Abstract
The proportion of elderly in the general population is rising, resulting in greater numbers of drivers with neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). These neurodegenerative disorders impair cognition, visual perception, and motor function, leading to reduced driver fitness and greater crash risk. Yet medical diagnosis or age alone is not reliable enough to predict driver safety or crashes, or revoke the driving privileges of these drivers. Driving research utilizes tools such as questionnaires about driving habits and history, driving simulators, standardized road tests utilizing instrumented vehicles, and state driving records. Research challenges include outlining the evolution of driving safety, understanding the mechanisms of driving impairment, and developing a reliable and efficient standardized test battery for prediction of driver safety in neurodegenerative disorders. This information will enable healthcare providers to advise their patients with neurodegenerative disorders with more certainty, affect policy, and help to develop rehabilitative measures for driving.
2. Introduction
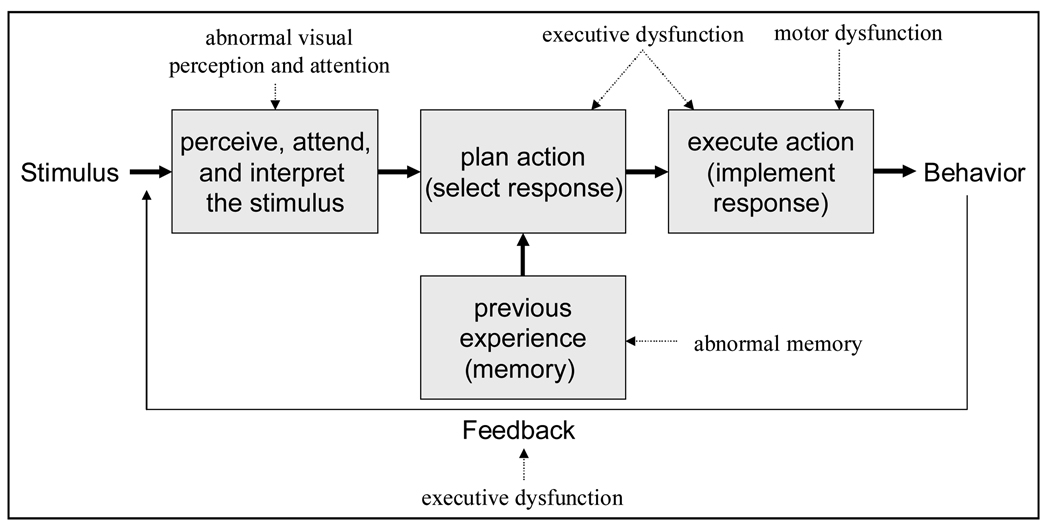
The number of older drivers at risk for neurodegenerative disorders is increasing with aging population trends. In this article, we will concentrate on Parkinson’s disease (PD) and Alzheimer’s disease (AD) due to their relative public health importance and large body of research on driving in these disorders. These conditions impair cognition, visual perception, and motor function, which can increase risk of crashes by interrupting driver information processing at several levels (Figure 1). These levels include: 1) Perception and attention to stimulus evidence (e.g., through sensory inputs) and interpretation of the situation on the road; 2) Formulation of a plan based on the particular driving situation and relevant previous experience or memory; 3) Execution of an action (e.g., by applying the accelerator, brake, or steering controls); and 4) Monitoring the outcome of the behavior as a source of potential feedback for subsequent corrective actions. The driver’s behavior is either safe or unsafe as a result of errors at one or more of these stages in the driving task. The risk of driver errors increases with deficits of attention, perception, executive and motor functions and awareness of cognitive and behavioral performance. Individuals with impairments in these domains are more likely than unimpaired drivers to commit errors that cause motor vehicle crashes. They are also less likely to monitor and recognize their errors or impairments, and less likely to take corrective actions [1].
Figure 1.
Information processing model for driver error in neurodegenerative disorders.
Establishing fair and accurate performance criteria to predict driving ability in AD and PD will help reduce the risk of motor vehicle crashes and protect the mobility and independence of these patients by avoiding unfair license revocation. Although a clinician may be able to identify potentially hazardous drivers, a clinician's assessment alone is not accurate enough to determine driving competence in drivers with mild cognitive impairment (e.g., CDR 0.5) or early dementia [2;3]. Type and degree of cognitive, visual, and motor impairments are better predictors of driving skills than age or medical diagnosis. The effects of in these performance impairments in AD and PD can be studied using driving records and experimental drives employing instrumented vehicles or simulators.
