SUMMARY
Abnormal Notch signaling in humans results in Alagille syndrome, a pleiotropic disease characterized by a paucity of intrahepatic bile ducts (IHBDs). It is not clear how IHBD paucity develops as a consequence of atypical Notch signaling, whether by a developmental lack of bile duct formation, a post-natal lack of branching and elongation or an inability to maintain formed ducts. Previous studies have focused on the role of Notch in IHBD development, and demonstrated a dosage requirement of Notch signaling for proper IHBD formation. In this study, we use resin casting and X-ray microtomography (microCT) analysis to address the role of Notch signaling in the maintenance of formed IHBDs upon chronic loss or gain of Notch function. Our data show that constitutive expression of the Notch1 intracellular domain in bi-potential hepatoblast progenitor cells (BHPCs) results in increased IHBD branches at post-natal day 60 (P60), which are maintained at P90 and P120. By contrast, loss of Notch signaling via BHPC-specific deletion of RBP-J (RBP KO), the DNA-binding partner for all Notch receptors, results in progressive loss of intact IHBD branches with age. Interestingly, in RBP KO mice, we observed a reduction in bile ducts per portal vein at P60; no further reduction had occurred at P120. Thus, bile duct structures are not lost with age; instead, we propose a model in which BHPC-specific loss of Notch signaling results in an initial developmental defect resulting in fewer bile ducts being formed, and in an acquired post-natal defect in the maintenance of intact IHBD architecture as a result of irresolvable cholestasis. Our studies reveal a previously unappreciated role for Notch signaling in the post-natal maintenance of an intact communicating IHBD structure, and suggest that liver defects observed in Alagille syndrome patients might be more complex than bile duct paucity.
INTRODUCTION
Mutations in JAGGED1, a ligand of the Notch pathway, are found in greater than 94% of patients with Alagille syndrome (AGS), with NOTCH2 receptor mutations identified in two families (McDaniell et al., 2006; Warthen et al., 2006). AGS is a pleiotropic, autosomal dominant disease characterized in the majority of cases by neonatal jaundice, cholestasis and paucity of intrahepatic bile ducts (IHBDs) (Emerick et al., 1999). Whether the paucity of IHBDs is due to a developmental defect in bile duct morphogenesis, a lack of post-natal branching and elongation or an inability to maintain formed ducts remains unclear (Perrault, 1981; Hadchouel, 1992; Libbrecht et al., 2005). In support of a bile duct maintenance defect, a subset of AGS patients, with clinical indications for progressive liver disease, demonstrate an increase in bile duct paucity from initial to subsequent liver biopsies (Emerick et al., 1999; Libbrecht et al., 2005). Although studies in mouse models have demonstrated a requirement for Notch signaling in IHBD development (Loomes et al., 2007; Geisler et al., 2008; Lozier et al., 2008; Zong et al., 2009; Sparks et al., 2010), the progressive paucity in AGS patients suggests an additional requirement for Notch signaling in the maintenance of IHBDs.
Notch signaling is a highly conserved intercellular communication pathway required for cell specification, lineage restriction, and maintenance of stem and progenitor populations during development and in adults (Chiba, 2006). Notch ligands, which are present on the cell surface, bind Notch receptors on the surface of an adjacent cell, resulting in a series of proteolytic cleavages culminating in the γ-secretase-dependent release of the Notch intracellular domain (NICD) from the cytoplasmic membrane. After release, the NICD translocates to the nucleus, where it interacts with the common DNA-binding partner for all Notch receptors, recombination signal binding protein for immunoglobulin kappa J region (RBP-J). This association converts RBP-J from a transcriptional co-repressor to a co-activator, resulting in target gene expression. In mammals, there are two families of canonical Notch ligands (Jagged1 and 2, and Delta-like-1, -3 and -4) and four Notch receptors (Notch1-4).
