Abstract
Background
The AIDS Drug Assistance Program (ADAP) provides antiretroviral medications to low-income individuals with HIV infection.
Methods
A prospective cohort study of ADAP utilization, measured using medication possession ratio (MPR), was conducted during the 2008 calendar year at the University of Alabama at Birmingham 1917 HIV Clinic. Multivariable ordinal logistic regression evaluated factors associated with ADAP utilization.
Results
Among 245 patients, MPR quartiles (Q) were the following: Q1<69 percent, Q2 = 69–83 percent, Q3 = 84–93 percent, Q4>93 percent. In ordinal logistic regression, younger age (OR = 0.59 per 10 years; 95 percent CI = 0.44–0.79), nonwhite males (2.18; 1.18–4.04), lower CD4 count (2.79 for <200 cells/mm3; 1.44–5.43), and a history of alcohol abuse (2.11; 1.02–4.37) were associated with poor ADAP utilization.
Conclusions
One quarter of ADAP enrollees had MPR below 69 percent, a level well below that associated with optimal HIV treatment outcomes, indicating a need for programmatic interventions to improve ADAP utilization.
Keywords: HIV, adherence, public health
The current paradigm of HIV/AIDS care calls for a lifetime of uninterrupted antiretroviral therapy (ART) (Finzi et al. 1999; Panel on Antiretroviral Guidelines for Adults and Adolescents 2009;). For many patients, the high cost of antiretroviral medications represents a significant barrier to continuous access to ART (Kates 2004; Jing et al. 2007;). In 1990, the Ryan White Comprehensive AIDS Resources Emergency (CARE) Act (RWCA) was authorized to provide funding for the care of low-income, uninsured, or underinsured individuals living with HIV/AIDS. To help address the high cost of therapy, AIDS Drug Assistance Programs (ADAPs) were included in the RWCA. Through these programs, the federal government provides individual states with block grants to administer local ADAPs to purchase life-saving antiretroviral and other medications at no cost to patients (Kaiser Family Foundation and the National Alliance of State and Territorial AIDS Directors 2009). The 2009 reauthorization of the Ryan White HIV/AIDS Treatment Modernization Act, a legislative extension of the RWCA, allocated U.S.$1.28 billion to Part B for fiscal year 2009, with ADAPs receiving approximately 75 percent of these funds to facilitate provision of ART (Ryan White HIV/AIDS Treatment Extension Act of 2009).
In addition to continuous ART receipt, high-level adherence to medications represents another principal tenet in the blueprint for HIV treatment success (Ulett et al. 2009). By providing medication for those without coverage from public or private insurers, ADAPs have succeeded in providing a critical service to vulnerable populations living with HIV/AIDS. One-third of patients engaged in HIV care in the United States receive reliable access to ART through these programs (Kaiser Family Foundation and the National Alliance of State and Territorial AIDS Directors 2009). While ADAPs have a clear beneficial effect on the numbers of HIV-infected individuals with access to ART, the degree of programmatic utilization by enrollees (i.e., consistent medication possession) is not well described. Evaluation of ADAP utilization by enrollees is important to understanding barriers to ART persistence and adherence among program enrollees.
The primary goals of this study were the following: (1) to characterize programmatic ADAP utilization at the University of Alabama at Birmingham 1917 HIV/AIDS Clinic (1917 Clinic) and (2) determine patient factors associated with ADAP underutilization among program enrollees at the 1917 Clinic.
MATERIALS AND METHODS
Conceptual Framework
The conceptual framework of this study was grounded in an adaptation of the Behavioral Model of Health Services utilization that evaluates the interplay between environmental factors and individual predisposing factors, enabling factors and perceived needs with regard to health services utilization and treatment outcomes (Andersen 1995; Mugavero 2008; Ulett et al. 2009;). This study specifically evaluated patient predisposing factors and environmental factors that influence the behaviors of ART utilization and adherence.
