SUMMARY
Breast cancer is the most common malignancy in women of the Western world. Even though a large percentage of breast cancer patients show pathological complete remission after standard treatment regimes, approximately 30–40% are non-responsive and ultimately develop metastatic disease. To generate a good preclinical model of invasive breast cancer, we have taken a tissue-specific approach to somatically inactivate p53 and E-cadherin, the cardinal cell-cell adhesion receptor that is strongly associated with tumor invasiveness. In breast cancer, E-cadherin is found mutated or otherwise functionally silenced in invasive lobular carcinoma (ILC), which accounts for 10–15% of all breast cancers. We show that mammary-specific stochastic inactivation of conditional E-cadherin and p53 results in impaired mammary gland function during pregnancy through the induction of anoikis resistance of mammary epithelium, resulting in loss of epithelial organization and a dysfunctional mammary gland. Moreover, combined inactivation of E-cadherin and p53 induced lactation-independent development of invasive and metastatic mammary carcinomas, which showed strong resemblance to human pleomorphic ILC. Dissemination patterns of mouse ILC mimic the human malignancy, showing metastasis to the gastrointestinal tract, peritoneum, lung, lymph nodes and bone. Our results confirm that loss of E-cadherin contributes to both mammary tumor initiation and metastasis, and establish a preclinical mouse model of human ILC that can be used for the development of novel intervention strategies to treat invasive breast cancer.
INTRODUCTION
Breast cancer affects a large number of females in the Western world, accounting for half a million deaths worldwide on an annual basis. Carcinoma of the breast is a heterogeneous disease based on pathological criteria, which is probably due to the multiplicity of genetic lesions that have accumulated during tumor development, resulting in distinct tumor types. The most frequently observed subtypes, invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC), are very distinct phenotypically as well as biochemically (Coradini et al., 2002; Korkola et al., 2003; Mathieu et al., 2004; Zhao et al., 2004; Stange et al., 2006). ILC is a subtype of breast cancer that accounts for 10–15% of all cases and has a greater tendency for multifocal development and bilateral presentation than other primary breast tumors (Newstead et al., 1992; Krecke and Gisvold, 1993; Helvie et al., 1993). Classical ILC is characterized by non-cohesive invasive cells that are arranged in trabecules without mass formation and calcification, a feature that hinders diagnosis using physical examination or mammography (Simpson et al., 2003). The vast majority of ILCs are mostly estrogen receptor (ER) positive and therefore responsive to endocrine therapy; however, they are mostly refractory to standard chemotherapy treatment once hormone receptor expression is lost (Gonzalez-Angulo et al., 2007), resulting in comparable survival rates for ILC and IDC (Molland et al., 2004; Tubiana-Hulin et al., 2006). Controversy still exists concerning the etiology of invasive breast cancer (IBC). Surprisingly, most clinicians do not regard lobular carcinoma in situ (LCIS) a precursor lesion for ILC, even though LCIS is regarded as a marker for progression to ipsilateral IBC, and 90% of LCIS-containing IBCs show a lobular phenotype (Wheeler et al., 1974; Rosen et al., 1978; Frykberg et al., 1987; Gump, 1993). Also, loss of E-cadherin, which we have postulated as the initiating and causal event in the development of mouse ILC (mILC) (Derksen et al., 2006), is a common feature of LCIS as well of the adjacent ILC cells (Vos et al., 1997).
E-cadherin is a key component of adherens junctions, structures that control the maintenance of epithelial integrity (Perez-Moreno et al., 2003). E-cadherin is a cell-cell adhesion molecule that functions as a scaffold in the formation of catenin-containing complexes that link E-cadherin to the actin and microtubule cytoskeleton (Hulsken et al., 1994; Takeichi, 1995; Perez-Moreno et al., 2003). In multiple types of cancer, loss of E-cadherin function through genetic or epigenetic mechanisms has been implicated in progression and metastasis (Frixen et al., 1991; Vleminckx et al., 1991; Cleton-Jansen et al., 1994; Hulsken et al., 1994; Oda et al., 1994; Berx et al., 1995; Graff et al., 1995; Takeichi, 1995; Yoshiura et al., 1995; Savagner et al., 1997; Perl et al., 1998; Batlle et al., 2000; Cano et al., 2000; Comijn et al., 2001; Fujita et al., 2003; Yang et al., 2004; Moody et al., 2005). Nonetheless, the molecular mechanisms that drive tumor development and progression upon loss of E-cadherin in breast cancer remain ill-defined.
To study all aspects of tumor pathology, mouse models are needed that mimic not only tumor phenotype, but also the initiating steps of human tumor development. In addition, it is important that mouse models reflect the events that are common in human ILC pathology such as lymphatic dissemination and subsequent distant metastasis, especially to bone. We have previously shown that tissue-specific inactivation of E-cadherin and p53 leads to the development of mILC (Derksen et al., 2006). While this mouse model mimics many aspects of human pathology, including metastatic dissemination, it is not attractive as a preclinical model because of the severity of skin-related problems due to cytokeratin 14 (K14) promoter driven Cre recombinase (K14cre) expression in multiple epithelial tissues. In addition, the stochastic activity of K14cre in mammary gland epithelium precludes in vivo analysis of the direct consequences of E-cadherin loss, alone or in combination with loss of p53. In this study, we have developed a new mouse model for human ILC, based on mouse whey acidic protein (Wap) gene promoter driven Cre recombinase (Wcre)-mediated conditional inactivation of E-cadherin and p53. Whereas mammary-specific loss of E-cadherin alone results in mammary epithelial cell death by apoptosis (Boussadia et al., 2002), combined loss of E-cadherin and p53 confers anchorage-independent survival of mammary cells, resulting in a nonfunctional mammary gland owing to aberrant lobulo-alveolar development and a severely disrupted ductal architecture. Wcre-mediated loss of E-cadherin and p53 resulted in a similar tumor spectrum and latency compared with the K14cre;Cdh1F/F;Trp53F/F model of human ILC. However, Wcre;Cdh1F/F;Trp53F/F mice showed increased rates of tumor cell invasion and metastasis compared with the previous model but without the development of skin-related problems, yielding a superior model of human invasive lobular breast cancer.
RESULTS
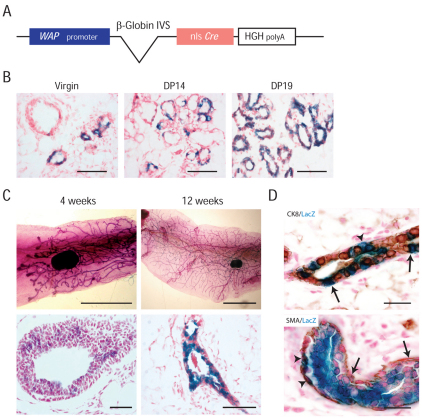
We have previously shown that somatic inactivation of E-cadherin and p53 in K14-expressing cells induces the formation of mILC (Derksen et al., 2006). Although this model recapitulates several aspects of human ILC, it is not very attractive as a preclinical model because of the induction of multiple skin lesions and tumors due to robust K14 expression in the epidermis. To circumvent this problem, we generated Wcre transgenic mice, in which Cre expression is controlled by the mouse Wap gene promoter (Hennighausen and Sippel, 1982), which is known to be expressed in the lumenal compartment of mammary epithelium (Fig. 1A). We examined the efficiency and tissue-specificity of Cre-mediated recombination by crossing Wcre founders with mice carrying the Rosa26-lacZ reporter (R26R mice) (Soriano, 1999). Bi-transgenic virgin and uniparous female littermates were analyzed for lacZ (β-galactosidase) activity using X-gal-stained tissue sections and whole mount preparations. We started by analyzing the Wcre-mediated recombinase activity upon lactation. Examination of whole mount preparations from uniparous female Wcre;R26R mice showed robust β-galactosidase activity throughout the mammary gland (Fig. 1B, right panel). Histological analyses suggested lacZ expression in both the myoepithelial and lumenal compartments of the uniparous mammary gland. In contrast to previously published results (Wagner et al., 1997), we could readily detect cells that had expressed Cre recombinase in mammary glands from virgin Wcre mice (Fig. 1B, left panel, and Fig. 1C). Examination of sectioned whole mounts revealed a weak and patchy staining pattern in 4-week-old virgin mammary glands, which was maintained throughout mammary gland development (Fig. 1C). The β-galactosidase staining patterns again suggested that Cre recombinase activity was not confined to lumenal cells of the mammary gland but was also present in myoepithelial cells (Fig. 1C, bottom right panel). To substantiate this, we performed immunohistochemistry for cytokeratin (CK)8, CK14 and smooth muscle actin (SMA) on sections from X-gal-stained Wcre;R26R virgin female mice. Unfortunately, CK14 immunohistochemistry did not yield interpretable results due to the incompatibility of the staining protocol with antigen retrieval (data not shown). However, although the majority of lacZ-positive (Cre-expressing) cells were coexpressing CK8, we could also detect lacZ positivity in CK8-negative cells (Fig. 1D, top panel). Moreover, we could detect activity of lacZ in SMA-positive cells (Fig. 1D, bottom panel), suggesting that Wcre is expressed in both lumenal and myoepithelial cells in Wcre;R26R female mice.