Previous studies have shown that Notch signaling regulates ductal plate formation and IHBD morphogenesis in mice (Geisler et al., 2008; Lozier et al., 2008; Zong et al., 2009). These studies have not revealed the intact IHBD structure, because the analyses have focused on two-dimensional (2D) morphology and immunohistochemistry in tissue section. Because the mammalian liver is not conducive to in vivo three-dimensional (3D) imaging, we have used a resin casting method to resolve the global 3D architecture of the mouse IHBD system. We previously demonstrated that loss of Notch signaling within the bi-potential hepatoblast progenitor cell (BHPC) lineage results in a dose-dependent reduction of peripheral IHBD branches, whereas activation of Notch1 within the BHPC lineage results in increased peripheral branches at post-natal day 120 (P120) (Sparks et al., 2010).
In this study we use resin casting in conjunction with X-ray microtomography (microCT) analysis to address the hypothesis that Notch signaling is required for post-natal maintenance of intact IHBD structure. IHBD resin casts were examined at P60, P90 and P120 in mice with chronic alterations in Notch signaling within the BHPC population. We used chronic alterations to reflect the changes in AGS patients, in which the normal protein function is not present during development nor in the adult. Activation of Notch signaling, via BHPC-specific expression of the intracellular domain of Notch1, resulted in increased peripheral IHBD branches that were maintained with age. Conversely, loss of Notch signaling, via BHPC-specific deletion of RBP-J, resulted in an age-dependent loss of intact peripheral IHBD branches in 3D resin cast. Surprisingly, in 2D histological section, there was an initially reduced number of bile ducts per portal vein and portal veins per millimeter (PV/mm) at P60, but no further reduction was observed at P120. Thus, chronic loss of Notch signaling in mice leads to an acquired, post-natal disruption of the intact communicating IHBD system.
RESULTS
An Albumin-Cre transgene (Alb-Cre), expressing Cre in the BHPC lineage, was used to delete RBP-J, the common DNA-binding partner required for gene transcription downstream from all Notch receptors, giving Alb-Cre;RBP-Jflox/flox (RBP KO) mice, or to activate Notch1 by conditional expression of the NICD (Alb-Cre; ROSA26Notch1; NICD mice). In a CD1 out-bred mouse strain, Alb-Cre-mediated recombination of the ROSA26 locus is complete at embryonic day 16.5 (E16.5), as indicated by reporter activity (Sparks et al., 2010). Immunofluorescent detection of GFP expressed via the internal ribosomal entry site within the NICD allele was observed at E16.5 (supplementary material Fig. S1A-D). The RBP-J locus begins to recombine as early as E14, as assessed by quantitative PCR analysis of genomic DNA isolated from whole liver. However, analysis of whole liver extract does not allow us to rule out the possibility that there are cells that escape recombination by Alb-Cre (supplementary material Fig. S2A). Analysis of ROSA26-YFP reporter (R26R-YFP) expression in Alb-Cre;RBP-Jflox/flox;R26R-YFP livers at P60 and P120 show very few cells that do not express reporter activity. These cells are only observed in hilar ducts and not in peripheral ducts, thus suggesting that the majority of cells have expressed Alb-Cre during early hepatoblast cell fate decisions (supplementary material Fig. S2B-E). Thus, Alb-Cre allows us to address the chronic requirement of Notch signaling within liver epithelial cell lineages derived from the BHPC, and thus infers the post-natal consequence of chronic alterations in Notch signaling within the BHPC lineage in AGS patients.
To visualize the macro-structural effect of gain or loss of Notch signaling on 3D IHBD architecture, resin casts were obtained from mice of each genotype (control, RBP KO and NICD) at P60, P90 and P120 (Fig. 1 and data not shown). Prior to P60, control mice are still rapidly growing (P30–P60 slope=8.43 vs P60–P120 slope=3.98; supplementary material Fig. S3B); therefore, we selected time points that allow us to specifically assay the maintenance of IHBDs subsequent to the post-natal branching and elongation period. Stereoscopic images of left lobe resin casts from control mice showed no noticeable change with age (Fig. 1A,B), whereas casts from RBP KO mice appeared to lose peripheral IHBDs (Fig. 1C,D). Casts from NICD mice appeared to have increased peripheral branches early in development, which are maintained with age (Fig. 1E,F).
Fig. 1.