Sample and Setting
Located in Birmingham, Alabama, the 1917 Clinic has provided comprehensive prevention, medical, and supportive care services for more than 7,000 HIV-infected individuals in the Southeastern United States since 1988. Currently, the clinic provides primary HIV care for over 1,700 active patients who participate in the UAB 1917 Clinic Cohort, a 100 percent quality controlled prospective clinical cohort study that has been described in detail elsewhere and has been recognized for excellence in information integrity (http://www.uab1917cliniccohort.org) (Chen et al. 2006; Mugavero et al. 2007; Willig et al. 2007;). Detailed sociodemographic, psychosocial, clinical, and pharmacy information across a wide range of domains is captured from patients receiving care at the clinic and recorded in the database. A cross-sectional study of program utilization among Alabama ADAP enrollees (patients who receive medication through ADAP) at the 1917 Clinic from January 1, 2008 through December 31, 2008 was undertaken. Patients continuously enrolled in the program by January 1, 2008 and for the entire calendar year were included in analyses. The UAB Institutional Review Board approved this study protocol.
1917 Clinic ADAP enrollees receive a 30-day supply of medications at each refill. Patients or designated surrogates sign for and pick up medications at the pharmacy colocated in the clinic. Patients who live outside the clinic's Ryan White catchment area have the option of having ADAP-funded medications mailed to them. Shipping costs are incurred by the clinic. Regardless of the method of delivery, enrollees do not receive reminders regarding medication refills and must initiate contact with the clinic pharmacy in person or by phone each time a medication refill is needed (i.e., every 30 days). Because all 1917 Clinic ADAP enrollees must use the clinic pharmacy, this setting provides an opportunity to systematically capture ADAP utilization data among all program enrollees engaged in care at the clinic. Analyses did not include patients (n = 39) who were enrolled in ADAP on January 1, 2008, but subsequently disenrolled during the study period. No formal criteria for disenrollment are in place at the clinic, and nonadherence to ART (n = 28) was the primary reason patients were dropped from ADAP during the study period.
Independent Variables
Variables were selected a priori and included patient sociodemographic information (age, sex, race, county of residence, and HIV risk factor), medical history (history of affective mental health disorder, substance abuse, alcohol abuse, hepatitis C virus, and opportunistic infections), and laboratory data (study-entry CD4 count and plasma HIV viral load [VL]). These data were measured at the start date of the study period (January 1, 2008) within a ± 90-day window.
Dependent Variable
Medication Possession Ratio (MPR): The MPR utilizes pharmacy claims data and is calculated by dividing the number of days an individual has access to medicine (i.e., has picked up a supply of medication) by the total number of days for which the medication was prescribed. The result is then multiplied by 100, providing the proportion of days during which a patient was in possession of medications during the period of observation (range 0–100 percent) (Weidle et al. 2006; Goldman et al. 2008;). Historically, MPR has been used as a measure of medication adherence. In previous studies, high MPR values have been correlated with plasma HIV VL suppression (Grossberg et al. 2004; Fairley, Permana, and Read 2005; Gross et al. 2006; Weidle et al. 2006; Nachega et al. 2007;) and improved patient survival in HIV-infected individuals (Hogg et al. 2002; Nachega et al. 2006;). However, MPR is noted to have limitations as a measure of ART adherence, including the likely overestimation of adherence because possession of medication does not equate with ingestion (Goldman et al. 2008). MPR represents an excellent measure of programmatic utilization because it fully captures patient participation in ADAP. Thus, MPR was primarily utilized as a measure of ADAP utilization and was calculated for all 1917 Clinic ADAP enrollees during the 2008 calendar year.
Statistical Analyses
Descriptive statistics were computed for all study variables to ensure assumptions of statistical tests were met. Patients were categorized into quartiles based upon ADAP utilization, as measured by MPR, during the study period. Univariate and multivariable ordinal logistic regression (proportional odds assumption test) were used to determine factors associated with ADAP underutilization; results reflect the odds of being in a lower MPR group. All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute).
RESULTS
Among 284 individuals enrolled in ADAP at the 1917 Clinic on January 1, 2008, 245 (86 percent) remained enrolled throughout the 2008 calendar year and were included in these analyses. The majority of patients were male (82 percent), nonwhite (55 percent), and had a history of affective mental health disorder (54 percent) (Table 1). Reasons for ADAP disenrollment (n = 39) included nonadherence (n = 28), obtained insurance (n = 9), medication holiday (n = 1), and death (n = 1). The 28 individuals removed from the program due to nonadherence likely represent the least adherent patients in our sample but were excluded from analyses owing to their limited enrollment time.
Table 1.