Fig. 1.
Cre expression in virgin and parous Wcre female mice. (A) Schematic representation of the Wcre transgenic construct. The mouse Wap gene promoter, the rabbit intron from the gene encoding β-globin, the nuclear localization signal (nls)-Cre coding sequences and the human growth hormone (HGH) poly(A) are represented. IVS, intervening sequence/intron. (B) Wcre activity in the mammary gland during gestation. 3-month-old Wcre;R26R mice were analyzed for β-galactosidase activity during pregnancy. Blue staining indicates Cre-expressing cells. Scale bars: 100 μm. (C) Cre expression in Wcre virgin and parous female mice. Shown is β-galactosidase activity of carmine-counterstained whole mount preparations (top) and fast-nuclear-red-counterstained sections (bottom) from 4-week- and 12-week-old Wcre;R26R heterozygous virgin mice. Scale bars: 10 mm (top panels); 100 μm (bottom panels). (D) Wcre is expressed in lumenal and myoepithelial cells. Whole mounts from Wcre;R26Rfemale mice were stained for lacZ activity, sectioned and analyzed for expression of CK8 (top panel) and SMA (bottom panel) using immunohistochemistry. Sections were counterstained with fast nuclear red. Arrowheads indicate non-lumenal lacZ-positive cells, whereas arrows point to CK8-positive lumenal cells (top panel) and SMA-expressing myoepithelial cells (bottom panel). Scale bars: 50 μm.
Mammary-gland-specific conditional inactivation of E-cadherin is not tolerated
To study the effects of E-cadherin loss during mammary gland development and tumor formation, we crossed our Wcre transgenic animals to conditional E-cadherin knockout (Cdh1F) mice (Derksen et al., 2006). Despite the extensive Cre-mediated recombination in the mammary gland of Wcre females (Fig. 1), we did not find morphological abnormalities in virgin, pregnant or parous Wcre;Cdh1F/F mice (Fig. 2A, left panels). Although Wcre;Cdh1F/F females lactated and were able to nurse their litters upon parturition, the produced quantity of milk and litters was markedly lower than in wild-type animals (data not shown). Upon histological examination, we did not observe abnormalities in mammary architecture, nor did we detect E-cadherin-negative ductal structures, indicating that – in agreement with previous studies (Boussadia et al., 2002; Derksen et al., 2006) – loss of E-cadherin is not tolerated in the mouse mammary gland (supplementary material Fig. S1). Moreover, Wcre-mediated loss of E-cadherin did not predispose mice to tumor formation, because none of the Wcre;Cdh1F/F animals developed mammary carcinomas during their live span, even after the induction of multiple pregnancies, which is consistent with previously published studies using MMTVcre and K14cre conditional E-cadherin knockout mice (Boussadia et al., 2002; Derksen et al., 2006).
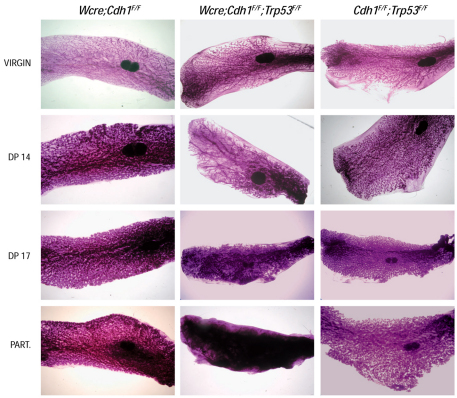
Fig. 2.
Developmental and lactational defects upon inactivation of E-cadherin and p53. Loss of E-cadherin and p53 leads to gross abnormalities in the mammary glands during gestation. Shown are carmine-stained whole mount preparations of mouse mammary glands from Wcre;Cdh1F/F mice (left panels), Wcre;Cdh1F/F;Trp53F/F mice (middle panels) and Cdh1F/F;Trp53F/F control animals (right panels). Part., parturition. Original magnification: 6.5×.
Developmental and lactational defects upon somatic inactivation of E-cadherin and p53
Mammary-specific loss of E-cadherin induces cell death by apoptosis (Boussadia et al., 2002). To investigate whether this apoptotic response to E-cadherin loss could be blocked by p53 inactivation, we introduced a conditional Trp53 allele (Jonkers et al., 2001) into the Wcre;Cdh1F/F mouse line. Next, we studied whether dual loss of E-cadherin and p53 would influence normal mammary gland development and function. To achieve effective Cre-mediated deletion of both Cdh1 and Trp53, we induced Wcre expression by mating and compared mammary gland morphology in Wcre;Cdh1F/F;Trp53F/F females with that in Wcre;Cdh1F/F and Cdh1F/F;Trp53F/F control mice. To this end, we examined carmine-stained whole mount preparations of fourth (inguinal) mammary glands, harvested at 14 and 17 days of pregnancy and at parturition (day 19.5). Mammary glands from virgin mice were used as a starting point. Whereas mammary glands from virgin Wcre;Cdh1F/F;Trp53F/F or Wcre;Cdh1F/F females showed no gross morphological abnormalities as compared with control female mice, Wcre;Cdh1F/F;Trp53F/F mammary glands harvested at day 14 of pregnancy displayed architectural abnormalities, showing severe ectasia (dilated ducts) and incomplete lobulo-alveolar development (Fig. 2). These effects became more pronounced as pregnancy progressed, with a filling of the mammary gland with nonfunctional tissue, resulting in complete disruption of the ductal structure at parturition (Fig. 2, bottom center panel). Wcre;Cdh1F/F and Cdh1F/F;Trp53F/F control animals showed no morphological mammary gland abnormalities during pregnancy (Fig. 2, left and right panels). Although Wcre;Cdh1F/F;Trp53F/F and Wcre;Cdh1F/F;Trp53F/+ female mice produced healthy newborn offspring, all pups fostered by Wcre;Cdh1F/F;Trp53F/F dams died before weaning age due to starvation. Also, pups from Wcre;Cdh1F/F;Trp53F/+ dams showed reduced survival rates due to the inhibition or absence of lactation. This phenotype could be rescued by fostering pups from Wcre;Cdh1F/F;Trp53F/+ dams to wild-type recipient dams (supplementary material Fig. S2). Furthermore, we did not detect significant differences in the sizes of newborn litters from Wcre;Cdh1F/F, Wcre;Cdh1F/+, Wcre;Cdh1F/F;Trp53F/+, Wcre;Cdh1F/+;Trp53F/F and Wcre;Cdh1F/F;Trp53F/F female mice (data not shown).
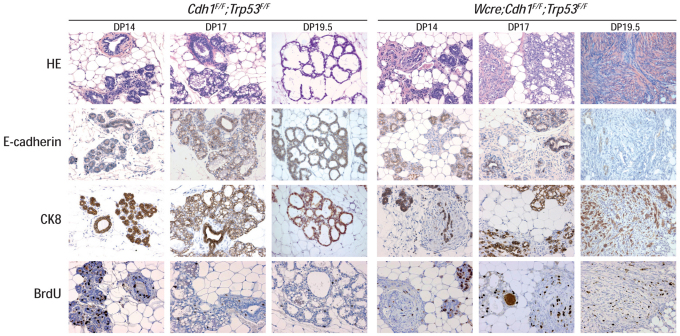
Next, we analyzed the histology of the mammary gland at different time points during pregnancy and at parturition. We stained for myoepithelial cells using CK14, for lumenal epithelial cells using CK8 and we analyzed Cre-mediated switching through E-cadherin expression. We also studied proliferation after incorporation of BrdU, and visualized apoptosis by means of cleaved caspase-3. During pregnancy, loss of E-cadherin was apparent multifocally in mammary glands from Wcre;Cdh1F/F;Trp53F/F mice at day-of-pregnancy (DP) 14, 17 and 19.5 (Fig. 3; supplementary material Fig. S3). Structures that showed an absence of E-cadherin expression had lost normal ductal architecture, showed no lobulo-alveolar development, and consisted of CK8-positive cells, interrupted by cells with a fibroblastic and/or mesenchymal appearance (Fig. 3; Wcre;Cdh1F/F;Trp53F/F, DP 14, 17 and 19.5). At parturition, Wcre;Cdh1F/F;Trp53F/F glands showed strong proliferation when compared with control glands (Fig. 3; DP 19.5), but no signs of involution-induced apoptosis were observed after weening (data not shown). Surprisingly, we did not detect histological abnormalities in mammary glands from Wcre;Cdh1F/F mice (supplementary material Fig. S1 and data not shown). Normal ductal architecture was maintained throughout pregnancy, and E-cadherin-negative ducts were not observed in these glands at either time point. These results show that loss of E-cadherin and p53 leads to aberrant lobulo-alveolar development during gestation. The developmental abnormalities are probably caused by loss of cell polarity and acquisition of anoikis resistance, resulting in loss of mammary gland organization and enhanced proliferative capacity of mammary epithelial cells, leading to a nonfunctional mammary gland.
