Abstract
The genome of Escherichia coli encodes two class I ribonucleotide reductases. The first, NrdAB, is a well-studied iron-dependent enzyme that is essential for aerobic growth. The second, NrdEF, is not functional under routine conditions, and its role is obscure. Recent studies demonstrated that NrdEF can be activated in vitro by manganese as well as iron. Since iron enzymes are potential targets for hydrogen peroxide, and since the nrdHIEF operon is induced during H2O2 stress, we hypothesized that H2O2 might inactivate NrdAB and that NrdEF might be induced to compensate. This idea was tested using E. coli mutants that are chronically stressed by H2O2. Contrary to expectation, NrdAB remained active. Its resistance to H2O2 depended upon YfaE, which helps to activate NrdB. The induction of NrdEF during H2O2 stress was mediated by the inactivation of Fur, an iron-dependent repressor. This regulatory arrangement implied that NrdEF has a physiological role during periods of iron starvation. Indeed, NrdEF supported cell replication in iron-depleted cells. Iron bound to NrdF when it was expressed in iron-rich cells, but NrdEF was functional only in cells that were both iron-depleted and manganese-rich. Thus NrdEF supports DNA replication when iron is unavailable to activate the housekeeping NrdAB enzyme.
Introduction
Ribonucleotide reductases provide the building blocks for DNA replication in all organisms. The reaction is chemically difficult, and it is achieved by free-radical chemistry that originates with an amino-acid radical in the protein active site. Life evolved in a world that for two billion years was anaerobic and iron-replete, and the prevailing ribonucleotide reductase isozyme was likely a class III enzyme of the glycyl-radical enzyme family. As in all members of this enzyme family, however, the glycyl radical of this ribonucleotide reductase is rapidly quenched by direct adduction with molecular oxygen; thus, the eventual oxygenation of the atmosphere forced the evolution of alternative enzymes. While anaerobes retain class III ribonucleotide reductases, organisms that dwell in oxic environments typically have been found to use either a B12-dependent (class II) enzyme or, more commonly, a di-ferric (class Ia) enzyme.
E. coli, which in its life cycle moves between the anaerobic gut and oxic surface waters, relies upon a class III enzyme (NrdD) for anoxic replication and a class Ia enzyme (NrdAB) for aerobic replication. Genetic experiments show that mutants lacking the former enzyme cannot grow in the absence of oxygen, while those lacking the latter enzyme cannot grow in its presence (Garriga et al., 1996; Jordan et al., 1996). The diferric NrdAB isozyme acquires iron in its ferrous state, and the NrdB subunit is then activated to its resting di-ferric/tyrosyl-radical form by molecular oxygen and an electron donor. In the resting enzyme the tyrosyl radical is buried in the protein interior, an arrangement that shields it from outside solutes and minimizes inadvertent inactivation. When substrate binds, the radical is transiently conducted to a cysteinyl residue within the NrdA active site, where the chemistry occurs. Thioredoxin is the coreactant that delivers the electrons that are channeled to the catalytic residues, ultimately reducing the ribonucleotide. NrdAB is oxygen-resistant and apparently sufficient to enable E. coli replication in aerobic habitats.
Thus it was puzzling when the genomic sequence of E. coli revealed the presence of a NrdAB homologue. It is denoted NrdEF, and these subunits are encoded within a four-gene operon (nrdHIEF). NrdH is a thiol-based redoxin, which evidently replaces thioredoxin as the electron donor for ribonucleotide reduction (Jordan et al., 1997). NrdI is a flavodoxin. In vitro studies confirmed that NrdEF can exhibit ribonucleotide reductase activity (Cotruvo & Stubbe, 2008). Members of this sequence group have been identified in a variety of bacterial genomes and are now denoted as class Ib enzymes. Although they comprise the sole ribonucleotide reductases is many microbes, the reason that they are used in preference to class Ia enzymes has been uncertain (Cotruvo & Stubbe, 2010).
The E. coli NrdEF is evidently not functional under standard aerobic growth conditions, since nrdAB mutants fail to grow (Jordan et al., 1996). This raises the question: What is its role? The nrdHIEF operon is somewhat induced in nrdR mutants, which lack the dATP-activated feedback repressor that controls nrdAB and nrdDG expression; thus a role in DNA replication, under some circumstance, seemed certain (Torrents et al., 2007).
A hypothesis emerged from several disparate observations. First, early in vitro experiments suggested that oxygen species such as hydrogen peroxide and superoxide might be able to disrupt either NrdAB activation or function (Fontecave et al., 1987a; Gaudu et al., 1996). Second, active NrdEF homologues that were purified from Corynebacterium species contained predominantly manganese rather than iron (Fieschi et al., 1998; Abbouni et al., 2009). Cotruvo and Stubbe showed that NrdEF from E. coli could be activated in vitro by manganese (as well as iron) (Cotruvo & Stubbe, 2010). And finally, Monje-Casas et al. discovered that the E. coli nrdHIEF operon was highly expressed during hydrogen peroxide stress (Monje-Casas et al., 2001). These observations would tentatively fit a notion that during oxidative stress E. coli prefers to employ a manganese-activated ribonucleotide reductase.
A reason that it might do so is suggested by studies with other iron-cofactored enzymes. Hydrogen peroxide rapidly oxidizes ferrous iron atoms in the Fenton reaction, and when cells are exposed to even micromolar H2O2, this reaction results in the inactivation of key iron-dependent enzymes and in iron-catalyzed DNA damage (Jang & Imlay, 2007; Park et al., 2005). Strikingly, when E. coli senses elevated levels of H2O2, it strongly induces the synthesis of its manganese importer, MntH (Kehres et al., 2002). The imported manganese has been proposed to replace iron in non-redox enzymes that require a mononuclear metal; since manganese does not react easily with H2O2, this substitution would ensure enzyme activity in the face of H2O2 stress (Anjem et al., 2009). Thus it seemed plausible that a similar situation might pertain to ribonucleotide reduction: H2O2 might interfere with the activation or activity of iron-dependent NrdAB, and the induction of putative manganese-dependent NrdEF might compensate. This study was undertaken to test that hypothesis.
Results
NrdEF is induced during H2O2 stress, but H2O2 does not inhibit NrdAB function
Previous workers demonstrated that the transcription of the nrdHIEF operon is elevated when a bolus of 100 μM H2O2 is added to exponentially growing cultures of E. coli (Monje-Casas et al., 2001). That dose substantially exceeds the amount of H2O2 that E. coli is likely to experience in natural environments (Imlay, 2008). To impose constant, low doses of H2O2 upon cells, we have used katE katG ahpCF (Hpx−) strains that lack catalase and peroxidase activities. These strains cannot degrade the H2O2 that is generated as a by-product of metabolism, and during aerobic growth their internal concentration of H2O2 rises to 0.5–1 micromolar (Seaver & Imlay, 2001). This dose is likely to represent a physiological degree of stress, since it exceeds the dose that triggers the OxyR response (0.2 μM) by only a moderate amount (Aslund et al., 1999; Seaver & Imlay, 2001). Measurements of transcript levels confirmed that all four genes of the nrdHIEF operon were induced in Hpx− mutants (Fig. S1). A PnrdHIEF – lacZ fusion was constructed and integrated at the phage attachment site. When the Hpx− mutant was grown in defined medium, the β-galactosidase activity was consistently elevated by two-fold over the level in unstressed wild-type cells. This effect is lower than previously reported (Monje-Casas et al., 2001), but differences arise from the effects of the culture medium (see below).
Hydrogen peroxide inactivates some mononuclear iron enzymes by oxidizing their exposed iron cofactor, triggering iron dissociation and/or covalent modification of the protein by the hydroxyl radical that is formed (Anjem et al., 2009; Sobota and Imlay, 2011). Thus we hypothesized (1) that NrdAB function might be inactivated by H2O2 and (2) that NrdEF might be induced to compensate.
Ribonucleotide reductase activity is essential for cell viability, but the roles of the aerobic isozymes can be genetically tested by the construction of null mutants in an anaerobic chamber. Under those conditions replication depends upon NrdD, an oxygen-sensitive glycyl-radical enzyme, whereas NrdAB is non-functional, since it requires oxygen for activation. When wild-type E. coli cultures are diluted into aerobic medium, NrdD immediately loses activity, while NrdAB is activated.
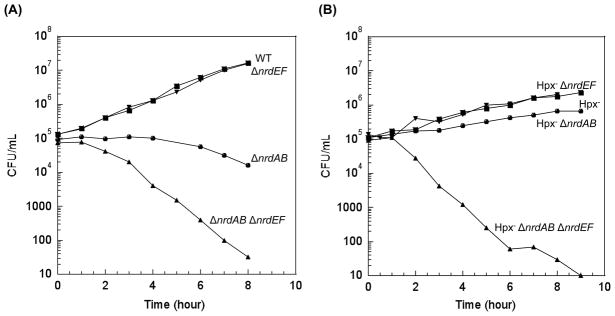
Once exposed to oxygen, a ΔnrdAB nrdEF+ mutant filamented and lost viability, while a nrdAB+ ΔnrdEF mutant grew as well as a wild-type strain (Fig. 1a). Thus, in unstressed E. coli, NrdAB is necessary and sufficient for aerobic DNA replication, whereas NrdEF has no apparent function. To determine if NrdAB remains functional during H2O2 stress, an E. coli Hpx− ΔnrdEF strain was constructed, and cell viability was monitored subsequent to aeration. This mutant grew as well as the wild-type strain. Thus, contrary to our expectation, NrdAB appeared to retain function during H2O2 stress (Fig. 1b).
Figure 1. NrdAB and NrdEF are both functional during protracted H2O2 stress.
Cells were pre-cultured in anaerobic glucose/aromatic medium and then diluted at time zero into the same aerobic medium. Viability was tracked by anaerobic plating. (A) Cell viability during routine aerobic growth. MG1655 (wild type), JEM84 (ΔnrdAB), JEM89 (ΔnrdEF), and JEM118 (ΔnrdAB ΔnrdEF). (B) Viability during protracted H2O2 stress (ca. 0.5 μM). LC106 (Hpx−), JEM86 (Hpx−ΔnrdAB), JEM90 (Hpx−ΔnrdEF), and JEM119 (Hpx−ΔnrdAB ΔnrdEF).