Demographics and Clinical Characteristics among ADAP Enrollees (n = 245) at the 1917 Clinic from January 1, 2008 to December 31, 2008
| Characteristic | Mean ± SD or N (%) |
|---|---|
| Age (years) | 42 ± 9 |
| Sex/race | |
| White female | 13 (5%) |
| Nonwhite female | 30 (12%) |
| White male | 96 (39%) |
| Nonwhite male | 106 (43%) |
| HIV risk factor | |
| MSM | 106 (43%) |
| Heterosexual | 65 (27%) |
| IVDU | 21 (9%) |
| Affective mental health disorder | |
| Yes | 132 (54%) |
| No | 113 (46%) |
| Substance abuse | |
| Yes | 61 (25%) |
| No | 184 (75%) |
| Alcohol abuse | |
| Yes | 33 (13%) |
| No | 212 (87%) |
| Study entry viral load value* | |
| <50 copies/mL | 128 (58%) |
| >50 copies/mL | 99 (42%) |
| Study entry CD4 value* (cells/mm3) | |
| <200 | 46 (20%) |
| 200–350 | 65 (28%) |
| >350 | 119 (52%) |
Study entry values obtained on 1/1/08 ± 90 days.
ADAP, AIDS Drug Assistance Program; IVDU, intravenous drug use; MSM, men who have sex with men.
A broad distribution of MPR was observed among study participants, with roughly one-third of patients having ART in their possession >90 percent of days in the 1-year study period according to pharmacy refill data (Figure 1A). The mean MPR was 77 percent, and the MPR quartiles were as follows: Q1<69 percent, Q2 = 69–83 percent, Q3 = 84–93 percent, and Q4>93 percent. The average age was 39 years for patients in the lowest MPR quartile compared with 45 years among those in the highest quartile. In contrast to white males, minority males were overrepresented in the lowest MPR quartile and underrepresented in the highest MPR quartile (Figure 1B).
Figure 1.
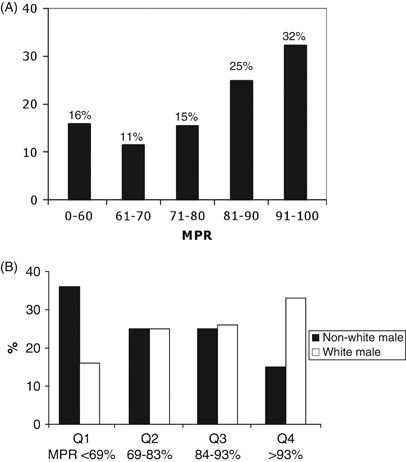
(A) Overall Medication Possession Ratio (MPR) Distribution among AIDS Drug Assistance Program (ADAP) Enrollees (n = 245) at the 1917 Clinic from January 1, 2008 to December 31, 2008. (B) MPR Quartiles of Nonwhite Males (n = 106) and White Males (n = 96) Enrolled in ADAP at the University of Alabama at Birmingham 1917 Clinic in 2008
(A) Overall distribution of calculated MPRs for study participants. Only 32 percent of patients achieved MPRs>90 percent. (B) Nonwhite males were disproportionately represented in the bottom quartile, while white males were disproportionately represented in the top quartile.Chi-square trend test. p-value was 0.002.
In multivariable ordinal logistic regression, older age (OR = 0.59 per 10 years; 95 percent CI = 0.44–0.79) was protective against ADAP underutilization. Lower CD4 count at study entry (OR = 2.79 for <200 cells/mm3; 95 percent CI = 1.44–5.43), nonwhite males (OR = 2.18; 95 percent CI = 1.18–4.04), and a history of alcohol abuse (OR = 2.11; 95 percent CI = 1.02–4.37) were associated with poor ADAP utilization (Table 2).
Table 2.