3. Main Body
3.1 Epidemiology
3.1.1 Parkinson’s disease
PD has a prevalence of ~0.3% in the general population and 3% in those over the age of 65[4]. PD has been recognized primarily as a motor disorder causing tremors, bradykinesia, rigidity, gait and balance problems. However, many patients with PD, including those in the early stages of the disease, suffer from cognitive (especially executive and visuospatial) dysfunction [5]. Besides motor, cognitive, and visual impairments that can affect driving, excessive daytime sleepiness (EDS) is common in PD, especially in those with advanced disease, cognitive impairment and hallucinations, and can occur with any dopaminergic medication [6]. A large survey using the modified Epworth Sleepiness Scale (ESS) scores found EDS in 51%, but sudden onset of sleep was rare [7].
There are no well established epidemiologic data on crash risk in PD [8], however, PD appears to be associated with decreased driving and increased crashes, especially in those with worse motor and cognitive dysfunction. A referral center based study compared driving history and habits of 150 patients with PD and 100 controls [9] and found that 20% of patients had stopped driving because of PD; increased disability was associated with decreased driving. The frequency of crashes in subjects with more severe PD (Hoehn and Yahr [HY] stage III) was five-fold higher than controls, whereas patients with mild PD (HY I) reported almost a twice as many crashes as the controls. The presence of cognitive impairment (i.e., a MMSE score of 23 or less) was associated with a three-fold increased crash rate. A large survey in Germany revealed that 82 % of the PD patients held a driving license, and 60% of them still participated in traffic. Of the patients holding a driving license, 15% had been involved in and 11% had caused at least one accident during the past 5 years. The risk of crashes significantly increased for patients who felt moderately impaired by PD, had an increased ESS score, and had experienced “sleep attacks” while driving. Those having retired from driving had a more advanced (subjective) disease severity, higher age, more frequently female gender, an increased ESS score, and longer disease duration. Real sleep attacks without any prior sleepiness were rare [10].
3.1.2 Alzheimer’s disease
Alzheimer's disease is the most common form of dementia, accounting for 50–60% of all cases, with exponentially increased prevalence with older age (24%–33% in ≥ 85 years old) [11]. Per projections based on the Ontario Ministry of Transportation driving data, census data, and dementia prevalence data, the number of senior drivers with dementia has grown from just under 15,000 in 1986 to about 34,000 in 2000 and will number nearly 100,000 in 2028. [12]. A survey of AD patients and age-matched, nondemented controls showed a mean of 0.091 reported crashes per year per person compared to 0.040 in matched controls in the same period of time. The average number of crashes per year in AD increased dramatically after the first 3 years [13], consistent with others reports showing a sharp decline in driving abilities 3 years after AD onset [14;15]. A review panel found that drivers with mild cognitive impairment (MCI) very mild AD (Clinical Dementia Rating Scale [CDR] = 0.5) have impairments similar to drivers aged 16–21 or those driving under the influence of alcohol at a blood alcohol concentration < 0.08%, and recommended to reassess dementia severity and driving fitness every 6 months in very mild AD, but discontinuation of driving in mild AD (CDR =1) due history of increased crashes and poor driving performance [16]. However this recommendation may be unduly restrictive [3].
Incipient AD may also contribute to fatal crashes of older drivers. A neuropathological study on the brains of 98 fatally injured aged drivers showed that 50% of drivers in the age group of 65–75 and 72% of drivers over 75 years of age had changes characteristic of AD (i.e. neuritic and diffuse plaques, and neurofibrillary tangles in the parietal and frontal cortices). In 14% of all killed drivers the number of neuritic plaques was diagnostic of AD, and in an additional 33%, the plaque count suggestive of AD. Neuropathological AD changes were most common in the brains of drivers killed in single vehicle crashes, followed by multivehicle crashes at intersections and least common in multivehicle crashes elsewhere. In a great majority (~85%) of cases the killed aged driver was the guilty party of the crash [17].
3.1.3 Driving performance
3.1.3.1 Road tests
3.1.3.1.1 Parkinson’s disease
A retrospective study of 154 consecutive drivers with PD referred to a driving assessment center revealed that 104 (66%) were able to continue driving although 46 individuals required an automatic transmission and 10 others needed car modifications [18]. Ability to drive was predicted by the severity of disease, age, presence of other associated medical conditions (particularly dementia), duration of disease, brake reaction time, and score on a driving test. The most important features in distinguishing safety to drive were motor severity of PD (HY stage III), reaction time, moderate disease (HY III) associated with another medical condition, and high score on the road testing.