Fig. 3.
Impaired lobulo-alveolar development upon dual inactivation of E-cadherin and p53. Comparative histochemistry during gestation of mammary glands from control (Cdh1F/F;Trp53F/F) and Wcre;Cdh1F/F;Trp53F/F animals. Lumenal cells were identified using CK8. Also shown are E-cadherin expression and proliferation by means of staining against incorporated BrdU. HE, hematoxylin and eosin. Original magnification: 100×.
E-cadherin loss collaborates with p53 loss in mammary tumorigenesis
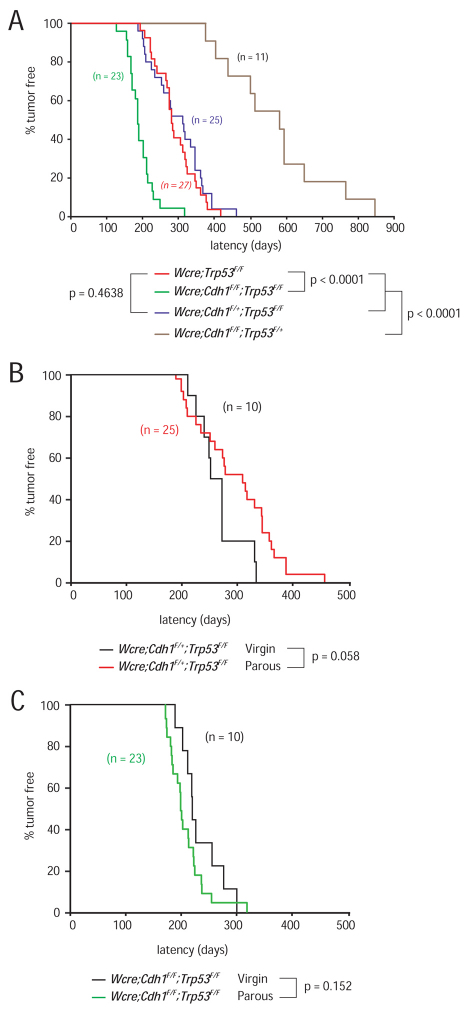
We have previously shown that K14cre-mediated somatic inactivation of p53 in epithelial tissues results in the development of noninvasive adenocarcinoma and carcinosarcoma tumor types with a median latency of ∼330 days (Liu et al., 2007). To target conditional loss of p53 to the mammary epithelial compartment, we crossed Trp53F/F conditional mutant mice (Liu et al., 2007) with our Wcre mice. All resulting Wcre;Trp53F/F female mice developed mammary tumors, with a median latency of 290 days (Fig. 4A). To study the tumor suppressor functions of E-cadherin, we crossed our Cdh1F conditional mice (Derksen et al., 2006) with the Wcre;Trp53F/F mouse model to produce cohorts of Wcre;Cdh1F/+;Trp53F/F and Wcre;Cdh1F/F;Trp53F/F female mice, which were mated once and monitored for subsequent tumor development. All mice were on a mixed genetic background of FVB/N and Ola129/sv (Derksen et al., 2006). Wcre;Cdh1F/+;Trp53F/F animals developed tumors with a similar median latency as Wcre;Trp53F/F females (Fig. 4A; P=0.4638), showing that E-cadherin is not haploinsufficient for suppression of mammary tumor formation in these mice. By contrast, Wcre;Cdh1F/F;Trp53F/F mice developed mammary tumors with a significantly reduced tumor-free survival age (T50) of 194 days (Fig. 4A; P<0.0001). Tumor onset and progression in these mice were relatively uniform, with most tumors arising between 150 and 250 days. These findings demonstrate that combined inactivation of E-cadherin and p53 contribute synergistically to mammary tumor formation in these mice. Subsequent genetic analyses revealed uniform loss of both mutant Cdh1 alleles in tumors derived from Wcre;Cdh1F/F;Trp53F/F females (data not shown). As in the K14cre mILC model, we also detected uniform loss of the conditional and wild-type Trp53 alleles in mammary tumors from Wcre;Cdh1F/+;Trp53F/F, Wcre;Cdh1F/F;Trp53F/+ and Wcre;Cdh1F/F;Trp53F/F females, indicating that loss of functional p53 is a prerequisite for mammary tumor formation in these mouse models (data not shown).
Fig. 4.
Synergistic tumor suppressor activity of E-cadherin and p53 in mammary tumorigenesis. Tumor incidence in Wcre uniparous females carrying conditional Cdh1 and Trp53 alleles. (A) E-cadherin and p53 synergize in mammary tumorigenesis. Shown is a Kaplan-Meier tumor-free survival curve for mammary tumors from Wcre;Trp53F/F (red) versus Wcre;Cdh1F/+;Trp53F/F (blue), Wcre;Cdh1F/F;Trp53F/F (green) and Wcre;Cdh1F/F;Trp53F/+ (brown) females. (B,C) Tumor onset and development in the Wcre;Cdh1F;Trp53F mouse models is lactation independent. Tumor-free survival curves (Kaplan-Meier) of virgin Wcre;Cdh1F/+;Trp53F/F (B; black) and Wcre;Cdh1F/F;Trp53F/F (C; black) female mice, versus uniparous Wcre;Cdh1F/+;Trp53F/F (B; red) and Wcre;Cdh1F/F;Trp53F/F (C; green) females. Mice were sacrificed when mammary tumors reached an average diameter of 10 mm.
Lactation does not affect tumor onset, incidence, latency or metastasis formation in Wcre;Cdh1F;Trp53F mice
Because our analysis of Wcre;R26R mice also revealed Cre recombinase activity in virgin females (Fig. 1), we set out to investigate whether Wcre;Cdh1F/+;Trp53F/F and Wcre;Cdh1F/F;Trp53F/F female mice would develop mammary tumors in the absence of lactation. To this end, we produced ten female mice of each genotype and monitored tumor development in virgin animals. Interestingly, tumors arose in Wcre;Cdh1F/+;Trp53F/F virgin females with identical incidence and latency when compared with uniparous females (Fig. 4B). In line with this finding, tumor-free latency was also similar in virgin and parous Wcre;Cdh1F/F;Trp53F/F female mice (Fig. 4C). Moreover, the tumor spectrum, invasiveness and metastatic dissemination were similar in virgin and parous Wcre;Cdh1F/+;Trp53F/F and Wcre;Cdh1F/F;Trp53F/F female mice (data not shown), indicating that Wcre expression levels in virgin female mice in the Wcre conditional E-cadherin and p53 mouse model (Wcre;Cdh1F;Trp53F) are sufficient to induce stochastic Cre-mediated loss of E-cadherin and p53.
