Abstract
Gene therapy has demonstrated the protective potential of a variety of genes against stroke. However, conventional gene therapy vectors are limited due to the inability to temporally control their expression, which can sometimes lead to deleterious side effects. Thus, an inducible vector that can be temporally controlled and activated by the insult itself would be advantageous. Using hypoxia responsive elements (HRE) and antioxidant responsive elements (ARE), we have constructed an insult-inducible vector activated by hypoxia and reactive oxygen species (ROS). In COS7 cells, the inducible ARE−HRE-luciferase vectors are highly activated by oxygen deprivation, hydrogen peroxide treatment, and the ROS-induced transcription factor NF-E2-related factor 2 (Nrf2). Using a defective herpes virus, the neuroprotective potential of this inducible vector was tested by over-expressing the transcription factor Nrf2. In primary cortical cultures, expression of the inducible ARE−HRE–Nrf2 protects against oxygen glucose deprivation, similar to that afforded by the constitutively expressed Nrf2. This ARE+HRE vector system is advantageous in that it allows the expression of a transgene to be activated not only during hypoxia but also maintained after reperfusion, thus prolonging the transgene expression during an ischemic insult. This insult-inducible vector system will be a valuable gene therapy tool for activating therapeutic/protective genes in cerebrovascular diseases.
Keywords: Insult-inducible, Gene therapy, Hypoxia, HIF1, Nrf2, Reactive oxygen species
Introduction
Stroke is a major acute neurological insult that disrupts brain function and causes neuron death. Ischemic injury can be categorized into two main phases, namely an energy-deficient hypoxic–ischemic phase and a reperfusion phase when blood and oxygen return. During the hypoxic–ischemic phase, the oxygen-sensitive hypoxia-inducible factor 1α (HIF1α) is stabilized by hypoxia and heterodimerizes with the constitutively expressed HIF1β [1–3]. The HIF1α/β heterodimer binds to hypoxia-responsive elements (HREs) to activate downstream genes such as vascular endothelial growth factor (VEGF), erythropoietin, nitric oxide synthase, and glycolytic enzymes [1, 2, 4]; this can be viewed as an adaptive defense. The initial energy crisis leads to the rapid release of glutamate in the extracellular synapse and causes increase in intracellular Ca2+, which in turn triggers the generation of reactive oxygen species (ROS) that crosslink and damage lipids, proteins, and nucleic acids [5, 6]. The bulk of ROS generation occurs during reperfusion [7], and this induces a second type of adaptive defense, namely the release of transcription factor NF-E2-related factor 2 (Nrf2) from Keap1 (negative regulator of Nrf2). The released Nrf2 subsequently activates the antioxidant responsive elements (AREs) to drive expression of a number of phase II detoxification enzymes and antioxidants [8–10].
Currently, the only United States approved therapy for the treatment of stroke is tissue plasminogen activator, which has a narrow 3-h time window and can result in secondary intracranial hemorrhage [11, 12]. Gene therapy is an exciting alternative therapeutic strategy that has demonstrated the protective potential of a variety of genes against the ischemic cascade, including genes that target the initial energy crisis (glucose transporter), antioxidant genes (catalase and glutathione (GSH) peroxidase), and anti-apoptotic gene (Bcl2) [6, 13–15]. Gene transfer of neurotrophic factors such as glial-derived neurotrophic factor, nerve growth factor, and brain-derived neurotrophic factor can also promote neuron survival following stroke [16, 17]. Conventional gene therapy vectors are driven by a constitutive promoter, whose expression is uncontrolled and can sometimes cause unexpected side effects. For example, over-expression of VEGF promotes angiogenesis and enhances survival in cerebral ischemia [18–20]; however, uncontrolled VEGF expression can result in hemangioma or tumor growth [18, 21]. One solution is to design an inducible vector such that the transgene expression can be temporally controlled and activated by the insult itself. An ideal insult-inducible vector should possess low basal transgene expression to minimize side effects and high insult-induced expression for adequate protection against ischemic injury. Previously, we have demonstrated the feasibility of insult-inducible vectors using glucocorticoid-response elements that are activated by stress [22]. Others have also utilized hypoxia-response elements (HREs) that are activated by HIF1α [19, 23, 24]. However, HIF1α has a short intracellular half-life [25, 26]; thus, activation of HREs is maximally active only during the initial hypoxic phase, and this activation is not well maintained into the post-reperfusion period.
In this study, we have constructed an insult-inducible vector that can be activated in both phases of the ischemic injury, namely the initial hypoxia–ischemia phase and the post-reperfusion period. This allows for prolonged inducible expression throughout the insult. This vector contains multiple copies of HREs and AREs, which are activated by HIF1α and by ROS generated during hypoxia–ischemia and reperfusion, respectively. We tested the inducibility of these vectors after oxygen deprivation and hydrogen peroxide treatment. Using primary cortical cultures, we examined the neuroprotective potential of the insult-inducible vector against oxygen glucose deprivation (OGD) by inclusion of the Nrf2 gene in a defective herpes virus (HSV).
Materials and Methods
Plasmid Construction
Oligonucleotides spanning the VEGF HREs (CCACAGTGCATACGTGGGCTCCAACAGGTCCTCTT) were synthesized and annealed, and multiple copies of HREs were cloned into a pGL3 vector (Promega) containing a minimal cytomegalovirus (CMV) promoter driving the luciferase gene. AREs (TAGCTTGGAAATGACATTGCTAATGGTGACAAAGCAACTTT) from the glutathione S-transferase were also cloned in a similar manner, and either 1XARE, 2XAREs, or 3XAREs were cloned into the 3XHREs vector, and 3XAREs were cloned into the 6XHREs or 9XHREs vectors. Mouse pEF-Nrf2 plasmid was kindly provided by Dr. Jawed Alam (Louisiana State University Health Sciences Center). The coding region of the mouse Nrf2 was cloned using PCR with these primers: forward 5′-ATGATGGACTTGGAGTTGCC-3′ and reverse 5′-CCGAACCTAGTTTTTCTTTGTATCTGG-3′. The Nrf2 PCR product was cloned into a replication defective HSV backbone obtained from Dr. Howard Federoff (Georgetown University).