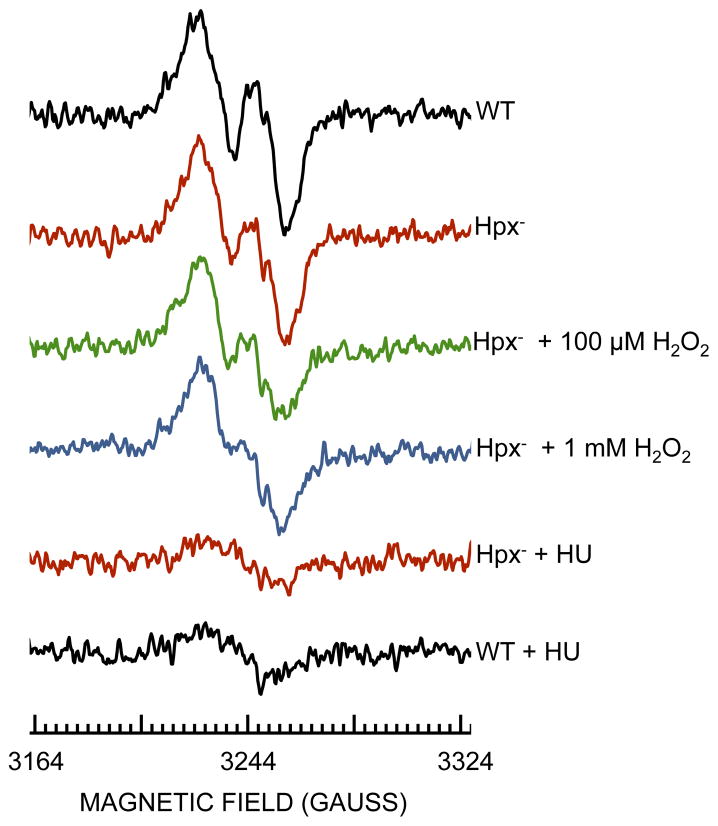
Measurements of total [3H]-thymidine incorporation (Drlica et al., 1980) confirmed that chromosomal replication continued to progress in the Hpx− ΔnrdEF strain (Figure S2). We were unable to reliably test the activity of ribonucleotide reductase by direct assay, since these methods are problematic in crude extracts, and purification steps can perturb the enzyme activity status. Therefore to directly test the stability of the NrdB iron center, we overexpressed nrdB and used whole-cell EPR to visualize its di-ferric/tyrosyl-radical signal (Hristova et al., 2008). A characteristic signal was visible, and it was quenched when cells were treated by hydroxyurea, an established scavenger of the tyrosyl radical (Ehrenberg & Reichard, 1972) (Fig. 2). However, the NrdB signal was not abolished when the Hpx− strain was exposed to up to 1 mM H2O2 (Fig. 2).
Figure 2. H2O2 does not eliminate the tyrosyl radical of NrdB.
The expression of nrdB was induced in aerobic LB medium for two hours in wild-type (JEM914) or Hpx− (JEM915) strains harboring pBAD-N-S-nrdB. Cells were washed and concentrated, and where indicated H2O2 was added for 5 min at 37°C. The intact cells were then transferred to EPR tubes and analyzed. HU: hydroxyurea was added to quench the NrdB tyrosyl radical.
Finally, we tested whether the NrdR repressor is deactivated during H2O2 stress. When cellular ribonucleotide reductase activity is sufficient, NrdR binds dATP and/or dADP and, as a complex, represses expression of ribonucleotide reductases (Grinberg et al., 2009, Torrents et al., 2007). In a wild-type cell, deletion of nrdR caused a 3-fold increase in PnrdH-lacZ expression (Fig. S3). A similar effect resulted from a ΔnrdAB deletion, and minimal further induction was observed when nrdR was additionally deleted, implying that in this strain the repressive effect of NrdR depends upon the function of NrdAB (Fig. S3). We then observed that the addition of the ΔnrdR allele to Hpx− mutants caused a similar degree of PnrdH-lacZ induction (Fig. S3). This finding shows that NrdR still actively represses nrdHIEF transcription during H2O2 stress and affirms that NrdAB is not inactivated by H2O2.
Some in vitro experiments had suggested that NrdAB might be directly poisoned by superoxide, an oxidant whose reactivity with metal centers resembles that of H2O2 (Fontecave et al., 1987b; Gaudu et al., 1996). However, we observed no replication defect when ΔsodA ΔsodB ΔnrdEF mutants were cultured in aerobic medium. Further, overproduced NrdB exhibited a normal tyrosyl radical in a SOD− strain (Fig. S4). The superoxide stress in these cells is sufficient to inactivate a variety of iron-sulfur enzymes and the metabolic pathways to which they belong (Imlay, 2008); we conclude, therefore, that NrdAB is not a significant target of superoxide stress in vivo.
NrdEF is functional during H2O2 stress
Mutants that lacked both NrdAB and NrdEF lost viability more quickly than did mutants that lacked only NrdAB (Fig. 1a), which indicated that under these circumstances NrdEF must provide some ribonucleotide reductase function, albeit not enough to enable normal aerobic growth. In fact, previous work indicated that the engineered overexpression of nrdEF could substantially compensate for nrdAB deficiency (Jordan et al., 1994; Jordan et al., 1996; Gon et al., 2006). Since nrdEF is induced during H2O2 stress, it seemed plausible that NrdEF activity might rise to a level that would allow DNA replication. Indeed, the Hpx− ΔnrdAB strain was able to successfully replicate under aerobic conditions (Fig. 1b), in contrast to the congenic Hpx+ΔnrdAB strain (Fig. 1a). Thus during oxidative stress the native nrdEF operon can generate enough ribonucleotide reductase activity to permit cell replication.
Fur and IscR regulate nrdHIEF transcription
We sought to identify the regulatory mechanism that triggers expression of nrdHIEF during oxidative H2O2 stress. Many of the proteins involved in protection against H2O2 stress are positively regulated by the OxyR transcription factor, which is fully activated by the H2O2 that accumulates in Hpx− mutants (Zheng et al., 2001). However, a constitutive oxyR2 mutant allele had no effect on the level of PnrdH-lacZ expression in an otherwise wild-type strain, indicating that this operon is not part of the OxyR regulon (data not shown). Others had made a similar conclusion from complementary experiments (Monje-Casas et al., 2001; Pueyo et al., 2002).
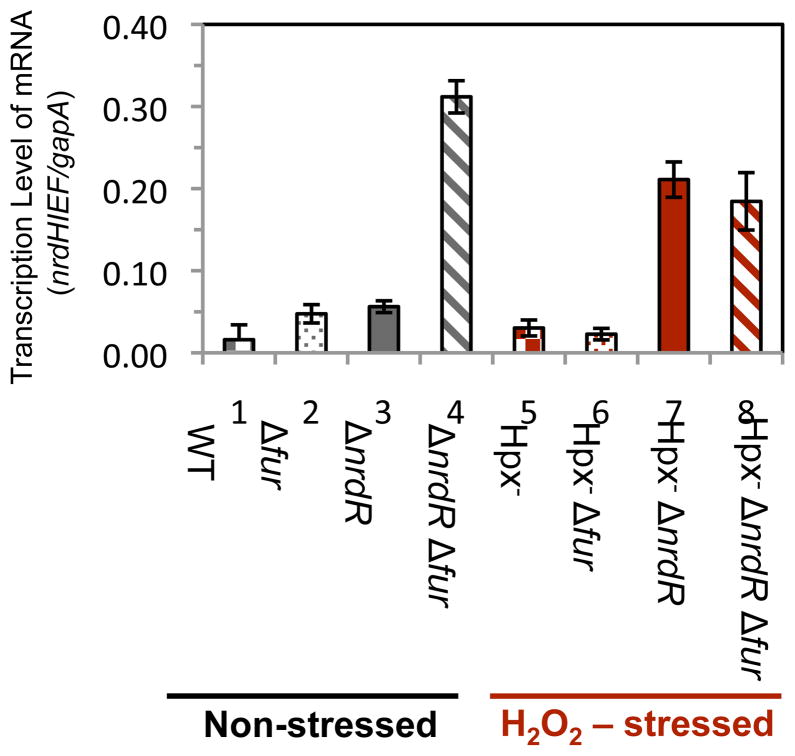
Fur and IscR are the other global regulators that are perturbed by H2O2 stress (Varghese et al., 2007; Yeo et al., 2006; Lee et al., 2008). In iron-replete cells Fur binds ferrous iron, and the Fur:Fe2+ complex binds to the promoter regions of iron-import genes, inhibiting their expression. It also represses the transcription of the small RNA RyhB, which otherwise targets for degradation mRNAs that encode iron-requiring enzymes; this system ensures that non-essential iron enzymes are only synthesized when iron is available to activate them (Varghese et al., 2007). Earlier work showed that the micromolar level of H2O2 inside Hpx− mutants can deactivate Fur, presumably due to the oxidation of its iron co-factor (Varghese et al., 2007). We believed this to be a plausible mechanism of nrdHIEF induction in Hpx− mutants, since several groups have reported that the transcription of nrdHIEF is elevated in cells devoid of fur (Vassinova & Kozyrev, 2000; McHugh et al., 2003). To assess the effect of Fur on the transcription of nrdHIEF, quantitative measurements of mRNA levels were made using RT-PCR. In a wild-type background we detected elevated nrdHIEF mRNA levels in both Δfur mutants and ΔnrdR single mutants (Fig. 3, left), thus confirming that under these growth conditions each regulator inhibits nrdHIEF transcription (Torrents et al., 2007). The combination of Δfur and ΔnrdR alleles resulted in a synergistic effect on transcription, indicating that Fur and NrdR repress nrdHIEF independently of one another.
Figure 3. During H2O2 stress the expression of nrdHIEF is due to derepression of the Fur regulon.
RNA was isolated from cells grown in aerobic LB medium. Data are representative of two independent experiments each measured in triplicate. Strains were SJ130 (wild type), JEM275 (Δfur), JEM406 (ΔnrdR), JEM286 (ΔnrdRΔfur), SJ108 (Hpx−), JEM309 (Hpx−Δfur), JEM407 (Hpx−ΔnrdR), and JEM311 (Hpx−Δfur ΔnrdR).
In the H2O2-stressed Hpx− background, NrdR still exerted a repressive effect on transcription, but Fur did not (Fig. 3, right). These data indicated that the induction of nrdHIEF by H2O2 occurs primarily because H2O2 inactivates the Fur repressor.
