, Bermudez E, Scaramuzzino D. Rat hepatocyte primary cell cultures. III. Improved dissociation and attachment techniques and the enhancement of survival by culture medium. In Vitro. 1977;13:809–17. doi: 10.1007/BF02615128. [DOI] [PubMed] [Google Scholar]
- Wilson ZE, Rostami-Hodjegan A, Burn JL, Tooley A, Boyle J, Ellis SW, Tucker GT. Inter-individual variability in levels of human microsomal protein and hepatocellularity per gram of liver. Br J Clin Pharmacol. 2003;56:433–40. doi: 10.1046/j.1365-2125.2003.01881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf KK, Bridges AS, Lee JK, Polli JW, Brouwer KR, Pollack GM, Brouwer KLR. Hepatobiliary Disposition of Morphine and Generated Morphine 3-Glucuronide in Sandwich-Cultured Rat Hepatocytes. 15th Annual ISSX North American Regional Meeting; San Diego, CA. 2008. [Google Scholar]
- Wolf KK, Vora S, Webster LO, Generaux GT, Polli JW, Brouwer KL. Use of cassette dosing in sandwich-cultured rat and human hepatocytes to identify drugs that inhibit bile acid transport. Toxicol In Vitro. 2009 doi: 10.1016/j.tiv.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Paine MF, Tidwell RR, Hall JE, Brouwer KLR. Comparison of the Hepatobiliary Disposition of Two Orally Active Pentamidine Analogues. American Association of Pharmaceutical Scientist National Meeting; Atlanta, GA. 2008. [Google Scholar]
- Yasuda K, Lan LB, Sanglard D, Furuya K, Schuetz JD, Schuetz EG. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J Pharmacol Exp Ther. 2002;303:323–32. doi: 10.1124/jpet.102.037549. [DOI] [PubMed] [Google Scholar]
- Ye ZW, Augustijns P, Annaert P. Cellular accumulation of cholylglycylamido-fluorescein in sandwich-cultured rat hepatocytes: kinetic characterization, transport mechanisms, and effect of human immunodeficiency virus protease inhibitors. Drug Metab Dispos. 2008;36:1315–21. doi: 10.1124/dmd.107.019398. [DOI] [PubMed] [Google Scholar]
- Yue W, Abe K, Brouwer KL. Knocking down breast cancer resistance protein (Bcrp) by adenoviral vector-mediated rna interference (RNAi) in sandwich-cultured rat hepatocytes: a novel tool to assess the contribution of Bcrp to drug biliary excretion. Mol Pharm. 2009;6:134–43. doi: 10.1021/mp800100e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CH, Okerholm RA, Guengerich FP. Oxidation of the antihistaminic drug terfenadine in human liver microsomes. Role of cytochrome P-450 3A(4) in N-dealkylation and C-hydroxylation. Drug Metab Dispos. 1993;21:403–9. [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM, Brouwer KL. Pharmacokinetics of 5 (and 6)-carboxy-2',7'-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther. 2003;304:801–9. doi: 10.1124/jpet.102.044107. [DOI] [PubMed] [Google Scholar]
- Zhang P, Swift B, Tian X, Brouwer KR. Optimization of Transporter Expression and Function in Sandwich-Cultured Human Hepatocytes (B-CLEAR®-HU). 13th North American ISSX Meeting; Maui, Hawaii. 2005a. [Google Scholar]
- Zhang P, Swift B, Tian X, Brouwer KR. Interspecies Variability of Transport Protein Functions in Sandwich-Cultured Rat, Dog, Monkey and Human Hepatocytes (B-CLEAR®). 13th North American ISSX Meeting; Maui, Hawaii. 2005. [Google Scholar]
- Zhang P, Tian X, Chandra P, Brouwer KL. Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol Pharmacol. 2005b;67:1334–41. doi: 10.1124/mol.104.004481. [DOI] [PubMed] [Google Scholar]
- Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717–27. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]