Cell Culture
For virus production, E5 and Vero cells (CCL81, ATCC, African green monkey kidney fibroblasts) were grown in Dulbecco’s modified Eagle’s media (DMEM, Invitrogen) with 10% NuSerum (Collaborative Research) and 1% penicillin/streptomycin (pen/strep). For the luciferase assay and Western blot studies, COS7 cells (CRL1651, ATCC, African green monkey kidney fibroblasts) were grown in DMEM with 10% fetal bovine serum (Invitrogen) and 1% pen/strep. Cells were grown in 37°C incubators containing 5% CO2.
Primary Cortical Cultures
Primary cortical cultures were obtained from E18 rat embryos as described previously [27]. Cells were plated into poly-d-lysine-treated 96-well plates at a density of 60,000 cells per well and grown in minimum essential media (Invitrogen) with 10% horse serum (HyClone), TC mix (glucose and l-glutamine), and 1% pen/strep. Experiments were done on days 10–12. These mixed cultures are typically 20–30% neurons and 70–80% glia.
Generation of HSV Amplicons
We use herpes simplex virus (HSV)-based vector to express mouse Nrf2. Protocols used for the generation of HSV amplicons have been previously described [28]. Briefly, E5 cells were transfected with plasmids expressing either the inducible Nrf2 (HSV-3A6H-Nrf2), the constitutive Nrf2 (HSV-Nrf2), or control vector expressing GFP alone (HSV-GFP), using Lipofectamine (Invitrogen). Sixteen hours after transfection, E5 cells were superinfected with the helper virus d120 (multiplicity of infection=0.03). The cells were harvested 56 h later, when the cytopathic effect reached 100%. Viruses were then extracted, concentrated, and purified. The ratio of vector to helper virus ratio was 1:1.
Luciferase Assay
For inducibility studies, these pGL3-luciferase vectors were tested in COS7 cells (3H, 6H, 9H, 1A3H, 2A3H, 3A3H, 3A6H, 3A9H, or basic). Cells were transfected with these constructs in 48-well culture plates using Lipofectamine 2000 (Invitrogen), according to manufacturer’s instructions. Cell lysates were collected after 24 h of hypoxia or hydrogen peroxide treatment (from 250 to 500 µM), using the Luciferase Cell Culture Lysis Reagent (Promega). For co-transfection studies with Nrf2, pEF-Nrf2 was co-transfected with the various inducible vectors and cell lysates were collected 24 h after transfection. Luciferase activities were measured using Luciferase Assay System (Promega) and a luminometer (UV Max kinetic microplate reader, Molecular Devices).
Western Blot
COS7 cells in 6-well plates were transfected with the following vectors using Lipofectamine 2000: HSV-GFP, HSV-6H-GFP, HSV-3A3H-GFP, HSV-3A6H-GFP, or HSV-3A9H-GFP. Cells were exposed to 24 h of oxygen deprivation by placement in a modular hypoxia chamber. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 10% gels containing 0.1% SDS and then transferred to immobilon-P polyvinylidene difluoride (PVDF) membrane for Western analysis by a Bio-Rad Trans-blot System. The membrane was probed with anti-GFP (sc9996, 1:1000, Santa Cruz Biotechnology) or anti-actin (A5060, 1:3000, Sigma). The protein level of actin was examined to ensure equal protein loading. The antibody reactivity was detected by enhanced chemiluminescent substrate (Amersham Pharmacia Biosciences).
Oxygen Glucose Deprivation and ABTS-ELISA Assay
Twenty-four hours before oxygen glucose deprivation (OGD), cultures were infected with HSV viruses expressing either the reporter gene, green fluorescent protein (HSV-GFP) or constitutive HSV-Nrf2 or inducible HSV-3A6H-GFP or inducible HSV-3A6H-Nrf2 vector (20000 active virions per well). On the day of OGD, cultures were washed once with balanced salt solution (without glucose) to remove trace amounts of glucose, and hypoxia was induced by placing the cultures in a modular hypoxic chamber for 6 h. Control cultures were treated the same way except placement under normoxia conditions and maintenance of normal glucose levels (5.5 mM) in the media. After 6 h of OGD, cells were reperfused with 5.5 mM glucose and returned to normoxic conditions. After cultures were reperfused for 24 h, cells were fixed with cold methanol followed by 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)-ELISA assay, described previously [27]. Briefly, cells were incubated with a neuron-specific marker microtubule-associated protein 2 (MAP2, M4403, Sigma), followed by incubation with a biotinylated anti-mouse antibody. A VECTASTAIN ABC standard kit (Vector labs, PK-6100) and an ABTS Peroxidase Substrate Kit (Vector labs, SK-4500) were then used to develop the colorimetric reaction, and the survival of cortical neurons was quantitated using a microplate reader at 405 nm. Percentage of neuron survival was determined by dividing the absorbance values of each group over GFP normal.
Immunocytochemistry
A separate group of cortical culture was treated with the same OGD insult described above. Cells were fixed with 4% paraformaldehyde and then stained with MAP2 followed by an Alexa 590-conjugated goat-anti-mouse secondary antibody to examine MAP2 staining under the fluorescent microscope. Pictures were taken using the Metamorph program, converted to grayscale, and exported to Photoshop for preparation into figures.
Statistics and Data Analysis
For all the studies, one-way or two-way ANOVA was used to examine statistical differences, followed by Bonferroni’s posthoc test. All statistics were performed using the Prism 5 program.