Resin casts of adult mice reveal IHBD changes upon altered Notch signaling. Resin casting was performed by manual, retrograde injection of a modified acrylic into the common bile duct, followed by tissue maceration. (A–F) Representative stereoscopic images of the peripheral branches of left lobe resin casts. Casts from control (A,B), RBP KO (C,D) and NICD (E,F) mice were obtained at P60 and P120. RBP KO IHBD casts are unable to maintain intact IHBD structure with age, whereas NICD IHBD casts maintain an excess of branches. Scale bar: 2 mm.
To quantify changes in the structure of the IHBD system with age, we quantified the volume, thickness and number of branches in left lobe resin casts using microCT (Fig. 2A). Analysis of microCT scans was achieved by adapting trabecular bone analysis software from Scanco (Perrien et al., 2007). Left lobe casts of control, RBP KO and NICD mice were microCT scanned and analyzed at P60 (n=4, 5 and 3, respectively), P90 (n=4, 6 and 3, respectively) and P120 (n=4, 4 and 3, respectively). The same casts were analyzed throughout this manuscript. The total volume of the IHBD casts did not change significantly with age in the control mice. Interestingly, the total volume of IHBD casts from RBP KO mice was reduced at P120, as compared with age-matched control mice (P≤0.01) and P60 RBP KO mice (P≤0.05). Conversely, NICD IHBD casts showed consistent, but not significant, increases in cast volume with age compared with control (Fig. 2B).
Fig. 2.
MicroCT analysis reveals that IHBD average volume is modulated with age upon deletion of RBP-J or activation of Notch1. Reconstruction of microCT scans provides a high-resolution global representation of resin casts. (A) Representative image from Scanco microCT 40 scanning of P120 control IHBD resin cast at 20 μm resolution. Red square demonstrates area in A’. (B) Total IHBD cast volume was calculated using software adapted from trabecular bone analysis (see Methods). At P60, RBP KO cast volume is equivalent to control; however, as age increases, the volume decreases when compared with control IHBD casts. This decrease is statistically significant at P120. The trend in NICD IHBD casts shows an increase in cast volume. For each genotype (control, RBP KO, NICD), casts at P60 (n=4, 5 and 3, respectively), P90 (n=4, 6 and 3, respectively) and P120 (n=4, 4 and 3, respectively) were scanned. Error bars represent s.e.m. *P≤0.05, **P≤0.01. Scale bars: 1 mm.
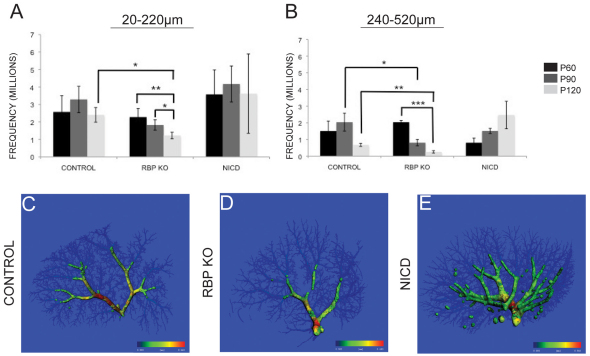
To further define the structural changes that are visually apparent in the resin casts, we calculated the distribution of branch thickness throughout the IHBD casts (Figs 3, 4; supplementary material Fig. S4). The frequency of branch diameter distribution of the resin casts is displayed in Fig. 3A,B. Owing to variations in maximum thickness resulting from the imprecise method of individual lobe separation (i.e. separated manually), the analysis included only diameters less than 520 μm, which consistently incorporates the main branches regardless of genotype. Small branches, as defined by a diameter of <15 μm (Glaser et al., 2009), are not resolved by this method due to detection limitations imposed by cast size; however, we define intermediate (20–220 μm) and main (240–520 μm) branches for our analysis. As shown in Fig. 3C–E, branches with a diameter between 240 and 520 μm highlight main branches, whereas branches with a diameter between 20 and 220 μm compose intermediate branches. Control IHBD casts demonstrate a high frequency of intermediate branches, and this frequency changed little with age. By contrast, RBP KO IHBD casts showed a significant decrease in intermediate branch frequency at P120 compared with control and RBP KO casts at P60 and P90. Intermediate branch diameter frequency in NICD IHBD casts did not significantly change with time (Fig. 3A).
Fig. 3.