Prospective studies found that, while there were individuals within normal range, drivers with PD as a group performed worse on various driving tasks compared to controls of similar age. Usually, a combination of cognitive, visual, and sometimes motor impairments predicted the outcome. A multidisciplinary evaluation of 20 patients with idiopathic PD and 20 age- and gender-matched healthy control subjects showed that drivers with PD performed worse than the controls both in the neuropsychologic tests and in the driving test on the road. Driving in a traffic flow was a considerably more difficult task for the participants with PD, as well as turning across traffic. There was a high correlation between performance in the neuropsychology battery and performance in the driving test. In PD patients, information processing was markedly slow and correlated with faults observed while driving on the road. Disease duration and the motor stage of the disease, and MMSE were not associated with the driving test. Patients themselves were not capable of reliably evaluating their own driving ability. The neurologist overestimated the driving ability in 35% of patients with PD [19].
The driving performance of 25 patients with idiopathic PD and 21 age matched controls was assessed on a standardized open road route by an occupational therapist and driving instructor. The drivers with PD were rated as significantly less safe than controls, and more than half of the drivers with PD would not have passed a state based driving test. Drivers with PD made significantly more errors than the control group during maneuvers that involved changing lanes and lane keeping, monitoring their blind spot, reversing, car parking, and traffic light controlled intersections. The driving instructor also had to intervene to avoid an incident significantly more often for drivers with PD than for controls [20]. The tests on motor performance (Purdue Pegboard test), contrast sensitivity (Pelli-Robson test), and cognitive function (verbal version of Symbol Digit Modalities test), and time since diagnosis predicted passes with relatively high sensitivity and specificity [21].
Using a modified version of the Washington University Road Test, 11 of 25 drivers with PD were found to be marginally safe or unsafe [22]. Poorer driving performance was associated with worse performance on measures of CS, visuospatial constructions, set shifting, and attention; a composite measure of executive functioning and visuospatial abilities was the most important predictor.
Using an instrumented vehicle (IV), Uc et al. compared drivers with PD and controls on various driving tasks. IVs permit quantitative assessments (of driver performance in the field, in a real car, under actual road conditions. These natural or naturalistic measurements are not subject to the type of human bias that affects inter-rater reliability on a standard road test. Seventy-seven subjects with mild-moderate PD and 152 neurologically normal elderly adults, all active and licensed drivers, were tested with a battery of visual, cognitive and motor tests of abilities. All group comparisons were adjusted for age, gender, education and familiarity with the region. Drivers with PD performed significantly worse on cognitive, visual and motor tests compared to controls, and took longer to finish the route on a navigation task. Higher proportions of these drivers made incorrect turns, got lost, or committed at-fault safety errors [23]. These drivers were also asked to report sightings of specific landmarks and traffic signs along a four-lane commercial strip to assess the ability for visual search and recognition of roadside targets. The drivers with PD identified significantly fewer targets and committed more at-fault safety errors during the task than control subjects, even after adjusting for baseline errors [24]. During the same IV drive, at-fault safety errors and vehicle control measures were determined on an uneventful baseline segment and during an auditory-verbal distracter task (Paced Auditory Serial Addition Task-PASAT) while they drove on a four-lane interstate freeway. The odds of increase in safety errors due to distraction was higher in the PD group even after adjusting for baseline errors, level of engagement in PASAT, sex, and education [25]. Within the PD group, the task performance and safety errors during these 3 experiments were predicted by poor performances on cognitive and visual tests such, but not by the severity of motor dysfunction.
3.1.3.1.2 Alzheimer’s disease
A prospective longitudinal study assessed on-road driving performance in 58 healthy older adults and those with 21 very mild (CDR=0.5) and 29 mild (CDR=1) AD. Mild AD group progressed faster on receiving a rating of not safe on the driving test than healthy controls; very mild AD fell between those of these two groups [26]. A similar observation was made in 128 older drivers, including 84 with early AD and 44 age-matched control subjects, who underwent repeated assessments of their cognitive, neurologic, visual, and physical function over 3 years [3]. At baseline, subjects with AD had experienced more accidents and performed more poorly on the road test compared to controls; however, the majority of them passed the road test. Over time, both groups declined in driving performance on the road test, with subjects with AD declining more than controls. Greater severity of dementia, increased age, and lower education at baseline were associated with higher rates of failure and poor performance [3].
Thirty-two subjects with mild AD and 136 neurologically normal older adults performed a route-finding task administered on the road in an IV. The drivers with mild AD made significantly more incorrect turns, got lost more often, and made more at-fault safety errors than control subjects, although their basic vehicular control abilities were normal [27]. These drivers also identified significantly fewer landmarks and traffic signs and made more at-fault safety errors during the task than control subjects during a visual search task [28]. The navigational abilities and the performance on visual search and recognition of roadside targets, and safety errors were predicted by scores on standardized tests of visual and cognitive function.
A meta-analysis of 27 primary studies to examine the relationship between cognition and driving ability for adults with dementia showed significant relationship between cognitive measures and on-road or non-road driving measures for all reported domains when control groups were included in the correlation analyses. Caregiver reports of driving ability and cognitive variables were correlated significantly only on measures of mental status and visuospatial skills. Only moderate correlations were observed between performance on tests of visuospatial skills and attention/concentration, the road test outcomes within the demented group [29].