Loss of E-cadherin induces a shift from noninvasive adenocarcinoma to ILC with pleomorphic features
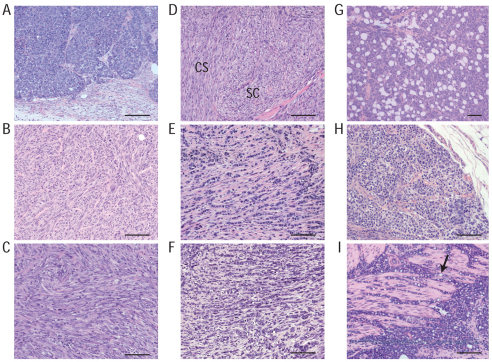
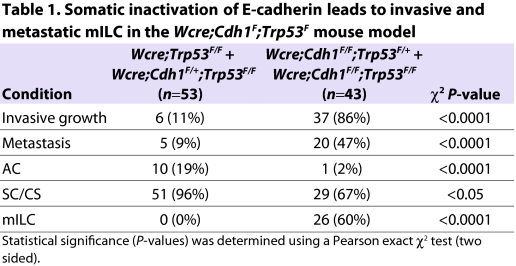
Mammary tumors of Wcre;Trp53F/F females were categorized into two groups and diagnosed as intermediate-grade adenocarcinoma (AC) or solid carcinoma/carcinosarcoma (SC/CS) tumor types, both characterized by expansive growth patterns. Tumors displayed benign noninvasive features and consisted of large epithelial cells forming solid nests or irregular glands. ACs showed membranous expression of E-cadherin and displayed a mixed but exclusive expression pattern of CK8 and CK14, but lacked expression of vimentin and SMA (Fig. 5A; supplementary material Table S1). SC/CS lesions were characterized by a metaplastic and biphasic histology that consisted of epithelial and mesenchymal elements (Fig. 5B–D), showing a heterogeneous and mutually exclusive expression pattern for CK8 and CK14, occasionally expressing vimentin and mostly lacking expression of E-cadherin (supplementary material Table S1). Expansive growth patterns were seen in the vast majority of tumors, which only sporadically metastasized to the lung (Table 1; supplementary material Tables S1 and S2).
Fig. 5.
Tumor spectrum in the Wcre;Trp53F and Wcre;Cdh1F;Trp53F mouse models. Comparative histology of mammary tumors. Shown are H&E-stained sections of: (A) adenocarcinoma (AC), (B) solid carcinoma/carcinosarcoma (SC/CS), epithelial type, (C) SC/CS, mesenchymal type, (D) SC/CS, mixed epithelial-mesenchymal type, (E) mILC, classical type (note the typical trabecular ‘Indian file’-type invasive growth patterns), (F) mILC, pleomorphic type, (G) mILC, solid type, (H) mouse LCIS, (I) metastatic mILC, signet-ring cell type [note the characteristic presence of intracellular lumina containing mucin (arrow)]. Scale bars: 100 μm.
Table 1.
Somatic inactivation of E-cadherin leads to invasive and metastatic mILC in the Wcre;Cdh1F;Trp53F mouse model
Because most mammary tumors in Wcre;Trp53F/F animals were neither invasive nor metastatic, we investigated the phenotypic consequences of loss of E-cadherin. Mammary-specific somatic loss of E-cadherin and p53 in Wcre;Cdh1F/F;Trp53F/F females resulted in a significant shift from expansive to invasive mammary tumors (P<0.0001; Table 1), which showed strong phenotypic similarities to human pleomorphic ILC (PILC). These tumors, which we have previously designated mILC (Derksen et al., 2006), developed with high incidence multifocally in several mammary glands (P<0.0001; Table 1). mILC cells grew in a non-cohesive manner in a trabecular fashion, were small in size, relatively pleomorphic in appearance and diagnosed as high grade (Fig. 5E,F). We could also detect signet ring cells, a typical occasional trait of human ILC (Fig. 5I). Like the adenocarcinomas from Wcre;Trp53F/F females, lobular carcinomas showed a mixed CK8 and CK14 expression pattern, but did not express vimentin nor SMA (supplementary material Table S1), indicating that mILC cells display epithelial properties. Although most mILCs from Wcre;Cdh1F/F;Trp53F/F and Wcre;Cdh1F/F;Trp53F/+ females were estrogen receptor (ER) negative, we occasionally found mILC lesions that weakly expressed ER. ER was mostly expressed by low-grade elements in mILC lesions, suggesting that ER expression is inversely correlated with tumor grade (supplementary material Table S1 and Fig. S4).
Wcre;Cdh1F/F;Trp53F/F and Wcre;Cdh1F/F;Trp53F/+ females also developed SC/CS. Although displaying mILC components, these tumors predominantly exhibited a mixed epithelial and mesenchymal or spindle-shaped cell morphology, presenting large cells with pleomorphic nuclei, coarsely clumped chromatin and sparse cytoplasm. SC/CS tumor cells showed both expansive and invasive growth patterns, an exclusive and heterogeneous expression of CK8 and CK14, and predominantly expressed vimentin, but lacked expression of E-cadherin and SMA (Table 1; supplementary material Table S1).
Loss of E-cadherin induces invasiveness and metastasis
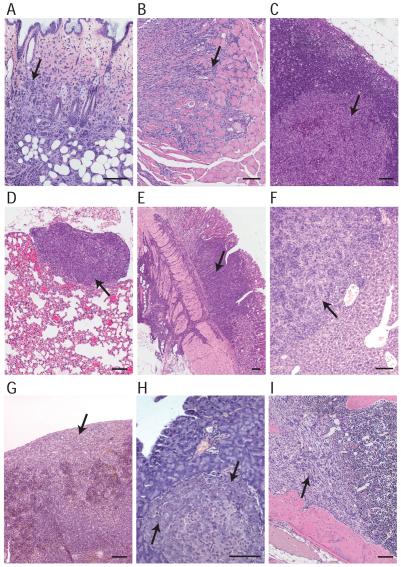
To explore whether the metastatic spread of mILC in the Wcre;Cdh1F;Trp53F model mimics the metastatic pattern of human ILC, we performed a detailed histological survey of various organs from tumor-bearing mice. Approximately 74% of the Wcre;Cdh1F/F;Trp53F/F and 70% of the Wcre;Cdh1F/F;Trp53F/+ females that presented mammary tumors of approximately 1 cm in diameter showed extensive local invasion and metastases to draining and distant lymph nodes (Fig. 6 and Table 1). Discohesive or loosely clustered mILC cells were detected in organs such as skin, lungs, liver, gastrointestinal tract, pancreas and spleen, or were diffusely disseminated throughout the peritoneal cavity (Fig. 6A–H), indicating that mILC in the Wcre;Cdh1F;Trp53F model recapitulates the histopathology and tumor biology of human ILC. In addition, several mice developed bone metastases, a feature that we have not observed in the K14cre;Cdh1F;Trp53F mouse model, exemplifying the additional value of the Wcre;Cdh1F;Trp53F model (Fig. 6I). mILC metastases showed a mixed and mutually exclusive expression pattern of CK8 and CK14, and displayed a cellular morphology similar to that of the primary tumor (Fig. 6; supplementary material Fig. S5).
Fig. 6.
The metastatic spectrum of mILC. (A) Local invasion of mILC, showing infiltration of the skin dermis by tumor cells. (B) Infiltration of the striated muscle of the hind limb by mILC cells, which originated from a primary tumor present in the adjacent fifth mammary gland. (C,D) Distant metastasis of mILC. Carcinoma cells are infiltrating an axillary lymph node (C) and lungs (D). (E–H) Peritoneal involvement in mILC. Sections showing invasion of mILC cells into the muscularis externa of the stomach (E), metastasis of mILC to liver (F) and spleen (G), and colonization by mILC cells of a pancreatic islet of Langerhans (H). (I) Bone metastasis in mILC. Metastatic mILC cells infiltrating the right femur of a Wcre;Cdh1F/F;Trp53F/+ female mouse. Arrows point to metastatic mILC cells. Scale bars: 100 μm.
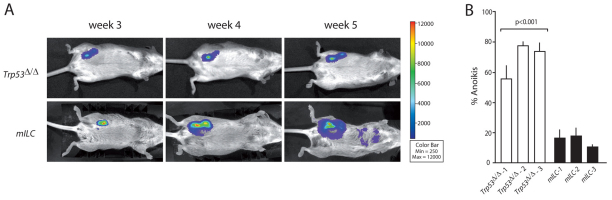
From the primary tumors that developed in our mouse model, we isolated tumor cells, which were then cultured and subsequently transduced with luciferase-encoding lentiviruses to enable bioluminescence imaging. To further characterize the metastatic spectrum of mILC, we orthotopically transplanted recipient animals with approximately 10,000 luciferase-expressing Cdh1Δ/Δ;Trp53Δ/Δ (mILC) tumor cells. Using noninvasive bioluminescence imaging, we could image tumor growth and distant metastases, which developed approximately 5 weeks post transplantation (Fig. 7A; P<0.001). Metastases were detected in the peritoneal and thoracic cavity, contralateral mammary glands, lungs and bone (data not shown), illustrating that the full metastatic spectrum of human ILC is recapitulated upon orthotopic transplantation of mILC cell lines derived from the Wcre;Cdh1F;Trp53F model. By contrast, orthotopic transplantation of luciferase-marked Trp53Δ/Δ tumor cells showed a mixed growth pattern, depending on the cell line studied. Most E-cadherin-proficient tumor cells showed tumor growth, but did not metastasize (Fig. 7A). The difference in metastatic capacity between Trp53Δ/Δ and mILC tumor cells in situ correlated well with the acquisition of anchorage independence upon loss of E-cadherin (Fig. 7B), in analogy to tumor cells derived from the K14cre;Cdh1F;Trp53F mILC model (Derksen et al., 2006). In the absence of cell-matrix interactions, E-cadherin-expressing Trp53Δ/Δ tumor cells were not able to survive, whereas mILC cells readily survived and proliferated in an anchorage-independent fashion (Fig. 7B). Anoikis resistance was observed for cell lines derived from four independent mILC primary tumors, whereas cell lines from three independent Trp53Δ/Δ tumors showed anoikis induction under non-adherent culture conditions, indicating that loss of E-cadherin mediates survival of mammary tumor cells in the absence of cell-matrix interactions. In conclusion, in vitro anoikis resistance is a reliable prognosticator of in vivo metastatic dissemination, using cell lines derived from the Wcre;Cdh1F;Trp53F mouse model.
