Abstract
In 2005, several groups identified a single gain-of-function point mutation in the JAK2 kinase that was present in the majority of patients with myeloproliferative neoplasms (MPNs). Since this discovery, much effort has been dedicated to understanding the molecular consequences of the JAK2V617F mutation in the haematopoietic system. Three waves of mouse models have been produced recently (bone marrow transplantation, transgenic and targeted knock-in), which have facilitated the understanding of the molecular pathogenesis of JAK2V617F-positive MPNs, providing potential platforms for designing and validating novel therapies in humans. This Commentary briefly summarises the first two types of mouse models and then focuses on the more recently generated knock-in models.
Introduction
The myeloproliferative neoplasms (MPNs) are a group of haematological diseases in which cells of the myelo-erythroid lineage are overproduced (Dameshek, 1951). Apart from chronic myelogenous leukaemia, the three most common MPNs are polycythaemia vera (PV), essential thrombocythaemia (ET) and primary myelofibrosis (PMF). The clinical features of these diseases are the overproduction of mature, functional blood cells, specifically characterised by an increased red-cell mass in PV, a high platelet count in ET and bone marrow fibrosis in PMF (Levine et al., 2007; Spivak et al., 2003). In 2005, several groups reported a somatically acquired gain-of-function mutation (V617F) in the JAK2 tyrosine kinase in MPN patients (Baxter et al., 2005; James et al., 2005; Jones et al., 2005; Kralovics et al., 2005; Levine et al., 2005; Zhao et al., 2005). The V617F mutation was found in virtually all PV patients and ∼50–60% of ET and PMF patients. It was also detected at low incidence in other MPNs, but not in solid cancers (Scott et al., 2005; Steensma et al., 2005).
The V617F mutation disrupts the inhibitory activity of the JAK2 pseudokinase domain, leading to constitutive JAK2 kinase activity and hyperactivation of multiple downstream targets (James et al., 2005). It remains unclear how this single mutation can associate with three different diseases. Cell-extrinsic factors, such as serum erythropoietin levels and depleted iron stores, have been suggested to constrain erythropoiesis in ET patients bearing the JAK2V617F mutation (Campbell et al., 2005). Cell-intrinsic factors, such as differential STAT1 activation in ET compared with PV patients, have also been identified (Chen et al., 2010). In addition, inherited genetic modifiers have been suggested to have a role in varying the disease phenotype (Campbell et al., 2005; Pardanani et al., 2006). Interestingly, most PV patients harbour subclones that are homozygous for the JAK2V617F mutation, whereas this is extremely rare in ET (Scott et al., 2006). Consistent with this, dosage and strength of signalling of the JAK2V617F allele have been implicated in the polycythaemic phenotype seen in PV patients (Passamonti and Rumi, 2009; Tiedt et al., 2008).
Early X-linked polymorphism studies indicated that MPNs were clonally derived and that their pathogenesis was probably the result of a transforming event in a haematopoietic stem cell (HSC) or early multipotent progenitor (Fialkow, 1979). Through improved functional assays and advances in techniques for HSC isolation via multiparameter flow cytometry, the JAK2V617F mutation has been detected in primitive Lin−CD34+CD38−CD90+ cells (Jamieson et al., 2006), several types of haematopoietic progenitors (e.g. GMP, BFU-e) (Baxter et al., 2005) and in CD34+CD38− cells, which are capable of reconstituting immunodeficient mice (James et al., 2008). Despite its clear role in MPNs, JAK2V617F is not always the initiating lesion, because it has been shown in some patients to be preceded by other genetic changes (i.e. mutations in TET2 or deletion of chromosome 20) (Delhommeau et al., 2009; Schaub et al., 2009). However, given the prevalence and specificity of the JAK2V617F mutation to the MPNs, substantial effort has been made in the last few years to create mouse models in which JAK2V617F is introduced in an effort to evaluate the functional consequences of the mutation in vivo and to understand mechanisms underlying the initiation and progression of MPNs. This Commentary briefly summarises the early attempts to model JAK2V617F-associated diseases in mice using bone marrow transplantation approaches and mouse transgenesis (Table 1), and then discusses in more detail the more recently published homologous recombination-derived knock-in models (Table 2).
Table 1.