3.1.3.2 Driving Simulator
Driving simulators provide optimal stimulus and response control in a challenging but safe environment to analyze the differing patterns of driver and vehicle behaviors of normal elderly and those with AD and PD that would be too unsafe to study on the road. However, driving simulators may vary widely in their characteristics (e.g., motion base vs. fixed base, interactivity, resolution, and field of view) and their validity against actual road driving, which provides more and balanced visual, tactile, vibratory, and vestibular cues to the driver for the control of the vehicle.
3.1.3.2.1 Parkinson’s disease
Madeley et al. (1990) found in 10 drivers with PD and 10 age-sex matched controls that PD drivers had significantly impaired steering accuracy, slower driving reaction times, and missed more red lights. There was a significant correlation between the motor severity of PD measured by Webster’s rating scale and simulated driving reaction time, steering accuracy and simple reaction time, but no significant correlation with the number of red lights missed [30]. In a similar study drivers with PD (n-=28, median age = 65) were more likely to fail to react to stimuli such as a red light and to have a high frequency of directional errors, reduced speed, and prolonged reaction times compared to younger controls (n=109, median age = 49). Unlike the study by Madeley et al., the Webster score was not a predictor of the simulator performance in the PD group [31].
Moller et al. (2002) evaluated 6 PD patients with history of sudden onset of sleep using a sleep-wake diary, the ESS, polysomnography, multiple sleep latency tests (MSLT), a vigilance test, and driving simulation. In all patients, ESS scores were increased and polysomnography showed sleep problems. Pathological results in the MSLT or the vigilance test were obtained in five cases. Three of these five patients had increased mean SDLP (standard deviation of lateral lane position) values, suggesting poor vehicle control [32].
Zesiewicz et al. (2002) compared the driving ability of 39 PD drivers with 25 control participants using a driving simulator. Within the PD group, 7 reported having stopped driving, 10 reported a decrease in the amount of driving, and 22 reported no change in driving habits. PD drivers who stopped driving had significantly lower MMSE scores than currently active PD drivers or controls. The PD group (including those who limited their driving and those who stopped driving) had more collisions on the driving simulator than control drivers. PD drivers who were still driving (including those who had no change in driving and those who had limited their driving) had more simulator collisions than control drivers. When considering only those PD drivers who reported no change in driving, a trend was observed for these drivers to have more simulator collisions compared to control drivers. Collisions correlated with higher motor severity and lower MMSE score [33].
Stolwyk et al. (2005) examined the impact of impaired internal cueing in PD on specific driving behaviors using a simulator in 18 current drivers in the mild-to-moderate stages of PD and 18 matched controls. Eight high curvature curves and 8 low curvature curves, and 3 traffic signals were included in each simulation. All traffic signals were programmed to display a red signal on approach, requiring participants to stop at each traffic signal.. Arrow road signs were placed 20 m before upcoming curves, and were yellow for low curvature curves and red for high curvature curves. A black diamond road sign was visible 50 m before each traffic signal. Participants navigated through different driving conditions where the opportunity to use internal and external cues was manipulated. Drivers with PD tended to approach traffic signals at lower speeds, decelerated significantly later, and experienced significantly more difficulty stopping in time. They drove around curves significantly slower, with a tendency for less variance in speed. Although mean lateral lane position did not vary from that of controls, they exhibited significantly more difficulty maintaining a constant lateral position. People with PD exhibited difficulties using internal cues to regulate driving behavior around traffic signals and curves. Instead of using internal cues, participants with PD were more reliant on external cues to regulate driving behavior [34]. The same group of subjects were compared using a concurrent task was manipulated between conditions during simulated driving. Dependent variables again included several driving behaviors measuring two key driving events: traffic signals and road curves, as with the study described above. Although groups were similarly affected by the concurrent task on most driving measures, participants with PD were disproportionately affected on operational level driving behavior, as manifested by closer deceleration points in PD drivers before traffic signals with distraction despite sacrificing concurrent task performance to maintain driving performance [35]. Within the PD group, executive dysfunction affecting working memory, planning and set shifting were associated with reduced tactical level driving performance such as speed adaptation and complex curve navigation. Impaired information processing, visual attention and visual perception in people with PD was associated with reduced operational level driving performance, such as reacting to road obstacles and maintaining constant lane position. Few correlations were found between measures of physical mobility and psychomotor speed with driving measures [36].
Addition of driving simulation assessment to a clinical screening battery (disease duration, contrast sensitivity, Clinical Dementia Rating, and motor part of the Unified Parkinson's Disease Rating Scale) increased the sensitivity and specificity of off-road testing in predicting pass/fail status of drivers with PD on an official road test [37].