Fig. 7.
mILC anoikis resistance correlates with in vivo metastasis. (A) mILC metastasis imaging in vivo. Bioluminescence imaging of recipient animals, which were orthotopically transplanted with luciferase-transduced Trp53Δ/Δ cells (top panels) or mILC cells (bottom panels). The color bar represents bioluminescence intensity counts. Transplantations were performed using two different mILC and Trp53Δ/Δ cell lines, in a minimum of five recipient animals. (B) Loss of E-cadherin induces anoikis resistance in the absence of p53. Tumor cell lines established from Wcre;Trp53F/F (AC) and Wcre;Cdh1F/F;Trp53F/F (mILC) female mice were plated onto non-coated low cluster wells and the percentage of cells expressing phosphatidylserine was determined using binding to FITC-conjugated annexin-V. Dead cells were detected using DNA binding to ToPro-3. In the presence of E-cadherin, tumor cells are not able to survive in the absence of anchorage (white bars), whereas E-cadherin-deficient Cdh1Δ/Δ;Trp53Δ/Δ (mILC) cells (black bars) show anoikis resistance (P>0.001). Error bars represent the standard deviation of triplicate measurements.
DISCUSSION
Research on breast cancer metastasis has greatly profited from recent advances in modeling metastatic disease in mice, using both transplantation techniques and genetic modification (Khanna and Hunter, 2005; Ottewell et al., 2006). Owing to the complex nature of the metastatic process, models that mimic both de novo tumor development and spontaneous metastasis formation are scarce, but nevertheless emerging. Here, we have used Cre-loxP-based conditional mutagenesis to develop a lactation-independent and mammary-gland-specific mouse model of human PILC.
Wcre-mediated inactivation of E-cadherin and p53 in mice induced simultaneous development of multiple tumors in several mammary glands. This high penetrance implies that only one or a few additional hits are required to induce tumor formation of predisposed E-cadherin- and p53-deficient mammary progenitors. Wcre-mediated loss of E-cadherin and p53 induced numerous biphasic tumors in several mammary glands that displayed both epithelial and mesenchymal characteristics, suggesting that conditional inactivation based on Wap promoter activity occurs in a progenitor cell that is not yet fully committed to a distinct epithelial lineage. Although Wap is known to be transcribed during gestation and lactation (Pittius et al., 1988; Dale et al., 1992), we found that a substantial amount of Wcre mammary epithelial cells show Cre activity independent of gestation and lactation. Moreover, mammary glands from 4-week-old Wcre;R26R female mice already displayed β-galactosidase activity, indicating that the Wap promoter used in our studies might be activated by prolactin in young virgin animals at to the onset of estrus (Topper and Freeman, 1980; Whittingham and Wood, 1983; Clarke et al., 1993). Nonetheless, the majority of mammary reconstitution assays that we have performed with mammary epithelial cells from 3-week-old Wcre;Cdh1F/F;Trp53F/F mice yielded phenotypically wild-type mammary glands (Eva J. Vlug and P.W.B.D., unpublished data), implying that the majority of mammary epithelial stem and/or progenitor cells in 3-week-old Wcre conditional mice have retained a non-switched (floxed) configuration and that the majority of Wcre-mediated recombination occurs upon the onset the first estrous cycle, which commences after approximately 28 days in mice (Caligioni, 2009). Because multiple copies of the Wcre transgene will have concatemerized during genomic integration, we envisage that physiological prolactin levels in the virgin animals might be sufficient to drive stochastic Wcre-mediated loss of E-cadherin and p53 in the absence of a lactational pulse.
It is likely that dependency on E-cadherin function changes during differentiation of mammary stem and/or progenitor cells towards lumenal and myoepithelial descendants. Cells that differentiate into lumenal-type cells will become dependent on E-cadherin-based adherens junctions, and succumb to apoptosis upon loss of E-cadherin as a result of the absence of a redundant classical cadherin (Boussadia et al., 2002; Derksen et al., 2006). Following this event, cells will be cleared from the fat-pad, resulting in the absence of E-cadherin-deficient glandular structures in MMTVcre;Cdh1F/F and Wcre;Cdh1F/F mice (Boussadia et al., 2002) (and this study). Myoepithelial cells do not express E-cadherin, and hence will not be influenced by Cdh1 deletion, mainly because P-cadherin is the predominant classical cadherin that will maintain the junctional integrity in these cells (Radice et al., 1997). We therefore hypothesize that mammary stem and/or progenitor cells tolerate the absence of E-cadherin until differentiation into lumenal descendants is commenced.
Our results show that loss of p53 confers resistance to the proapoptotic signals that are initiated upon E-cadherin inactivation, resulting in the survival of lumenal cells in the absence of a functional adherens junction. In Wcre;Cdh1F/F;Trp53F/F female mice, inactivation of E-cadherin and p53 during gestation resulted in the accumulation of mostly CK8-positive epithelial cells and mesenchymal-type cells that fill the periductal environment, thereby inhibiting formation of a functional mammary gland. Interestingly, Wcre-mediated gene switching did not result in accelerated tumor development in parous females when compared with the incidence in virgin females or the tumor-free survival in our models, indicating that Cre recombinase activity during gestation and lactation is mostly restricted to differentiated cells that do not contribute to mammary tumor formation. By contrast, lactation-independent inactivation of p53 and E-cadherin in mammary stem and/or progenitor cells will give rise to transformed epithelial cell types that display enhanced survival and growth and, in addition, harbor stem-cell-like characteristics that might facilitate progression into carcinomas with a non-cohesive growth pattern typical for mouse and human ILC. In the rare event that the myoepithelial compartment is not affected by somatic inactivation of the mutant alleles and the integrity of the basement membrane remains unaffected, this might give rise to LCIS, as in the human situation. Nonetheless, most early mILC lesions show a disturbed myoepithelial and/or basement membrane architecture, which is most probably causal to their highly invasive character. Also, the stromal component that is abundantly present in the periductal regions might contribute to tumor cell invasiveness through deposition of extracellular matrix, as has been recently proposed (Levental et al., 2009).
Although human ILCs are mostly ER positive, we only detected sporadic ER-positive cells in low- or intermediate-grade mILC lesions that still display features of glandular and/or ductal architecture. High-grade invasive and metastatic mILC never showed ER expression, which is in line with previous observations that mouse mammary tumors in general show physiological differences compared with human breast cancer with respect to hormone receptor expression (Yoshidome et al., 2000; Jonkers et al., 2001; Derksen et al., 2006; Alvarez et al., 2006; Liu et al., 2007). We assume that the vast majority of the mammary tumors that form in Wcre;Cdh1F/F;Trp53F/F female mice have developed from an ER-negative progenitor cell. Occasionally, preneoplastic cells will express ER, but will either not develop into a high-grade invasive tumor, or will transit to an ER-signaling independent mILC.