Alterations in Notch signaling affect the frequency of intermediate and main IHBD branches. (A,B) The frequency of intermediate branches (20–220 μm; A) and main branches (240–520 μm; B) for each genotype are shown. The frequency of a diameter was ‘counted’ using the direct thickness determination model and summed for the indicated diameter range (see Methods). (A) Control IHBD casts show a consistent frequency of intermediate branches with age. RBP KO IHBD casts demonstrate a significant reduction in intermediate branch frequency at P120 compared with control. Additionally, whereas control mice show no significant change with age within the genotype, RBP KO mice show a significant decrease in frequency with age when compared with each other. NICD IHBD casts do not significantly change with this analysis. (B) Main branch frequency does not change significantly with age in control or NICD IHBD casts. However, there is a significant reduction in main branch frequency in RBP KO IHBD casts at P90 and P120 compared with control. Error bars represent s.e.m. *P≤0.05, **P≤0.01, ***P≤0.001. (C–E) A representative scan from control (C), RBP KO (D) and NICD (E) IHBD casts, with branch thicknesses from 240 μm to 520 μm highlighted. We have defined this thickness range as sufficient to incorporate all main branches. Colored bars indicate heat map thickness range; blue=20 μm to red=520 μm.
Fig. 4.
Notch signaling affects the frequency of different branch diameters in proportion to the original contribution. Individual diameter frequencies (A–C) represented as a percent contribution to the total structure (D–F). (A,D) In control IHBD casts, 80–100 μm branch diameters represent the greatest frequency and percent contribution. (B,E) RBP KO IHBD casts show decreased frequency of all diameters with age, but the percent contribution per branch diameter was consistent with age. Therefore, all intermediate branches are lost in proportion to their original contribution. (C,F) NICD IHBD casts demonstrate consistent contribution of branch diameter at P60 and P90; however, at P120 there is a shift towards the main branch contribution. Vertical dotted line represents division between intermediate and main branches. Horizontal grey line represents 1 million (A–C) and 25% (D–F). Error bars represent s.e.m.
For all genotypes, the frequency of branches with a diameter of 240–520 μm (main branches) was reduced compared with the frequency of intermediate branches. Main branch frequency remained consistent in control and NICD IHBD casts with age, but was reduced in RBP KO IHBD casts at P90 and P120 compared with control (P≤0.05 and P≤0.01, respectively), and at P120 compared with P60 RBP KO (P≤0.001) (Fig. 3B). Further dissection of the frequency of individual diameters revealed that 80–100 μm diameters were the most frequent regardless of genotype or age (Fig. 4; supplementary material Fig. S4). To visualize the relative contribution of each intermediate diameter branch to the total cast, frequency was represented as a percentage of all diameters (Fig. 4D–F). Consistent with the raw data, the percent contribution of intermediate branches in control IHBD casts was the greatest of all of the branch diameters and remained consistent with age (Fig. 4A,D). The percent contribution of each diameter in RBP KO IHBD casts did not change with age, indicating that RBP KO IHBD casts are losing intermediate and main branch diameters at the same rate (Fig. 4B,E). NICD IHBD casts demonstrated a shift in contribution towards main branches at P120, which could be attributed to the inability to resolve individual intermediate branches with increased cast density at later time points with the current scanning resolution (branch separation <20 μm; Fig. 4C,F).
For RBP KO IHBD casts, a reduction in the frequency of a specific diameter could be due to (1) branch shortening and/or (2) branch loss (Fig. 5A). In order to ascertain which possibility is more likely, we determined the total number of segments within the IHBD casts. A segment is defined as the portion between two branch points. The total number of segments in control IHBD casts with age was not significantly changed. In RBP KO IHBD casts, there was a significant reduction of segment number at P90 (P≤0.05) that was further reduced by P120 (P≤0.05). NICD IHBD casts had a consistently, but not statistically significant, increased number of segments compared with control (Fig. 5B). These data suggest that changes in branch diameter frequency in RBP KO are due, at least in part, to cast branch loss. However, we cannot rule out that branch loss is due to excessive branch shortening. Resin casting and microCT analysis demonstrated that intact intermediate and main branches were not maintained with age in RBP KO mice.
Fig. 5.