Retroviral overexpression, transplantation and transgenic mouse model phenotypes A. Retroviral overexpression and bone marrow transplantation models
Table 2.
Summary of JAK2V617F knock-in mouse models
Bone marrow transplantation models of JAK2V617F-associated MPNs
Using retroviruses to overexpress a gene of interest is a highly versatile method for modelling haematopoietic malignancies. It is relatively quick compared with other genetic techniques and allows targeting of a specific cell population. However, there are also several conceptual shortfalls with the approach – for example, the ubiquitous and/or non-physiological expression levels obtained, as well as position-effect differences in expression between transgene integration sites. Nevertheless, generating models using this method is a powerful and efficient approach for defining the impact of specific genetic changes on the phenotypes in vivo.
Several groups have reported that transplantation of mice with bone marrow cells retrovirally transduced with JAK2V617F results in a myeloproliferative phenotype with many characteristics of PV, including marked erythrocytosis, splenomegaly, extramedullary haematopoiesis and cytokine-independent progenitor growth (Bumm et al., 2006; Lacout et al., 2006; Wernig et al., 2006; Zaleskas et al., 2006) (for a review, see Morgan and Gilliland, 2008). Interestingly, platelet counts were reported to be normal in most of these studies, despite abundant transgene expression in megakaryocytes. Many mice also appeared to undergo evolution to a myelofibrosis-like disease with associated anaemia, leukocytosis and marrow fibrosis with neutrophilia, although the extent of disease transformation was variable depending on the strain background of the transplanted mice. Additional factors compounding the variable disease presentation could include differences in viral integration sites, the level of transgene expression and the cell population targeted by the viruses in different cohorts. Nevertheless, these bone marrow transplantation studies provided the first data demonstrating that JAK2V617F expression is sufficient to induce an MPN-like phenotype in mice.
Transgenic models of JAK2V617F-associated MPNs
The next series of mouse models were derived by pronuclear injection, creating stable transgenic lines of mice expressing JAK2V617F, the first of which was generated by Shide and coworkers (Shide et al., 2008). In this study, ectopic expression of the cDNA encoding mouse JAK2V617F under the control of the H2Kb promoter was associated with erythrocytosis, thrombocytosis and eventual myelofibrosis. Similar findings were later reported following overexpression of the cDNA encoding human JAK2V617F under the control of the Vav promoter (Xing et al., 2008). In the latter study, a second transgenic line exhibited only a mild increase in these myelo-erythroid lineages. Interestingly, this was associated with similar expression levels of the human JAK2V617F transgene compared with the level of endogenous mouse Jak2.
Soon thereafter, Tiedt and colleagues took an innovative approach to drive variable levels of human JAK2V617F expression in an inducible manner (Tiedt et al., 2008). A mouse line was generated by microinjection that carries nine copies of a bacterial artificial chromosome (BAC) (containing human JAK2 exons 1–12 with the 5′ regulatory region) followed by a cDNA fragment (exons 13–25 with SV40 polyA, encoding the mutant human JAK2 kinase domains) placed in the inverse orientation and flanked by antiparallel lox66 and lox71 sites. This line expresses the human JAK2V617F under the control of the minimal human JAK2 promoter when crossed with mice that express Cre recombinase, giving rise to different JAK2V617F expression levels depending on the amount of Cre activity. Specifically, mice in which JAK2V617F expression was induced by a haematopoietic Vav-Cre transgene expressed lower levels of human mutant JAK2V617F than endogenous wild-type mouse JAK2 and developed thrombocytosis with strongly elevated platelet counts, moderate neutrophilia and unchanged haematocrit. However, mice in which JAK2V617F expression was induced by Mx1-Cre [where Cre expression is transient and the levels of Cre expression can be controlled by dose-dependent activation of the Mx1 promoter with polyinosine-polycytosine (pIpC)] generally retained higher JAK2V617F transgene copy number than in the Vav-Cre model and displayed increased erythrocytosis as well as thrombocytosis. These results are consistent with the hypothesis that the level of JAK2V617F expression correlates with MPN subtype, where higher doses of JAK2V617F expression are usually associated with PV.