In contrast to human classical ILC, mILC depends on inactivation of p53, which is thought to occur in a minority (4–25%) of human cases (Marchetti et al., 1993; Rosen et al., 1995; Soslow et al., 2000; Coradini et al., 2002; Arpino et al., 2004). Most studies have employed immunohistochemical detection of mutant p53 protein, leaving the possibility of functional p53 loss through alternative mechanisms. Supporting this are recent studies showing that 30–40% of human lobular carcinomas have lost the TP53 locus on chromosome 17p13 (Mohsin et al., 2005; Stange et al., 2006). Most mILC lesions showed atypical nuclear morphology and would be classified as PILC in human pathology. PILC shows a more aggressive clinical behavior than ILC but is thought to share a common genetic pathway (Buchanan et al., 2008; Orvieto et al., 2008; Simpson et al., 2008; Gudlaugsson et al., 2009). The basis for the poorer prognosis of PILC when compared with ILC is thought to emanate from the fact that PILC is characterized by a higher frequency of ER and/or progesterone receptor negativity (Bentz et al., 1998) and loss of p53 function (Middleton et al., 2000; Kaya et al., 2002; Sneige et al., 2002; Reis-Filho et al., 2005).
In conclusion, we have generated a novel Wcre transgenic mouse line that displays Cre recombinase activity in mammary stem and/or progenitor cells. Consequently, Wcre-driven somatic inactivation of E-cadherin and p53 results in mammary tumor formation with similar incidence and latency in both virgin and parous mice. Combined inactivation of p53 and E-cadherin leads to mILC, which is highly invasive and shows (lymph)angiogenic and diffuse dissemination with metastasis to the gastrointestinal tract and bone, similar to the human situation. Our mouse model therefore represents an excellent preclinical model to test novel intervention strategies for invasive and metastatic breast cancer.
METHODS
Generation of Wcre transgenic mice
We constructed the Wcre transgene from an expression cassette that includes a 4.5-kb genomic BamHI-SalI mouse Wap gene promoter fragment followed by a 0.65-kb rabbit β-globin intron and a 0.63-kb transcription termination/polyadenylation fragment derived from the human growth hormone gene. Cre coding sequences were inserted between the intron and the poly(A) fragment (Fig. 1A). Next, we separated the 7.8-kb transgene fragment from vector sequences, purified and injected it into the pronuclei of one-cell-stage embryos of FVB/N mice. Microinjected eggs were transferred at the two-cell stage into the oviducts of pseudopregnant recipient females. Heterozygous transgenic animals were identified using PCR analysis.
DNA analysis and genotyping
Genomic DNA isolation and Southern blot analysis were performed as described (Jonkers et al., 2001; Derksen et al., 2006). Detection of the Trp53F, Trp53Δ, Cdh1F and Cdh1Δ alleles was done by PCR as described (Derksen et al., 2006). Transgenic Wcre animals were identified by PCR using primers 5′-ACAGCCATCAGT-CACTTGCC-3′ and 5′-CATCACTCGTTGCATCGACC-3′, yielding an amplification product of 432 bp.
Antibodies
Antibodies used were: mouse anti-E-cadherin (1:300; BD Biosciences), mouse anti β-catenin (1:150; BD Biosciences), rat anti-CK8 (Troma-1; 1:125; Developmental Studies Hybridoma Bank), rabbit anti-CK14 (1:10,000; BabCo), guinea pig anti-vimentin (1:400; RDI), rabbit anti-SMA (1:350; Lab Vision), mouse anti-BrdU (1:1000; DAKO), rabbit anti-ERα (1:1000; Santa Cruz Biotechnology). Secondary antibodies were: biotin-conjugated anti-mouse, anti-rat and anti-rabbit antibodies (DAKO), and biotin-conjugated anti-guinea pig (Jackson ImmunoResearch).
Stainings of whole mount preparations
Reconstituted mammary glands were dissected and stretched on a glass slide. The glands were fixed in a mixture of 6:3:1 methanol:1,1,1-trichloroethane:acetic acid (all Sigma) for 4 hours and processed as whole mounts, which were stained overnight with carmine aluminum staining solution [2 g/l carmine (Sigma), 5 g/l aluminum potassium sulphate dissolved in H2O]. After stepwise dehydration in 70%, 95% and 100% ethanol, the glands were cleared in xylene (Sigma) for 10 minutes before taking pictures. For the β-galactosidase staining, we fixed glands in 4% paraformaldehyde (Sigma), 2 mM MgCl2 (Merck) and 5 mM EGTA (Sigma) for 4 hours at 4°C. After washing with 2 mM MgCl2, 0.02% NP-40, 0.01% Na-deoxycholate in PBS, glands were stained overnight with X-Gal staining solution [5 mM ferro-cyanide (K4Fe(CN)6), 5 mM ferri-cyanide (K3Fe(CN)6), 2 mM MgCl2, 0.02% NP-40 and 1 mg/ml X-Gal (Biosolve)] at 37°C in the dark. After dehydration in ethanol, glands were cleared in xylene (Sigma) for 10 minutes. For determination of cellular proliferation, mice were injected intraperitoneally with 2 mg/ml BrdU (Sigma) 90 minutes prior to harvest of the mammary glands.
Histological analysis
Tissues were isolated, fixed and processed as described (Derksen et al., 2006).
Cell culture
Cells were isolated and cultured as described previously (Derksen et al., 2006)
Lentiviral production and transduction of cells
Lentiviral particles were produced by seeding 106 293T cells onto a 10 cm Petri dish and performing transient transfection after 24 hours with third-generation packaging constructs and a luciferase-encoding transfer vector (LV-luc) (Derksen et al., 2006). Supernatant containing lentiviral particles were harvested and concentrated tenfold by ultra centrifugation at 75,000 g for 2.5 hours. Tumor cells were infected for 16 hours in the presence of 4μg/ml polybrene.
Orthotopic transplantations and bioluminescence imaging
3-week-old Rag2–/−;IL2Rγc–/− BALB/c female recipient mice (Gimeno et al., 2004) were anaesthetized by intraperitoneal injection of a mixture containing 25 μl fentanyl citrate/fluanisone (hypnorm; Janssen Pharmaceutica), 25 μl midazolam (dormicum; Roche) and 50 μl water. The fourth mammary gland was exposed and endogenous mammary epithelial tissue was removed. Next, approximately 10,000 luciferase-transduced tumor cells were injected in the cleared fat-pad using a 10 μl Hamilton syringe, after which the animals were sutured. After a recovery period of 2 weeks, mice were anesthetized with isofluorane (Janssen Pharmaceutica), injected intraperitoneally with 225 μg/g body weight n-luciferin (potassium salt; Biosynth AG) and imaged on an IVIS-200 bioluminescence imager (Xenogen). All animal experiments were performed in accordance with institutional guidelines and national regulations.
TRANSLATIONAL IMPACT.
Clinical issue
Metastatic disease is the major cause of mortality in breast cancer patients. A hallmark of invasive and metastatic cells is inhibition of E-cadherin function. Invasive lobular carcinoma (ILC; the second most common type of primary breast cancer) is characterized by early loss of the epithelial cell-cell adhesion molecule E-cadherin. The classical form of ILC is characterized by non-cohesive and invasive cells but without mass formation, often resulting in a false-negative diagnosis using physical examination or mammography. Although most cases of ILC are estrogen receptor (ER) positive and therefore responsive to initial endocrine therapy, the disease is generally refractory to standard chemotherapy treatments once ER expression is lost at later stages.
Results
In this paper, the authors establish a new mammary-specific conditional knockout mouse model to show that combined stochastic inactivation of E-cadherin and p53 results in impaired mammary gland function. Phenotypically, mammary tumors in this model exhibit multiple features of human pleomorphic ILC, both in terms of their appearance and their metastatic behavior. The data show that somatic inactivation of E-cadherin renders mammary epithelial cells anoikis resistant in the context of p53 deficiency. Importantly, the female mice in this model develop highly invasive and metastatic mammary tumors for which the onset, incidence and metastasis are lactation independent.
Implications and future directions
The lactation-independent and mammary-gland-specific mouse model of human pleomorphic ILC presented in this paper provides a new tool to gain insight into the role of E-cadherin loss of function in mammary tumor initiation, progression and metastasis. The study also addresses how E-cadherin and p53 might cooperate in mammary tumor development. Given the similarities between mouse and human ILC, this model might ultimately contribute to the development of novel clinical intervention strategies for the treatment of metastatic breast cancer.
Anoikis assay
Cells were plated at a density of 75,000 cells per well in a six-well ultra-low cluster polystyrene culture dish (Corning). FITC-conjugated annexin-V (1:20; IQ Products) and ToPro-3 (1:2000; Molecular Probes) were added and annexin-V-positive apoptotic cells were analyzed by FACS as described (Derksen et al., 2003). Statistical significance was calculated using the Student’s t-test.