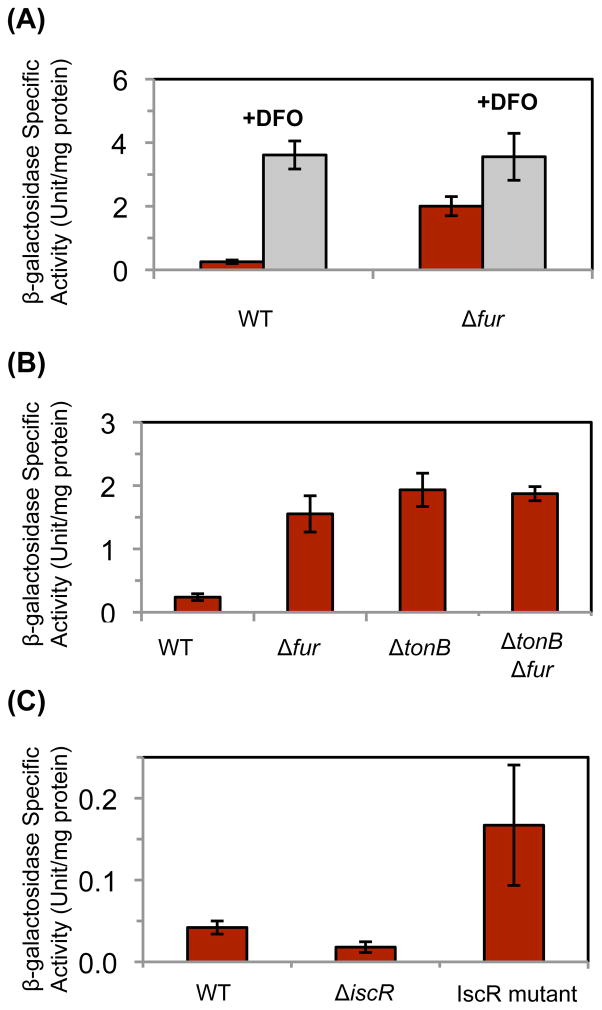
These results suggested that NrdEF is likely to respond to a diminution of cellular iron levels. We wished to test this idea without the involvement of H2O2 stress or fur mutations, which each exert physiological effects that would not be triggered by iron restriction. When wild-type cells were grown in the presence of the iron-chelator desferroxamine (DFO), a 17-fold increase in nrdHIEF levels occurred (Fig. 4a). Interestingly, DFO addition had a modest but consistent effect even upon a Δfur mutant, potentially suggesting that iron starvation diminished NrdAB activity and thereby partially inactivated NrdR. A similar inducing effect was achieved by the deletion of tonB, thereby eliminating the energy source that drives the import of iron chelates (Fig. 4b). These approaches confirm that iron starvation triggers nrdHIEF induction. Jordan and coworkers mapped the initiation of basal nrdHIEF transcription to a start site 67 bp upstream of the first translated gene (Jordan et al., 1996). We established by 5 RLM-RACE that the same start site is used when transcription is elevated in a Δfur ΔnrdR strain.
Figure 4. Fur and IscR both regulate the expression of nrdHIEF.
Cells bearing a nrdH’-‘lacZ translational fusion were grown in aerobic media. Data represent the mean of three independent cultures. (A) JEM648 (wild type) and JEM657 (Δfur) were grown in glucose/amino acids medium with or without 100 μM of the iron chelator DFO. (B) JEM648 (wild type), JEM657 (Δfur), JEM707 (ΔtonB) and JEM709 (ΔtonB Δfur) were grown in LB medium. (C) Expression of a nrdH’-lac+ transcriptional fusion in LB medium from JEM646 (wild type), JEM762 (ΔiscR), and JEM776 (iscR-C92A/C98A/C104A).
Genetic approaches have been used to locate Fur binding sites in E. coli, and a positive result was reported for clones carrying DNA upstream of the nrdHIEF operon (Stojiljkovic et al., 1994; Vassinova & Kozyrev, 2000). In vitro studies indicated that Fur protein can bind to this region of the Salmonella gene (Panosa et al., 2010). A region of similarity to a Fur binding box (GTAATTTCGACCACTATT) was reported 61 bp upstream of the transcriptional start site, near the transcriptional start of the divergently transcribed ygaC gene. However, we found that Fur continued to regulate nrdHIEF transcription when this sequence was removed (Fig. S5a). Regulation also persisted in mutants lacking ryhB and hfq, which appears to rule out post-transcriptional regulation by a small RNA (Fig. S5b); further, the regulatory effect of Fur disappeared when the promoter was replaced by the tetRA expression system (Fig. S5c). We deduce that Fur regulates nrdHIEF transcription through an alternate binding site, either directly or through an intermediary; we did not further map this site.
Hydrogen peroxide also affects the function of IscR, a transcriptional regulator that can coordinate a [2Fe-2S] cluster (Yeo et al., 2006; Lee et al., 2008). In its holoprotein form, IscR[2Fe-2S] represses a broad set of genes that includes those that encode the housekeeping iron-sulfur-cluster assembly system (iscSUA-fdx-hscAB) (Schwartz et al., 2001; Giel et al., 2006). In its apoprotein form, IscR is a positive activator of expression of other genes, including the alternative iron-sulfur-cluster assembly system that is encoded by the sufABCDSE operon. The level of H2O2 that accumulates in Hpx− mutants is sufficient to disrupt Isc-mediated cluster assembly, leading to the conversion of substantial IscR to its apoprotein form (Jang and Imlay, 2010), with consequent effects on the operons it regulates. We tested whether nrdHIEF might lie within its regulon. In fact, we observed a two-fold decrease in nrdHIEF transcription when iscR was deleted from wild-type cells (Fig. 4c), while overexpression of iscR did not affect nrdHIEF regulation (data not shown). These data suggested that IscR might activate nrdHIEF transcription. To test this idea further, the native iscR gene was replaced with iscR-(C92A/C98A/C104A) mutant allele, which is incapable of binding an iron-sulfur cluster and thereby forms apo-iscR protein (Nesbit et al., 2009). A four-fold increase in nrdHIEF transcription resulted (Fig. 4c). The presence of the native iscR gene did not affect this increase, since up-regulation of nrdHIEF was still observed in a meroploid strain containing both alleles (data not shown). It appears that apo-IscR activates transcription of nrdHIEF. The effect of apo-IscR persisted in strains with deletions in fur and/or nrdR (Fig. S6). Thus these three regulatory proteins act independently of one another: NrdR:dATP and Fur:Fe2+ repress nrdHIEF transcription, while apo-IscR activates it.
Expression of nrdHIEF is not sufficient for function
The induction of nrdHIEF in the Δfur mutants was substantially higher than that which occurred in the Hpx− background, and so we anticipated that the Δfur mutants might similarly produce enough NrdEF to compensate for a ΔnrdAB mutation. However, unlike the Hpx− ΔnrdAB strain, the ΔnrdAB Δfur mutant did not grow in aerobic medium (Fig. S7). Nor did a ΔnrdAB Δfur iscR-(C92A/C98A/C104A) mutant, despite the simultaneous deactivation of NrdR, absence of Fur, and presence of apo-IscR (Fig. S7). Indeed, expression of nrdHIEF from the tet promoter also failed to enable growth (data not shown); thus transcription of nrdHIEF is not sufficient for NrdEF to become functional. Since NrdEF becomes functional during H2O2 stress (Fig. 1b), we considered that another element of the cellular response to H2O2 might be essential to activate the enzyme.
NrdEF requires manganese to function
Superoxide dismutase, like ribonucleotide reductase, is a non-heme/non-metal-cluster enzyme that requires a redox-active metal. When cells are replete with iron, the iron-containing superoxide dismutase is synthesized. When iron is scant, a manganese-dependent isozyme is induced as a replacement (Compan & Touati, 1993). The fact that NrdEF is induced in iron-poor cells suggested that it might similarly be manganese-dependent. In fact, when iron levels are low, the manganese transporter gene, mntH, is upregulated through derepression of the Fur regulon (Patzer & Hantke, 2001; Kehres et al., 2002), potentially facilitating this adjustment. The induction of mntH is especially pronounced during during H2O2 stress, since OxyR directly activates its transcription (Kehres et al., 2002; Anjem et al., 2009). Furthermore, in vitro studies have suggested that Corynebacterium glutamicum and Corynebacterium ammoniagenes use manganese for ribonucleotide reductase function (Abbouni et al., 2009; Fieschi et al., 1998). If E. coli NrdEF were manganese-dependent, then the failure of this enzyme to function when the nrdHIEF operon was artificially expressed would be understandable, since manganese is not imported into E. coli under normal growth conditions (Anjem et al., 2009).
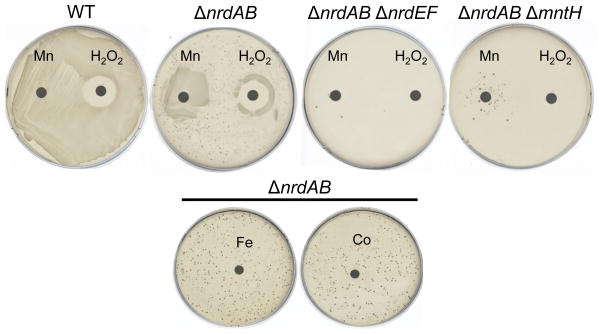
A disk diffusion assay was employed to test whether NrdEF required manganese to function (Fig. 5). Unlike wild-type cells, ΔnrdAB mutants were unable to grow on this base aerobic medium. However, the ΔnrdAB mutants showed growth near a manganese-impregnated disk. Similarly, they grew in a halo close to a H2O2-containing disk. This growth was abolished by the addition of either nrdEF or mntH null mutations (Fig. 5). Neither iron nor cobalt could substitute for manganese (Fig. 5, bottom). These data suggest that manganese is requisite for NrdEF to function.
Figure 5. NrdEF function requires manganese import.
Anaerobically grown cells were spread onto aerobic glucose/aromatic plates. Sterile disks impregnated with 5 μmoles H2O2 or 5 nmoles metal solution (MnCl2, Fe(NH4)2SO4, or CoCl2) were placed on each plate. Results are shown after 48 hours of aerobic incubation at 37°C. Strains were MG1655 (wild type), JEM84 (ΔnrdAB), JEM118 (ΔnrdAB ΔnrdEF), and JEM128 (ΔnrdAB ΔmntH). Note: the scattered suppressor colonies were not stable and may represent the amplification of the nrdHIEF locus.