Knock-in models of JAK2V617F-associated MPNs
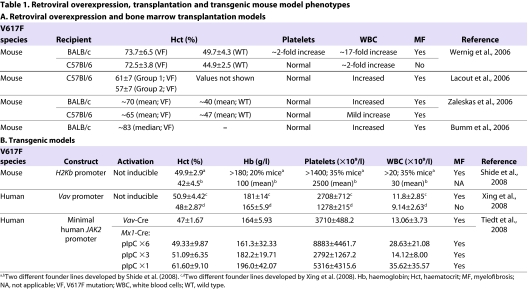
Increasing evidence has indicated that the timing of oncogene activation, the cell type(s) in which it is activated and the level at which it is expressed are crucial to modelling human leukaemic malignancies (Ren, 2004). Analysis of patient samples has indicated that the dosage of JAK2V617F expression correlates with different disease subtypes; in particular, homozygous mutations are mostly detected in PV and rarely in ET (Barbui et al., 2004; De Stefano et al., 2010; Scott et al., 2006). The retroviral transduction and transgenic models discussed above have clearly demonstrated the importance of JAK2V617F in myeloid malignancies, and the observed phenotypic variability probably reflects the positional effect of the transgene integration sites and consequences of high transgene copy number. To dissect precisely the pathogenesis of MPN initiation and progression, it is important to express JAK2V617F at a physiological level and in the correct spatio-temporal expression pattern. This can be achieved by introducing the JAK2V617F mutation under the control of the endogenous Jak2 promoter via homologous recombination in embryonic stem (ES) cells. This approach allows JAK2V617F to be expressed at a physiological level and only in haematopoietic tissues, thereby mimicking the acquisition of the JAK2V617F mutation in human disease. Recently, several such knock-in models have been generated via homologous recombination in ES cells and express JAK2V617F from the endogenous Jak2 promoter (Fig. 1A) (Akada et al., 2010; Mullally et al., 2010; Marty et al., 2010; Li et al., 2010).
Fig. 1.
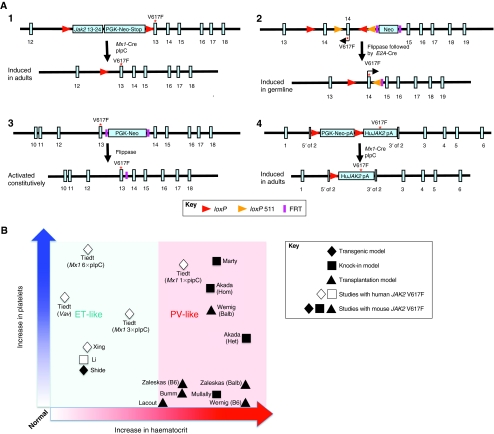
Genetic strategies for generating mouse models of JAK2V617F-positive MPNs, and the phenotypic characteristics of each. (A) Schematic representation of the targeting strategies of four knock-in models. In the Akada et al. (Akada et al., 2010) model (1), the conditional knock-in allele was generated by introducing a V617F mutation construct including the floxed Jak2 cDNA (exons 13–24) with a PGK-Neo-stop cassette in intron 12 of the mouse Jak2 gene. Upon Cre recombination and removal of the floxed sequence, mouse JAK2V617F was expressed under the control of the endogenous mouse Jak2 regulatory elements. In the model by Mullally et al. (Mullally et al., 2010) (2), the conditional knock-in allele was engineered to have a point mutation in exon 13 of the mouse Jak2 gene (numbered 14 in the figure) and, upon flippase (removal of Neo) and Cre (removal of wild-type exon 14) recombination, the mouse JAK2V617F was expressed under the control of the endogenous mouse Jak2 regulatory elements. In the Marty et al. (Marty et al., 2010) model (3), the constitutive knock-in allele was engineered to have a point mutation in exon 13 of the mouse Jak2 gene with a PGK-Neo cassette flanked by flippase recognition target (FRT) in intron 13. The PGK-Neo cassette can be removed by flippase recombination. In the Li et al. (Li et al., 2010) model (4), the conditional knock-in allele was generated by inserting a floxed PGK-Neo-polyA-stop cassette preceding the human JAK2V617F cDNA into the translation start site in exon 2 of the mouse Jak2 locus. Upon Cre recombination, human JAK2V617F was expressed under the control of the endogenous mouse Jak2 regulatory elements. Red asterisks indicate where the mutation is introduced. (B) Approximate increase (relative to normal control) in haematocrit (x-axis) and platelet counts (y-axis) for the transplantation, transgenic models and knock-in mouse models. Open data points indicate studies using human JAK2V617F, whereas filled symbols are studies that use mouse JAK2V617F. The shaded red area indicates models that exhibit a PV-like phenotype and the shaded blue area indicates models that resemble an ET-like phenotype. See reference list for full references.