Results
Oxygen Deprivation Activates the Insult-Inducible Vectors
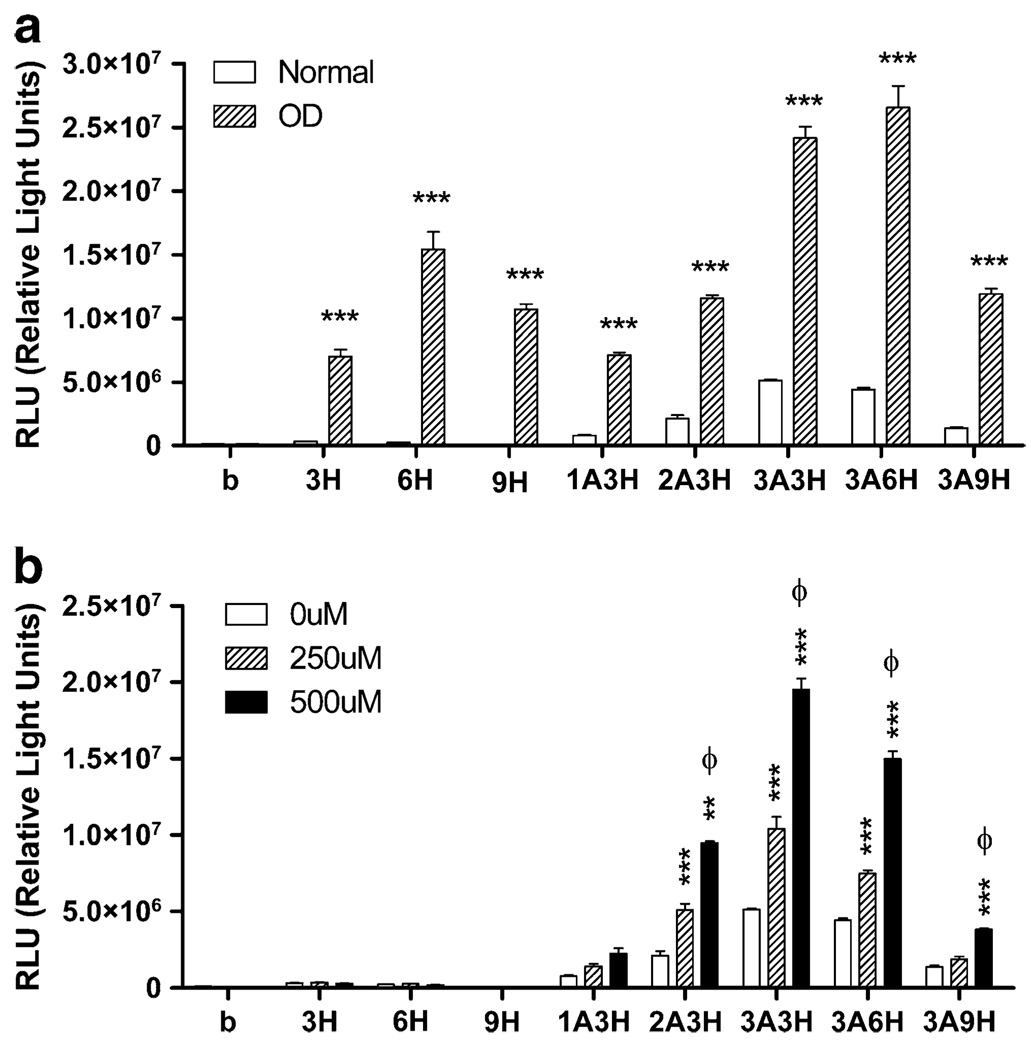
Several combinations of insult-inducible cassettes were cloned into a pGL3 luciferase reporter vector (Fig. 1a). Using a quantitative luciferase reporter assay, we tested whether hypoxia can activate these vectors. Inducible vectors were first transfected in COS7 cells, followed by exposure to 24 h of oxygen deprivation (OD). The expressions of both the vectors containing HREs alone or vectors containing ARE+HRE vectors were induced by OD (Fig. 2a). In HRE-alone vectors (containing 3, 6, or 9 copies of the HREs), basal expression was very low, and OD significantly increased signal (p<0.001), with the greatest effect in 6H vector (Fig. 2a). Increasing numbers of HRE did not result in a copy-dependent increase in the inducibility, as 9H exhibited lower luciferase activity. However, addition of the AREs greatly enhanced the inducibility, with 3A6H (i.e., 3 copies of the ARE and 6 of the HRE) exhibiting the highest luciferase activity after OD (Fig. 2a).
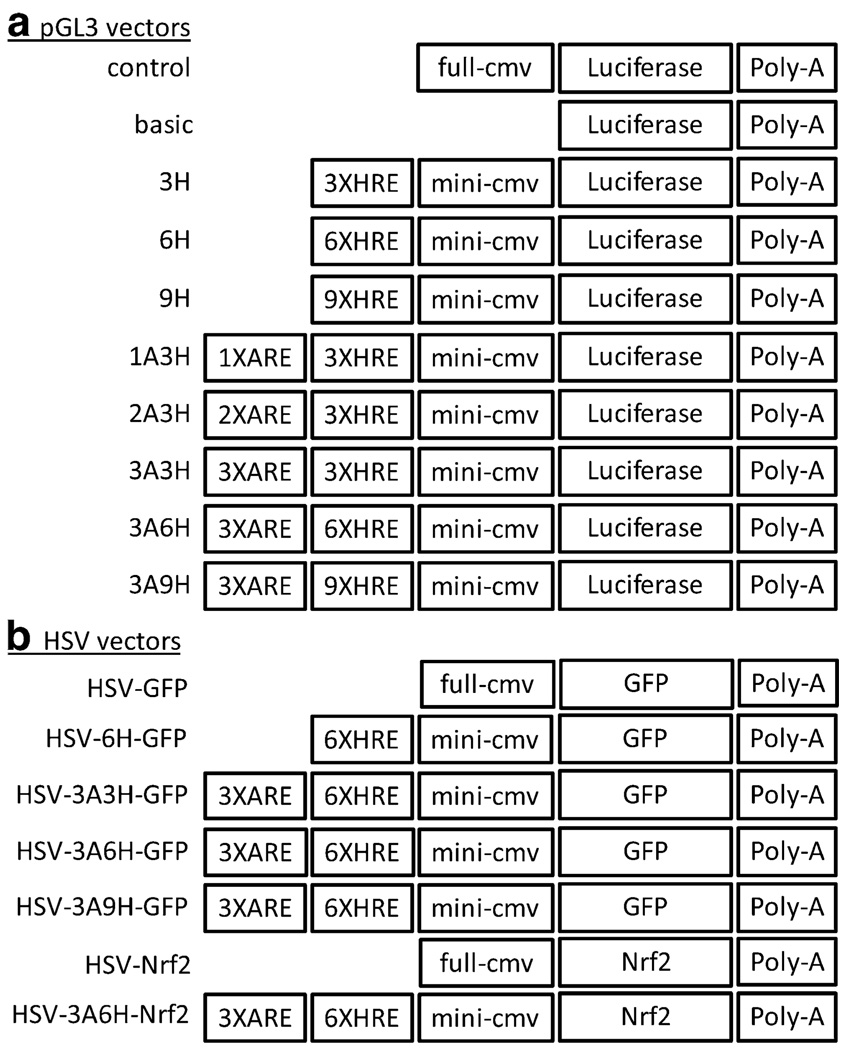
Fig. 1.
