Abstract
Sirenomelia, also known as sirenomelia sequence, is a severe malformation of the lower body characterized by fusion of the legs and a variable combination of visceral abnormalities. The causes of this malformation remain unknown, although the discovery that it can have a genetic basis in mice represents an important step towards the understanding of its pathogenesis. Sirenomelia occurs in mice lacking Cyp26a1, an enzyme that degrades retinoic acid (RA), and in mice that develop with reduced bone morphogenetic protein (Bmp) signaling in the caudal embryonic region. The phenotypes of these mutant mice suggest that sirenomelia in humans is associated with an excess of RA signaling and a deficit in Bmp signaling in the caudal body. Clinical studies of sirenomelia have given rise to two main pathogenic hypotheses. The first hypothesis, based on the aberrant abdominal and umbilical vascular pattern of affected individuals, postulates a primary vascular defect that leaves the caudal part of the embryo hypoperfused. The second hypothesis, based on the overall malformation of the caudal body, postulates a primary defect in the generation of the mesoderm. This review gathers experimental and clinical information on sirenomelia together with the necessary background to understand how deviations from normal development of the caudal part of the embryo might lead to this multisystemic malformation.
Sirenomelia: a multisystemic malformation with wide phenotypic variability
In Greek mythology, the Sirens were three creatures with the head of a woman and the body of a bird from the wings down. They were dangerous to sailors, whom they narcotized with their enchanting music and voices to later kill them. Over time, these bird-women were portrayed as more aquatic creatures, and eventually with a full mermaid-like appearance. It is likely that creatures of classical and medieval mythology were inspired by the observation of real cases of human malformations (Kleiss, 1964; Stahl and Tourame, 2010), and it is likely that Sirens were similarly inspired. However, despite the present perception of Sirens as romantic and cute creatures, the sirenomelia human malformation is a severe condition.
Sirenomelia sequence, also known as sirenomelia, is a birth defect of the lower body characterized by the apparent fusion of the legs into a single lower limb (Fig. 1). This striking external phenotype is associated with a variable combination of severe visceral abnormalities, most commonly urogenital and gastrointestinal, making sirenomelia a multisystemic condition (Kampmeier, 1927; Duhamel, 1961; Stevenson et al., 1986; Stocker and Heifetz, 1987; Kallen et al., 1992). The analysis of several series of cases has demonstrated a wide phenotypic variability in this condition (Kampmeier, 1927; Stevenson et al., 1986; Stocker and Heifetz, 1987; Kallen et al., 1992).
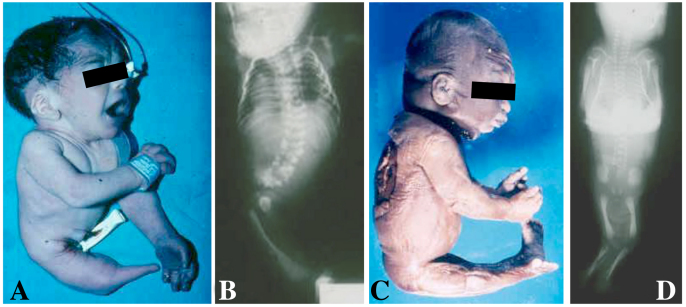
Fig. 1.
A newborn and a stillborn with sirenomelia. (A,B) Photograph (A) and corresponding radiography (B) of a newborn with type VII sirenomelia. (C,D) Photograph (C) and corresponding radiography (D) of a stillborn with type I sirenomelia. Classification of sirenomelia is according to Stocker and Heifetz (Stocker and Heifetz, 1987) (see Fig. 2).
Owing to visceral abnormalities, sirenomelia is usually incompatible with life; death occurs in the perinatal period. Recently, a few exceptional cases have survived owing to the presence of a functional kidney and reconstructive surgery to restore pelvic organs and separate the legs (Messineo et al., 2006). The babies that survived had normal neurological development.
The reported incidence of human sirenomelia varies between 1.1 and 4.2 per 100,000 births (Johnson, 1966; Martinez-Frias et al., 1992; Murphy et al., 1992). It has been reported in all ethnic groups around the world. The diagnosis, which is obvious at birth, is currently performed by prenatal ultrasonography. Antenatal ultrasonography clues include oligohydramnios, renal agenesis and a fibula positioned between the tibiae (see below) (van Zalen-Sprock et al., 1995; Valenzano et al., 1999).
Hindlimb fusion phenotypes
In humans, fusion of the legs in sirenomelia occurs in a spectrum of morphologies, ranging from the mildest cases in which all bones of the two fused lower limbs are discernible (Fig. 1C,D), to the most severe cases in which there is no indication that the single rudimentary lower limb that is present derives from the fusion of two (Fig. 1A,B).
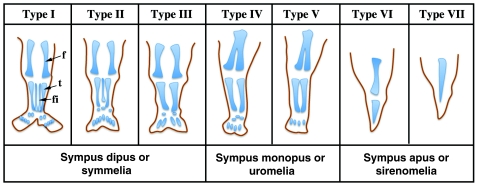
There have been several attempts to classify this spectrum of limb phenotypes, which is a difficult task owing to the wide variability observed. Stocker and Heifetz classified sirenomelia into type I to type VII, according mainly to the presence of skeletal elements in the thigh and leg (Fig. 2) (Stocker and Heifetz, 1987). In type I, the mildest form, all bones in the two fused limbs are present, and the fusion only affects superficial tissues (Fig. 1C,D and Fig. 2). In type VII, the most severe form, only a single bone is present, with no indication of legs or feet (Fig. 1A,B and Fig. 2). Other classifications, which have now been essentially abandoned, focused on the degree of development of the fused legs denoted by the presence of feet. Thus, sirenomelia used to be classified as sympus dipus or symmelia if the two feet were present, sympus monopus or uromelia when only one foot was discernible, and sympus apus or sirenomelia when there was no evidence of any distal foot element (Saint-Hilaire, 1836; Forster, 1865) (Figs 1, 2). In general, the severity of the fusion negatively correlates with the integrity of the feet, with the most severe cases typically presenting with fewer or even an absence of toes (Fig. 2).
Fig. 2.
Schematic depicting the seven types of sirenomelia. Classification is according to Stocker and Heifetz (Stocker and Heifetz, 1987). Alternative nomenclatures, used more frequently in the past, are indicated underneath. f, femur; fi, fibula; t, tibia. Adapted from Stocker and Heifetz (Stocker and Heifetz, 1987), with permission.
Clinical terms.
- Atresia:
congenital absence or closure of a normal body opening, such as the rectum (rectal atresia); can affect multiple structures.
- Blind-end colon:
closure of the terminal portion of colon.
- Dysplasia:
abnormality of development; e.g. in the case of renal and urethral dysplasia, this describes abnormal development of the kidneys and urethra, respectively.
- Fistula:
abnormal connection or passageway between two epithelium-lined organs or vessels that normally do not connect.
- Hypoplasia:
underdevelopment or incomplete development of a tissue or organ.
- Meconium:
dark fecal material that accumulates in the fetal intestines.
- Oligohydramnios:
decreased volume of amniotic fluid.
- Potter sequence (Potter’s phenotype, Potter’s face):
complex physical appearance of a fetus or neonate caused by oligohydramnios. Typical of bilateral renal agenesis in which the kidneys fail to produce the amniotic fluid.
- Renal agenesis:
failure of one or both of a fetus’ kidneys to develop.
- Sacral agenesis:
failure of the sacrum to develop.
There has been some debate about whether the genesis of the limb phenotype in sirenomelia is due to fusion or merging of the two bilateral hindlimb buds. Because the term fusion implies the breakdown of the epithelial lining, whereas the term merging does not, it is more appropriate to describe the generation of the sirenomelia leg phenotype as a merging of the early hindlimb fields (Chandebois and Brunet, 1987; Barr, 1988; O’Rahilly and Muller, 1989).
Owing to the original position of the leg fields in the lateral body wall, their approximation and subsequent merging would occur if the parts normally lying between them did not develop (Ballantyne, 1904; Barr, 1988; O’Rahilly and Muller, 1989). Therefore, a failure of midline structures to develop – either the cloacal and urogenital sinus at the ventral side, or the somites and neural tube at the dorsal side – can result in the approximation and merging of the hindlimb fields. The joining of the two anlagen occurs along the postaxial (posterior) region, indicating that the midline defect increases towards the caudal end (Fig. 1). Therefore, and despite the merging of the lower limbs giving the name to the sirenomelia condition, it might be secondary, and occur as a consequence of the approximation of the limb fields.