To assess crash risk of drivers with Parkinson s disease (PD) during driving simulation under low visual contrast conditions, 67 drivers with mild-moderate PD and elderly controls (n=51) drove on a 2-lane rural highway under foggy conditions in a high-fidelity simulator scenario in which the driver s approach to within 4.0 seconds of an intersection triggered an illegal incursion by another vehicle causing crash risk. 76.1% of drivers with PD crashed compared to 37.3 % in controls. The time to first reaction (releasing accelerator, braking, or steering away) of drivers with PD was slower than that of controls (median 2.7 vs. 2.1 seconds), which correlated with worse scores on tests of visual perception and memory, visuospatial abilities, executive functions, motor severity, and postural stability within the PD group. Prolonged reaction time and decreased performance on tests of contrast sensitivity, perception of structure from motion, visual speed of processing and attention (Useful Field of View), and visuospatial perception (Judgment of Line Orientation) predicted crashes within the PD group. Motor dysfunction contributed to slowing of time to first reaction [38].
3.1.3.2.2 Alzheimer’s disease
In a group of 21 licensed drivers with AD and 18 controls, 29% with AD experienced crashes vs. none of the control participants. Drivers with AD were more than twice as likely to experience close calls. Visuospatial impairment, reduction in the useful field of view, and reduced perception of 3-dimensional structure-from-motion predicted crashes [39]. In a follow up study, during an illegal intersection incursion scenario, 6 of 18 drivers with AD experienced crashes versus none of 12 nondemented drivers of similar age. Plots of control over steering wheel position, brake and accelerator pedals, vehicle speed, and vehicle position during the 5 seconds preceding a crash event showed inattention and inappropriate or slow control responses. Predictors of combined crashes in the illegal intersection incursion and rear-end collision (REC) avoidance scenarios included visuospatial impairment, disordered attention, reduced processing of visual motion cues, and overall cognitive decline. [40]
Comparison of 29 outpatients with probable AD with 21 age-matched control participants on an driving simulator revealed that patients with AD had cognitive difficulty operating the simulator, drove off the road more often, spent more time driving slower than the posted speed limit, applied less brake pressure in stop zones, spent more time negotiating left turns, and drove more poorly overall. The AD patients who could not drive the simulator because of confusion and disorientation or had stopped driving already had lower MMSE scores. The computed total driving score correlated significantly with MMSE scores [41].
A study of 70 drivers with mild AD and 132 elderly controls showed that the simulator composite score, reflecting overall driving ability, was significantly correlated with overall cognitive ability, as indexed by the neuropsychology composite score, as well as with individual cognitive tests of attention, memory, visuospatial and visuomotor abilities. Drivers who crashed during an intersection incursion scenario performed significantly worse on the composite measure of cognitive function than those who successfully steered around the incurring vehicle. Memory test performances for both verbal information and visual material were associated with subsequent on-road crashes over a follow-up period of 2 years as confirmed by DOT records [42].
REC avoidance was tested in 61 drivers with mild AD and 115 elderly controls using a high-fidelity interactive driving simulator. After a segment of uneventful driving, each driver suddenly encountered a lead vehicle stopped at an intersection, creating the potential for a collision with lead vehicle or with another vehicle following closely behind the driver. Eighty-nine percent of drivers with AD had unsafe outcomes, either an REC or an risky avoidance behavior (defined as slowing down abruptly or prematurely, or swerving out of the traffic lane) compared to 65% of controls. Crash rates were similar in AD (5%) and controls (3%), yet a greater proportion of drivers with AD slowed down abruptly (70% vs. 37%) or prematurely (66% vs. 45%). Abrupt slowing increased the odds of REC by the following vehicle. Unsafe outcomes were predicted by tests of visual perception, attention, memory, visuospatial/constructional abilities, and executive functions, as well as vehicular control measures during an uneventful driving segment [43].
Response to an emergency vehicle requires detection and recognition of an object in peripheral vision, situation recognition, and a rapid response to execute a safety maneuver to decrease the potential for crashing into the vehicle or striking people situated near it. Forty-eight drivers with AD and 101 controls were tested in a simulator scenario in which drivers encountered a police car on the shoulder of the road [44]. Compared with controls, drivers with Alzheimer’s reacted more slowly—with abrupt decelerations resulting—or failed to steer clear of the police car. Several older drivers stopped in the middle of the road. Poorer performance on tests of perception, attention, memory, and executive function predicted slower first reactions and increased the risk of inappropriate and potentially unsafe reactions. The findings suggest that there is decreased situation awareness or poor executive control over response implementation in drivers with AD.