Constructs of inducible promoters in pGL3-luciferase vector and HSV vector. Various combinations of HREs and AREs were first cloned into pGL3-luciferase vector to test for their insult inducibility (a). The inducible cassette was subsequently cloned into the HSV vector for neuroprotection studies (b)
Fig. 2.
Inducible pGL3-luciferase vectors are activated by hypoxia and ROS. a HREs alone vectors (3H, 6H, 9H), ARE+HRE vectors (1A3H, 2A3H, 3A3H, 3A6H, 3A9H) and basic control vector (b, promoterless) were transfected into COS7 cells and exposed to hypoxia (24 h of OD). Luciferase assay showed that hypoxic condition significantly increased luciferase activity in all inducible vectors. ***p<0.001, two-way ANOVA with Bonferroni’s posthoc test indicates significant difference between normal and OD condition in each vector group, n=4 per group. b Same inducible cassettes were transfected into COS7 cells and exposed to ROS for 24 h (hydrogen peroxide, 250 or 500 uM). Luciferase assay showed that hydrogen peroxide treatment significantly increased luciferase activity in ARE-containing vectors. Two-way ANOVA with Bonferroni’s posthoc test indicates significant difference between 0 and 250 uM groups (**p<0.01, ***p<0.001) and between 250 and 500 uM (φ<0.001), n=4 per group
Hydrogen Peroxide Treatment Dose-Dependently Activates the Insult-Inducible Vectors
We next tested whether these inducible vectors can be activated by hydrogen peroxide (H2O2), a form of ROS. As would be expected, hydrogen peroxide treatment (250 and 500 uM) only activated ARE-containing vectors and had no effect on vectors with HREs alone. Inducibility was observed in 1A3H vector, and increasing copies of ARE led to higher luciferase activity (Fig. 2b). The inducibility was the greatest with 3A3H and decreased when there were more HREs (3A6H and 3A9H). Hydrogen peroxide treatment (250 and 500 uM) caused a dose-dependent increase in the inducibility in most of the ARE-containing inducible vectors (Fig. 2b).
Nrf2 Specifically and Potently Activates ARE-Containing Inducible Vectors
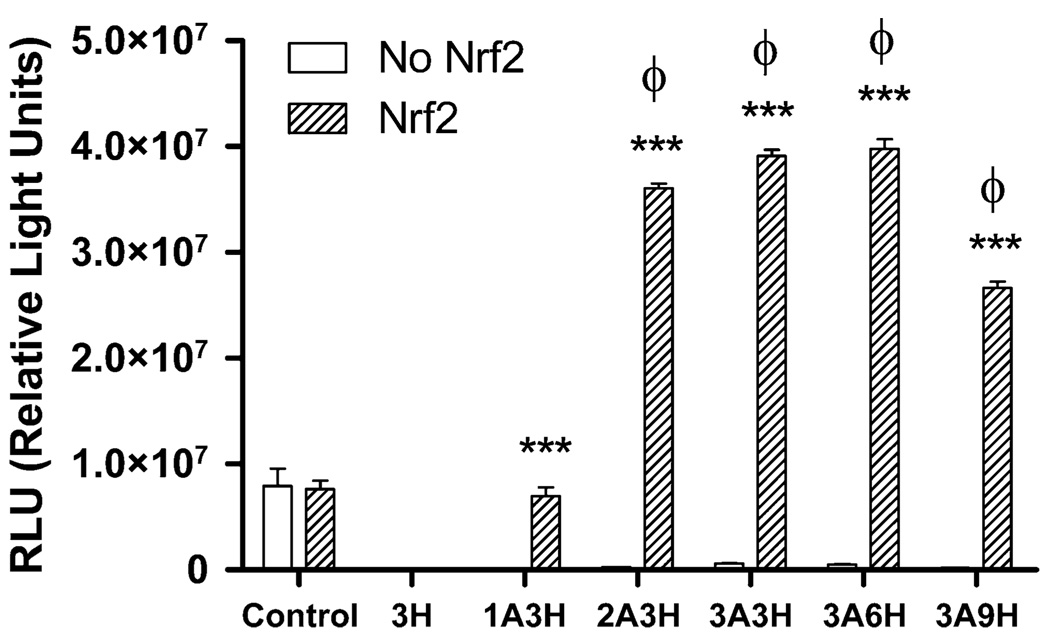
Nrf2 is a transcription factor activated by ROS [8–10]. To test the specificity and the potency of Nrf2 on our insult-inducible vectors, COS7 cells were transfected with both Nrf2 and the insult-inducible vectors. Co-transfection of Nrf2 caused a potent inducibility in all but the 1A3H vector. The highest inducibility was observed in 3A6H vector (Fig. 3); activation was ~400% higher than control vector (driven by constitutive CMV promoter). Nrf2 did not activate vectors with HREs alone. This indicates that Nrf2 can specifically activate the ARE promoters.
Fig. 3.