Manganese did not influence nrdHIEF expression (Fig. S8a). These data support the idea that NrdEF uses manganese as a metal cofactor. The plate phenotype could also be replicated in liquid medium: ΔnrdAB mutants were able to grow in aerobic defined medium if it was supplemented with 1 μM manganese, but ΔnrdAB ΔmntH mutants could not (Fig. S8b).
NrdEF becomes active during protracted iron starvation
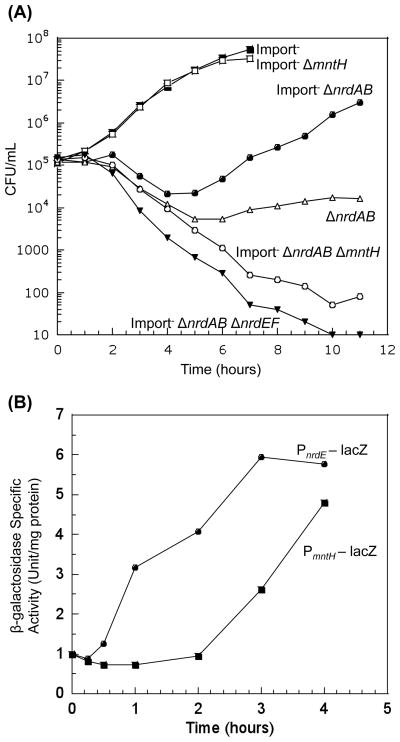
It is difficult to create protracted iron deficiency in lab cultures by simply minimizing iron in the medium, both because trace iron contaminates medium components and because the dense cultures that are used in lab studies will quickly drive low-iron levels to near zero. In the complete absence of iron, E. coli cannot grow, and so DNA replication cannot be monitored. In order to simulate persistent iron-poor environments that might be found in nature, we constructed E. coli strains that lack the primary iron uptake systems (ΔtonB ΔfeoABC ΔzupT) (Grass et al., 2005). The number of viable cells was tracked over time. Under these conditions, the low iron levels of unsupplemented medium enabled slow growth of the ΔtonB ΔfeoABC ΔzupT strain, presumably because iron inefficiently slips into cells through low-affinity systems that are dedicated to other polyvalent cations. Iron-limited cells devoid of nrdAB showed a brief lag before they were able to grow (Fig. 6a). This outgrowth depended upon the function of NrdEF, since iron-limited mutants lacking both aerobic ribonucleotide reductases were non-viable (Fig. 6a).
Figure 6. (A) NrdEF is functional when iron is limited and manganese is available.
Cells in anaerobic MOPS glucose/amino acids medium were diluted into aerobic medium at time zero, and viability was monitored. “Import-minus” strains contain ΔtonB ΔfeoABC ΔzupT null alleles and therefore have reduced iron import. Strains used were JEM609 (Import−), JEM1144 (Import− ΔmntH), JEM720 (Import− ΔnrdAB), JEM1137 (Import− ΔnrdAB ΔmntH), JEM722 (Import− ΔnrdAB ΔnrdHIEF), and JEM756 (ΔnrdAB). (B) Delayed expression of mntH during iron limitation coincides with delayed NrdEF function. Iron-limited ΔnrdAB strains bearing PnrdE-lacZ (JEM738) or PmntH-lacZ (JEM757) transcription fusions were grown in anaerobic MOPS glucose/amino acids medium and aerated at time zero. Data are normalized to the anaerobic expression level and is representative of at least two independent experiments.
Similar growth patterns were also observed with defined medium supplemented with manganese (data not shown). To test whether the initial growth lag was due to delays in the induction of mntH or nrdHIEF, we followed the expression of PmntH – lacZ and PnrdE – lacZover time. The iron-limited ΔnrdAB mutants showed that nrdHIEF was induced soon after aeration, presumably because NrdR was immediately derepressed; in contrast, mntH failed to be induced for several hours, which might be the time needed for depletion of cellular iron stores (Fig. 6b). The lag in mntH induction coincided with the lag in the outgrowth of the iron-limited ΔnrdAB mutants.
The native promoters of mntH and nrdHIEF were replaced with a tetracycline-inducible promoter in order to allow metal-independent control of nrdHIEF and mntH expression. Surprisingly, the over-expression of these genes still did not enable NrdEF to function in iron-replete cells, but it did allow it to function in the iron-limited mutants—after a residual delay (Fig. S9). These data indicated that low iron concentrations are needed not simply for nrdHIEF and mntH induction, but also for NrdEF polypeptide to become functional.
To verify our inference regarding intracellular metal availability, we monitored the activity of the cellular manganese superoxide dismutase, an enzyme that can bind either iron or manganese but is only active with the latter. By assaying the enzyme in cell extracts, partially denaturing and renaturing the protein in the presence of manganese, and assaying again, one can determine the fraction of enzyme that was initially active. In iron-replete cells, this enzyme is expressed, but metal-replacement experiments showed that less than 5% of the enzyme was active, due to lack of manganese in the active site (Anjem et al., 2009). In contrast, we found that 80% of this enzyme was active during outgrowth of the iron-limited ΔnrdAB mutants.
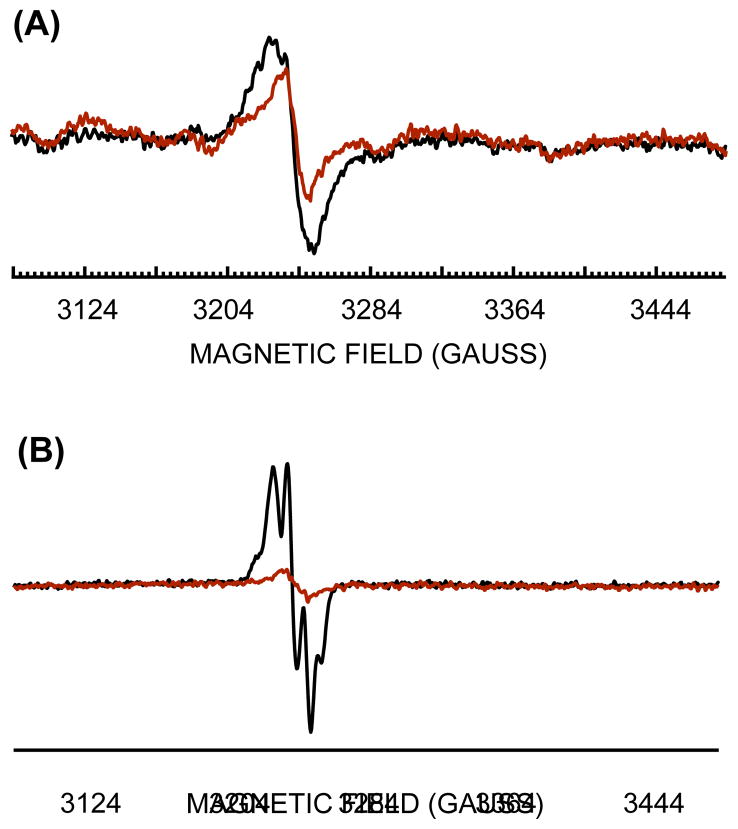
Recent in vitro studies have shown that NrdEF can form an active enzyme using either iron or manganese; however, in the studies that have been reported, the manganese-cofactored enzyme was about five-fold more active (Cotruvo & Stubbe, 2010). The two forms are distinguishable by the characteristic EPR spectra of the tyrosyl radical, which interacts with the metal cofactor. Whole-cell EPR of a NrdF-overproducing strain indicated that NrdF was loaded with manganese during the outgrowth of the iron-limited ΔnrdAB mutants (Fig. 7a).
Figure 7. EPR spectra of NrdF expressed in iron-deficient and iron-replete cells.
(A) Iron-deficient (ΔtonB ΔfeoABC ΔzupT) cells with (JEM1121, black) or without (JEM865, red) plasmid pBAD-N-S-2-nrdF. Cells were cultured in aerobic MOPS glucose/amino acids medium, and arabinose was added for 2 hours before harvesting and whole-cell EPR analysis. (B) Iron-replete wild-type cells harboring pBAD-N-S-2-nrdF (JEM1033) were cultured in aerobic LB medium, and arabinose was added for two hours before harvesting and whole-cell EPR analysis (black). Red: 160 mM hydroxyurea was added for 1 hour after nrdF expression prior to EPR analysis.
In contrast, when cells were grown in iron-rich conditions, a signal denoting iron-loaded NrdF was observed (Fig. 7b). The ΔnrdAB mutants do not grow under these conditions, suggesting that iron-loaded NrdEF is not sufficiently functional to enable normal replication in vivo.
The EPR data cannot be used to compare the relative amounts of NrdF activation under these two conditions, since interference from cellular metal pools compromises spin quantification. However, they do show that the identity of the metal that binds NrdF depends upon the relative availability of iron and manganese. More broadly, these data collectively show that metallation by manganese is necessary for NrdEF to be functional in vivo, while iron is inadequate.
The iron-import mutations that were used in these experiments still did not block iron import enough to inactivate NrdAB, and for this reason the nrdEF phenotype was apparent only in nrdAB-deficient strains. A more severe iron restriction can be created with chelators, but their affinity for manganese introduces complexity to the interpretation. Further work will be needed in order to reproduce in the laboratory the degree of iron restriction that microbes periodically experience in nature.
YfaE is required for continued NrdAB function during H2O2 stress
The competition between iron and manganese for the active site of NrdF suggested that the same might occur in NrdB. This would be problematic, since in vitro studies have shown that iron is required for NrdAB activity and manganese most likely cannot be used (Brown et al., 1968; Atta et al., 1992; Hogbom et al., 2001). Recent evidence suggests that NrdB activation can be a catalyzed process that involves YfaE, a ferredoxin that is encoded immediately downstream of nrdB (Wu et al., 2007; Hristova et al., 2008). We wondered whether the YfaE-mediated process excludes manganese.