Multivariable Ordinal Logistic Regression Model of Factors Associated with Poor ADAP Utilization per MPR Quartile (n = 245) among Program Enrollees at the 1917 Clinic in 2008
| Characteristic | Unadjusted OR (95%CI) | Adjusted OR (95%CI) |
|---|---|---|
| Age (per 10 years) | 0.60 (0.47–0.78) | 0.59 (0.44–0.79) |
| Race/sex | ||
| White male | 1.0 | 1.0 |
| White female | 0.96 (0.34–2.73) | 1.09 (0.33–3.62) |
| Nonwhite female | 1.36 (0.62–2.96) | 1.65 (0.62–4.40) |
| Nonwhite male | 2.46 (1.46–4.16) | 2.18 (1.18–4.04) |
| HIV risk factor | ||
| MSM | 1.0 | 1.0 |
| Heterosexual | 1.11 (0.62–1.97) | 0.87 (0.42–1.80) |
| IVDU | 1.31 (0.55–3.17) | 2.21 (0.87–5.63) |
| Unknown | 2.3 (1.23–4.28) | 2.01 (1.04–3.88) |
| Affective mental health disorder | ||
| No | 1.0 | 1.0 |
| Yes | 1.09 (0.68–1.74) | 1.47 (0.89–2.43) |
| Substance abuse | ||
| No | 1.0 | 1.0 |
| Yes | 1.08 (0.63–1.85) | 0.82 (0.45–1.48) |
| Alcohol abuse | ||
| No | 1.0 | 1.0 |
| Yes | 1.77 (0.90–3.51) | 2.11 (1.02–4.37) |
| County of residence | ||
| Jefferson County+RWCA | 1.0 | 1.0 |
| Outside RWCA | 1.83 (1.12–2.99) | 1.27 (0.74–2.19) |
| CD4 value (cells/mm3) | ||
| <200 | 2.51 (1.35–4.69) | 2.79 (1.44–5.43) |
| 200–350 | 1.42 (0.82–2.44) | 1.34 (0.76–2.35) |
| ≥350 | 1.0 | 1.0 |
Note. Bold values are statistically significant (p<0.05).
ADAP, AIDS Drug Assistance Program; IVDU, intravenous drug users; MPR, medication possession ratio; MSM, men who have sex with men; RWCA, Ryan White catchment area.
DISCUSSION
Over U.S.$1 billion is allocated to Ryan White ADAPs annually for the purpose of medication acquisition for vulnerable, socioeconomically disadvantaged individuals needing HIV/AIDS therapy. Despite the availability of free antiretroviral medications, one quarter of 1917 Clinic ADAP enrollees had medication possession below 69 percent, representing a level well below that associated with optimal treatment outcomes (Paterson et al. 2000). As a payer of last resort for vulnerable populations living with HIV, ADAP is well positioned to continue to have a large impact on public health and patient outcomes. Yet only 77 percent of medications reached the intended ADAP enrollees at the 1917 Clinic in 2008. The remaining medications, their cost, and the efforts involved in making them consistently available all represent significant opportunities to enhance program implementation and administration.
Currently, 97 percent of the ADAP budget is used to purchase prescription drugs (Kaiser Family Foundation and the National Alliance of State and Territorial AIDS Directors 2009). After basic administrative costs are included, there is no funding for programmatic infrastructure to facilitate delivery of medications to their intended recipients. In 2008, 23 percent of the medications (at an estimated cost of U.S.$500,000) delivered to the 1917 Clinic through ADAP did not reach the intended program enrollees. Therefore, the provision of free antiretroviral medications alone is not sufficient. Investment into the requisite infrastructures and resources informed by research and local needs assessments is critical to ensuring maximal ADAP utilization.
The median MPR was 84 percent among those who remained in the program, which at first glance seems encouraging owing to the generally accepted adherence threshold of 80 percent for other chronic conditions (Cramer et al. 2008; Sherman et al. 2009;). However, utilization at such levels in HIV-infected individuals has important medical and public health implications. Extremely high levels of medication adherence are required in HIV infection to achieve and sustain VL suppression. Suboptimal medication possession at levels below 84 percent, as observed in half of study patients, can play an important role in virologic failure, development of resistance to therapy, and ultimately the possibility of transmission of resistant virus (Montaner et al. 2006; Dieffenbach and Fauci 2009;). Indeed, recent emphasis on a “test and treat” approach to HIV prevention is predicated upon consistent receipt and adherence to ART to promote sustained virologic suppression.
Difficulties with utilization and adherence to HIV therapy are not unique to ADAP patients. One study of patients receiving antiretroviral medications through the Veteran's Administration demonstrated similar levels of utilization (overall adherence of 75 percent) to those seen among ADAP patients in the current study (Paterson et al. 2000). We propose that system and individual focused ART medication utilization and adherence interventions within ADAP could serve as meaningful pilot programs that may have application in other settings. Because funding for such interventions would limit the already stressed budgets designed to provide medications to patients in need, cost-effectiveness analyses evaluating the benefits of a specific interventions at the individual and public health levels against the potential costs would be essential.