Nrf2 specifically activates ARE-containing inducible pGL3-luciferase vectors. ARE–HRE vectors (1A3H, 2A3H, 3A3H, 3A6H, 3A9H), HRE- alone vector (3H), and constitutive control vector were co-transfected with a plasmid expressing Nrf2 (pEF-Nrf2). Luciferase assay showed that Nrf2 potently activates ARE-containing vectors, especially with two or more copies of AREs. ***p<0.001, two-way ANOVA indicates a significant difference between Nrf2 and no Nrf2 groups. φ p<0.001, one-way ANOVA indicates a significant difference between inducible vectors and control vector in the Nrf2 group, n=6 per group
Activation of Insult-Inducible Cassette in HSV Vectors
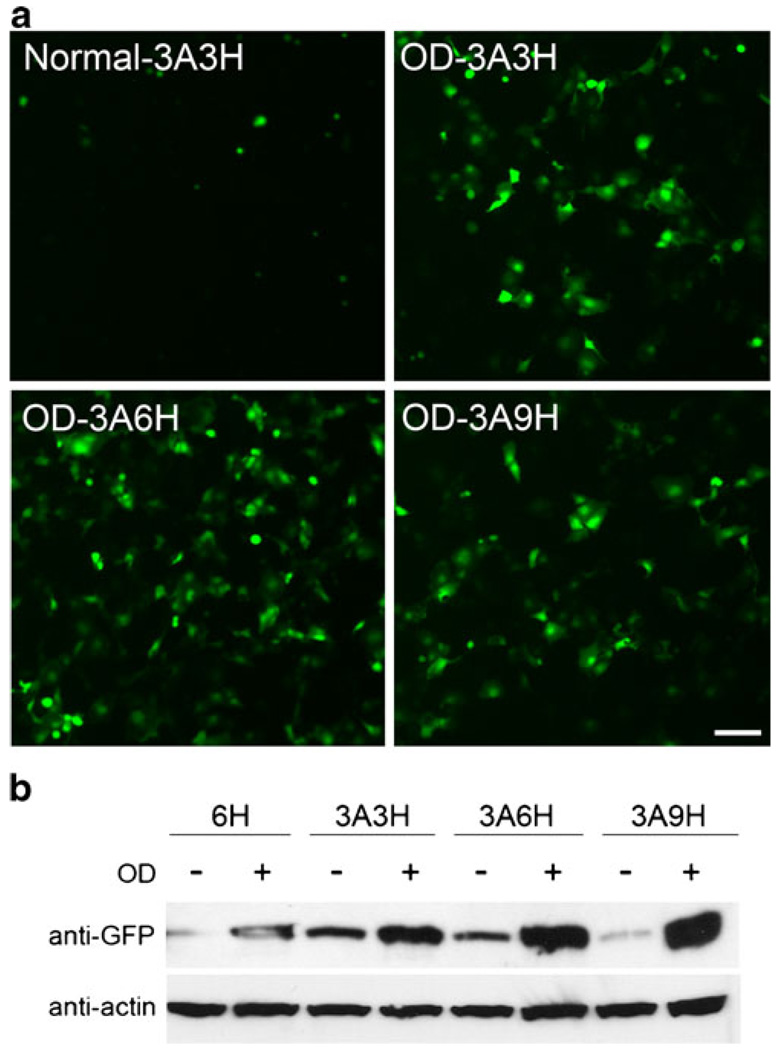
Next we cloned the inducible cassette (6H, 3A3H, 3A6H, or 3A9H) into a defective HSV backbone driving the green fluorescent protein (GFP) (Fig. 1b). We first tested whether the insult inducibility is maintained in the HSV backbone by examining GFP expression after 24 h of oxygen deprivation in COS7 cells. Figure 4a shows representative images of inducible HSV-3A3H, HSV-3A6H, and HSV-3A9H vectors driving GFP in normal and oxygen-deprived COS7 cells. Western blot analysis with anti-GFP antibody showed that these HSV inducible vectors can be highly induced after oxygen deprivation, especially in ARE+HRE vectors (Fig. 4b). The inducibility profile is very similar to what we observed in the pGL3-luciferase backbone (Fig. 2a).
Fig. 4.
Inducible cassettes in HSV vector driving GFP are activated by hypoxia. HRE-alone vector (6H) and ARE+HRE vectors (3A3H, 3A6H, 3A9H) were transfected in COS7 cells and exposed to hypoxia (24 h of OD). a Representative images of inducible HSV vectors during normal and hypoxia (OD). b Western analysis showed that hypoxia (OD) induced GFP expression in all vectors, with higher expression in 3A6H and 3A9H inducible vectors. The protein level of actin was assessed to ensure equal protein loading
Inducible Vector Driving Nrf2 Protects Neuron Death from Oxygen Glucose Deprivation
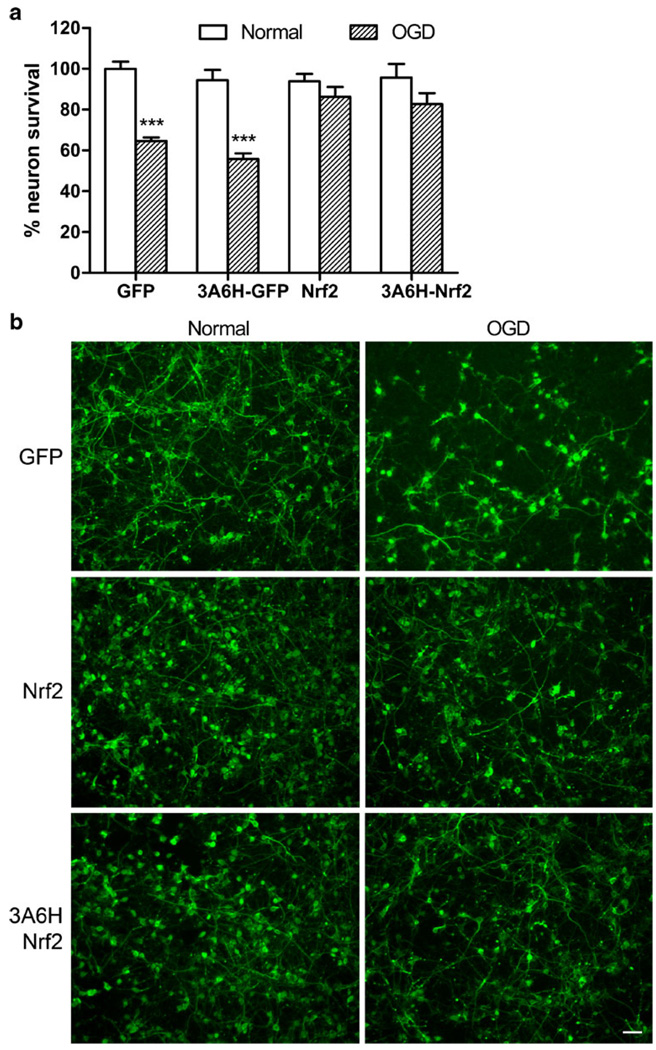
To test the neuroprotective potential of the insult-inducible vector, we cloned the inducible cassette 3A6H in a defective HSV backbone driving Nrf2 expression (HSV-3A6H-Nrf2). The neuroprotective potential of HSV-3A6H-Nrf2 was compared to the control constitutive vector driving GFP (HSV-GFP), the control inducible vector driving GFP (HSV-3A6H-GFP), and the constitutive vector driving Nrf2 (HSV-Nrf2) (Fig. 1b). The 3A6H vector was chosen because of its low basal expression and high inducibility. We chose to express Nrf2 under the inducible promoters for two reasons: (1) Nrf2 has been previously shown to protect against oxidative stress and cerebral ischemia [29–33], and (2) Nrf2 can also activate the inducible vector, resulting in a self-amplifying vector. Using primary cortical cultures, we tested whether the self-amplifying inducible HSV-3A6H-Nrf2 can protect neuron death in an oxygen glucose deprivation (OGD) model. HSV-3A6H-Nrf2 was able to protect against neuron death in OGD, when compared to the constitutive or inducible GFP controls. This protection was similar to the protective potential from the constitutive Nrf2 (HSV-Nrf2) (Fig. 5). We have also tested the HSV-3A9H-Nrf2 against OGD and it showed similar level of protection as the HSV-3A6H-Nrf2 (data not shown). Cortical neuron survival was measured using an ELISA with a neuron-specific marker microtubule-associated protein 2 (MAP2).
Fig. 5.
Inducible HSV vector expressing Nrf2 protects neuron death against OGD. Primary cortical cultures were infected with control HSV-GFP, inducible HSV-3A6H-GFP, constitutive HSV-Nrf2 and inducible HSV-3A6H-Nrf2 vectors. a Both the constitutive HSV-Nrf2 and inducible HSV-3A6H-Nrf2 vectors protect neuron death against OGD, using a quantitative ELISA assay with a MAP2 antibody. ***p<0.001, two-way ANOVA with Bonferroni’s posthoc indicates a significant difference in the GFP group (normal vs OGD), n=25–30 per group. b Representative images of the MAP2 staining in normal and OGD