Akada and colleagues first reported a conditional mouse model in which the mouse JAK2V617F expression was controlled by the endogenous Jak2 promoter and could be experimentally induced by pIpC treatment (Akada et al., 2010). The knock-in allele was generated by introducing a V617F mutation construct including the floxed Jak2 cDNA exons 13–24 with a PGK-Neo-stop cassette in intron 12 of mouse Jak2. Following pIpC treatment of adult mice, heterozygous mice developed a PV-like phenotype, including markedly increased haemoglobin, haematocrit, red blood cells, leukocytosis, thrombocytosis, splenomegaly, reduced serum erythropoietin (Epo) levels and Epo-independent erythroid colonies. Expression of mRNA encoding the JAK2V617F mutant was only 50% of wild-type levels. Mice homozygous for JAK2V617F also developed a PV-like disease associated with significantly greater reticulocytosis, leukocytosis, neutrophilia and thrombocytosis; marked expansion of erythroid progenitors and Epo-independent erythroid colonies; larger spleen size; and accelerated bone marrow fibrosis compared with heterozygous JAK2V617F mice. Of particular note, haemoglobin levels of mice homozygous for JAK2V617F were similar to, or even lower than, those of heterozygous mice, but the platelet counts were considerably increased compared with those in the heterozygous mice. These observations contrast with the current patient data suggesting that JAK2V617F homozygosity is associated with PV and not ET (Scott et al., 2006). Mice with expression of both heterozygous and homozygous JAK2V617F showed significantly expanded haematopoietic stem and progenitor cell compartments in bone marrow and spleen, as determined by increased frequency of Lin−Sca1+cKit+ (LSK) and myeloid progenitor populations.
Next, Marty and co-workers and Mullally and colleagues independently reported mouse JAK2V617F knock-in models with expression of mouse JAK2V617F under the control of the endogenous promoter. Both mouse lines were engineered to have a point mutation in exon 13 of the mouse Jak2 gene, with JAK2V617F germline expression in a constitutive knock-in (Marty et al., 2010), or in a conditional knock-in activated by breeding the mice with E2A transgenic Cre mice (Mullally et al., 2010). Both models displayed marked PV-like phenotypes characterised by erythrocytosis, leukocytosis, splenomegaly and reduced survival. However, distinct features were also observed between the two models (Table 2). The model reported by Marty and colleagues demonstrated markedly increased megakaryopoiesis, as determined by the presence of giant platelets and a 4.7-fold increase in overall platelet counts. Both bone marrow and spleen displayed myeloid tri-lineage hyperplasia, and there was an increase in erythroid (88-fold) and myeloid (82-fold) precursors in spleen. Most animals survived to develop advanced fibrosis in these organs at around 9 months of age. By contrast, the model reported by Mullally and colleagues displayed a lethal MPN with a median survival of 146 days. The mutant mice did not exhibit elevated platelet counts and no differences were observed in megakaryocyte ploidy, although a mild increase in megakaryocytes was observed in the spleen and CD41+ cells were increased in bone marrow. White blood cell counts were elevated, but there was not an obvious increase in the size of Mac1+Gr1+ or Mac1+ myeloid cell populations. Reticulin fibrosis was absent in both bone marrow and spleen, even in older cohorts (>6 months of age).
The final model, reported by Li and co-workers, was a conditional knock-in model that expresses the human JAK2V617F (Li et al., 2010). This allele was generated by inserting a floxed PGK-Neo-poly(A) transcriptional stop cassette preceding the cDNA encoding human JAK2V617F into the translation start site in exon 2 of the mouse Jak2 locus (JAK2F/+ allele). This targeted allele was designed to disrupt the wild-type Jak2 expression and result in a 1:1 expression ratio of mouse JAK2 to human JAK2V617F. Heterozygous mice expressing human JAK2V617F were obtained by crossing mice carrying the JAK2F/+ allele with Mx1-Cre mice. Six weeks following pIpC injection, mice with JAK2V617F expression displayed mildly elevated platelet counts together with significantly higher haemoglobin levels, but white blood cell counts were not significantly changed, which is reminiscent of human JAK2V617F-positive ET. These phenotypes were supported by increased terminal erythroid and megakaryocytic differentiation together with increased numbers of lineage-restricted progenitors in the bone marrow. Splenomegaly was not observed and there was no increase in reticulin staining in both spleen and bone marrow, even in older mice. Notably, as in human ET, approximately 10% of JAK2V617F mice observed for more than 26 weeks after pIpC treatment developed either PV-like disease with marked erythrocytosis or bone marrow fibrosis.