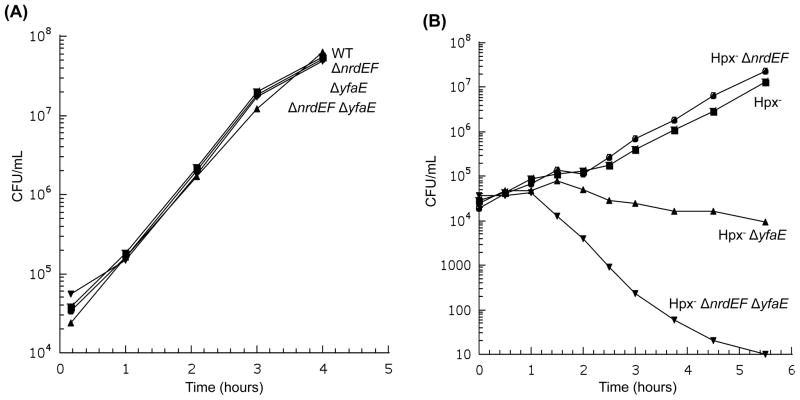
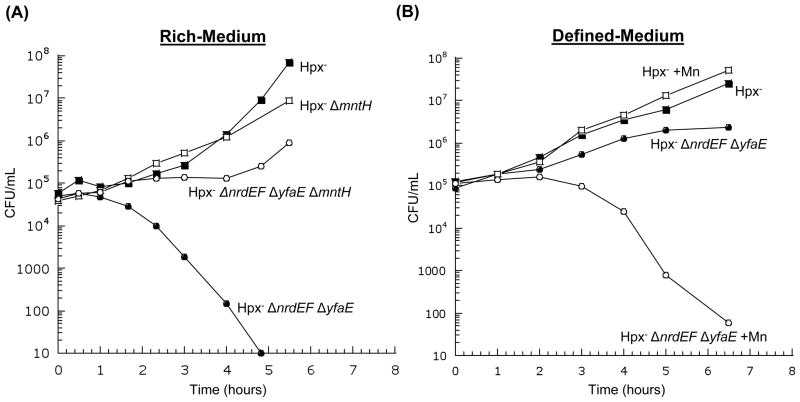
The deletion of yfaE did not compromise the function of NrdAB in iron-replete cells (Fig. 8a). However, in Hpx− mutants yfaE was essential (Fig. 8b). In LB medium, which has substantial manganese, the Hpx− ΔyfaE defect could be substantially suppressed by deletion of mntH (Fig. 9a). The defect was less pronounced in defined medium, but it re-emerged when manganese was added (Fig. 9b). Thus an apparent effect of YfaE is to discriminate between iron and manganese during NrdB activation.
Figure 8. NrdAB exhibits activity during H2O2 stress only if YfaE is active.
Cells in anaerobic LB medium were diluted into aerobic medium at time zero, and the number of viable cells was monitored. (A) Viable cells during routine (unstressed) aerobic growth. MG1655 (wild type), JEM273 (ΔnrdEF), JEM290 (ΔyfaE), and JEM296 (ΔnrdEF ΔyfaE). (B) Viable cells during protracted H2O2 stress. Strains were LC106 (Hpx−), JEM646 (Hpx− ΔnrdEF), JEM325 (Hpx− ΔyfaE), and JEM475 (Hpx− ΔnrdEF ΔyfaE).
Figure 9. In H2O2-stressed cells, imported manganese can poison NrdAB if YfaE is absent.
(A) Cells in anaerobic LB medium were diluted into aerobic medium at time zero. Strains were LC106 (Hpx−), AA30 (Hpx− ΔmntH), JEM475 (Hpx− ΔnrdEF ΔyfaE), and JEM504 (Hpx− ΔnrdEF ΔyfaE ΔmntH). (B) Cells in anaerobic glucose/amino acids medium were diluted into aerobic medium. Where indicated, 50 μM MnCl2 was included. Strains were LC106 (Hpx−) and JEM475 (Hpx− ΔnrdEF ΔyfaE).
The yfaE phenotype was not apparent during iron limitation, as the ΔtonB ΔfeoABC ΔzupT ΔnrdEF mutants continued to replicate their DNA even when yfaE was deleted (data not shown). It is plausible that H2O2 stress is a greater challenge for YfaE-independent metallation either because it oxidizes ferrous iron during the loading process or because it more strongly induces the manganese importer.
Discussion
DNA synthesis in E. coli has long been known to rely upon NrdD, an oxygen-sensitive ribonucleotide reductase, in anaerobic habitats, and NrdAB, an oxygen-dependent isozyme, in aerobic habitats. The goal of this work was to assess the physiological role of a NrdAB homologue, NrdEF. Its function was not apparent, as it is not active under standard laboratory growth conditions. Prior work had hinted that NrdAB might be vulnerable to oxidative stress and that NrdEF might be induced to compensate, but we found that this was not so. Instead, NrdEF is a manganese-dependent enzyme that becomes functional only when iron is scarce; by doing so, it allows DNA synthesis to proceed when NrdAB, an iron-dependent enzyme, cannot be activated.
As this work approached completion, we learned from JoAnne Stubbe that a member of her lab, Joseph Cotruvo, Jr., had isolated biochemically active NrdEF from severely iron-limited E. coli mutants {Cotruvo and Stubbe, 2011. These mutants, like those used in our study, contained mutations that eliminate the major iron-import systems (feo ent fec zupT mntH); in addition, they were cultured with dipyridyl, a cell-permeable iron chelator that further diminishes the availability of intracellular iron. Substantial manganese (100 μM) was added to the medium, and its import through non-specific metal channels presumably compensated for the lack of MntH. Combined EPR and western-blot analyses of cell extracts indicated that about 20% of the NrdEF recovered from these cells contained a tyrosyl radical, and metal-content measurements indicated that the NrdF subunit contained two manganese atoms. Although these culture conditions differ slightly from those used in our own experiments, it seems clear that their biochemical data affirm the conclusions of our physiological, genetic, and EPR results.
The physical features that determine the metal specificities of NrdB and NrdF are unclear; data from other workers established that each of these enzymes can bind either iron or manganese in vitro (Atta et al., 1992; Cotruvo & Stubbe, 2010). Binding constants have not been determined, but the results of this investigation indicate that they can be metallated with either metal in vivo, too. Surprisingly, while both iron and manganese can facilitate tyrosine activation in NrdF, biochemical measurements show that the iron-loaded radical enzyme is significantly less active than the manganese-loaded enzyme (Cotruvo & Stubbe, 2010). The data of Figure 7b and 1a support that conclusion: although the NrdF tyrosyl radical is generated in iron-loaded cells, the enzyme activity is evidently insufficient to drive replication. One possibility is that the electronic effect of the metal upon the radical—which is evidenced by the metal-dependency of the radical EPR spectrum—might control its reduction potential and thereby influence how adept the radical is at abstracting and then returning the cysteinyl electron during the course of the catalytic cycle. In addition, crystal structures of NrdF indicate that despite their similarities, the ligand sphere around iron does not exactly match that around manganese, a structural difference that might similarly degrade electron conduction (Boal et al., 2010). Broadly speaking, both physiological and structural parameters will determine which metals load and activate metalloenzymes in vivo; this question is gaining currency.
Ribonucleotide reductase and iron starvation
E. coli is routinely iron-dependent; in laboratory media in which all biological metals are available, this microbe accumulates high concentrations of total iron (ca. 1 mM) but scant manganese (15 μM) (Anjem et al., 2009). This preference may originate in the conditions of its usual habitat, the large intestine, which is anaerobic and relatively iron-replete. However, once E. coli is excreted into surface waters, iron sources are less reliable. To maintain metabolic activity, adaptations are made when environmental iron is scarce. Iron that has been stored in ferritins is released; siderophores are excreted; and, if the situation is especially dire, the synthesis of dispensable iron-requiring enzymes is suppressed. It is now becoming apparent that another adjustment is made: manganese is imported in order to preserve enzyme activities that might otherwise falter. Some non-redox enzymes rely upon iron as a mononuclear cofactor, and manganese can activate these very well (Ireton et al., 2002; Jain et al., 2005; Chai et al., 2008; Sobota and Imlay, 2011); presumably, the induction of MntH, the manganese transporter, rescues these enzymes. However, such a strategy cannot easily work for enzymes that use iron as a redox cofactor, since the inherent reduction potentials of these metals differ. A coordination environment that poises iron at the optimal potential for a given reaction is unlikely to succeed with manganese. A long-standing example is bacterial superoxide dismutase: enteric bacteria use an iron-SOD as the housekeeping enzyme, but when iron levels are low, its transcripts are degraded, and a distinct isozyme is induced that is functional only with manganese.
The present study reveals that a similar arrangement pertains to ribonucleotide reductase: the manganese-dependent isozyme is induced when iron is scarce. This adaptation is especially striking because it had seemed that the essentiality of ribonucleotide reduction would impose upon bacteria an absolute requirement for iron. One wonders, now, whether enteric bacteria might adapt to dwindling iron levels so efficiently that growth could be established without it. To date Borrelia burgdorferi is the sole organism known to grow under rigorously iron-deplete conditions (Posey & Gherardini, 2000); although it harvests its deoxynucleosides from its host, related Borrelia species employ a ribonucleotide reductase that is a homologue of the E. coli NrdEF enzyme. Lactic acid bacteria, which also eschew major iron-dependent pathways, including the TCA cycle and respiratory chains, also rely on NrdEF homologues.
Iron sequestration is a key host strategy used by mammals to suppress invasion by bacteria, and so would-be pathogens rely upon iron-acquisition and -sparing strategies. NrdEF is found in many of them. An initial interpretation was that its role might be in tolerating the oxidative stress that is created by macrophages; however, the results of the current study suggest that it may help pathogens to tolerate the iron restriction that is imposed by the host.
Ribonucleotide reduction during oxidative stress
It has long been suspected that oxidants might interfere with conventional ribonucleotide reductases. This idea arose when superoxide dismutase (SOD) was determined to be necessary for the aerobic activation of E. coli NrdAB in an in vitro system that utilized flavin reductase as an electron source (Fontecave et al., 1987a). Further investigation determined that the SOD requirement could be circumvented if the loading of ferrous iron occurred anaerobically (Fontecave et al., 1989). Flavin reductase (Fre) itself generates superoxide (Fontecave et al., 1987b), and since superoxide efficiently oxidizes ferrous iron, one explanation for the early data might be that the superoxide introduced in the system by Fre interfered with metal loading, by converting ferrous to ferric iron. A later study showed that superoxide might degrade the tyrosine radical of pre-activated NrdAB in vitro (Gaudu et al., 1996). However, the rate of this reaction was slow, with a half-time of ca. 10 minutes; in contrast, superoxide inactivates iron-sulfur dehydratases within seconds (Flint et al., 1993). Similarly, although H2O2 exposure was observed to gradually quench the tyrosine radical (Fontecave et al., 1987b), the conditions (5 mM × 30 min) far exceeded the micromolar doses that are physiological. Thus our observation that NrdAB retains function in Hpx− and SOD− mutants indicates that this enzyme is not a physiologically important target of these oxygen species.