Discussion
We have successfully constructed an insult-inducible gene therapy vector using a combination of HREs and AREs, which can be activated by both HIF1α and ROS generated during ischemia/reperfusion injury, respectively. In contrast to the HRE-alone vector, our ARE+HRE vector system is advantageous in that it allows the expression of a transgene to be activated not only during hypoxia but also maintained after reperfusion, thus prolonging the transgene expression during an ischemic insult. Among the various combinations of the HREs and AREs, 3A6H provided the highest insult inducibility. Other groups have previously demonstrated the use of multiple copies of HREs to drive expression of protective genes [19, 23, 24, 34]. A recent study reported the use of AREs from heme oxygenase-1 for oxidative stress-induced gene therapy in non-neuronal cells [35]. In our study, we constructed an insult-inducible vector that can be activated by both hypoxia and ROS. The addition of AREs to HRE constructs greatly enhanced their inducibility with low basal expression, which is an ideal feature of an insult-inducible gene therapy vector. Moreover, we generated a self-amplifying version of the inducible 3A6H vector by expressing the transcription factor Nrf2, such that Nrf2 can activate the ARE enhancer, resulting in further Nrf2 expression. Using primary cortical cultures, we showed that the HSV-3A6H-Nrf2 inducible vector can protect neurons against OGD.
We observed that increasing copy numbers of the enhancer does not guarantee higher inducibility. In both HRE-alone and ARE+HRE vectors, the inducibility increased from 3H to 6H, but expression began to drop in 9H (Figs. 2 and 3). It is likely that we have already reached maximal activation with 6H, such that any additional HRE could not increase further expression; it is not clear why there was, in fact, reduced expression with 9H. The inducibility of these constructs is also dependent on the insult model used. For example, although 3A6H had the greatest expression during OD (Fig. 2a), its inducibility was lower than the 3A3H after H2O2 treatment (Fig. 2b). The lower inducibility of 3A6H after H2O2 treatment could be due to the distance between the enhancer and the transgene. Since the constructs were built in the order of ARE−HRE–transgene (Fig. 1), it is possible that the additional HREs increased the distance between the ARE and the transgene, thereby making the ARE less effective as a transcriptional enhancer.
During normoxia, HIF1α is quickly degraded by ubiquitination and proteasomal digestion [1, 2, 4, 36]. Hypoxia inhibits this degradation and stabilizes HIF1α to activate HREs and drive hypoxia responsive genes. Similar to HIF1α, ROS also cause the activation of a stretch of DNA known as the ARE, which drives a number of phase II detoxification genes and antioxidants [8–10, 29]. Nrf2 is considered as one of the major transcription factors for the ARE [8, 10]. Over-expression of Nrf2 or application of Nrf2-inducing agent tert-butyl-hydroquinone has been shown to protect neuron death from glutamate excitotoxicity, oxidative stress, ischemic injury, and neurodegenerative diseases [30–33, 37, 38]. Nrf2 knockout mice also had larger infarcts and greater neurological deficit, and this was partly due to the accumulation of ROS in these Nrf2-deficient mice [39]. Thus, we chose Nrf2 as our protective gene because it can protect against a number of insults, as well as capable of boosting its own expression via an Nrf2-ARE positive feedback.