A striking characteristic of the sirenomelia phenotype is that the merged lower limbs show an abnormal position that corresponds to a 180° rotation with respect to the position of a normal leg (Fig. 1C). During normal limb development, the hindlimb buds rotate medially so that the ventral surface eventually faces dorsally (Schoenwolf et al., 2009). However, because of early abnormal fusion in the midline along their posterior margins, the fused hindlimbs cannot accomplish this rotation (Fig. 1C). This is strikingly evidenced by the soles facing anteriorly, by the abnormal flexion of the knees and by the fibulae adopting a medial position between the tibiae (Fig. 1C,D and Fig. 2).
Visceral malformations
All human cases of sirenomelia analyzed thus far show a variable degree of renal and urethral dysplasia, with total renal agenesis being very frequently reported (Kallen and Winberg, 1974; Stocker and Heifetz, 1987; Kallen et al., 1992; Sikandar and Munim, 2009; Thottungal et al., 2010). Curiously, ectopic renal tissue has been described in the pelvis of affected individuals (Stocker and Heifetz, 1987), probably reflecting a defect in the normal migration of the metanephric tissue.
Malformation of the urinary tract is consistently associated with genital malformations. These mainly affect the external genitalia, which are either absent or represented by an indistinct tag of tissue, whereas the gonads are usually unaffected (de Jonge et al., 1984; Goodlow et al., 1988; Kallen et al., 1992). The presence of gastrointestinal anomalies in sirenomelia is also common, the most frequent being a blind-ending colon, rectal atresia and imperforate anus (Kallen et al., 1992; Thottungal et al., 2010).
Case study.
A 16-year-old diabetic adolescent primigravida with an unsupervised pregnancy of 28 weeks was admitted with severe abdominal pain. Ultrasonography revealed a dead fetus, oligohydramnios and renal agenesis. Severe caudal malformation of the fetus, including fused lower extremities, was presumed, although detailed evaluation was not possible owing to the presence of severe oligohydramnios.
Until that date, the concealed pregnancy was uneventful except for the woman’s poorly controlled diabetes mellitus, and a history of moderate exposure to tobacco (ten cigarettes per day) and alcohol (up to 20 g per weekend). There was no declared exposure to other drugs.
She underwent vaginal delivery of a dead, malformed male fetus of 1600 g. The stillborn had Potter’s face, fused lower limbs and absence of anus. Postmortem radiography showed sacral agenesis, a hypoplastic pelvis, and fused lower extremities with paired femurs and tibias, but absent fibulae. Autopsy examination confirmed the prenatal diagnosis of renal agenesis and revealed hypoplastic lungs, testes in the inguinal area, absence of anus and the terminal portion of the colon full of meconium. A single umbilical artery branching off the abdominal aorta was present. The karyotype of the fetus was normal.
Four years later, the woman asked for genetic counseling and was informed of the sporadic incidence and negligible recurrence risk of human sirenomelia, and also of the fetal risks of maternal diabetes. She was encouraged to access a specialized prenatal center before attempting a new pregnancy.
Vascular malformations
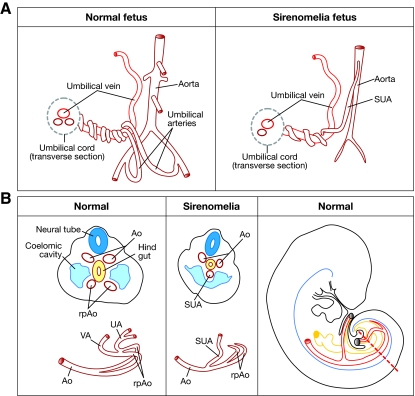
The vascular abnormalities in sirenomelia deserve particular mention owing to their possible relevance to pathogenesis (see Box 1 for a description of how disturbance in the development of vitelline and umbilical arteries might give rise to an aberrant vascular pattern). The human umbilical cord normally contains two umbilical arteries (returning deoxygenated blood from the fetus to the placenta) and a single umbilical vein (supplying oxygenated blood to the fetus). However, fetuses with sirenomelia almost invariably exhibit a single umbilical artery (SUA) instead of the normal two (Heifetz, 1984; Stevenson et al., 1986) (Fig. 3A). Moreover, this SUA has an abnormal origin, branching off the abdominal aorta quite high in the abdomen, usually immediately below the celiac branch. Below the origin of the SUA, the aorta becomes abnormally narrow and lacks a considerable number of the branches that normally supply the kidneys, large intestine and genitalia. The SUA has also been referred to as the persistent vitelline artery to indicate its possible derivation from the vitelline plexus, and to distinguish it from other cases of SUA that are found in about 1% of newborns but that have normal origin and are not related to other malformations (Martinez-Frias et al., 2008).
Box 1. Development of vitelline and umbilical arteries.
Vascular development begins in extraembryonic tissues (the yolk sac in mammals and the area opaca in birds) when mesodermal cell progenitors initially coalesce and then differentiate into endothelial and blood cells to form so-called ‘blood islands’. These blood islands later fuse to form a primitive vascular plexus that connects with the intraembryonic plexus, and this, with the onset of circulatory flow, undergoes heavy remodeling into the branched circulatory network (Adams and Alitalo, 2007; Jin and Patterson, 2009).
Vascular development has been extensively analyzed in mice (i.e. Walls et al., 2008). In the early mouse embryo, at embryonic day (E)8, the paired dorsal aortas become progressively organized towards the caudal growing region of the embryo (Coffin et al., 1991; Walls et al., 2008). At the caudal end of the embryo the dorsal aortas curve towards the anterior (ventral) region, where they merge to form the vitelline or omphalomesenteric artery (Fig. 3B). The caudal curved parts of the dorsal aortas are called the recurved distal portions, although they are also referred to as the primary umbilical arteries (Fig. 3B). By E8.5, the umbilical artery, which originally forms in association with the allantois, fuses to the recurved distal portions of the dorsal aortas, temporally connecting the vitelline and umbilical vasculature. Subsequently, the vitelline artery discontinues its connection with the umbilical artery while connecting dorsally with the abdominal aorta (Monie and Khemmani, 1973; Garcia-Porrero et al., 1995). Consequently, the umbilical artery pumps blood from the embryo to the placenta while the vitelline artery carries blood from the embryo to the yolk sac and eventually becomes remodeled to form the intestinal arteries.
Fig. 3.
Vascular pattern in a normal versus sirenomelia fetus. (A) Schematic drawing of the fetal umbilical cord vasculature in a normal and a sirenomelia fetus. Note the abnormally high origin of the SUA in sirenomelia and the hypoplasia of the aorta caudal to its origin. (B) Left two panels: schematic of transverse sections at the caudal level of an early normal and sirenomelia embryo. Below each section, the corresponding vascular pattern is shown in red. Ao, dorsal aorta; rpAo, recurved distal portions of aorta; UA, umbilical arteries; UV, umbilical vein; SUA, single umbilical artery; VA, vitelline artery. Right panel: a schematic lateral view of an embryo in which the great caudal vessels are shown in red and the hindgut in yellow. The broken red line indicates the level of the sections shown in the two panels to the left.
The presence of an SUA of vitelline origin has been considered as characteristic or even pathognomonic of sirenomelia and was proposed to be used for the differential diagnosis of other malformations of the lower body such as caudal dysgenesis (CD; see Box 2) (Heifetz, 1984; Stevenson et al., 1986). However, there are occasional cases of sirenomelia with two symmetrical umbilical arteries, although they are of abnormal origin (Opitz et al., 2002; Thottungal et al., 2010), whereas others have only one umbilical artery but that is of normal origin (Jaiyesimi et al., 1998). Reciprocally, aberrant umbilical arteries have also been described in individuals with CD (Duesterhoeft et al., 2007).
Box 2. Sirenomelia versus other defects of the caudal body.
Caudal dysgenesis (CD; OMIM 600145; formerly referred to as caudal regression syndrome) describes a heterogeneous variable association of malformations of the lower body that always include some degree of sacral agenesis (Duhamel, 1961; Boulas, 2009). CD is characterized by, in variable proportions, lumbosacral vertebral, cardiac, anorectal and genitourinary malformations, as well as some type of dysgenesis of the lower limbs.