Supplementary Material
Acknowledgments
We thank Miranda van Amersfoort, Hermien Boerhout and Sabine Vishnudatt for technical support. Members of the Jonkers lab are acknowledged for reagents, help and fruitful discussions. We are also indebted to the animal facility and the animal pathology lab. Joost Vermaat is kindly acknowledged for the statistical analyses. This work was supported by grants from the Netherlands Organization for Scientific Research (ZonMw VIDI 917.36.347) and the Dutch Cancer Society (NKI 2002-2635 and NKI 2006-3486). P.W.B.D. was supported by grants from the Netherlands Organization for Scientific Research (ZonMw VENI 916.56.135 and VIDI 917.96.318).
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
P.W.B.D. and J.J. designed the experiments. P.K. generated the Wcre transgenic mice. P.W.B.D., T.M.B., M.H. and E.v.d.B. performed the experiments. E.M. executed the immune histochemistry on tissue sections and J.W. diagnosed mouse pathology. P.W.B.D. wrote the paper with assistance from J.J.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.006395/-/DC1
REFERENCES
- Alvarez J. V., Perez D., Chodosh L. A. (2006). MILC-ing the mouse mammary gland: A model for invasive lobular carcinoma. Cancer Cell 10, 347–349 [DOI] [PubMed] [Google Scholar]
- Arpino G., Bardou V. J., Clark G. M., Elledge R. M. (2004). Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 6, R149–R156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E., Sancho E., Franci C., Dominguez D., Monfar M., Baulida J., Garcia De, Herreros A. (2000). The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84–89 [DOI] [PubMed] [Google Scholar]
- Bentz J. S., Yassa N., Clayton F. (1998). Pleomorphic lobular carcinoma of the breast: clinicopathologic features of 12 cases. Mod. Pathol. 11, 814–822 [PubMed] [Google Scholar]
- Berx G., Cleton-Jansen A. M., Nollet F., de Leeuw W. J., van de Vijver M., Cornelisse C., van Roy F. (1995). E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 14, 6107–6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia O., Kutsch S., Hierholzer A., Delmas V., Kemler R. (2002). E-cadherin is a survival factor for the lactating mouse mammary gland. Mech. Dev. 115, 53–62 [DOI] [PubMed] [Google Scholar]
- Buchanan C. L., Flynn L. W., Murray M. P., Darvishian F., Cranor M. L., Fey J. V., King T. A., Tan L. K., Sclafani L. M. (2008). Is pleomorphic lobular carcinoma really a distinct clinical entity? J. Surg. Oncol. 98, 314–317 [DOI] [PubMed] [Google Scholar]
- Caligioni C. S. (2009). Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. Appendix 4, Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A., Perez-Moreno M. A., Rodrigo I., Locascio A., Blanco M. J., del Barrio M. G., Portillo F., Nieto M. A. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 [DOI] [PubMed] [Google Scholar]
- Clarke D. L., Arey B. J., Linzer D. I. (1993). Prolactin receptor messenger ribonucleic acid expression in the ovary during the rat estrous cycle. Endocrinology 133, 2594–2603 [DOI] [PubMed] [Google Scholar]
- Cleton-Jansen A. M., Moerland E. W., Kuipers-Dijkshoorn N. J., Callen D. F., Sutherland G. R., Hansen B., Devilee P., Cornelisse C. J. (1994). At least two different regions are involved in allelic imbalance on chromosome arm 16q in breast cancer. Genes Chromosomes Cancer 9, 101–107 [DOI] [PubMed] [Google Scholar]
- Comijn J., Berx G., Vermassen P., Verschueren K., van Grunsven L., Bruyneel E., Mareel M., Huylebroeck D., van Roy F. (2001). The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 7, 1267–1278 [DOI] [PubMed] [Google Scholar]
- Coradini D., Pellizzaro C., Veneroni S., Ventura L., Daidone M. G. (2002). Infiltrating ductal and lobular breast carcinomas are characterised by different interrelationships among markers related to angiogenesis and hormone dependence. Br. J. Cancer 87, 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale T. C., Krnacik M. J., Schmidhauser C., Yang C. L., Bissell M. J., Rosen J. M. (1992). High-level expression of the rat whey acidic protein gene is mediated by elements in the promoter and 3′ untranslated region. Mol. Cell Biol. 12, 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen P. W., de Gorter D. J., Meijer H. P., Bende R. J., van Dijk M., Lokhorst H. M., Bloem A. C., Spaargaren M., Pals S. T. (2003). The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia 17, 764–774 [DOI] [PubMed] [Google Scholar]
- Derksen P. W., Liu X., Saridin F., Van Der Gulden H., Zevenhoven J., Evers B., van Beijnum J. R., Griffioen A. W., Vink J., Krimpenfort P., et al. (2006). Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 [DOI] [PubMed] [Google Scholar]
- Frixen U. H., Behrens J., Sachs M., Eberle G., Voss B., Warda A., Lochner D., Birchmeier W. (1991). E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113, 173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykberg E. R., Santiago F., Betsill W. L., Jr, O’Brien P. H. (1987). Lobular carcinoma in situ of the breast. Surg. Gynecol. Obstet. 164, 285–301 [PubMed] [Google Scholar]
- Fujita N., Jaye D. L., Kajita M., Geigerman C., Moreno C. S., Wade P. A. (2003). MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113, 207–219 [DOI] [PubMed] [Google Scholar]
- Gimeno R., Weijer K., Voordouw A., Uittenbogaart C. H., Legrand N., Alves N. L., Wijnands E., Blom B., Spits H. (2004). Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood 104, 3886–3893 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo A. M., Morales-Vasquez F., Hortobagyi G. N. (2007). Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. 608, 1–22 [DOI] [PubMed] [Google Scholar]
- Graff J. R., Herman J. G., Lapidus R. G., Chopra H., Xu R., Jarrard D. F., Isaacs W. B., Pitha P. M., Davidson N. E., Baylin S. B. (1995). E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res. 55, 5195–5199 [PubMed] [Google Scholar]
- Gudlaugsson E., Skaland I., Janssen E. A., van Diest P. J., Voorhorst F. J., Kjellevold K., Hausen A. Z., Baak J. P. (2009). Prospective multicenter comparison of proliferation and other prognostic factors in lymph node negative lobular invasive breast cancer. Breast Cancer Res. Treat. 121, 35–40 [DOI] [PubMed] [Google Scholar]
- Gump F. E. (1993). Lobular carcinoma in situ (LCIS): pathology and treatment. J. Cell. Biochem. Suppl. 17, 53–58 [PubMed] [Google Scholar]
- Helvie M. A., Paramagul C., Oberman H. A., Adler D. D. (1993). Invasive lobular carcinoma. Imaging features and clinical detection. Invest. Radiol. 28, 202–207 [DOI] [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. (1982). Mouse whey acidic protein is a novel member of the family of ‘four-disulfide core’ proteins. Nucleic Acids Res. 10, 2677–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsken J., Birchmeier W., Behrens J. (1994). E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J. Cell Biol. 127, 2061–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J., Meuwissen R., Van Der Gulden H., Peterse H., van der Valk M., Berns A. (2001). Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418–425 [DOI] [PubMed] [Google Scholar]
- Kaya H., Aribal E., Yegen C. (2002). Apocrine differentiation in invasive pleomorphic lobular carcinoma with in situ ductal and lobular apocrine carcinoma: case report. Pathol. Oncol. Res. 8, 151–152 [DOI] [PubMed] [Google Scholar]
- Khanna C., Hunter K. (2005). Modeling metastasis in vivo. Carcinogenesis 26, 513–523 [DOI] [PubMed] [Google Scholar]
- Korkola J. E., DeVries S., Fridlyand J., Hwang E. S., Estep A. L., Chen Y. Y., Chew K. L., Dairkee S. H., Jensen R. M., Waldman F. M. (2003). Differentiation of lobular versus ductal breast carcinomas by expression microarray analysis. Cancer Res. 63, 7167–7175 [PubMed] [Google Scholar]
- Krecke K. N., Gisvold J. J. (1993). Invasive lobular carcinoma of the breast: mammographic findings and extent of disease at diagnosis in 184 patients. AJR Am. J. Roentgenol. 161, 957–960 [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F., Csiszar K., Giaccia A., Weninger W., et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Holstege H., van der Gulden H., Treur-Mulder M., Zevenhoven J., Velds A., Kerkhoven R. M., van Vliet M. H., Wessels L. F., Peterse J. L., et al. (2007). Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc. Natl. Acad. Sci. USA 104, 12111–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti A., Buttitta F., Pellegrini S., Campani D., Diella F., Cecchetti D., Callahan R., Bistocchi M. (1993). P53 mutations and histological type of invasive breast carcinoma. Cancer Res. 53, 4665–4669 [PubMed] [Google Scholar]