Black males at our clinic were disproportionately represented in the lowest quartile of program utilization (Figure 1B) and were at higher risk for ADAP underutilization. Poor rates of ADAP utilization are of particular concern among racial/ethnic minority males, a group that continues to bear a disproportionate burden of new HIV infections (Center for Disease Control 2003, 2007). Previous studies document worse ART use in racial/ethnic minority patients when compared with whites, though cross-sectional, dichotomous measures of ART use were often used (Ammassari et al. 2002; Asad et al. 2008;). Previous studies point to medication plan benefit limits, mistrust of government, and stigma related to HIV as factors negatively impacting programmatic adherence (Morin et al. 2002).
Younger age was also associated with reduced ADAP utilization, with a mean age of 39 years in the lowest quartile and 45 years in the highest. The reason for increased program utilization with older age is unclear, but some studies suggest due to increased familiarity with taking medications, consistent utilization requiring fewer lifestyle changes for older individuals (Barclay et al. 2007). Alcohol abuse was similarly associated with poor ADAP programmatic utilization. Alcohol abuse is common among individuals with HIV (Chander, Himelhoch, and Moore 2006) and is recognized as a barrier to optimal ART use in numerous studies (Gordillo et al. 1999; Ammassari et al. 2002; Conen et al. 2009;). On a positive note, other studies report that patients who quit using alcohol and are actively engaged in alcohol abuse treatment demonstrate improved rates of ART utilization that approach that of the general population (Hicks et al. 2007). Even recent abstinence from alcohol significantly improves ART use (Samet et al. 2004). Accordingly, focus on early identification and treatment of alcohol abuse in HIV primary care settings may favorably impact ADAP utilization.
Our findings should be interpreted with respect to the limitations of our study. As a single HIV cohort study with a modest sample size, our findings may not be generalizable to other national settings, though our analysis may provide insights applicable to such settings. As with all observational studies, we were able to identify associations but cannot attribute causality. While we controlled for potential confounders using multivariable models, there is potential for unmeasured confounding inherent to observational studies. Further, several variables of interest (e.g., educational level) were not systematically available for analyses. However, to our knowledge this study is the first comprehensive evaluation of ADAP utilization in a contemporary HIV clinic setting and provides a first look at an issue that is likely to be shared in similar treatment settings across the United States.
In conclusion, our findings highlight that ADAP utilization falls alarmingly short at our site, particularly among younger patients, racial/ethnic minority males, and those with alcohol abuse disorders. We suspect these findings are not unique to our setting but rather beset vulnerable ADAP enrollees throughout the United States. Further study into the structural and individual barriers and facilitators to ADAP utilization is needed to inform interventions and policy reform needed to maximize the effectiveness of ADAP. The provision of free ART to low-income individuals through the Ryan White ADAP was a transformative moment in the care of people living with HIV/AIDS in the United States. While ADAP continues to have a tremendous impact in providing vulnerable patients with needed antiretroviral medications, better understanding of both programmatic and patient factors associated with suboptimal ADAP utilization may improve delivery of life-saving medications, enhance the effective use of federal funds, improve clinical outcomes, and benefit public health by ultimately decreasing HIV transmission.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: Data presented in part at 4th International Conference on HIV Treatment Adherence, Miami, FL, April 5–7, 2009.
This study was supported by a Lister Hill Policy grant from the University of Alabama at Birmingham and by grant number K23MH082641 from the National Institute of Mental Health (MJM). The UAB 1917 HIV/AIDS Clinic Cohort Observational Database project receives financial support from the following: UAB Center for AIDS Research (grant P30-AI27767), CFAR-Network of Integrated Clinical Systems, CNICS (grant 1 R24 AI067039-1), and the Mary Fisher CARE Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, or any other agency providing support for this study.
Disclosures: Noah Godwin, Christa R. Nevin, Hui-Yi Lin, Jeroan Allison, Kathy Gaddis, Jennifer Peterson, and James L. Raper have no conflicts of interest related to this manuscript. James H. Willig has received research funding and/or consulted for Bristol-Myers Squibb, Gilead, Merck, and Tibotec. Michael S. Saag has received research funding and/or consulted for Adrea Pharmaceuticals, Avexa, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram Biosciences, Panacos, Pfizer, Progenics, Roche, Serono, Tanox, Tibotec, Trimeris, and Vertex. Michael J. Mugavero has received research funding and/or consulted for Tibotec Therapeutics, Bristol-Myers Squibb, and Gilead.