A specific set of the malformations that are typical of CD forms the VACTERL association (OMIM 192350; acronym of Vertebral anomalies, Anal atresia, Cardiac defect, TracheoEsophageal fistula with esophageal atresia, Renal abnormalities and Limb abnormalities). The easily recognizable VACTERL association is found in many clinical entities and syndromes of known causal factors, reflecting a heterogeneous etiology (Martinez-Frias et al., 1990; Kim et al., 2001; Martinez-Frias et al., 2001).
The considerable degree of overlap in the abnormalities present in CD, VACTERL association and sirenomelia, together with the poorly understood etiology of each, has led to debate about whether these three entities might in fact be different manifestations of a common pathogenic process (Stocker and Heifetz, 1987; Duncan et al., 1991; Duesterhoeft et al., 2007). It is conceivable that a defect during blastogenesis, regardless of its nature, could result in abnormal development of caudal structures ranging from mild forms of CD to sirenomelia – the two ends of the same malformative spectrum. It should be stressed that disruption of various different factors that function in the same pathway could result in identical or very similar alterations in blastogenesis, therefore explaining why VACTERL association can be found in entities with different etiologies.
Other malformations associated with sirenomelia
Among other abnormalities that are commonly observed in sirenomelia are lumbosacral and pelvic malformations, including sacral agenesis, malformed vertebrae and hemivertebrae (Fig. 1), and corresponding anomalies of the central nervous system (Stocker and Heifetz, 1987; Kallen et al., 1992; Chen et al., 1998; Kjaer et al., 2003). Finally, it should be mentioned that, albeit at a much lower frequency, sirenomelia is also associated with malformations of the upper part of the body, including cleft palate, upper thoracic and cervical vertebral abnormalities, pulmonary hypoplasia, and cardiac defects (Kallen and Winberg, 1974; Rodriguez et al., 1991; Kallen et al., 1992; Rodriguez and Palacios, 1992; Drossou-Agakidou et al., 2004). In summary, sirenomelia comprises a variable combination of malformations, predominantly of the lower body. In the remainder of this article, we discuss experimental evidence that provides insight into the still-uncertain causes and mechanisms of this malformation, which collectively suggest a global defect in the development of the caudal end of the embryo.
Hypotheses regarding etiology, based on clinical observations
Although the primary molecular defect underlying sirenomelia remains unknown, clinical studies have given rise to several hypotheses to explain the causal mechanisms. The two main pathogenic hypotheses are the vascular steal hypothesis and the defective blastogenesis hypothesis. Other mechanical hypotheses that have been proposed to cause sirenomelia, such as lateral compression of the caudal body by amniotic folds and medial compression by overdistension of the neural tube, are not considered here (Gardner, 1980; Orr et al., 1982; Stevenson et al., 1986).
The vascular steal hypothesis
The vascular steal hypothesis is based on the aberrant vasculature pattern of the caudal body present in most individuals with sirenomelia (Kampmeier, 1927; Kallen and Winberg, 1974; Stevenson et al., 1986). This hypothesis posits a vascular origin for the defect: it proposes that the SUA of vitelline origin diverts blood flow to the placenta, leaving the lower part of the body with a severely deficient circulation (Fig. 3A,B, Box 1).
It is unknown how the abnormal vasculature is established in sirenomelia, but it seems reasonable to assume that the aberrant SUA of apparent vitelline origin derives from the original connection between the vitelline and umbilical vasculature (Fig. 3B, Box 1). This pattern might be favored in the remodeling of the very early capillary plexus if there is a defect in the formation of the caudal part of the aortas.
According to the vascular steal hypothesis, fusion of the limbs results from a deficient blood flow and nutrient supply to the caudal mesoderm, which in turn would result in agenesis of midline structures and subsequent abnormal approximation of both lower limb fields. In this view, the variable lower limb phenotype depends on the variable development of the sciatic artery, a branch of the recurved distal portions of the dorsal aorta that initially supplies the leg bud and that, with variable development, is normally present in sirenomelia.
Vascular mechanisms have been proposed to underlie the pathogenesis of several other defects as well as sirenomelia (Sadler and Rasmussen, 2010). However, in most cases, including sirenomelia, the evidence gathered is not sufficient to unequivocally establish the abnormal vasculature pattern as the primary cause, and the possibility of an earlier interference with a basic developmental event cannot be ruled out.
The defective blastogenesis hypothesis
This hypothesis was initially shaped in the 19th century, when several investigators noted the overall abnormal caudal body in individuals with sirenomelia and correlated its origin with defective development of the caudal somites and tailbud [see Stocker and Heifetz (Stocker and Heifetz, 1987) and references therein]. Later, Duhamel identified the frequent and nonrandom association between different types of malformations of the lower body, affecting the hindgut and other systems, and he introduced the notion of caudal regression syndrome to refer to a variable combination of defects of the caudal part of the body (Duhamel, 1961). Caudal regression syndrome is presently designated by the more accurate term of CD (Opitz et al., 2002; Thottungal et al., 2010) (Box 2).
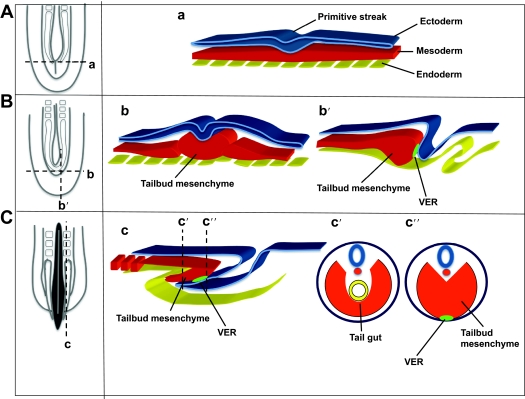
The vertebrate body plan goes through a series of events during gastrulation. In amniotes, this involves massive movements of epiblast cells through the primitive streak, transforming a two-layered blastocyst into an embryo consisting of three germ layers, with the formation of the endomesoderm in a rostrocaudal sequence (Voiculescu et al., 2007). At late gastrulation, after massive ingression movements terminate, the remnant of the regressing primitive streak forms the caudal eminence, or tailbud, posterior to the site of the former neurenteric canal [Fig. 4A,B, based on Mills and Bellairs (Mills and Bellairs, 1989)]. The caudal eminence has a bulb-like appearance and consists of a mass of loose mesenchyme covered by the ectoderm [Fig. 4B; for a human description see O’Rahilly and Muller (O’Rahilly and Muller, 1989)].
Fig. 4.
Diagrams summarizing the normal development of the caudal region of the vertebrate embryo before, during and after tailbud formation. For each stage [before (A), during (B) and after (C) tailbud formation] a dorsal representation of the caudal end of the embryo is shown on the left; the structures that would be seen underneath the ectoderm are shown. Transversal (a,b,c′ and c″) and parasagittal (b′,c) sections highlight the relationship between the ectoderm (blue), the mesoderm (red) and the endoderm (yellow). Panels b′, c and c″ show the position of the VER (light green) at the contact point between the ectoderm and the mesoderm. Adapted from Mills and Bellairs (Mills and Bellairs, 1989), with permission.
As a consequence of the growth and elongation of the tailbud, the remnants of the primitive streak progressively involute towards the ventral region of the embryo [see figure 5 from Mills and Bellairs (Mills and Bellairs, 1989)]. This morphogenetic process is accompanied by the formation of a thickened ectodermal area on the ventral (distal) side called the ventral ectodermal ridge (VER) (Gruneberg, 1956) (Fig. 4B,C). The VER is considered the continuation of the posterior primitive streak. In fact, mesoderm precursors on the surface continue to internalize and to contribute to tail elongation and formation of caudal structures (Wilson and Beddington, 1996; Knezevic et al., 1998). This continuity has been confirmed in both chick and mouse embryos through the analysis of molecular markers whose expression is continuous from the primitive streak cells into subpopulations of cells in the tailbud region (Gofflot et al., 1997; Knezevic et al., 1998).
The VER has been compared to another ectodermal thickening, the apical ectodermal ridge of the developing limb (Gruneberg, 1956), and has attracted much attention as a putative signaling center responsible for tail elongation by controlling cell proliferation in the underlying mesoderm. Although experiments of VER ablation in mice did not confirm a role for the VER in promoting proliferation, studies in chick reported that VER cells undergo an epithelial-mesenchymal transition process, accounting for the accumulation of mesoderm in the lateral and ventral tailbud region (Gofflot et al., 1997; Goldman et al., 2000; Ohta et al., 2007).