- Mathieu M. C., Rouzier R., Llombart-Cussac A., Sideris L., Koscielny S., Travagli J. P., Contesso G., Delaloge S., Spielmann M. (2004). The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur. J. Cancer 40, 342–351 [DOI] [PubMed] [Google Scholar]
- Middleton L. P., Palacios D. M., Bryant B. R., Krebs P., Otis C. N., Merino M. J. (2000). Pleomorphic lobular carcinoma: morphology, immunohistochemistry, and molecular analysis. Am. J. Surg. Pathol. 24, 1650–1656 [DOI] [PubMed] [Google Scholar]
- Mohsin S. K., O’Connell P., Allred D. C., Libby A. L. (2005). Biomarker profile and genetic abnormalities in lobular carcinoma in situ. Breast Cancer Res. Treat. 90, 249–256 [DOI] [PubMed] [Google Scholar]
- Molland J. G., Donnellan M., Janu N. C., Carmalt H. L., Kennedy C. W., Gillett D. J. (2004). Infiltrating lobular carcinoma-a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast 13, 389–396 [DOI] [PubMed] [Google Scholar]
- Moody S. E., Perez D., Pan T. C., Sarkisian C. J., Portocarrero C. P., Sterner C. J., Notorfrancesco K. L., Cardiff R. D., Chodosh L. A. (2005). The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 [DOI] [PubMed] [Google Scholar]
- Newstead G. M., Baute P. B., Toth H. K. (1992). Invasive lobular and ductal carcinoma: mammographic findings and stage at diagnosis. Radiology 184, 623–627 [DOI] [PubMed] [Google Scholar]
- Oda T., Kanai Y., Oyama T., Yoshiura K., Shimoyama Y., Birchmeier W., Sugimura T., Hirohashi S. (1994). E-cadherin gene mutations in human gastric carcinoma cell lines. Proc. Natl. Acad. Sci. USA 91, 1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto E., Maiorano E., Bottiglieri L., Maisonneuve P., Rotmensz N., Galimberti V., Luini A., Brenelli F., Gatti G., Viale G. (2008). Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single institution. Cancer 113, 1511–1520 [DOI] [PubMed] [Google Scholar]
- Ottewell P. D., Coleman R. E., Holen I. (2006). From genetic abnormality to metastases: murine models of breast cancer and their use in the development of anticancer therapies. Breast Cancer Res. Treat. 96, 101–113 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M., Jamora C., Fuchs E. (2003). Sticky business: orchestrating cellular signals at adherens junctions. Cell 112, 535–548 [DOI] [PubMed] [Google Scholar]
- Perl A. K., Wilgenbus P., Dahl U., Semb H., Christofori G. (1998). A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 392, 190–193 [DOI] [PubMed] [Google Scholar]
- Pittius C. W., Sankaran L., Topper Y. J., Hennighausen L. (1988). Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol. Endocrinol. 2, 1027–1032 [DOI] [PubMed] [Google Scholar]
- Radice G. L., Ferreira-Cornwell M. C., Robinson S. D., Rayburn H., Chodosh L. A., Takeichi M., Hynes R. O. (1997). Precocious mammary gland development in P-cadherin-deficient mice. J. Cell Biol. 139, 1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Filho J. S., Simpson P. T., Jones C., Steele D., Mackay A., Iravani M., Fenwick K., Valgeirsson H., Lambros M., Ashworth A., et al. (2005). Pleomorphic lobular carcinoma of the breast: role of comprehensive molecular pathology in characterization of an entity. J. Pathol. 207, 1–13 [DOI] [PubMed] [Google Scholar]
- Rosen P. P., Kosloff C., Lieberman P. H., Adair F., Braun D. W., Jr (1978). Lobular carcinoma in situ of the breast. Detailed analysis of 99 patients with average follow-up of 24 years. Am. J. Surg. Pathol. 2, 225–251 [DOI] [PubMed] [Google Scholar]
- Rosen P. P., Lesser M. L., Arroyo C. D., Cranor M., Borgen P., Norton L. (1995). P53 in node-negative breast carcinoma: an immunohistochemical study of epidemiologic risk factors, histologic features, and prognosis. J. Clin. Oncol. 13, 821–830 [DOI] [PubMed] [Google Scholar]
- Savagner P., Yamada K. M., Thiery J. P. (1997). The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J. Cell Biol. 137, 1403–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. T., Gale T., Fulford L. G., Reis-Filho J. S., Lakhani S. R. (2003). The diagnosis and management of pre-invasive breast disease: pathology of atypical lobular hyperplasia and lobular carcinoma in situ. Breast Cancer Res. 5, 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. T., Reis-Filho J. S., Lambros M. B., Jones C., Steele D., Mackay A., Iravani M., Fenwick K., Dexter T., Jones A., et al. (2008). Molecular profiling pleomorphic lobular carcinomas of the breast: evidence for a common molecular genetic pathway with classic lobular carcinomas. J. Pathol. 215, 231–244 [DOI] [PubMed] [Google Scholar]
- Sneige N., Wang J., Baker B. A., Krishnamurthy S., Middleton L. P. (2002). Clinical, histopathologic, and biologic features of pleomorphic lobular (ductal-lobular) carcinoma in situ of the breast: a report of 24 cases. Mod. Pathol. 15, 1044–1050 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- Soslow R. A., Carlson D. L., Horenstein M. G., Osborne M. P. (2000). A comparison of cell cycle markers in well-differentiated lobular and ductal carcinomas. Breast Cancer Res. Treat. 61, 161–170 [DOI] [PubMed] [Google Scholar]
- Stange D. E., Radlwimmer B., Schubert F., Traub F., Pich A., Toedt G., Mendrzyk F., Lehmann U., Eils R., Kreipe H., et al. (2006). High-resolution genomic profiling reveals association of chromosomal aberrations on 1q and 16p with histologic and genetic subgroups of invasive breast cancer. Clin. Cancer Res. 12, 345–352 [DOI] [PubMed] [Google Scholar]
- Takeichi M. (1995). Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 7, 619–627 [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. (1980). Multiple hormone interactions in the developmental biology of the mammary gland. Physiol. Rev. 60, 1049–1106 [DOI] [PubMed] [Google Scholar]
- Tubiana-Hulin M., Stevens D., Lasry S., Guinebretiere J. M., Bouita L., Cohen-Solal C., Cherel P., Rouesse J. (2006). Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann. Oncol. 17, 1228–1233 [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet L., Jr, Mareel M., Fiers W., van Roy F. (1991). Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66, 107–119 [DOI] [PubMed] [Google Scholar]
- Vos C. B., Cleton-Jansen A. M., Berx G., de Leeuw W. J., ter Haar N. T., van Roy F., Cornelisse C. J., Peterse J. L., van de Vijver M. J. (1997). E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br. J. Cancer 76, 1131–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K. U., Wall R. J., St Onge L., Gruss P., Wynshaw-Boris A., Garrett L., Li M., Furth P. A., Hennighausen L. (1997). Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25, 4323–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler J. E., Enterline H. T., Roseman J. M., Tomasulo J. P., McIlvaine C. H., Fitts W. T., Jr, Kirshenbaum J. (1974). Lobular carcinoma in situ of the breast. Long-term followup. Cancer 34, 554–563 [DOI] [PubMed] [Google Scholar]
- Whittingham D. G., Wood M. J. (1983). The Mouse in Biomedical Research, Reproductive Physiology (Foster H. L., Small J. D., Fox J. G., eds), pp. 137–164 New York: Academic Press [Google Scholar]
- Yang J., Mani S. A., Donaher J. L., Ramaswamy S., Itzykson R. A., Come C., Savagner P., Gitelman I., Richardson A., Weinberg R. A. (2004). Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 [DOI] [PubMed] [Google Scholar]
- Yoshidome K., Shibata M. A., Couldrey C., Korach K. S., Green J. E. (2000). Estrogen promotes mammary tumor development in C3(1)/SV40 large T-antigen transgenic mice: paradoxical loss of estrogen receptoralpha expression during tumor progression. Cancer Res. 60, 6901–6910 [PubMed] [Google Scholar]
- Yoshiura K., Kanai Y., Ochiai A., Shimoyama Y., Sugimura T., Hirohashi S. (1995). Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc. Natl. Acad. Sci. USA 92, 7416–7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Langerod A., Ji Y., Nowels K. W., Nesland J. M., Tibshirani R., Bukholm I. K., Karesen R., Botstein D., Borresen-Dale A. L., et al. (2004). Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol. Biol. Cell 15, 2523–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.