JAK2V617F in stem and progenitor cells
Importantly, Mullally and colleagues and Li and colleagues further characterised the haematopoietic hierarchy to clarify the impact of JAK2V617F on the various stages of haematopoietic differentiation. In the model generated by Mullally et al., quantitative evaluation of bone marrow stem and progenitor populations showed an increase in myeloid progenitors (Lin−Sca1−cKit+), which was largely attributed to an expansion of the megakaryocytic and erythroid progenitor (MEP) subpopulation. However, the frequency of HSC-enriched LSK (Lin−Sca1+cKit+) cells was not altered, which is in contrast to what was observed in the first model reported by Akada and colleagues. There were also no changes in the frequencies of long-term HSCs (CD150+CD48−LSK). Although the JAK2V617F mutation has been detected in HSC-enriched populations of human MPN patients (Jamieson et al., 2006), there were no observed functional consequences in LSK cells, such as differences in cell cycling (as measured by BrdU and 7AAD incorporation) or JAK-STAT signalling (as measured by flow cytometric analysis of phosphorylated STAT5). Furthermore, microarray analysis showed that mutant LSK cells were highly similar to wild-type LSK cells with respect to gene expression, although an enrichment of the erythroid, myeloid and megakaryocytic differentiation pathways was noted, suggesting an increase in the likelihood of myeloid differentiation by cells in the LSK compartment. Bone marrow transplantation experiments demonstrated that the disease phenotypes were transplantable, and that the disease-initiating cell population is contained within the HSC-enriched LSK population but not in the committed myeloid progenitors. Competitive transplantation experiments demonstrated that JAK2V617F LSK cells confer, at most, a minor selective advantage (Mullally et al., 2010).
By contrast, Li and colleagues found that JAK2V617F mice aged over 6 months after pIpC injection had reduced numbers of LSK cells, which exhibited increased DNA damage, reduced cell cycling and reduced apoptosis. This finding contrasts with the notion that, in patients, following acquisition of the JAK2V617F mutation, HSCs might acquire a subtle selective advantage that allows clonal outgrowth over time. Furthermore, in the model generated by Li et al., non-competitive bone marrow transplantation experiments showed a progressive reduction of donor chimerism of JAK2V617F in recipients, indicating a decreased stem cell activity in cells expressing JAK2V617F compared with wild-type littermate controls. Consistent with this, direct comparison of the wild-type versus JAK2V617F bone marrow cells in competitive transplantation experiments demonstrated that JAK2V617F confers a mild but significant disadvantage to HSCs, an effect that was most striking in secondary transplantations.
In summary, all four models involving a heterozygous knock-in allele displayed a transplantable myeloproliferative disease. The models expressing a heterozygous mouse JAK2V617F all had a PV-like phenotype, whereas the model expressing heterozygous human JAK2V617F was associated with ET with a low-level progression to PV and myelofibrosis. As summarised in Table 2, these models demonstrate many similar features but also have interesting differences that impact our understanding of haematopoiesis and MPNs.