Disclaimers: None.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, Vecchiet J, D'Arminio Monforte A, Wu AW, Antinori A, and the AdICoNA Study Group Correlates and Predictors of Adherence to Highly Active Antiretroviral Therapy: Overview of Published Literature. Journal of Acquired Immune Deficiency Syndrome. 2002;31(suppl 3):S123–7. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- Andersen RM. Revisiting the Behavioral Model and Access to Medical Care: Does It Matter? Journal of Health and Social Behavior. 1995;36:1–10. [PubMed] [Google Scholar]
- Asad S, Hulgan T, Raffanti SP, Daugherty J, Ray W, Sterling TR. Sociodemographic Factors Predict Early Discontinuation of HIV Non-Nucleoside Reverse Transcriptase Inhibitors and Protease Inhibitors. Journal of the National Medical Association. 2008;100:1417–24. doi: 10.1016/s0027-9684(15)31541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, Levine AJ, Durvasula RS. Age-Associated Predictors of Medication Adherence in HIV-Positive Adults: Health Beliefs, Self-Efficacy, and Neurocognitive Status. Health Psychology. 2007;26:40–9. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2009. HIV/AIDS Surveillance Report, 2007. Vol. 19. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Chander G, Himelhoch S, Moore RD. Substance Abuse and Psychiatric Disorders in HIV-Positive Patients: Epidemiology and Impact on Antiretroviral Therapy. Drugs. 2006;66:769–89. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- Chen RY, Accortt NA, Westfall AO, Mugavero MJ, Raper JL, Cloud GA, Stone BK, Carter J, Call S, Pisu M, Allison J, Saag MS. Distribution of Health Care Expenditures for HIV-Infected Patients. Clinical Infectious Diseases. 2006;42:1003–10. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- Conen A, Fehr J, Glass TR, Furrer H, Weber R, Vernazza P, Hirschel B, Cavassini M, Bernasconi E, Bucher HC, Battegay M the Swiss HIV Cohort Study. Self-Reported Alcohol Consumption and Its Association with Adherence and Outcome of Antiretroviral Therapy in the Swiss HIV Cohort Study. Antiviral Therapy. 2009;14:349–57. [PubMed] [Google Scholar]
- Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The Significance of Compliance and Persistence in the Treatment of Diabetes, Hypertension and Dyslipidaemia: A Review. International Journal of Clinical Practice. 2008;62:76–87. doi: 10.1111/j.1742-1241.2007.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieffenbach CW, Fauci AS. Universal Voluntary Testing and Treatment for Prevention of HIV Transmission. Journal of the American Medical Association. 2009;301:2380–2. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- Fairley CK, Permana A, Read TR. Long-Term Utility of Measuring Adherence by Self-Report Compared with Pharmacy Record in a Routine Clinic Setting. HIV Medicine. 2005;6:366–9. doi: 10.1111/j.1468-1293.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, Pierson T, Smith K, Lisziewicz J, Lori F, Flexner C, Quinn TC, Chaisson RE, Rosenberg E, Walker B, Gange S, Gallant J, Siliciano RF. Latent Infection of CD4+T Cells Provides a Mechanism for Lifelong Persistence of HIV-1, Even in Patients on Effective Combination Therapy. Nature Medicine. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- Goldman JD, Cantrell RA, Mulenga LB, Tambatamba BC, Reid SE, Levy LW, Limbada M, Taylor A, Saag MS, Vermund SH, Stringer JS, Chi BH. Simple Adherence Assessments to Predict Virologic Failure among HIV-Infected Adults with Discordant Immunologic and Clinical Responses to Antiretroviral Therapy. AIDS Research and Human Retroviruses. 2008;24:1031–5. doi: 10.1089/aid.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and Psychological Variables Influencing Adherence to Antiretroviral Therapy. AIDS. 1999;13:1763–9. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- Gross R, Yip B, Lo Re V, III, Wood E, Alexander CS, Harrigan PR, Bangsberg DR, Montaner JS, Hogg RS. A Simple, Dynamic Measure of Antiretroviral Therapy Adherence Predicts Failure to Maintain HIV-1 Suppression. Journal of Infectious Diseases. 2006;194:1108–14. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- Grossberg R, Zhang Y, Gross R. A Time-to-Prescription-Refill Measure of Antiretroviral Adherence Predicted Changes in Viral Load in HIV. Journal of Clinical Epidemiology. 2004;57:1107–10. doi: 10.1016/j.jclinepi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, Korthuis PT, Hellinger J, Gaist P, Gebo KA, the HIV Research Network The Impact of Illicit Drug Use and Substance Abuse Treatment on Adherence to HAART. AIDS Care. 2007;19:1134–40. doi: 10.1080/09540120701351888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, Montaner JS. Intermittent Use of Triple-Combination Therapy Is Predictive of Mortality at Baseline and after 1 Year of Follow-Up. AIDS. 2002;16:1051–8. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- H.R. 3792—111th Congress: Ryan White HIV/AIDS Treatment Extension Act of 2009. 2009. In GovTrack.us (database of federal legislation). Retrieved January 20, 2010, from http://www.govtrack.us/congress/bill.xpd?bill=h111-3792.