This finding is chemically reasonable. Hydrogen peroxide does not react with organic radicals; and, although superoxide may be chemically capable of doing so, the tyrosine radical is not solvent-exposed. Ferric iron itself is poorly reactive with H2O2, and we have found no evidence that H2O2 stress oxidizes the thioredoxin/glutaredoxin pool, the electron source for NrdAB. Thus the most obvious opportunity for H2O2 to disrupt NrdAB function might be through interference with the iron-loading stage of NrdB activation. This process is catalyzed, and YfaE is a participant (Hristova et al., 2008). Our data indicate that in wild-type cells iron is delivered to NrdB in a way that precludes problems from H2O2. In contrast, the persistent activity of NrdAB in yfaE mutants presumably arises from the passive, uncatalyzed binding of ferrous iron to nascent apo-NrdB. The data show that H2O2 interferes. In part this is due to the MntH-driven import of manganese, which apparently competes with iron to bind NrdB. The situation is likely aggravated by the action of Dps, an ferritin that sequesters cellular iron as a device to minimize Fenton chemistry (Park et al., 2005; Anjem et al., 2009). Further, the uncatalyzed (YfaE-independent) NrdB-activation process may be slow enough that H2O2 might interfere directly by oxidizing the newly bound ferrous iron, thereby aborting the activation cycle. We observed that YfaE was not essential for NrdAB activation in iron-starved cells absent H2O2 stress (data not shown); it is the combination of H2O2 stress and high manganese that imposes an absolute requirement that YfaE catalyze NrdB activation.
In contrast to NrdB, the wild-type NrdF activation process appears not to effectively exclude its non-cognate metal, iron. Since iron-loaded NrdEF is poorly active, the repression of nrdHIEF by Fur:Fe2+ makes sense. However, what is the physiological logic for nrdHIEF induction by apo-IscR? Plausibly this stems from the role of YfaE, a [2Fe-2S] enzyme, in activating NrdAB. When iron-sulfur synthesis is impeded—the situation that is sensed by IscR—YfaE may become unavailable to activate NrdAB, and so a shift to NrdEF could be salutary. Alternatively, apo-IscR may serve as an adjunct indicator of iron deficiency (Outten et al., 2004). This may be useful in manganese-rich cells, since abundant manganese can restore the repressor activity of Fur protein.
Materials and methods
Reagents
All antibiotics, manganese (II) chloride tetrahydrate, ferrous ammonium sulfate, colbalt(II) chloride hexahydrate, 8-hydroxyquinoline-5-sulphonic acid, desferroxamine, 30% H2O2, E. coli manganese-containing superoxide dismutase, amino acids, casein acid hydrolysate, o-nitrophenyl-β-D-galactopyranoside (ONPG), xanthine, bovine xanthine oxidase, and thymidine were purchased from Sigma. Guanidine hydrochloride, nitroblue tetrazolium, 3-(N-morpholino) propane-sulfonic acid (MOPS), and RNase inhibitor were from Fisher Scientific. Riboflavin and tricine were from Aldrich Chemical Co. Thymidine (methyl-3H) was from MP biomedicals, Inc. DEPC water, hexamer random primer, and superscript II reverse transcriptase were from Invitrogen. Na-acetate, 20% SDS solution, EDTA, saturated phenol, acid phenol-chloroform, nuclease free water, and TE buffer were from Ambion.
Growth conditions
Anaerobic cultures were grown in an anaerobic chamber (Coy Laboratory Products Inc.) under an atmosphere of 85% nitrogen, 10% hydrogen, and 5% carbon dioxide. Aerobic cultures were grown with vigorous shaking. All cultures were grown at 37°C unless specified elsewhere. To ensure that cells were growing exponentially before they were exposed to oxygen, anaerobic overnight cultures of oxygen-sensitive strains were diluted to OD600 = 0.005 into fresh anaerobic medium. After ~5 generations of anaerobic growth, cells were diluted into fresh aerobic medium.
LB medium contained (per liter) 10 g of tryptone, 10 g of NaCl, and 5 g of yeast extract. Standard glucose/amino acids medium contained minimal A salts (Miller, 1972), 1 mM MgSO4, 5 mg/liter thiamine, 0.2 % glucose, 0.2% casamino acids, and 0.5 mM tryptophan. Glucose/aromatic medium contained 0.5 mM aromatic amino acids and 0.5 mM histidine in place of casamino acids and tryptophan. For low-iron experiments, defined medium contained MOPS salts (Neidhardt et al., 1974), 1.32 mM K2HPO4, 5 mg/liter thiamine, 0.2 % glucose, 0.2% casamino acids, and 0.5 mM tryptophan. The MOPS medium was adjusted to pH 7.2 and filter-sterilized before use. Antibiotics were used at concentrations of 100 μg/ml ampicillin, 20 μg/ml chloramphenicol, 30 μg/ml kanamycin, and 12.5 μg/ml tetracycline.
Bacterial strain and strain construction
The strains and plasmids used in this study are described in Table S1. All oxygen-sensitive strains were constructed under anaerobic conditions to ensure that suppressor mutations were not selected during outgrowth. The λ red recombinase method was used to create null mutations (Datsenko & Wanner, 2000). Mutations were introduced into new strains by P1-transduction (Miller, 1972). The resulting mutations were confirmed by PCR analysis or blue/white selection with Xgal. When necessary, the antibiotic cassette was removed by transformation with the temperature-sensitive plasmid pCP20, which encodes FLP recombinase (Datsenko & Wanner, 2000).
Single-copy lacZ transcriptional fusions to the nrdHIEF promoter region were integrated into the λ attachment site, while the wild-type genes remained at their native positions (Haldimann & Wanner, 2001). The promoter region was amplified using the forward primer 5′-CACTGTCTGCAGCAGCTGCGGTAGGTAGGTA-3′ or 5′-GTCCGTTCGACCTGCAGTTGCTATATATTGTGTGGTT-3′ and the reverse primers 5′-GTGCATAGGAATTCGCATGATTCGTATTTCCG-3′ or 5′-AGCATCGAATTCAGCGCGTGGTAATCCATCGT-3′ that were designed with PstI and EcoR1 restriction sites. The plasmid pAH125 was modified by replacing the kanamycin-resistance cassette with a chloramphenicol-cassette flanked by FLP sites, to permit antibiotic selection under anaerobic conditions. The promoter region was inserted into pSJ501, and the resulting plasmid was confirmed by restriction analysis and sequencing. After chromosomal integration and P1-transduction into recipient strains, the chloramphenicol-cassette was removed via pCP20.
Targeted single-copy transcriptional and translational lac fusions to the genes of the nrdHIEF operon were made using λ red and FLP-mediated site-specific recombination (Ellermeier et al., 2002). Chromosomal mutations were created using the λ red recombinase method and pKD13, which contains a FRT flanked kanamycin-resistant cassette for in frame deletions. After confirmation and P1-transduction into a Lac− strain, the kanamycin-cassette was removed via pCP20 at 30°C. The resultant kanamycin-sensitive strain contained a single FRT site in the desired chromosomal location and retained pCP20. Construction of lac fusions were made by transformation and integration of pKG137 (transcriptional lac fusion) or pCE40 (in frame translational lac fusion) into the chromosome at the FRT site. Transformants were recovered in LB broth and selected on LB kanamycin agar plates at 37°C. Growth at 37°C blocks the replication of the temperature-sensitive plasmid pCP20. Isolates that contained stable integration of the fusion plasmid were kanamycin-resistant, ampicillin-sensitive, and Lac+ on Xgal. Colony PCR was used to confirm that single plasmid integrants were in the correct orientation and chromosomal location. P1-transduction was used to move the lac fusions into recipient strains.
For the construction of tetracycline inducible genes, we used the λ red recombinase method to replace the nrdHIEF and mntH promoter regions with tetRA (Merighi et al., 2005). The coding sequence of tetRA was amplified with the following primers 5′-GCTATGTTGTGTATGGAAGCTGAAAGTTATGTAAATGTGCTAGGTGGGTACGTTC-3′ and 5′-GCTGCTACTCTCAACGCGATAGTTCGTCATCTTCTCCTTGGTGACGAAATAA-3 or 5′-CACTGTTAAGCATGCGCAAATCGTAGTGCAAAAATGATATAGGTGGGTACGTTG-3′ and 5′-TACGGCGCGATGATACGCGTCGGGTTGTCTTCTCTGTTGATTTGGTGACGAAATAA-3′ which are flanked by sequences from the mntH or nrdHIEF promoter regions, respectively. Products were electrophoresed on a 1% agarose gel. Resulting bands were excised, purified, and transformed into strains harboring the temperature-sensitive plasmid pKD46. Transformants were selected on LB containing tetracycline, the resulting mutations were confirmed by PCR analysis, and P1-transduction was used to move Ptet – fusions into recipient strains.
Cell viability
Anaerobic overnight cultures were diluted to OD600 = 0.005 into fresh anaerobic medium. After ~5 generations of anaerobic growth, cells were diluted to OD600 = 0.0025 into fresh aerobic medium and grown aerobically at 37°C with vigorous shaking. At intervals, aliquots of cells were removed and serially-diluted into aerobic medium. The diluted samples were mixed with anaerobic top agar and poured onto anaerobic medium agar plates. Colonies formed were counted after 24 (LB medium) or 48 hours (defined medium) of anaerobic incubation at 37°C.
Enzyme assays
Aerobic cultures were grown to an OD600 of 0.25 in fresh medium, after which cells were centrifuged, washed, resuspended, and lysed by French press in cold 50 mM Tris-HCl buffer, pH 8. β-galactosidase activities in cell extracts were determined by ONPG hydrolysis (Miller, 1972).