Moving forward
Tremendous efforts have been undertaken to model the JAK2V617F mutation in mice since its identification in human MPNs in 2005. To better understand disease initiation, progression, and even transformation, it is informative to compare the phenotypes of JAK2V617F mice between these models, in particular the recent knock-in models that were designed to express the mutant protein at a pathophysiological level. Through these models, it is clear that JAK2V617F mutation is sufficient to drive the disease phenotypes observed in patients and that the severity of the phenotype is sensitive to the levels of JAK2V617F expression (Fig. 1B). The bone marrow retroviral transduction and transplantation studies were perhaps most instructive in their demonstration of a clear PV-like phenotype, showing the impact that this single point mutation can have on the haematopoietic system (Bumm et al., 2006; Lacout et al., 2006; Wernig et al., 2006; Zaleskas et al., 2006). Importantly, transgenic mice that brought the dose closer to normal physiological levels, with JAK2V617F expression under the control of either the minimal human JAK2 promoter, or the H2Kb or Vav promoters, gave the field important information on how JAK2V617F produces variable phenotypes resembling ET, PV or both, depending on the levels of JAK2V617F expression (Shide et al., 2008; Tiedt et al., 2008; Xing et al., 2008). The mechanisms responsible for these differences are not entirely clear, but might include integration-site-related position effects, clonal variation in transgene copy number or loss of 3′ Jak2 cis-regulatory elements.
The recent knock-in mouse models all demonstrated a myeloproliferative phenotype: all three models expressing the mouse heterozygous JAK2V617F exhibited a PV-like phenotype, whereas the model expressing the human JAK2V617F exhibited an ET-like phenotype. The reasons for these phenotypic differences are currently unclear, but could be related to inherent differences in the human and mouse JAK2V617F protein, differences between the mouse and human environments, or the different targeting strategies used.
First, there might be inherent differences in kinase activity between the two species when V617F is introduced. Although expression of the mouse JAK2V617F results in a ligand-independent phosphorylation of downstream pathways [i.e. erythroblasts (Akada et al., 2010) and bone marrow (Marty et al., 2010)], expression of the human JAK2V617F only gave rise to Epo-hypersensitive cells [as measured by phosphorylated STAT5 in erythroblasts (Li et al., 2010)]. It is possible, therefore, that the V617F mutation dysregulates kinase function to a different extent when introduced into the human versus the mouse protein. Second, human and mouse might differ with respect to JAK2V617F-interacting partners or binding and/or activation properties (e.g. receptors, signal transducers or transcription factors) or the downstream response to the aberrant JAK2 signal, resulting in stronger signalling consequences in mouse models compared with patients, resulting in a PV-like rather than an ET-like phenotype in mice. Also, differences in mouse strains and targeting strategies might be responsible for the differences observed.
Interestingly, Li and co-workers reported a relatively mild phenotype in the human JAK2V617F-expressing model, which is consistent with many aspects of human ET, including a 5–10% rate of disease transformation to PV or myelofibrosis (Li et al., 2010). In those mice that transformed to PV or myelofibrosis, it is presumed that an additional genetic event must occur to drive enhanced signalling and/or other cellular processes. Indeed, other genetic alterations have been found in human MPNs in addition to JAK2V617F, such as mutations in TET2, MPL, ASXL1, CBL, EZH2 and IDH (Tefferi, 2010). Therefore, it will be of considerable interest to uncover additional genetic events acquired in the mice that transform to PV or myelofibrosis. Furthermore, if it turns out that the human JAK2V617F protein is fully functional in the mouse environment, then it would be predicted that mice expressing homozygous human JAK2V617F would produce a PV-like phenotype.