4. Conclusions
Many people with early AD are capable of driving safely, with evidence suggesting that the risk of crashes in drivers with dementia is low for up to three years after disease onset [45]. Medical diagnosis or a clinician's assessment alone are not accurate enough adequate to determine driving competence in those with dementia [2;3]. Although neuropsychologic testing helps to understand associations of driver performance with cognitive impairment, a general lack of validated cutoff scores makes it impossible to employ these tests in a standardized fashion to advise patients [46]. Furthermore, there are no established guidelines on the timing of follow-up of drivers with mild dementia; recommendations range from 6 months or less to a full year [47].
Although typically identified as a motor disorder, impairments in cognition and visual perception and increased daytime sleepiness are present in drivers with PD and contribute substantially to driving problems. Further research needs to done to establish the natural course of driving abilities and to develop a reliable and efficient evaluation battery to predict driver safety in PD. For both AD and PD, there are no evidence based useful methods for driver rehabilitation.
Reporting requirements for impaired drivers are not uniform across the US. The American Academy of Neurology (AAN) “supports optional reporting of individuals with medical conditions that may impact one’s ability to drive safety, especially in cases where public safety has already been compromised, or it is clear that the person no longer has the skills needed to drive safely” [48]. The AAN also supports “the development and promotion of better evaluation tools to assess driver safety, both in terms of helping physicians recognize when a driver should be referred for evaluation, and assisting state officials in conducting such an evaluation” and “clarification of physician immunity policies, to make it apparent that a physician should be granted immunity both for reporting and not reporting a patient’s condition when such action is taken in good faith, when the patient is reasonably informed of his or her driving risks, and when such actions are documented by the physician in good faith” [48].
Acknowledgments
SUPPORTED BY: NIH/National Institute of Neurological Disorders and Stroke, R01 NS044930 (E.Y.U.); National Institute on Aging, R01 AG 17717 and 15071 (M.R.).
Reference List
- 1.Anderson SW, Rizzo M, Skaar N, Stierman L, Cavaco S, Dawson J, Damasio H. Amnesia and driving. J.Clin.Exp.Neuropsychol. 2007;29:1–12. doi: 10.1080/13803390590954182. [DOI] [PubMed] [Google Scholar]
- 2.Ott BR, Anthony D, Papandonatos GD, D'Abreu A, Burock J, Curtin A, Wu CK, Morris JC. Clinician assessment of the driving competence of patients with dementia. J.Am.Geriatr.Soc. 2005;53:829–833. doi: 10.1111/j.1532-5415.2005.53265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ott BR, Heindel WC, Papandonatos GD, Festa EK, Davis JD, Daiello LA, Morris JC. A longitudinal study of drivers with Alzheimer disease. Neurology. 2008;70:1171–1178. doi: 10.1212/01.wnl.0000294469.27156.30. Recent article on evolution of driving in AD using a large sample.
- 4.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N.Engl.J.Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 5.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- 6. Comella CL. Sleep disorders in Parkinson's disease: An overview. Mov Disord. 2007;22:S367–S373. doi: 10.1002/mds.21682. Good review of sleepiness issues in PD.
- 7.Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden-onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–463. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- 8.Homann CN, Suppan K, Homann B, Crevenna R, Ivanic G, Ruzicka E. Driving in Parkinson's disease - a health hazard? J.Neurol. 2003;250:1439–1446. doi: 10.1007/s00415-003-0239-5. [DOI] [PubMed] [Google Scholar]
- 9.Dubinsky RM, Gray C, Husted D, Busenbark K, Vetere-Overfield B, Wiltfong D, Parrish D, Koller WC. Driving in Parkinson's disease. Neurology. 1991;41:517–520. doi: 10.1212/wnl.41.4.517. [DOI] [PubMed] [Google Scholar]
- 10.Meindorfner C, Korner Y, Moller JC, Stiasny-Kolster K, Oertel WH, Kruger HP. Driving in Parkinson's disease: mobility, accidents, and sudden onset of sleep at the wheel. Mov Disord. 2005;20:832–842. doi: 10.1002/mds.20412. [DOI] [PubMed] [Google Scholar]