A potential defense against ROS is the induction of phase II detoxification genes and antioxidants, which are downstream genes activated by Nrf2 binding to the ARE [8, 10]. This antioxidant response preferentially occurs in glia, yet can protect neurons against oxidative stress, possibly by the release of glutathione (GSH) and/or GSH precursors from glia [31, 33]. Although the level of Nrf2 is lower in neurons, the ARE and its downstream pathways are still intact [31, 33]. In this study, we used a neurotrophic herpes simplex virus (HSV) to over-express Nrf2, thus increasing the level of Nrf2 in neurons to activate the antioxidant pathways in neurons. As HSV mainly infects neurons [40, 41], the protection we observed against OGD is likely due to the effect of Nrf2-ARE pathways in neurons. However, a small percentage of the glia is infected (~5%), which could also contribute to the protection we observed.
An ideal insult-inducible system should provide the following advantages: (1) the inducible vector should have low or no expression when there is no insult, minimizing unexpected side effects caused by uncontrolled constitutive expression. Some protective genes such as VEGF [21] can have adverse effects when introduced during normal conditions; (2) the inducible vector should be quickly activated when endogenous signals are released during an insult, and expression should decrease when the endogenous signals subside; (3) the extent of inducible expression should be high enough to afford protection. Our insult-inducible 3A6H vector provides most of these advantages. It possesses low or no expression when there is no insult, and its expression can be activated by endogenous signals released during hypoxia and oxidative stress. Most importantly, the inducible Nrf2 expression is sufficient to decrease neuron death after OGD. The expression of inducible vectors should be quickly activated during hypoxia since HIF1α can be released within a few hours after hypoxia onset [26, 42, 43]. Using the luciferase reporter system, we observed that our ARE−HRE inducible cassette can induce luciferase expression after several hours of hypoxia (data not shown). It is important that a protective gene can be activated in the early phase of neuron death after an insult, as it increases not only the chance of a neuron surviving but of its function being maintained as well [44]. As the incidence of stroke is unpredictable, it would be ideal to administer such insult-inducible vector prior to an ischemic event in individuals who are at high risk for cerebrovascular diseases or even individuals undergoing surgical procedures that may cause reduction of cerebral blood flow. The insult-induced protective gene should then be quickly activated to provide neuroprotection. Future studies will assess the inducibility and neuroprotective potential of this inducible vector using an in vivo hypoxic–ischemic model.
Conclusion
We have constructed a novel insult-inducible self-amplifying gene therapy vector that is highly activated by both hypoxia and oxidative stress. Using an in vitro oxygen glucose deprivation model, our inducible vector system can protect against neuron death and achieve similar neuroprotective potential as constitutive vectors, while being advantageous because its expression can be controlled by endogenous signals released during ischemia/reperfusion injury. This insult-inducible vector can be a potential therapeutic gene therapy tool for activating therapeutic/protective genes in cerebrovascular diseases.
Acknowledgments
We would like to thank Dr. Jewed Alam and Dr. Howard Federoff for kindly providing the pEF-Nrf2 plasmid and the replication defective HSV plasmid, respectively. This work was supported by the NIH-Center for Cerebrovascular Disease no. 2P01 NS37520. MYC was supported by the American Heart Association (no. 0525025Y).
Contributor Information
Michelle Y. Cheng, Email: mycheng@stanford.edu, Department of Biology, Stanford University, 371 Serra Mall, Stanford, CA 94305-5020, USA; Department of Neurosurgery, Stanford University School of Medicine, 300 Pasteur Drive, Rm 281A, Stanford, CA 94305-5327, USA.
I-Ping Lee, Department of Biology, Stanford University, 371 Serra Mall, Stanford, CA 94305-5020, USA.
Michael Jin, Department of Biology, Stanford University, 371 Serra Mall, Stanford, CA 94305-5020, USA.
Guohua Sun, Department of Neurosurgery, Stanford University School of Medicine, 300 Pasteur Drive, Rm 281A, Stanford, CA 94305-5327, USA.
Heng Zhao, Department of Neurosurgery, Stanford University School of Medicine, 300 Pasteur Drive, Rm 281A, Stanford, CA 94305-5327, USA.
Gary K. Steinberg, Department of Neurosurgery, Stanford University School of Medicine, 300 Pasteur Drive, Rm 281A, Stanford, CA 94305-5327, USA
Robert M. Sapolsky, Department of Biology, Stanford University, 371 Serra Mall, Stanford, CA 94305-5020, USA Department of Neurosurgery, Stanford University School of Medicine, 300 Pasteur Drive, Rm 281A, Stanford, CA 94305-5327, USA.