In summary, it is clear that each wave of JAK2V617F mouse models (transplantation, transgenic and knock-in) has provided numerous insights into the cellular process of disease pathogenesis and enhanced our understanding of the disease-initiating stem cells. Most recently, the knock-in models have provided a system with pathophysiological levels of JAK2V617F and have permitted higher-resolution studies of particular cell compartments (e.g. stem and progenitor cells). In particular, resolution of the different stem and progenitor cell biology described in the papers by Mullally and colleagues, and Li et al. will be key to determining how MPNs develop and to designing future therapies. Also, as recently proposed by Skoda, it is important to uncover why the MPN knock-in model with the mildest phenotype appears to have a compromised stem cell compartment (Skoda, 2010). These models are valuable tools to further understand the molecular consequences of MPNs, to test novel therapies and/or to identify additional genetic events for disease progression.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Akada H., Yan D., Zou H., Fiering S., Hutchison R. E., Mohi M. G. (2010). Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 115, 3589–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui T., Barosi G., Grossi A., Gugliotta L., Liberato N. L., Marchetti M. (2004). Practice guidelines for the therapy of essential thrombocythemia. A statement from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 89, 215–232 [PubMed] [Google Scholar]
- Baxter E. J., Scott L. M., Campbell P. J., East C., Fourouclas N., Swanton S., Vassiliou G. S., Bench A. J., Boyd E. M., Curtin N., et al. (2005). Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 [DOI] [PubMed] [Google Scholar]
- Bumm T. G., Elsea C., Corbin A. S., Loriaux M., Sherbenou D., Wood L., Deininger J., Silver R. T., Druker B. J., Deininger M. W. (2006). Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 66, 11156–11165 [DOI] [PubMed] [Google Scholar]
- Campbell P. J., Scott L. M., Buck G., Wheatley K., East C. L., Marsden J. T., Duffy A., Boyd E. M., Bench A. J., Scott M. A., et al. (2005). Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet 366, 1945–1953 [DOI] [PubMed] [Google Scholar]
- Chen E., Beer P. A., Godfrey A. L., Ortmann C. A., Li J., Costa-Pereira A. P., Ingle C. E., Dermitzakis E. T., Campbell P. J., Green A. R. (2010). Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell 18, 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameshek W. (1951). Some speculations on the myeloproliferative syndromes. Blood 6, 372–375 [PubMed] [Google Scholar]
- De Stefano V., Za T., Rossi E., Vannucchi A. M., Ruggeri M., Elli E., Mico C., Tieghi A., Cacciola R. R., Santoro C., et al. (2010). Increased risk of recurrent thrombosis in patients with essential thrombocythemia carrying the homozygous JAK2 V617F mutation. Ann. Hematol. 89, 141–146 [DOI] [PubMed] [Google Scholar]
- Delhommeau F., Dupont S., Della Valle V., James C., Trannoy S., Masse A., Kosmider O., Le Couedic J. P., Robert F., Alberdi A., et al. (2009). Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 360, 2289–2301 [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. (1979). Clonal origin of human tumors. Annu. Rev. Med. 30, 135–143 [DOI] [PubMed] [Google Scholar]
- James C., Ugo V., Le Couedic J. P., Staerk J., Delhommeau F., Lacout C., Garcon L., Raslova H., Berger R., Bennaceur-Griscelli A., et al. (2005). A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434, 1144–1148 [DOI] [PubMed] [Google Scholar]
- James C., Mazurier F., Dupont S., Chaligne R., Lamrissi-Garcia I., Tulliez M., Lippert E., Mahon F. X., Pasquet J. M., Etienne G., et al. (2008). The hematopoietic stem cell compartment of JAK2V617F-positive myeloproliferative disorders is a reflection of disease heterogeneity. Blood 112, 2429–2438 [DOI] [PubMed] [Google Scholar]
- Jamieson C. H., Gotlib J., Durocher J. A., Chao M. P., Mariappan M. R., Lay M., Jones C., Zehnder J. L., Lilleberg S. L., Weissman I. L. (2006). The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc. Natl. Acad. Sci. USA 103, 6224–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. V., Kreil S., Zoi K., Waghorn K., Curtis C., Zhang L., Score J., Seear R., Chase A. J., Grand F. H., et al. (2005). Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood 106, 2162–2168 [DOI] [PubMed] [Google Scholar]
- Kralovics R., Passamonti F., Teo S. S., Buser A. S., Tiedt R., Tichellie A., Cazzola M., Skoda R. C. (2005). A gain of function mutation in Jak2 is frequently found in patients with myeloproliferative disorders. N. Engl. J. Med. 352, 1779–1790 [DOI] [PubMed] [Google Scholar]