According to the defective blastogenesis hypothesis, sirenomelia is a primary defect of blastogenesis that occurs during the final stages of gastrulation at the tailbud stage, corresponding to the third gestational week in humans (Opitz et al., 2002; Duesterhoeft et al., 2007; Davidson, 1993). The phenotypic variability depends on the intensity, time of initiation and duration of the underlying event. In this view, sirenomelia can be considered a particular manifestation of CD, in contrast to the still persistent debate in the clinic regarding whether sirenomelia is a distinct entity. Debate has been based on the observation that sirenomelia presents with characteristics that are distinct from CD: these characteristics include the nearly constant presence of an SUA of vitelline origin, the occurrence of cases without dorsal defects of the neural tube and spine, and the lack of a clear association with maternal insulin-dependent diabetes mellitus, as is observed for CD (see Boxes 2, 3) (Passarge and Lenz, 1966; Stocker and Heifetz, 1987; Duncan and Shapiro, 1993; Lynch and Wright, 1997).
Box 3. Genetic and environmental aspects of human caudal malformations.
In humans, a genetic etiology for congenital caudal anomalies has only been confirmed for the Currarino syndrome (Currarino et al., 1981), a sacral agenesis caused by mutations in the homeobox-containing gene HLXB9 (Ross et al., 1998; Merello et al., 2006). By contrast, to date, all reported incidences of sirenomelia in humans have been sporadic cases (Castilla et al., 2008; Thottungal et al., 2010). This is in marked contrast to the well-established genetic basis for sirenomelia-like phenotypes in mice. Although it is possible that, in humans, sirenomelia is an autosomal-dominant genetic condition, with every single case caused by a new spontaneous mutation, it seems more likely that it has a combined genetic and environmental component. The absence of familial cases might rely, at least in part, on insufficient documentation and classification of spontaneous or induced abortions (Castilla et al., 2008). However, it should be noted that there are two reported cases of diabetic mothers that had children with sirenomelia and CD, or children with sirenomelia and VACTERL (Assimakopoulos et al., 2004; Castori et al., 2010). If sirenomelia, CD and VACTERL are indeed different degrees of the same entity, the above mentioned cases could represent familial cases.
RA, maternal diabetes and heavy metals have been described as important environmental risk factors for caudal malformations. The involvement of RA signaling in the genesis of sirenomelia is well established in experimental models and represents a potentially interesting connection to the environment, because RA levels can be modified by genetic, nutritional and iatrogenic causes. However, to date, sirenomelia has not been reported among the malformations of RA-exposed human fetuses. Maternal diabetes is considered as a causative environmental factor for CD because 10–15% of affected children have diabetic mothers (Passarge and Lenz, 1966; Kalter, 1993; Twickler et al., 1993; Lynch and Wright, 1997; Assimakopoulos et al., 2004; Castori et al., 2010). However, this association remains controversial for sirenomelia because only 0.5%–3.7% of reported cases have diabetic mothers (Stocker and Heifetz, 1987; Duncan and Shapiro, 1993; Lynch and Wright, 1997; Duesterhoeft et al., 2007). Finally, exposure to heavy metals is associated with CD and sirenomelia in both experimental models (Ferm and Carpenter, 1968; Hilbelink and Kaplan, 1986) and in humans (Castilla et al., 2008; Orioli et al., 2009).
Although the vascular steal hypothesis and the deficient blastogenesis hypothesis do not exclude one other, it is reasonable to assume that deficient blastogenesis would concomitantly affect organ and vessel development.
Genetic aspects of sirenomelia and animal models
Although sirenomelia has been known to occur in humans since ancient times (Saint-Hilaire, 1836; Stocker and Heifetz, 1987), this malformation was only recently documented in other animals, including mice (Gluecksohn-Schoenheimer and Dunn, 1945). Interestingly, and in marked contrast to the human condition in which all the cases reported so far have been sporadic, sirenomelia in the mouse has been shown to have a genetic basis.
The first cases of mouse sirenomelia were reported in the progeny of mice carrying different mutations at or near the T locus [in the brachyury gene (short-tail strain) and in the axin1 gene (fused strain)] (Gluecksohn-Schoenheimer and Dunn, 1945). The mutations in these mice were known to affect tail and caudal body development. Although, at the time of this study, the genotypes could not be fully established, it was clear that at least one copy of the short-tail allele was involved in the phenotype. A spontaneous mutation called sirenomelia (srn) was also identified but it was unfortunately lost (Hoornbeek, 1970). Remarkably, all of these mice exhibited the combination of malformations typical of human sirenomelia.
More recently, a sirenomelia-like phenotype has been observed in several genetically modified mouse strains with either gain-of-function of retinoic acid (RA) signaling or loss-of-function of bone morphogenetic protein (Bmp) signaling.
Sirenomelia occurs when RA signaling is increased in the caudal end of the embryo
Cyp26a1 is an enzyme that degrades RA (the active metabolite of vitamin A) and that is expressed in the caudal region of the embryo and transiently in the developing vascular network (Abu-Abed et al., 2001; Abu-Abed et al., 2003). Disruption of Cyp26a1 results in a gain of RA function that leads to several caudal defects, including sirenomelia with 20% penetrance. Consistently, disruption of Cdx2, which encodes a transcription factor that activates the Cyp26a1 promoter, or the disruption of Por, which encodes an enzyme that is required for the function of the Cyp26 family of enzymes, both cause sirenomelia (Ribes et al., 2007b; Savory et al., 2009; Young et al., 2009; Pennimpede et al., 2010). A decrease in the production of RA by reducing the level of the enzyme that synthesizes it (haploinsufficiency of Raldh2) is sufficient to rescue the Cyp26a1 phenotype (Niederreither et al., 2002).
Kochhar (Kochhar, 1967) and Yasuda et al. (Yasuda et al., 1990) were the first to report that excess administration of RA to pregnant mice could lead to caudal malformations in the offspring, and Padmanabhan (Padmanabhan, 1998) later reported that this treatment could also cause sirenomelia. Therefore, in mice, sirenomelia is clearly associated with excess RA signaling in the caudal region of the embryo. Importantly, because RA levels can be influenced by both genetic and nutritional factors, this metabolite has the potential to act as a genetic and/or environmental cause of sirenomelia.
Sirenomelia occurs when Bmp signaling is decreased in the caudal end of the embryo
An important discovery was the finding that sirenomelia was an invariable phenotypic trait in the double Bmp7;Tsg (Twisted gastrulation) mutant mouse (Zakin et al., 2005). Bmp7 is a member of the Bmp family of multifunctional secreted signaling proteins, which belongs to the transforming growth factor-β (TGFβ) superfamily. Tsg, which can function as an activator or inhibitor of Bmp signaling, depending on the context, acts as a positive modulator in the caudal embryonic region (Larrain et al., 2001). The mermaid phenotype in the Bmp7;Tsg double mutant results from a reduction in Bmp signaling in the ventral caudal mesoderm; this reduction is more marked than that caused by the loss of Bmp7 alone, because the single Bmp7 mutant does not exhibit a mermaid phenotype (Zakin et al., 2005). The contribution of reduced Bmp signaling to the malformation has been confirmed in loss-of-function experiments (Bmp7-morpholino and Tsg-morpholino injections) in Xenopus laevis (Zakin et al., 2005). In zebrafish, deficient Bmp signaling after the mid-gastrula stage similarly causes deficient ventral mesoderm formation, with defects in kidney and excretory system morphogenesis (e.g. aberrant cloaca) (Pyati et al., 2006). Therefore, the association between reduced Bmp signaling at the caudal level and the occurrence of sirenomelia seems to be conserved across species.
Out of the mutant mice that have been characterized to date, the Cyp26a1 and Bmp7;Tsg engineered mouse mutants exhibit a phenotype that is most similar to the human condition, making them the most suitable models for studying and understanding the pathogenesis of this human malformation.