- Jing Y, Klein P, Kelton CM, Li X, Guo JJ. Utilization and Spending Trends for Antiretroviral Medications in the U.S. Medicaid Program from 1991 to 2005. AIDS Research and Therapy. 2007;4:22. doi: 10.1186/1742-6405-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation and the National Alliance of State and Territorial AIDS Directors. 2009. National ADAP Monitoring Project Annual Report.
- Kates J. Financing HIV/AIDS Care: A Quilt with Many Holes. 2004. (HIV/AIDS policy issue brief; no. 1607-02). Menlo Park, CA: Henry J. Kaiser Family Foundation. [accessed on December 27, 2010]. Available at http://www.kff.org/hivaids/upload/Financing-HIV-AIDS-Care-A-Quilt-with-Many-Holes.pdf.
- Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, Harrigan PR. The Case for Expanding Access to Highly Active Antiretroviral Therapy to Curb the Growth of the HIV Epidemic. Lancet. 2006;368:531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- Morin SF, Sengupta S, Cozen M, Richards TA, Shriver MD, Palacio H, Kahn JG. Responding to Racial and Ethnic Disparities in Use of HIV Drugs: Analysis of State Policies. Public Health Reports. 2002;117:263–72. doi: 10.1016/S0033-3549(04)50160-7. discussion 231–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ. Improving Engagement in HIV Care: What Can We Do? Topics in HIV Medicine. 2008;16:156–61. [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Allison JJ, Willig JH, Chang PW, Marler M, Raper JL, Schumacher JE, Pisu M, Saag MS. Failure to Establish HIV Care: Characterizing the “No Show” Phenomenon. Clinical Infectious Diseases. 2007;45:127–30. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, Chaisson RE, Maartens G. Adherence to Highly Active Antiretroviral Therapy Assessed by Pharmacy Claims Predicts Survival in HIV-Infected South African Adults. Journal of Acquired Immune Deficiency Syndrome. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to Nonnucleoside Reverse Transcriptase Inhibitor-Based HIV Therapy and Virologic Outcomes. Annals of Internal Medicine. 2007;146:564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-infected Adults and Adolescents. Department of Health and Human Services; 2009. [accessed on December 27, 2010]. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to Protease Inhibitor Therapy and Outcomes in Patients with HIV Infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol Consumption and Antiretroviral Adherence among HIV-Infected Persons with Alcohol Problems. Alcoholism: Clinical and Experimental Research. 2004;28:572–7. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Sherman BW, Frazee SG, Fabius RJ, Broome RA, Manfred JR, Davis JC. Impact of Workplace Health Services on Adherence to Chronic Medications. American Journal of Managed Care. 2009;15:e53–9. [PubMed] [Google Scholar]
- Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, Chatham A, Raper JL, Saag MS, Mugavero MJ. The Therapeutic Implications of Timely Linkage and Early Retention in HIV Care. AIDS Patient Care STDS. 2009;23:41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidle PJ, Wamai N, Solberg P, Liechty C, Sendagala S, Were W, Mermin J, Buchacz K, Behumbiize P, Ransom RL, Bunnell R. Adherence to Antiretroviral Therapy in a Home-Based AIDS Care Programme in Rural Uganda. Lancet. 2006;368:1587–94. doi: 10.1016/S0140-6736(06)69118-6. [DOI] [PubMed] [Google Scholar]
- Willig JH, Westfall AO, Allison J, Van Wagoner N, Chang PW, Raper J, Saag MS, Mugavero MJ. Nucleoside Reverse-Transcriptase Inhibitor Dosing Errors in an Outpatient HIV Clinic in the Electronic Medical Record Era. Clinical Infectious Diseases. 2007;45:658–61. doi: 10.1086/520653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