- Lacout C., Pisani D. F., Tulliez M., Gachelin F. M., Vainchenker W., Villeval J. L. (2006). JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood 108, 1652–1660 [DOI] [PubMed] [Google Scholar]
- Levine R. L., Wadleigh M., Cools J., Ebert B. L., Wernig G., Huntly B. J., Boggon T. J., Wlodarska I., Clark J. J., Moore S., et al. (2005). Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7, 387–397 [DOI] [PubMed] [Google Scholar]
- Levine R. L., Pardanani A., Tefferi A., Gilliland D. G. (2007). Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat. Rev. Cancer 7, 673–683 [DOI] [PubMed] [Google Scholar]
- Li J., Spensberger D., Ahn J. S., Anand S., Beer P. A., Ghevaert C., Chen E., Forrai A., Scott L. M., Ferreira R., et al. (2010). JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 116, 1528–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty C., Lacout C., Martin A., Hasan S., Jacquot S., Birling M. C., Vainchenker W., Villeval J. L. (2010). Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood 116, 783–787 [DOI] [PubMed] [Google Scholar]
- Morgan K. J., Gilliland D. G. (2008). A role for JAK2 mutations in myeloproliferative diseases. Annu. Rev. Med. 59, 213–222 [DOI] [PubMed] [Google Scholar]
- Mullally A., Lane S. W., Ball B., Megerdichian C., Okabe R., Al-Shahrour F., Paktinat M., Haydu J. E., Housman E., Lord A. M., et al. (2010). Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 17, 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanani A., Lasho T., McClure R., Lacy M., Tefferi A. (2006). Discordant distribution of JAK2V617F mutation in siblings with familial myeloproliferative disorders. Blood 107, 4572–4573 [DOI] [PubMed] [Google Scholar]
- Passamonti F., Rumi E. (2009). Clinical relevance of JAK2 (V617F) mutant allele burden. Haematologica 94, 7–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R. (2004). Modeling the dosage effect of oncogenes in leukemogenesis. Curr. Opin. Hematol. 11, 25–34 [DOI] [PubMed] [Google Scholar]
- Schaub F. X., Jager R., Looser R., Hao-Shen H., Hermouet S., Girodon F., Tichelli A., Gisslinger H., Kralovics R., Skoda R. C. (2009). Clonal analysis of deletions on chromosome 20q and JAK2-V617F in MPD suggests that del20q acts independently and is not one of the predisposing mutations for JAK2-V617F. Blood 113, 2022–2027 [DOI] [PubMed] [Google Scholar]
- Scott L. M., Campbell P. J., Baxter E. J., Todd T., Stephens P., Edkins S., Wooster R., Stratton M. R., Futreal P. A., Green A. R. (2005). The V617F JAK2 mutation is uncommon in cancers and in myeloid malignancies other than the classic myeloproliferative disorders. Blood 106, 2920–2921 [DOI] [PubMed] [Google Scholar]
- Scott L. M., Scott M. A., Campbell P. J., Green A. R. (2006). Progenitors homozygous for the V617F mutation occur in most patients with polycythemia vera, but not essential thrombocythemia. Blood 108, 2435–2437 [DOI] [PubMed] [Google Scholar]
- Shide K., Shimoda H. K., Kumano T., Karube K., Kameda T., Takenaka K., Oku S., Abe H., Katayose K. S., Kubuki Y., et al. (2008). Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia 22, 87–95 [DOI] [PubMed] [Google Scholar]
- Skoda R. C. (2010). JAK2 impairs stem cell function? Blood 116, 1392–1393 [DOI] [PubMed] [Google Scholar]
- Spivak J. L., Barosi G., Tognoni G., Barbui T., Finazzi G., Marchioli R., Marchetti M. (2003). Chronic myeloproliferative disorders. Hematology Am. Soc. Hematol. Educ. Program 2003, 200–224 [DOI] [PubMed] [Google Scholar]
- Steensma D. P., Dewald G. W., Lasho T. L., Powell H. L., McClure R. F., Levine R. L., Gilliland D. G., Tefferi A. (2005). The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood 106, 1207–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tefferi A. (2010). Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 24, 1128–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedt R., Hao-Shen H., Sobas M. A., Looser R., Dirnhofer S., Schwaller J., Skoda R. C. (2008). Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 111, 3931–3940 [DOI] [PubMed] [Google Scholar]
- Wernig G., Mercher T., Okabe R., Levine R. L., Lee B. H., Gilliland D. G. (2006). Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood 107, 4274–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S., Wanting T. H., Zhao W., Ma J., Wang S., Xu X., Li Q., Fu X., Xu M., Zhao Z. J. (2008). Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood 111, 5109–5117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleskas V. M., Krause D. S., Lazarides K., Patel N., Hu Y., Li S., Van Etten R. A. (2006). Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS ONE 1, e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Xing S., Li Z., Fu X., Li Q., Krantz S. B., Zhao Z. J. (2005). Identification of an acquired JAK2 mutation in polycythemia vera. J. Biol. Chem. 280, 22788–22792 [DOI] [PMC free article] [PubMed] [Google Scholar]