For SOD activity measurements, aliquots of aerobic cultures grown in MOPS glucose medium supplemented with casamino acids/tryptophan were taken at intervals over time, after which cells were centrifuged, washed, resuspended, and lysed by French press in cold 50 mM potassium phosphate buffer (pH 7.8). SOD activity was measured in cell extracts using the xanthine oxidase/cytochrome c method ((McCord & Fridovich, 1969)). To track the activity of the MnSOD, we used a sodB mutant. The fraction of MnSOD that was active in the cell extracts was determined after extracts were subjected to partial denaturation and renaturation in the presence of manganese to ensure full activation of MnSOD protein (Kirby et al., 1980; Anjem et al., 2009). Briefly, MnSOD was denatured at pH 3.8 in the presence of 5 mM Tris, 2.5 M guanidinium chloride, 20 mM 8-hydroxyquinoline-5-sulphonic acid, and 0.1 mM EDTA, then renatured at pH7.8 in the presence of 5 mM Hepes and 0.1 mM MnCl2. Excess metal was removed by dialysis at pH 7.8 in 5 mM Tris and 0.1 mM EDTA. Purchased E. coli manganese-containing SOD was used as a control for the reconstitution procedure. For both enzymes assays, total protein content was determined using the Coomassie blue dye-binding assay (Thermo Scientific).
Disk diffusion assay
Anaerobic overnight cultures were diluted to OD600 = 0.005 into fresh anaerobic minimal A glucose medium supplemented with aromatic amino acids/histidine. After ~5 generations of anaerobic growth, cells were harvested by centrifugation. The cells were suspended in 1/10 the original volume with minimal A glucose medium supplemented with aromatic amino acids/histidine. Concentrated cells were spread plated aerobically onto minimal A glucose agar plates supplemented with aromatic amino acids/histidine. Sterile paper disks impregnated with 5 μmoles of H2O2 or 5 nmoles metal solution were placed on each plate. The plates were incubated for 48 hours aerobically at 37°C.
Total thymidine incorporation
Thymine auxotrophs (thyA null mutants) were constructed, in order to incorporate thymidine linearly for extended periods of time. P1 transduction was used to move the thyA-null mutation into recipient strains. Transductants were selected on minimal A glucose medium supplemented with casamino acids/tryptophan, 50 μg/ml thymidine, and chloramphenicol. The resultant thyA mutants were unable to grow on defined medium unless supplemented with thymidine.
Anaerobic overnight cultures were diluted to OD600 = 0.005 into fresh anaerobic minimal A glucose medium supplemented with aromatic amino acids/histidine and 50 μg/ml thymidine. After ~5 generations of anaerobic growth, cells were harvested by centrifugation and suspended in 1/10 the original volume with warm medium. Concentrated cells were diluted to OD600 = 0.025 into aerobic minimal A glucose medium supplemented with aromatic amino acids/histidine and 32 μM thymidine/3H-thymidine (1 μCi/ml) with vigorous shaking. At intervals, aliquots of cells were mixed with cold 5% trichloroacetic acid (TCA) to stop the labeling reaction and stored on ice until all intervals were collected. Lysed cells were filtered through a G6 glass fiber filter using a manifold. Filters were washed with cold 5% TCA followed by cold 100% ethanol, then spotted with 0.1 M KOH to quench intrinsic fluorescence during counting. Filters were air dried and the radioactivity was counted using a Coulter multipurpose scintillation counter (Beckman, #LS6500).
Whole-cell EPR measurements
In vivo EPR samples were prepared with strains that overproduce NrdB or NrdF (Hristova et al., 2008). To overexpress the structural genes, 5 mM (for LB) or 20 mM arabinose (for defined medium) was added to aerobic cultures when cells reached OD600 = 0.25. After another 2 hours of incubation, the cells were harvested by centrifugation and washed two times in cold buffer A [100 mM Tris, 150 mM NaCl, and 5% glycerol (pH 7.6)]. The cell pellet was resuspended in 1/250of the original volume in buffer A. A cell suspension (250 μl) was incubated with H2O2 for 5 minute at 37°C. As a control, a cell suspension was incubated with 160 mM hydroxyurea for 1 hour at 37°C (Hristova et al., 2008). The cell suspension was then transferred into an EPR tube and frozen in dry ice/ethanol. EPR spectra of the NrdB tyrosyl radical were obtained at the following settings: 9.08 GHz frequency; 110 K temperature; 0.2 mW power, 4 G modulation amplitude, 0.032 s time constant, and 10 scans at 30 s per scan. Spectra of the NrdF signal were obtained at 0.5 mW power. Due to interactions of the metal centers with the tyrosyl radicals, the spectra of manganese- and iron-loaded enzymes differ substantially in field position and sharpness (Cotruvo and Stubbe, 2010).
RNA isolation
Anaerobic overnight cultures were diluted to OD600 = 0.005 into LB medium. After ~5 generations of anaerobic growth, cells were moved to aerobic conditions and vigorously shaken until cells reached OD600 = 0.45. Total RNA was isolated from cells by hot phenol extraction. Briefly, an aliquot of cells was mixed with pre-warmed fresh 8x lysis solution (0.32 M Na-acetate pH 5.5, 0.4% SDS, 16 mM EDTA pH 8 in DEPC water) and incubated at 65°C for 15 minutes with vigorous shaking. After microcentrifugation at room temperature for 10 minutes, the aqueous-layer was transferred to a clean tube with phenol-chloroform. Repeated phenol-chloroform extractions were performed until the interface was clean. The aqueous-layer was then transferred to ethanol and incubated at −80°C overnight. Samples were microcentrifuged at max speed for 10 minutes at 4°C. The supernatant was discarded and the precipitated RNA was rinsed with cold 75% ethanol by gentle palpitation. The RNA pellet was air dried, suspended in 10mM Tris/1mM EDTA (RNase free) buffer, pH 7, and stored at −80°C.
Contaminating DNA was removed from isolated RNA by rigorous DNase treatment using the Turbo DNA-free™ kit (Ambion, #AM1907) according to the manufacturer’s directions. The product was amplified by PCR using 2 μl aliquot and subjected to gel-electrophoresis. The absence of product confirmed that the RNA samples were DNA-free. Clean RNA was then converted to cDNA by reverse-transcription. Briefly, RNA (1 μg) was mixed with 20 nmoles dNTPs in 12 μl ddH2O and incubated at 65°C for 5 minutes, then quenched on ice. DTT (9mM), 5x first strand buffer, 40 units RNase inhibitor, and 3 ng of hexamer random primer were added to the RNA solution and incubated at room temperature for 2 minutes. 200 units superscript II reverse transcriptase was added and the reaction was incubated for 10 minutes at room temperature, and then moved to 42°C for 50 minutes. The reaction was inactivated at 70°C for 15 minutes and stored at −20°C.
Quantitative real-time PCR
PCR amplification was carried out using the primer pairs listed in Table S2. gapA served as the housekeeping gene. PCR amplification was performed in a mixture (25 μl final volume) containing 2x iQ™ Sybr green supermix (Qiagen, #170-8882), 800 nM each primer, and 1–10 ng cDNA. Cycling conditions were as follows: 95°C/3 minutes; 40 cycles of 94°C/1 minute, 55°C/45 s, 70°C/45 s. The cDNA was amplified using primers listed in Table S2. PCR outcomes were normalized to the gapA gene and relative transcription levels were calculated by comparison of the ratio of treated to non-treated cells.
5′ RLM-RACE
5′ RNA ligase mediated-rapid amplification of cDNA ends (RLM-RACE) was carried out using the First Choice RLM-RACE kit (Ambion, #AM1700) according to the manfacturer’s directions. RNA was extracted from cells and treated with DNase as described earlier. However, the reaction was terminated by bringing the reaction volume up to 200 μl with DEPC water and the addition of an equal amount of acid phenol-chloroform. The samples were microcentrifuged at maximum speed for 5 minutes at room temperature. The aqueous-layer was transferred to a clean tube and the RNA was precipitated in 100% ethanol containing 80 mM Na-Acetate at −80°C for 10 minutes. Precipitated RNA was pelleted by microcentrifugation, air dried, and then resuspended in 15 μl nuclease free water. Approximately 1.2 μg of DNA-free RNA was reserved for the ‘Minus-Tobacco Acid Pyrophosphatase (TAP)’ control reaction which was used to assess whether the products produced by 5′RLM-RACE are true. The remaining DNA-free RNA (3 μg) was treated with TAP to remove the phosphate from full length mRNA. The 5′RACE adapter was ligated to monophosphate mRNA, and then reverse transcribed to make cDNA. Two subsequent nested PCRs were performed using the 5′RACE adapter primers and a gene-specific reverse primer 5′-TGTGGCGCTGGATGCAGACGGTTAAT-3′, 5′-AATCACTACCGGCAACTGACGAAAGC-3′, 5′-TCTACCTGAATCCGTTCCCGCTCATT-3′, and 5′-GTGTTTTCGGAGCTGCTGGAGAAGTA-3′. Cycling conditions were as follows: 94°C/3 minutes; 35 cycles of 94°C/30 s, 65°C/30 s, 72°C/60 s; 72°C/7 minutes. Products were run on a 2% nusieve agarose gel. Resulting bands were excised, gel purified using Qiaquick gel extraction kit (Qiagen, #28704), and sequenced.
Supplementary Material
Acknowledgments
We are grateful to Chris Rensing (University of Arizona), Jim Slauch (University of Illinois at Urbana-Champaign), and Tricia Kiley (University of Wisconsin-Madison) for providing key strains and insights and to Mark Nilges for his assistance with EPR analyses. We greatly appreciate the willingness of Joseph Cotruvo, Jr., and JoAnne Stubbe to allow us to cite their unpublished results, as well as their helpful comments on this manuscript. This work was supported by NIH GM49640. J.M. is supported by an NIH Chemistry-Biology Interface Training Program (GM070421).