Integration of clinical and experimental data
Sirenomelia, as well as other malformations that affect multiple organs, arises very early during development and could therefore result from disturbances of a basic developmental event that would later have a broad impact in different systems. For example, a single pathophysiological mechanism affecting the formation of the caudal mesoderm could explain the abnormal vascular pattern as well as the variable organ hypoplasia (Duesterhoeft et al., 2007). The alternative possibility that several pathological mechanisms, acting independently on different biological functions, lead to the sirenomelia phenotype seems less likely.
Clinical data suggest that sirenomelia might result from a defect, at the late gastrula stage, in the formation or remodeling of the early embryonic vasculature or in the generation of the mesodermal precursors. In both situations, the resulting development of the caudal region would be impaired. Experimental data suggest that sirenomelia has a genetic basis resulting from a defect in RA or Bmp signaling in the caudal embryonic region (Abu-Abed et al., 2001; Zakin et al., 2005). Therefore, it seems plausible that there is a connection between the cellular mechanisms identified in the clinic and the molecular signals identified in animal studies. As such, the use of new and existing animal models will be of great value in elucidating the definite cause of sirenomelia.
Bmp signaling in caudal development and vasculogenesis
Bmp signaling performs multiple important roles during early embryogenesis, including the control of gastrulation. During late gastrulation, several Bmp ligands and their extracellular antagonists and modulators are concomitantly expressed in dynamic and partially overlapping domains (Winnier et al., 1995; Hogan, 1996; Godin et al., 1998; Fujiwara et al., 2001). For example, in the mouse, Bmp2 and Bmp7 are expressed in the VER, whereas Bmp4, Bmp7 and the Bmp antagonist Noggin are expressed in the underlying mesoderm (Ohta et al., 2007). Although this makes it difficult to assign a specific role to each particular factor, a role for Bmp signaling in the growth of the tailbud and in promoting the formation of caudal mesoderm in the VER has been shown (Ohta et al., 2007; Suzuki et al., 2009). Consistently, in the chick, termination of cell movements through the VER coincides with the attenuation of Bmp signaling at this level (Goldman et al., 2000; Ohta et al., 2007). Furthermore, the sirenomelia phenotype of the double Bmp7;Tsg mutant strongly supports the involvement of Bmp signaling in caudal development (Zakin et al., 2005; Suzuki et al., 2009).
Bmp signaling also plays a crucial role in angiogenesis and vasculogenesis by promoting endothelial cell activation, migration and proliferation, three processes that are central to the establishment and remodeling of the vasculature. Bmp signaling also directs maturation of the primitive capillary plexus into the mature vasculature (Park et al., 2004; Reese et al., 2004; Moser and Patterson, 2005; Astorga and Carlsson, 2007; Garriock et al., 2010). Therefore, defective Bmp signaling is a solid candidate cause for sirenomelia, given that this pathway is important for the normal formation of the mesoderm and the differentiation of hematopoietic and endothelial precursor cells.
RA signaling in caudal development and vasculogenesis
Similar to Bmp, RA signaling also plays crucial roles during early embryogenesis, particularly in influencing the development of caudal structures. The spatiotemporal distribution of RA in the early embryo is tightly controlled by the balanced expression of its synthesizing and catabolizing enzymes (Duester, 2008; Niederreither and Dolle, 2008). A multitude of experiments that modified the level of RA signaling by genetic and nutritional means have demonstrated that the embryo is particularly sensitive to deviations from normal levels of RA during gastrulation (Fujii et al., 1997; Niederreither et al., 1997; Niederreither et al., 1999; Abu-Abed et al., 2001; Sakai et al., 2001; Niederreither et al., 2002).
The expression of Cyp26a1, which occurs at the early gastrula stage in the primitive streak and its newly formed mesoderm, shifts to the open neuropore, hindgut endoderm and tailbud mesoderm at the late gastrula stage (Fujii et al., 1997; Abu-Abed et al., 2001; Ribes et al., 2007a; Pennimpede et al., 2010), where it participates in the caudal development of the embryo. The elongation of the trunk is controlled by a balance between the amount of fibroblast growth factor 8 (Fgf8) produced at the tail tip and RA signaling generated by Raldh2 in the somites (Diez del Corral et al., 2003; Olivera-Martinez and Storey, 2007; Ribes et al., 2009; Young et al., 2009). Fgf8 maintains an undifferentiated zone of precursor cells (Akai et al., 2005), whereas RA promotes the differentiation of these precursors. Cyp26a1 expression at the caudal level is thus crucial for RA clearance and for maintenance of the appropriate balance between proliferation and differentiation. However, and despite the implication of Cyp26a1 in sirenomelia, a mutational screening of the CYP26A1 gene in patients with CD did not provide evidence that it is involved in this human malformation (De Marco et al., 2006).
RA is also involved in vascular development. Animal models that carry mutations conferring a gain of RA signaling because of reduced degradation (Ribes et al., 2007a), or with loss of RA function (Lai et al., 2003), show severe and strikingly similar vascular defects. These mutants have severe cardiac malformations and an absence of major vessels owing to defective formation of the primitive capillary plexus. Interestingly, the administration of RA to pregnant rats causes abnormal development of the umbilical and vitelline arteries that are comparable to those reported in human sirenomelia (Monie and Khemmani, 1973). Further studies on the function of RA in chick and mouse embryos (Hochgreb et al., 2003; Lai et al., 2003) showed that RA negatively regulates endothelial cell proliferation, allowing the premature coalescence and differentiation of precursors, thereby interfering with vascular remodeling.
Therefore, defective RA signaling is another well-supported causative candidate for sirenomelia, because the role of this pathway in normal development is strongly supported by the phenotype of the Cyp26a1 mutant mouse.
Crosstalk between the Bmp and RA signaling pathways
The activity of a signaling pathway can be modulated by other signaling pathways either synergistically or antagonistically, depending on the biological context. Because the Bmp and RA pathways both participate in caudal development, it is reasonable to speculate that they might regulate each other. It has been recently shown that RA decreases Bmp signal duration by reducing the level of phosphorylated Smad1, an intracellular component of the Bmp signaling pathway (Hogan, 1996; Kawabata et al., 1998; Macias-Silva et al., 1998; Sheng et al., 2010). If this crosstalk, which was identified in the developing neural tube, also operates in the caudal region of the early embryo, it would suggest that increasing RA levels act by decreasing Bmp signaling levels. Reciprocally, Bmp signaling has been shown to negatively regulate RA signaling during chondrogenesis (Hoffman et al., 2006).
In summary, to fully understand the etiology of sirenomelia, future research should focus on the study of the RA and Bmp signaling components during early embryonic development, and on the identification of possible crosstalk between these two signaling cascades. The use of the animal models of sirenomelia should permit close examination of the participation and possible interconnection between these signaling pathways in the generation of this malformation.
Concluding remarks
Sirenomelia is a multisystemic human malformation of unknown etiology. Clinical observations over the years have provided important insights into the problem and given rise to causal hypotheses that can now be tested and analyzed at the molecular level owing to the availability of animal models that reliably reproduce the human malformation. Much work remains to be done to define the precise role of the candidate factors in causing sirenomelia malformations. The future should include careful analyses of the role of Bmp and RA signaling pathways, as well as their potential interaction, in the generation of this malformation.
Clinical and basic research opportunities.
To determine the genetic and epigenetic roots of sirenomelia.
To characterize the involvement of abnormal caudal mesoderm formation and vasculogenesis in sirenomelia.
To develop additional murine models of sirenomelia.
To obtain a better understanding of the role of the RA and Bmp signaling pathways in the development of sirenomelia.
To study the effects of teratogens (RA and others) as causal factors of sirenomelia.
Acknowledgments
We are very grateful to members of the Ros lab for helpful discussions, particularly to Marisa Junco for her invaluable assistance. Supported by grant BFU2008-00397 (to M.A.R.) and grant BFU2010-19656 (to F.B.) from the Spanish Ministry of Science and Innovation, and by I+D+I cooperative projects in Biomedicine and Biotechnology from the Government and University of Cantabria.