- 11.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 12. Hopkins RW, Kilik L, Day DJ, Rows C, Tseng H. Driving and dementia in Ontario: a quantitative assessment of the problem. Can.J.Psychiatry. 2004;49:434–438. doi: 10.1177/070674370404900704. Interesting article about the scope of potential future problems in drivers with dementia
- 13.Drachman DA, Swearer JM. Driving and Alzheimer's disease: the risk of crashes. Neurology. 1993;43:2448–2456. doi: 10.1212/wnl.43.12.2448. [DOI] [PubMed] [Google Scholar]
- 14.Friedland RP, Koss E, Kumar A, Gaine S, Metzler D, Haxby JV, Moore A. Motor vehicle crashes in dementia of the Alzheimer type. Ann Neurol. 1988;24:782–786. doi: 10.1002/ana.410240613. [DOI] [PubMed] [Google Scholar]
- 15.Gilley DW, Wilson RS, Bennett DA, Stebbins GT, Bernard BA, Whalen ME, Fox JH. Cessation of driving and unsafe motor vehicle operation by dementia patients. Arch Intern Med. 1991;151:941–946. [PubMed] [Google Scholar]
- 16.Dubinsky RM, Stein AC, Lyons K. Practice parameter: risk of driving and Alzheimer's disease (an evidence-based review): report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2000;54:2205–2211. doi: 10.1212/wnl.54.12.2205. [DOI] [PubMed] [Google Scholar]
- 17.Viitanen M, Johansson K, Bogdanovic N, Berkowicz A, Druid H, Eriksson A, Krantz P, Laaksonen H, Sandler H, Saukko P, Thiblin I, Winblad B, Kalimo H. Alzheimer changes are common in aged drivers killed in single car crashes and at intersections. Forensic Sci Int. 1998;96:115–127. doi: 10.1016/s0379-0738(98)00114-5. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Pentland B, Hunter J, Provan F. Parkinson's disease and driving ability. J.Neurol Neurosurg.Psychiatry. 2007;78:363–366. doi: 10.1136/jnnp.2006.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heikkila VM, Turkka J, Korpelainen J, Kallanranta T, Summala H. Decreased driving ability in people with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;64:325–330. doi: 10.1136/jnnp.64.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood JM, Worringham C, Kerr G, Mallon K, Silburn P. Quantitative assessment of driving performance in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2005;76:176–180. doi: 10.1136/jnnp.2004.047118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Worringham CJ, Wood JM, Kerr GK, Silburn PA. Predictors of driving assessment outcome in Parkinson's disease. Mov Disord. 2006;21:230–235. doi: 10.1002/mds.20709. [DOI] [PubMed] [Google Scholar]
- 22.Amick MM, Grace J, Ott BR. Visual and cognitive predictors of driving safety in Parkinson's disease patients. Arch.Clin.Neuropsychol. 2007;22:957–967. doi: 10.1016/j.acn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Impaired navigation in drivers with Parkinson's disease. Brain. 2007;130:2433–2440. doi: 10.1093/brain/awm178. Investigates how PD affects a common daily driving task in a large sample of drivers with PD.
- 24.Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson's disease. Ann.Neurol. 2006;60:407–413. doi: 10.1002/ana.20958. [DOI] [PubMed] [Google Scholar]
- 25.Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Driving with distraction in Parkinson disease. Neurology. 2006;67:1774–1780. doi: 10.1212/01.wnl.0000245086.32787.61. [DOI] [PubMed] [Google Scholar]
- 26.Duchek JM, Hunt L, Ball K, Buckles V, Morris JC. Attention and driving performance in Alzheimer's disease. J Gerontol B Psychol Sci Soc Sci. 1998;53:130–141. doi: 10.1093/geronb/53b.2.p130. [DOI] [PubMed] [Google Scholar]
- 27.Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver route-following and safety errors in early Alzheimer disease. Neurology. 2004;63:832–837. doi: 10.1212/01.wnl.0000139301.01177.35. [DOI] [PubMed] [Google Scholar]
- 28.Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Driver landmark and traffic sign identification in early Alzheimer's disease. J.Neurol Neurosurg.Psychiatry. 2005;76:764–768. doi: 10.1136/jnnp.2004.049338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reger MA, Welsh RK, Watson GS, Cholerton B, Baker LD, Craft S. The relationship between neuropsychological functioning and driving ability in dementia: a meta-analysis. Neuropsychology. 2004;18:85–93. doi: 10.1037/0894-4105.18.1.85. [DOI] [PubMed] [Google Scholar]
- 30.Madeley P, Hulley JL, Wildgust H, Mindham RH. Parkinson's disease and driving ability. J Neurol Neurosurg Psychiatry. 1990;53:580–582. doi: 10.1136/jnnp.53.7.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lings S, Dupont E. Driving with Parkinson's disease. A controlled laboratory investigation. Acta Neurol Scand. 1992;86:33–39. doi: 10.1111/j.1600-0404.1992.tb08050.x. [DOI] [PubMed] [Google Scholar]