References
- Abbouni B, Oehlmann W, Stolle P, Pierik AJ, Auling G. Electron paramagnetic resonance (EPR) spectroscopy of the stable-free radical in the native metallo-cofactor of the manganese-ribonucleotide reductase (Mn-RNR) of Corynebacterium glutamicum. Free Radic Res. 2009;43:943–950. doi: 10.1080/10715760903140568. [DOI] [PubMed] [Google Scholar]
- Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcriptional factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta M, Nordlund P, Aberg A, Eklund H, Fontecave M. Substitution of manganese for iron in ribonucleotide reductase from Escherichia coli. Spectroscopic and crystallographic characterization. J Biol Chem. 1992;267:20682–20688. [PubMed] [Google Scholar]
- Boal AK, Cotruvo JA, Jr, Stubbe J, Rosenzweig AC. Structural basis for activation of class Ib ribonucleotide reductase. Science. 2010;329:1526–1530. doi: 10.1126/science.1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NC, Eliasson R, Reichard P, Thelander L. Nonheme iron as a cofactor in ribonucleotide reductase from Escherichia coli. Biochem Biophys Res Commun. 1968;30:522–527. doi: 10.1016/0006-291x(68)90083-1. [DOI] [PubMed] [Google Scholar]
- Chai SC, Wang WL, Ye ZQ. Fe(II) is the native cofactor for Escherichia coli methionine aminopeptidase. J Biol Chem. 2008;283:26879–26885. doi: 10.1074/jbc.M804345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan I, Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993;175:1687–1696. doi: 10.1128/jb.175.6.1687-1696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotruvo JA, Jr, Stubbe J. NrdI, a flavodoxin involved in maintenance of the diferric-tyrosyl radical cofactor in Escherichia coli class Ib ribonucleotide reductase. Proc Natl Acad Sci USA. 2008;105:14383–14388. doi: 10.1073/pnas.0807348105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotruvo JA, Jr, Stubbe J. An active dimanganese(III)-tyrosyl radical cofactor in Escherichia coli class Ib ribonucleotide reductase. Biochemistry. 2010;49:1297–1309. doi: 10.1021/bi902106n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotruvo JA, Jr, Stubbe J. Escherichia coli class Ib ribonucleotide reductase contains a dimanganese(III)-tyrosyl radical cofactor in vivo. Biochemistry. 2011 doi: 10.1021/bi101881d. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K, Engle EC, Manes SH. DNA gyrase on the bacterial chromosome: possibility of two levels of action. Proc Natl Acad Sci USA. 1980;77:6879–6883. doi: 10.1073/pnas.77.11.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg A, Reichard P. Electron spin resonance of the iron-containing protein B2 from ribonucleotide reductase. J Biol Chem. 1972;247:3485–3488. [PubMed] [Google Scholar]
- Ellermeier CD, Janakiraman A, Slauch JM. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene. 2002;290:153–161. doi: 10.1016/s0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- Fieschi F, Torrents E, Toulokhonova L, Jordan A, Hellman U, Barbe J, Gibert I, Karlsson M, Sjoberg BM. The manganese-containing ribonucleotide reductase of Corynebacterium ammoniagenes is a class Ib enzyme. J Biol Chem. 1998;273:4329–4337. doi: 10.1074/jbc.273.8.4329. [DOI] [PubMed] [Google Scholar]
- Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- Fontecave M, Eliasson R, Reichard P. NAD(P)H:flavin oxidoreductase of Escherichia coli. A ferric iron reductase participating in the generation of the free radical of ribonucleotide reductase. J Biol Chem. 1987b;262:12325–12331. [PubMed] [Google Scholar]
- Fontecave M, Eliasson R, Reichard P. Enzymatic regulation of the radical content of the small subunit of Escherichia coli ribonucleotide reductase involving reduction of its redox centers. J Biol Chem. 1989;264:9164–9170. [PubMed] [Google Scholar]
- Fontecave M, Graslund A, Reichard P. The function of superoxide dismutase during the enzymatic formation of the free radical of ribonucleotide reductase. J Biol Chem. 1987a;262:12332–12336. [PubMed] [Google Scholar]
- Garriga X, Eliasson R, Torrents E, Jordan A, Barbe J, Gibert I, Reichard P. nrdD and nrdG genes are essential for strict anaerobic growth of Escherichia coli. Biochem Biophys Res Commun. 1996;229:189–192. doi: 10.1006/bbrc.1996.1778. [DOI] [PubMed] [Google Scholar]
- Gaudu P, Niviere V, Petillot Y, Kauppi B, Fontecave M. The irreversible inactivation of ribonucleotide reductase from Escherichia coli by superoxide radicals. FEBS Lett. 1996;387:137–140. doi: 10.1016/0014-5793(96)00480-2. [DOI] [PubMed] [Google Scholar]
- Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. IscR-dependent gene espression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol Microbiol. 2006;60:1058–1075. doi: 10.1111/j.1365-2958.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- Gon S, Faulkner MJ, Beckwith J. In vivo requirement for glutaredoxins and thioredoxins in the reduction of the ribonucleotide reductases of Escherichia coli. Antiox Redox Signal. 2006;8:735–742. doi: 10.1089/ars.2006.8.735. [DOI] [PubMed] [Google Scholar]
- Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol. 2005;187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg I, Shteinberg T, Hassan AQ, Aharonowitz Y, Borovok I, Cohen G. Functional analysis of the Streptomyces coelicolor NrdR ATP-cone domain: role in nucleotide binding, oligomerization, and DNA interactions. J Bacteriol. 2009;191:1169–1179. doi: 10.1128/JB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann A, Wanner BL. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol. 2001;183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogbom M, Andersson ME, Nordlund P. Crystal structures of oxidized dinuclear manganese centres in Mn-substituted class I ribonucleotide reductase from Escherichia coli: carboxylate shifts with implications for O2 activation and radical generation. J Biol Inorg Chem. 2001;6:315–323. doi: 10.1007/s007750000205. [DOI] [PubMed] [Google Scholar]
- Hristova D, Wu CH, Jiang W, Krebs C, Stubbe J. Importance of the maintenance pathway in the regulation of the activity of Escherichia coli ribonucleotide reductase. Biochemistry. 2008;47:3989–3999. doi: 10.1021/bi702408k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton GC, McDermott G, Black ME, Stoddard BL. The structure of Escherichia coli cytosine deaminase. J Mol Biol. 2002;315:687–697. doi: 10.1006/jmbi.2001.5277. [DOI] [PubMed] [Google Scholar]
- Jain R, Hao B, Liu RP, Chan MK. Structures of Escherichia coli peptide deformylase bound to formate: insight into the preference for Fe2+ over Zn2+ as the active site metal. J Am Chem Soc. 2005;127:4558–4559. doi: 10.1021/ja0503074. [DOI] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol. 2010;78:1448–1467. doi: 10.1111/j.1365-2958.2010.07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A, Aragall E, Gibert I, Barbe J. Promoter identification and expression analysis of Salmonella typhimurium and Escherichia coli nrdEF operons encoding one of two class I ribonucleotide reductases present in both bacteria. Mol Microbiol. 1996;19:777–790. doi: 10.1046/j.1365-2958.1996.424950.x. [DOI] [PubMed] [Google Scholar]
- Jordan A, Aslund F, Pontis E, Reichard P, Holmgren A. Characterization of Escherichia coli NrdH. A glutaredoxin-like protein with a thioredoxin-like activity profile. J Biol Chem. 1997;272:18044–18050. doi: 10.1074/jbc.272.29.18044. [DOI] [PubMed] [Google Scholar]
- Jordan A, Gibert I, Barbe J. Cloning and sequencing of the genes from Salmonella typhimurium encoding a new bacterial ribonucleotide reductase. J Bacteriol. 1994;176:3420–3427. doi: 10.1128/jb.176.11.3420-3427.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+) J Bacteriol. 2002;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T, Blum J, Kahane I, Fridovich I. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Arch Biochem Biophys. 1980;201:551–555. doi: 10.1016/0003-9861(80)90544-5. [DOI] [PubMed] [Google Scholar]
- Lee KC, Yeo WS, Roe JH. Oxidant-responsive induction of the suf operon, encoding a Fe-S assembly system, through Fur and IscR in Escherichia coli. J Bacteriol. 2008;190:8244–8247. doi: 10.1128/JB.01161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- McHugh JP, Rodrigues-Quinones F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, Andrews SC. Global iron-dependent gene regulation in Escherichia coli. J Biol Chem. 2003;278:29478–29486. doi: 10.1074/jbc.M303381200. [DOI] [PubMed] [Google Scholar]
- Merighi M, Ellermeier CD, Slauch JM, Gunn JS. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J Bacteriol. 2005;187:7407–7416. doi: 10.1128/JB.187.21.7407-7416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. [Google Scholar]
- Monje-Casas F, Jurado J, Prieto-Alamo MJ, Holmgren A, Pueyo C. Expression analysis of the nrdHIEF operon from Escherichia coli. Conditions that trigger the transcript level in vivo. J Biol Chem. 2001;276:18031–18037. doi: 10.1074/jbc.M011728200. [DOI] [PubMed] [Google Scholar]
- Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbit AD, Giel JL, Rose JC, Kiley PJ. Sequence-specific binding to a subset of IscR-regulated promoters does not require IscR Fe-S cluster ligation. J Mol Biol. 2009;387:28–41. doi: 10.1016/j.jmb.2009.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- Panosa A, Roca I, Gibert I. Ribonucleotide reductases of Salmonella typhimurium: transcriptional regulation and differential role in pathogenesis. PLoS ONE. 2010;5:e11328. doi: 10.1371/journal.pone.0011328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc Natl Acad Sci USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- Pueyo C, Jurado J, Prieto-Alamo MJ, Monje-Casas F, Lopez-Barea J. Multiplex reverse transcription-polymerase chain reaction for determining transcriptional regulation of thioredoxin and glutaredoxin pathways. Methods Enzymol. 2002;347:441–451. doi: 10.1016/s0076-6879(02)47044-9. [DOI] [PubMed] [Google Scholar]
- Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, Beinert H, Kiley PJ. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci USA. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojiljkovic I, Baumler AJ, Hantke K. Fur regulon in Gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- Torrents E, Grinberg I, Gorovitz-Harris B, Lundstrom H, Borovok I, Aharonowitz Y, Sjoberg BM, Cohen G. NrdR controls differential expression of the Escherichia coli ribonucleotide reductase genes. J Bacteriol. 2007;189:5012–5021. doi: 10.1128/JB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S, Wu A, Park S, Imlay KRC, Imlay JA. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol. 2007;64:822–830. doi: 10.1111/j.1365-2958.2007.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassinova N, Kozyrev D. A method for direct cloning of Fur-regulated genes: identification of seven new Fur-regulated loci in Escherichia coli. Microbiology. 2000;146(Pt 12):3171–3182. doi: 10.1099/00221287-146-12-3171. [DOI] [PubMed] [Google Scholar]
- Wu CH, Jiang W, Krebs C, Stubbe J. YfaE, a ferredoxin involved in diferric-tyrosyl radical mainenance in Escherichia coli ribonucleotide reductase. Biochemistry. 2007;46:11577–11588. doi: 10.1021/bi7012454. [DOI] [PubMed] [Google Scholar]
- Yeo WS, Lee JH, Lee KC, Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol Microbiol. 2006;61:206–218. doi: 10.1111/j.1365-2958.2006.05220.x. [DOI] [PubMed] [Google Scholar]
- Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.