REFERENCES
- Abu-Abed S., Dolle P., Metzger D., Beckett B., Chambon P., Petkovich M. (2001). The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 15, 226–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abed S., Dolle P., Metzger D., Wood C., MacLean G., Chambon P., Petkovich M. (2003). Developing with lethal RA levels: genetic ablation of Rarg can restore the viability of mice lacking Cyp26a1. Development 130, 1449–1459 [DOI] [PubMed] [Google Scholar]
- Adams R. H., Alitalo K. (2007). Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478 [DOI] [PubMed] [Google Scholar]
- Akai J., Halley P. A., Storey K. G. (2005). FGF-dependent Notch signaling maintains the spinal cord stem zone. Genes Dev. 19, 2877–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assimakopoulos E., Athanasiadis A., Zafrakas M., Dragoumis K., Bontis J. (2004). Caudal regression syndrome and sirenomelia in only one twin in two diabetic pregnancies. Clin. Exp. Obstet. Gynecol. 31, 151–153 [PubMed] [Google Scholar]
- Astorga J., Carlsson P. (2007). Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development 134, 3753–3761 [DOI] [PubMed] [Google Scholar]
- Ballantyne J. W. (1904). Malformations of the limb. In Manual and Antenatal Pathology and Hygiene: The Embryo (ed. Sons W. G.), pp. 568–594 Edinburgh: William Green and Sons [Google Scholar]
- Barr M., Jr (1988). Comments on ‘Origin of abnormality in a human simelian foetus as elucidated by our knowledge of vertebrate development’. Teratology 38, 487–491 [DOI] [PubMed] [Google Scholar]
- Boulas M. M. (2009). Recognition of caudal regression syndrome. Adv. Neonatal Care 9, 61–69; quiz 70–71 [DOI] [PubMed] [Google Scholar]
- Castilla E. E., Mastroiacovo P., Lopez-Camelo J. S., Saldarriaga W., Isaza C., Orioli I. M. (2008). Sirenomelia and cyclopia cluster in Cali, Colombia. Am. J. Med. Genet. 146A, 2626–2636 [DOI] [PubMed] [Google Scholar]
- Castori M., Silvestri E., Cappellacci S., Binni F., Sforzolini G. S., Grammatico P. (2010). Sirenomelia and VACTERL association in the offspring of a woman with diabetes. Am. J. Med. Genet. 152A, 1803–1807 [DOI] [PubMed] [Google Scholar]
- Chandebois R., Brunet C. (1987). Origin of abnormality in a human simelian foetus as elucidated by our knowledge of vertebrate development. Teratology 36, 11–22 [DOI] [PubMed] [Google Scholar]
- Chen C., Shih S. L., Jan S. W., Lin Y. N. (1998). Sirenomelia with an uncommon osseous fusion associated with a neural tube defect. Pediatr. Radiol. 28, 293–296 [DOI] [PubMed] [Google Scholar]
- Coffin J. D., Harrison J., Schwartz S., Heimark R. (1991). Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev. Biol. 148, 51–62 [DOI] [PubMed] [Google Scholar]
- Currarino G., Coln D., Votteler T. (1981). Triad of anorectal, sacral, and presacral anomalies. AJR Am. J. Roentgenol. 137, 395–398 [DOI] [PubMed] [Google Scholar]
- Davidson E. H. (1993). Later embryogenesis: regulatory circuitry in morphogenetic fields. Development 118, 665–690 [DOI] [PubMed] [Google Scholar]
- de Jonge H. J., Los J. A., Knipscheer R. J., Frensdorf E. L. (1984). Sirenomelia (‘mermaid’). Eur. J. Obstet. Gynecol. Reprod. Biol. 18, 85–93 [DOI] [PubMed] [Google Scholar]
- De Marco P., Merello E., Mascelli S., Raso A., Santamaria A., Ottaviano C., Calevo M. G., Cama A., Capra V. (2006). Mutational screening of the CYP26A1 gene in patients with caudal regression syndrome. Birth Defects Res. A Clin. Mol. Teratol. 76, 86–95 [DOI] [PubMed] [Google Scholar]
- Diez del Corral R., Olivera-Martinez I., Goriely A., Gale E., Maden M., Storey K. (2003). Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40, 65–79 [DOI] [PubMed] [Google Scholar]
- Drossou-Agakidou V., Xatzisevastou-Loukidou C., Soubasi V., Kostopoulou E., Laporda A., Pantzaki A., Agelidou S., Kremenopoulos G. (2004). Rare manifestations of sirenomelia syndrome: a report of five cases. Am. J. Perinatol. 21, 395–401 [DOI] [PubMed] [Google Scholar]
- Duester G. (2008). Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesterhoeft S. M., Ernst L. M., Siebert J. R., Kapur R. P. (2007). Five cases of caudal regression with an aberrant abdominal umbilical artery: further support for a caudal regression-sirenomelia spectrum. Am. J. Med. Genet. 143A, 3175–3184 [DOI] [PubMed] [Google Scholar]
- Duhamel B. (1961). From the mermaid to anal imperforation: the syndrome of caudal regression. Arch. Dis. Child. 36, 152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P. A., Shapiro L. R. (1993). Interrelationships of the hemifacial microsomia-VATER, VATER, and sirenomelia phenotypes. Am. J. Med. Genet. 47, 75–84 [DOI] [PubMed] [Google Scholar]
- Duncan P. A., Shapiro L. R., Klein R. M. (1991). Sacrococcygeal dysgenesis association. Am. J. Med. Genet. 41, 153–161 [DOI] [PubMed] [Google Scholar]
- Ferm V. H., Carpenter S. J. (1968). The relationship of cadmium and zinc in experimental mammalian teratogenesis. Lab. Invest. 18, 429–432 [PubMed] [Google Scholar]
- Forster A. (1865). Die Missbildungen des Menschen, Systematisch Dargestellt, Nebst Atlas, Part 2. Jena: Manke [Google Scholar]
- Fujii H., Sato T., Kaneko S., Gotoh O., Fujii-Kuriyama Y., Osawa K., Kato S., Hamada H. (1997). Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. EMBO J. 16, 4163–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Dunn N. R., Hogan B. L. (2001). Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc. Natl. Acad. Sci. USA 98, 13739–13744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Porrero J. A., Godin I. E., Dieterlen-Lievre F. (1995). Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat. Embryol. (Berl.) 192, 425–435 [DOI] [PubMed] [Google Scholar]
- Gardner W. J. (1980). Hypothesis; overdistention of the neural tube may cause anomalies of non-neural organs. Teratology 22, 229–238 [DOI] [PubMed] [Google Scholar]
- Garriock R. J., Czeisler C., Ishii Y., Navetta A. M., Mikawa T. (2010). An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development 137, 3697–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S., Dunn L. C. (1945). Sirens, aprosopi and intestinal abnormalities in the house mouse. Anat. Rec. 92, 201–213 [Google Scholar]
- Godin R. E., Takaesu N. T., Robertson E. J., Dudley A. T. (1998). Regulation of BMP7 expression during kidney development. Development 125, 3473–3482 [DOI] [PubMed] [Google Scholar]
- Gofflot F., Hall M., Morriss-Kay G. M. (1997). Genetic patterning of the developing mouse tail at the time of posterior neuropore closure. Dev. Dyn. 210, 431–445 [DOI] [PubMed] [Google Scholar]
- Goldman D. C., Martin G. R., Tam P. P. (2000). Fate and function of the ventral ectodermal ridge during mouse tail development. Development 127, 2113–2123 [DOI] [PubMed] [Google Scholar]
- Goodlow O. G., Sibley R. I., Allen B. G., Kamanda W. S., Gullattee A. C., Rayfield W. C. (1988). Sirenomelia: mermaid syndrome. J. Natl. Med. Assoc. 80, 343–346 [PMC free article] [PubMed] [Google Scholar]
- Gruneberg H. (1956). A ventral ectodermal ridge of the tail in mouse embryos. Nature 177, 787–788 [DOI] [PubMed] [Google Scholar]
- Heifetz S. A. (1984). Single umbilical artery. A statistical analysis of 237 autopsy cases and review of the literature. Perspect. Pediatr. Pathol. 8, 345–378 [PubMed] [Google Scholar]
- Hilbelink D. R., Kaplan S. (1986). Sirenomelia: analysis in the cadmium- and lead-treated golden hamster. Teratog. Carcinog. Mutagen. 6, 431–440 [DOI] [PubMed] [Google Scholar]