- 32.Moller JC, Stiasny K, Hargutt V, Cassel W, Tietze H, Peter JH, Kruger HP, Oertel WH. Evaluation of sleep and driving performance in six patients with Parkinson's disease reporting sudden onset of sleep under dopaminergic medication: a pilot study. Mov Disord. 2002;17:474–481. doi: 10.1002/mds.10020. [DOI] [PubMed] [Google Scholar]
- 33.Zesiewicz TA, Cimino CR, Malek AR, Gardner N, Leaverton PL, Dunne PB, Hauser RA. Driving safety in Parkinson's disease. Neurology. 2002;59:1787–1788. doi: 10.1212/01.wnl.0000035636.83680.c6. [DOI] [PubMed] [Google Scholar]
- 34.Stolwyk RJ, Triggs TJ, Charlton JL, Iansek R, Bradshaw JL. Impact of internal versus external cueing on driving performance in people with Parkinson's disease. Mov Disord. 2005;20:846–857. doi: 10.1002/mds.20420. [DOI] [PubMed] [Google Scholar]
- 35.Stolwyk RJ, Triggs TJ, Charlton JL, Moss S, Iansek R, Bradshaw JL. Effect of a concurrent task on driving performance in people with Parkinson's disease. Mov Disord. 2006;21:2096–2100. doi: 10.1002/mds.21115. [DOI] [PubMed] [Google Scholar]
- 36.Stolwyk RJ, Charlton JL, Triggs TJ, Iansek R, Bradshaw JL. Neuropsychological function and driving ability in people with Parkinson's disease. J.Clin.Exp.Neuropsychol. 2006;28:898–913. doi: 10.1080/13803390591000909. [DOI] [PubMed] [Google Scholar]
- 37.Devos H, Vandenberghe W, Nieuwboer A, Tant M, Baten G, De Weerdt W. Predictors of fitness to drive in people with Parkinson disease. Neurology. 2007;69:1434–1441. doi: 10.1212/01.wnl.0000277640.58685.fc. [DOI] [PubMed] [Google Scholar]
- 38.Uc EY, Rizzo M, Sparks JD, Anderson SW, Rodnitzky R, Dawson J. Increased crash risk in drivers with Parkinson's disease under low visual contrast lighting conditions. Neurology. 2007;68:A208. [Google Scholar]
- 39.Rizzo M, Reinach S, McGehee D, Dawson J. Simulated car crashes and crash predictors in drivers with Alzheimer disease. Arch Neurol. 1997;54:545–551. doi: 10.1001/archneur.1997.00550170027011. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo M, McGehee DV, Dawson JD, Anderson SN. Simulated car crashes at intersections in drivers with Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15:10–20. doi: 10.1097/00002093-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Cox DJ, Quillian WC, Thorndike FP, Kovatchev BP, Hanna G. Evaluating driving performance of outpatients with Alzheimer disease. J Am Board Fam Pract. 1998;11:264–271. doi: 10.3122/jabfm.11.4.264. [DOI] [PubMed] [Google Scholar]
- 42.Anderson SW, Rizzo M, Shi Q, Uc EY, Dawson JD. Cognitive abilities related to driving performance in a simulator and crashing on the road. University of Iowa. Proceedings of the Third International Driving Symposium on Human Factors in Driver Assessment, Training and Vehicle Design; 2005. pp. 286–292. 2005. [Google Scholar]
- 43.Uc EY, Rizzo M, Anderson SW, Shi Q, Dawson JD. Unsafe rear-end collision avoidance in Alzheimer's disease. J.Neurol Sci. 2006;251:35–43. doi: 10.1016/j.jns.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Rizzo M, Shi Q, Dawson JD, Anderson SW, Kellison I, Pietras T. Stops for Cops: Impaired Response Implementation for Older Drivers with Cognitive Decline. Transportation Research Record. 2005;1992:1–8. [Google Scholar]
- 45. Breen DA, Breen DP, Moore JW, Breen PA, O'Neill D. Driving and dementia. BMJ. 2007;334:1365–1369. doi: 10.1136/bmj.39233.585208.55. A good guide for general practitioner.
- 46.Molnar FJ, Patel A, Marshall SC, Man-Son-Hing M, Wilson KG. Clinical utility of office-based cognitive predictors of fitness to drive in persons with dementia: A systematic review. J.Am.Geriatr.Soc. 2006;54:1809–1824. doi: 10.1111/j.1532-5415.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 47.Molnar FJ, Patel A, Marshall SC, Man-Son-Hing M, Wilson KG. Systematic review of the optimal frequency of follow-up in persons with mild dementia who continue to drive. Alzheimer Dis.Assoc.Disord. 2006;20:295–297. doi: 10.1097/01.wad.0000213843.43871.c7. [DOI] [PubMed] [Google Scholar]
- 48. Bacon D, Fisher RS, Morris JC, Rizzo M, Spanaki MV. American Academy of Neurology position statement on physician reporting of medical conditions that may affect driving competence. Neurology. 2007;68:1174–1177. doi: 10.1212/01.wnl.0000259514.85579.e0. Important position paper by the AAN.