References
- 1.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;884:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 2.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev. 2004;56:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 3.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;9212:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res. 2001;495:614–617. doi: 10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;3996738 Suppl:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 6.Sapolsky RM. Neuroprotective gene therapy against acute neurological insults. Nat Rev. 2003;41:61–69. doi: 10.1038/nrn1006. [DOI] [PubMed] [Google Scholar]
- 7.Peters O, Back T, Lindauer U, Busch C, Megow D, Dreier J, et al. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;182:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;108:879–891. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Meth Enzymol. 2004;378:273–286. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- 10.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;1011:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;663:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 12.Dubinsky R, Lai SM. Mortality of stroke patients treated with thrombolysis: analysis of nationwide inpatient sample. Neurology. 2006;6611:1742–1744. doi: 10.1212/01.wnl.0000218306.35681.38. [DOI] [PubMed] [Google Scholar]
- 13.Sapolsky RM, Steinberg GK. Gene therapy using viral vectors for acute neurologic insults. Neurology. 1999;539:1922–1931. doi: 10.1212/wnl.53.9.1922. [DOI] [PubMed] [Google Scholar]
- 14.Yenari MA, Dumas TC, Sapolsky RM, Steinberg GK. Gene therapy for treatment of cerebral ischemia using defective herpes simplex viral vectors. Neurol Res. 2001;235:543–552. doi: 10.1179/016164101101198802. [DOI] [PubMed] [Google Scholar]
- 15.Linnik MD, Zahos P, Geschwind MD, Federoff HJ. Expression of bcl-2 from a defective herpes simplex virus-1 vector limits neuronal death in focal cerebral ischemia. Stroke J Cereb Circ. 1995;269:1670–1674. doi: 10.1161/01.str.26.9.1670. discussion 1675. [DOI] [PubMed] [Google Scholar]
- 16.Hermann DM, Kilic E, Kugler S, Isenmann S, Bahr M. Adenovirus-mediated GDNF and CNTF pretreatment protects against striatal injury following transient middle cerebral artery occlusion in mice. Neurobiol Dis. 2001;84:655–666. doi: 10.1006/nbdi.2001.0399. [DOI] [PubMed] [Google Scholar]
- 17.Andsberg G, Kokaia Z, Klein RL, Muzyczka N, Lindvall O, Mandel RJ. Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol Dis. 2002;92:187–204. doi: 10.1006/nbdi.2001.0456. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;4387070:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 19.Shen F, Su H, Fan Y, Chen Y, Zhu Y, Liu W, et al. Adeno-associated viral-vector-mediated hypoxia-inducible vascular endothelial growth factor gene expression attenuates ischemic brain injury after focal cerebral ischemia in mice. Stroke J Cereb Circ. 2006;3710:2601–2606. doi: 10.1161/01.STR.0000240407.14765.e8. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Investig. 2003;11112:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;1028:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa CR, Ho JJ, Tsai DJ, Ho DY, Sapolsky RM. Neuroprotective potential of a viral vector system induced by a neurological insult. Proc Natl Acad Sci USA. 2000;9716:9270–9275. doi: 10.1073/pnas.160503997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Post DE, Van Meir EG. Generation of bidirectional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 2001;823:1801–1807. doi: 10.1038/sj.gt.3301605. [DOI] [PubMed] [Google Scholar]
- 24.Reinblatt M, Pin RH, Bowers WJ, Federoff HJ, Fong Y. Herpes simplex virus amplicon delivery of a hypoxia-inducible soluble vascular endothelial growth factor receptor (sFlk-1) inhibits angiogenesis and tumor growth in pancreatic adenocarcinoma. Ann Surg Oncol. 2005;1212:1025–1036. doi: 10.1245/ASO.2005.03.081. [DOI] [PubMed] [Google Scholar]
- 25.Qutub AA, Popel AS. A computational model of intracellular oxygen sensing by hypoxia-inducible factor HIF1 alpha. J Cell Sci. 2006;119Pt(16):3467–3480. doi: 10.1242/jcs.03087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;2754(Pt 1):L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 27.Brooke SM, Bliss TM, Franklin LR, Sapolsky RM. Quantification of neuron survival in monolayer cultures using an enzyme-linked immunosorbent assay approach, rather than by cell counting. Neurosci Lett. 1999;2671:21–24. doi: 10.1016/s0304-3940(99)00315-8. [DOI] [PubMed] [Google Scholar]
- 28.Ho DY, Fink SL, Lawrence MS, Meier TJ, Saydam TC, Dash R, et al. Herpes simplex virus vector system: analysis of its in vivo and in vitro cytopathic effects. J Neurosci Meth. 1995;572:205–215. doi: 10.1016/0165-0270(94)00150-f. [DOI] [PubMed] [Google Scholar]
- 29.Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, et al. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;2014:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- 30.Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, et al. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem. 2005;28024:22925–22936. doi: 10.1074/jbc.M414635200. [DOI] [PubMed] [Google Scholar]
- 31.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;238:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;2544:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Erb H, Murphy TH. Coordinate regulation of glutathione metabolism in astrocytes by Nrf2. Biochem Biophys Res Commun. 2005;3262:371–377. doi: 10.1016/j.bbrc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Lee YS, Kim JM, Kim KL, Lee JS, Jang HS, et al. A novel chimeric promoter that is highly responsive to hypoxia and metals. Gene Ther. 2006;1310:857–868. doi: 10.1038/sj.gt.3302728. [DOI] [PubMed] [Google Scholar]
- 35.Hurttila H, Koponen JK, Kansanen E, Jyrkkanen HK, Kivela A, Kylatie R, et al. Oxidative stress-inducible lentiviral vectors for gene therapy. Gene Ther. 2008;1518:1271–1279. doi: 10.1038/gt.2008.75. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, et al. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- 37.Shih AY, Erb H, Murphy TH. Dopamine activates Nrf2-regulated neuroprotective pathways in astrocytes and meningeal cells. J Neurochem. 2007;1011:109–119. doi: 10.1111/j.1471-4159.2006.04345.x. [DOI] [PubMed] [Google Scholar]
- 38.Siebert A, Desai V, Chandrasekaran K, Fiskum G, Jafri MS. Nrf2 activators provide neuroprotection against 6-hydroxydopamine toxicity in rat organotypic nigrostriatal cocultures. J Neurosci Res. 2009;877:1659–1669. doi: 10.1002/jnr.21975. [DOI] [PubMed] [Google Scholar]
- 39.Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, et al. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;1471:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink DJ, DeLuca NA, Goins WF, Glorioso JC. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 41.Glorioso JC, Goins WF, Fink DJ, DeLuca NA. Herpes simplex virus vectors and gene transfer to brain. Dev Biol Stand. 1994;82:79–87. [PubMed] [Google Scholar]
- 42.Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR. Induction of hypoxia-inducible factor-1 (HIF-1) and its target genes following focal ischaemia in rat brain. Eur J Neurosci. 1999;1112:4159–4170. doi: 10.1046/j.1460-9568.1999.00845.x. [DOI] [PubMed] [Google Scholar]
- 43.Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Investig Ophthalmol Vis Sci. 1999;401:182–189. [PubMed] [Google Scholar]
- 44.Dumas TC, Sapolsky RM. Gene therapy against neurological insults: sparing neurons versus sparing function. Trends Neurosci. 2001;2412:695–700. doi: 10.1016/s0166-2236(00)01956-1. [DOI] [PubMed] [Google Scholar]