- Hochgreb T., Linhares V. L., Menezes D. C., Sampaio A. C., Yan C. Y., Cardoso W. V., Rosenthal N., Xavier-Neto J. (2003). A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development 130, 5363–5374 [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Garcha K., Karamboulas K., Cowan M. F., Drysdale L. M., Horton W. A., Underhill T. M. (2006). BMP action in skeletogenesis involves attenuation of retinoid signaling. J. Cell Biol. 174, 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L. (1996). Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 10, 1580–1594 [DOI] [PubMed] [Google Scholar]
- Hoornbeek F. K. (1970). A gene producing symmelia in the mouse. Teratology 3, 7–10 [DOI] [PubMed] [Google Scholar]
- Jaiyesimi F., Gomathinayagam T., Dixit A., Amer M. (1998). Sirenomelia without vitelline artery steal. Ann. Saudi Med. 18, 542–544 [DOI] [PubMed] [Google Scholar]
- Jin S. W., Patterson C. (2009). The opening act: vasculogenesis and the origins of circulation. Arterioscler. Thromb. Vasc. Biol. 29, 623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. L. (1966). Sirenomelia (Mermaid Fetus). Br. J. Clin. Pract. 20, 198–201 [Google Scholar]
- Kallen B., Winberg J. (1974). Caudal mesoderm pattern of anomalies: from renal agenesis to sirenomelia. Teratology 9, 99–111 [DOI] [PubMed] [Google Scholar]
- Kallen B., Castilla E. E., Lancaster P. A., Mutchinick O., Knudsen L. B., Martinez-Frias M. L., Mastroiacovo P., Robert E. (1992). The cyclops and the mermaid: an epidemiological study of two types of rare malformation. J. Med. Genet. 29, 30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter H. (1993). Case reports of malformations associated with maternal diabetes: history and critique. Clin. Genet. 43, 174–179 [DOI] [PubMed] [Google Scholar]
- Kampmeier O. F. (1927). On sireniform monsters, with a consideration of the causation and predominance of the male sex among them. Anat. Rec. 34, 365 [Google Scholar]
- Kawabata M., Imamura T., Miyazono K. (1998). Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 9, 49–61 [DOI] [PubMed] [Google Scholar]
- Kim J., Kim P., Hui C. C. (2001). The VACTERL association: lessons from the Sonic hedgehog pathway. Clin. Genet. 59, 306–315 [DOI] [PubMed] [Google Scholar]
- Kjaer K. W., Keeling J. W., Opitz J. M., Gilbert-Barness E., Hartling U., Hansen B. F., Kjaer I. (2003). Sirenomelia sequence according to the distance between the first sacral vertebra and the ilia. Am. J. Med. Genet. 120A, 503–508 [DOI] [PubMed] [Google Scholar]
- Kleiss E. (1964). Historia de la Embriología y Teratología en la Antigüedad y Épocas Pre-Colombinas. Merida, Venezuela: Universidad de ls Andes, Facultad de Medicina [Google Scholar]
- Knezevic V., De Santo R., Mackem S. (1998). Continuing organizer function during chick tail development. Development 125, 1791–1801 [DOI] [PubMed] [Google Scholar]
- Kochhar D. M. (1967). Teratogenic activity of retinoic acid. Acta Pathol. Microbiol. Scand. 70, 398–404 [DOI] [PubMed] [Google Scholar]
- Lai L., Bohnsack B. L., Niederreither K., Hirschi K. K. (2003). Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development 130, 6465–6474 [DOI] [PubMed] [Google Scholar]
- Larrain J., Oelgeschlager M., Ketpura N. I., Reversade B., Zakin L., De Robertis E. M. (2001). Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development 128, 4439–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. A., Wright C. (1997). Sirenomelia, limb reduction defects, cardiovascular malformation, renal agenesis in an infant born to a diabetic mother. Clin. Dysmorphol. 6, 75–80 [PubMed] [Google Scholar]
- Macias-Silva M., Hoodless P. A., Tang S. J., Buchwald M., Wrana J. L. (1998). Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 273, 25628–25636 [DOI] [PubMed] [Google Scholar]
- Martinez-Frias M. L., Frias J. L., Salvador J. (1990). Clinical/epidemiological analysis of malformations. Am. J. Med. Genet. 35, 121–125 [DOI] [PubMed] [Google Scholar]
- Martinez-Frias M. L., Cucalon F., Urioste M. (1992). New case of limb body-wall complex associated with sirenomelia sequence. Am. J. Med. Genet. 44, 583–585 [DOI] [PubMed] [Google Scholar]
- Martinez-Frias M. L., Bermejo E., Frias J. L. (2001). The VACTERL association: lessons from the Sonic hedgehog pathway. Clin. Genet. 60, 397–398 [DOI] [PubMed] [Google Scholar]
- Martinez-Frias M. L., Bermejo E., Rodriguez-Pinilla E., Prieto D. (2008). Does single umbilical artery (SUA) predict any type of congenital defect? Clinical-epidemiological analysis of a large consecutive series of malformed infants. Am. J. Med. Genet. 146A, 15–25 [DOI] [PubMed] [Google Scholar]
- Merello E., De Marco P., Mascelli S., Raso A., Calevo M. G., Torre M., Cama A., Lerone M., Martucciello G., Capra V. (2006). HLXB9 homeobox gene and caudal regression syndrome. Birth Defects Res. A Clin. Mol. Teratol. 76, 205–209 [DOI] [PubMed] [Google Scholar]
- Messineo A., Innocenti M., Gelli R., Pancani S., Lo Piccolo R., Martin A. (2006). Multidisciplinary surgical approach to a surviving infant with sirenomelia. Pediatrics 118, e220–e223 [DOI] [PubMed] [Google Scholar]
- Mills C. L., Bellairs R. (1989). Mitosis and cell death in the tail of the chick embryo. Anat. Embryol. (Berl.) 180, 301–308 [DOI] [PubMed] [Google Scholar]
- Monie I. W., Khemmani M. (1973). Absent and abnormal umbilical arteries. Teratology 7, 135–141 [DOI] [PubMed] [Google Scholar]
- Moser M., Patterson C. (2005). Bone morphogenetic proteins and vascular differentiation: BMPing up vasculogenesis. Thromb. Haemost. 94, 713–718 [DOI] [PubMed] [Google Scholar]
- Murphy J. J., Fraser G. C., Blair G. K. (1992). Sirenomelia: case of the surviving mermaid. J. Pediatr. Surg. 27, 1265–1268 [DOI] [PubMed] [Google Scholar]
- Niederreither K., Dolle P. (2008). Retinoic acid in development: towards an integrated view. Nat. Rev. Genet. 9, 541–553 [DOI] [PubMed] [Google Scholar]
- Niederreither K., McCaffery P., Drager U. C., Chambon P., Dolle P. (1997). Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech. Dev. 62, 67–78 [DOI] [PubMed] [Google Scholar]
- Niederreither K., Subbarayan V., Dolle P., Chambon P. (1999). Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444–448 [DOI] [PubMed] [Google Scholar]
- Niederreither K., Abu-Abed S., Schuhbaur B., Petkovich M., Chambon P., Dolle P. (2002). Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nat. Genet. 31, 84–88 [DOI] [PubMed] [Google Scholar]
- O’Rahilly R., Muller F. (1989). Interpretation of some median anomalies as illustrated by cyclopia and symmelia. Teratology 40, 409–421 [DOI] [PubMed] [Google Scholar]
- Ohta S., Suzuki K., Tachibana K., Tanaka H., Yamada G. (2007). Cessation of gastrulation is mediated by suppression of epithelial-mesenchymal transition at the ventral ectodermal ridge. Development 134, 4315–4324 [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I., Storey K. G. (2007). Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development 134, 2125–2135 [DOI] [PubMed] [Google Scholar]
- Opitz J. M., Zanni G., Reynolds J. F., Jr, Gilbert-Barness E. (2002). Defects of blastogenesis. Am. J. Med. Genet. 115, 269–286 [DOI] [PubMed] [Google Scholar]
- Orioli I. M., Mastroiacovo P., Lopez-Camelo J. S., Saldarriaga W., Isaza C., Aiello H., Zarante I., Castilla E. E. (2009). Clusters of sirenomelia in South America. Birth Defects Res. A Clin. Mol. Teratol. 85, 112–118 [DOI] [PubMed] [Google Scholar]
- Orr B. Y., Long S. Y., Steffek A. J. (1982). Craniofacial, caudal, and visceral anomalies associated with mutant sirenomelic mice. Teratology 26, 311–317 [DOI] [PubMed] [Google Scholar]
- Padmanabhan R. (1998). Retinoic acid-induced caudal regression syndrome in the mouse fetus. Reprod. Toxicol. 12, 139–151 [DOI] [PubMed] [Google Scholar]
- Park C., Afrikanova I., Chung Y. S., Zhang W. J., Arentson E., Fong Gh G., Rosendahl A., Choi K. (2004). A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development 131, 2749–2762 [DOI] [PubMed] [Google Scholar]
- Passarge E., Lenz W. (1966). Syndrome of caudal regression in infants of diabetic mothers: observations of further cases. Pediatrics 37, 672–675 [PubMed] [Google Scholar]
- Pennimpede T., Cameron D. A., MacLean G. A., Li H., Abu-Abed S., Petkovich M. (2010). The role of CYP26 enzymes in defining appropriate retinoic acid exposure during embryogenesis. Birth Defects Res. A Clin. Mol. Teratol. 88, 883–894 [DOI] [PubMed] [Google Scholar]
- Pyati U. J., Cooper M. S., Davidson A. J., Nechiporuk A., Kimelman D. (2006). Sustained Bmp signaling is essential for cloaca development in zebrafish. Development 133, 2275–2284 [DOI] [PubMed] [Google Scholar]
- Reese D. E., Hall C. E., Mikawa T. (2004). Negative regulation of midline vascular development by the notochord. Dev. Cell 6, 699–708 [DOI] [PubMed] [Google Scholar]
- Ribes V., Fraulob V., Petkovich M., Dolle P. (2007a). The oxidizing enzyme CYP26a1 tightly regulates the availability of retinoic acid in the gastrulating mouse embryo to ensure proper head development and vasculogenesis. Dev. Dyn. 236, 644–653 [DOI] [PubMed] [Google Scholar]
- Ribes V., Otto D. M., Dickmann L., Schmidt K., Schuhbaur B., Henderson C., Blomhoff R., Wolf C. R., Tickle C., Dolle P. (2007b). Rescue of cytochrome P450 oxidoreductase (Por) mouse mutants reveals functions in vasculogenesis, brain and limb patterning linked to retinoic acid homeostasis. Dev. Biol. 303, 66–81 [DOI] [PubMed] [Google Scholar]
- Ribes V., Le Roux I., Rhinn M., Schuhbaur B., Dolle P. (2009). Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development 136, 665–676 [DOI] [PubMed] [Google Scholar]
- Rodriguez J. I., Palacios J. (1992). Craniorachischisis totalis and sirenomelia. Am. J. Med. Genet. 43, 732–736 [DOI] [PubMed] [Google Scholar]
- Rodriguez J. I., Palacios J., Razquin S. (1991). Sirenomelia and anencephaly. Am. J. Med. Genet. 39, 25–27 [DOI] [PubMed] [Google Scholar]
- Ross A. J., Ruiz-Perez V., Wang Y., Hagan D. M., Scherer S., Lynch S. A., Lindsay S., Custard E., Belloni E., Wilson D. I., et al. (1998). A homeobox gene, HLXB9, is the major locus for dominantly inherited sacral agenesis. Nat. Genet. 20, 358–361 [DOI] [PubMed] [Google Scholar]
- Sadler T. W., Rasmussen S. A. (2010). Examining the evidence for vascular pathogenesis of selected birth defects. Am. J. Med. Genet. 152A, 2426–2436 [DOI] [PubMed] [Google Scholar]
- Saint-Hilaire I. (1836). Des monstres doubles hétéraliens. In Histoire Générale et Particulière des Anomalies de L’organisation chez L’homme et les Animaux. Traite de Teratologie, Atlas (ed. Baillière J.-B.), pp. 239–264 Paris [Google Scholar]
- Sakai Y., Meno C., Fujii H., Nishino J., Shiratori H., Saijoh Y., Rossant J., Hamada H. (2001). The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 15, 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savory J. G., Bouchard N., Pierre V., Rijli F. M., De Repentigny Y., Kothary R., Lohnes D. (2009). Cdx2 regulation of posterior development through non-Hox targets. Development 136, 4099–4110 [DOI] [PubMed] [Google Scholar]
- Schoenwolf G., Bleyl S., Brauer P., Francis-West P. (2009). Development of the limbs. In Larsen’s Human Embryology (ed. Schoenwolf G., Bleyl S., Brauer P., Francis-West P.), pp. 617–644 Philadelphia, Pennsylvania: Churchill Livingstone/Elsevier [Google Scholar]
- Sheng N., Xie Z., Wang C., Bai G., Zhang K., Zhu Q., Song J., Guillemot F., Chen Y. G., Lin A., et al. (2010). Retinoic acid regulates bone morphogenic protein signal duration by promoting the degradation of phosphorylated Smad1. Proc. Natl. Acad. Sci. USA 107, 18886–18891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikandar R., Munim S. (2009). Sirenomelia, the Mermaid syndrome: case report and a brief review of literature. J. Pak. Med. Assoc. 59, 721–723 [PubMed] [Google Scholar]
- Stahl A., Tourame P. (2010). From teratology to mythology: Ancient legends. Arch. Pediatr. 17, 1716–1724 [DOI] [PubMed] [Google Scholar]
- Stevenson R. E., Jones K. L., Phelan M. C., Jones M. C., Barr M., Jr, Clericuzio C., Harley R. A., Benirschke K. (1986). Vascular steal: the pathogenetic mechanism producing sirenomelia and associated defects of the viscera and soft tissues. Pediatrics 78, 451–457 [PubMed] [Google Scholar]
- Stocker J. T., Heifetz S. A. (1987). Sirenomelia. A morphological study of 33 cases and review of the literature. Perspect. Pediatr. Pathol. 10, 7–50 [PubMed] [Google Scholar]
- Suzuki K., Economides A., Yanagita M., Graf D., Yamada G. (2009). New horizons at the caudal embryos; coordinated urogenital/reproductive organ formation by growth factor signaling. Curr. Opin. Genet. Dev. 19, 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thottungal A. D., Charles A. K., Dickinson J. E., Bower C. (2010). Caudal dysgenesis and sirenomelia-single centre experience suggests common pathogenic basis. Am. J. Med. Genet. 152A, 2578–2587 [DOI] [PubMed] [Google Scholar]
- Twickler D., Budorick N., Pretorius D., Grafe M., Currarino G. (1993). Caudal regression versus sirenomelia: sonographic clues. J. Ultrasound Med. 12, 323–330 [DOI] [PubMed] [Google Scholar]
- Valenzano M., Paoletti R., Rossi A., Farinini D., Garlaschi G., Fulcheri E. (1999). Sirenomelia. Pathological features, antenatal ultrasonographic clues, and a review of current embryogenic theories. Hum. Reprod. Update 5, 82–86 [DOI] [PubMed] [Google Scholar]
- van Zalen-Sprock M. M., van Vugt J. M., van der Harten J. J., van Geijn H. P. (1995). Early second-trimester diagnosis of sirenomelia. Prenat. Diagn. 15, 171–177 [DOI] [PubMed] [Google Scholar]
- Voiculescu O., Bertocchini F., Wolpert L., Keller R. E., Stern C. D. (2007). The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature 449, 1049–1052 [DOI] [PubMed] [Google Scholar]
- Walls J. R., Coultas L., Rossant J., Henkelman R. M. (2008). Three-dimensional analysis of vascular development in the mouse embryo. PLoS ONE 3, e2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V., Beddington R. S. (1996). Cell fate and morphogenetic movement in the late mouse primitive streak. Mech. Dev. 55, 79–89 [DOI] [PubMed] [Google Scholar]
- Winnier G., Blessing M., Labosky P. A., Hogan B. L. (1995). Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 9, 2105–2116 [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Konishi H., Kihara T., Tanimura T. (1990). Discontinuity of primary and secondary neural tube in spina bifida induced by retinoic acid in mice. Teratology 41, 257–274 [DOI] [PubMed] [Google Scholar]
- Young T., Rowland J. E., van de Ven C., Bialecka M., Novoa A., Carapuco M., van Nes J., de Graaff W., Duluc I., Freund J. N., et al. (2009). Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell 17, 516–526 [DOI] [PubMed] [Google Scholar]
- Zakin L., Reversade B., Kuroda H., Lyons K. M., De Robertis E. M. (2005). Sirenomelia in Bmp7 and Tsg compound mutant mice: requirement for Bmp signaling in the development of ventral posterior mesoderm. Development 132, 2489–2499 [DOI] [PubMed] [Google Scholar]