Loss of RBP-J results in a reduction of segments due to total branch loss with age. (A) Schematic illustrating a model of branch reduction in RBP KO IHBD casts. Upper panel represents a portion of the IHBD at a younger time point. This portion contains one originating branch with two side branches emanating from it. In this simplified example, the younger time point has five segments (S) and an initial frequency of a given diameter of 5 counts (C). As seen in Figs 3 and 4, there is a reduction in all branch diameter frequencies in RBP KO mice with age. This might be due to branch shortening (Option #1) and/or branch loss (Option #2). In either case, the frequency of branch diameter is reduced to 4C. To elucidate the difference between these two options, the number of segments must be examined. In the case of branch shortening, the number of segments would not be affected (5S); however, in the case of branch loss, there would be a reduced number of segments (3S). (B) The average number of segments, where a segment is defined as the portion between branch points, is consistent with age in control IHBD casts. In RBP KO IHBD casts, there is a progressive loss of segments compared with control. Conversely, NICD IHBD casts show a consistent, but not significant, increase in segments with age. Error bars represent s.e.m. *P≤0.05.
We hypothesize that Notch signaling might modulate maintenance of intact IHBD structure by (1) IHBD loss or gain, (2) functional blockage of the duct due to obstruction, or (3) physical collapse of the IHBD structure. To address the underlying physiology leading to structural loss, we assessed the number of bile ducts per portal vein in control and RBP KO mice at P60 and P120. For counting purposes, bile ducts were defined as cytokeratin19 (CK19)-positive structures containing a lumen. As expected, there was a reduction in the number of bile ducts per portal vein in P60 and P120 RBP KO mice as compared with control (P≤0.0001 and P≤0.05, respectively). However, the number of bile ducts per portal vein did not change between P60 and P120 in RBP KO mice (Fig. 6A). It remains a possibility that entire portal tracts are lost, reducing the overall number of ducts without affecting the number of bile ducts per portal vein. However, consistent with the bile ducts per portal vein analysis, there is an initially reduced number of PV/mm in P60 RBP KO compared with control, but no further reduction at P120 (Fig. 6B). Together, 3D and 2D analyses indicate that intact branches are not maintained with age in RBP KO mice, but 2D ductal structures are maintained. Conversely, NICD mice maintain an excess of intermediate branches with age.
Fig. 6.

RBP KO mice have a reduction in bile ducts per portal vein and portal vein per area (mm) ratios at P60, which are not further reduced at P120. (A,B) Regions from the left or medial lobe were counted for peripheral bile ducts per portal vein and PV/mm. (A) RBP KO mice demonstrate significant reduction in bile ducts per portal vein at P60 (n=5) and P120 (n=4) compared with control (n=6 and 6, respectively). There is no difference in the number of bile ducts per portal vein in RBP KO mice at each age. (B) RBP KO mice demonstrate reduced PV/mm at P60 and P120 compared with age-matched controls. There is no difference in the number of PV/mm in RBP KO mice between P60 and P120. Error bars represent s.e.m. *P≤0.05, ****P≤0.0001.
DISCUSSION
Mechanistically, Notch signaling is linked to the regulation of cell fate, proliferation and/or death, and progenitor cell maintenance (Chiba, 2006). Previous studies have demonstrated a requirement of Notch signaling in the specification and morphogenesis of the IHBD (Geisler et al., 2008; Lozier et al., 2008; Zong et al., 2009; Sparks et al., 2010), but the post-natal consequence of this improper development has not been studied. To date, many studies have evaluated ‘ductal cells’ in 2D following chronic or acute genetic manipulations (Mishra, 2004). However, these prior approaches have not revealed the 3D nature of the IHBD system, which has limited our understanding of how intercellular and intracellular signals regulate the intact communicating IHBD structure. In this study, we set out to investigate the cell-autonomous requirement of chronic Notch signaling in liver epithelial cells to maintain an intact IHBD structure. We describe an innovative approach to evaluate 3D structural changes within the IHBD in adult mice. Our results establish that Notch signaling is essential for the maintenance of an intact IHBD architecture. However, subsequent to the initial establishment of the IHBD system, Notch is not required for the maintenance of the number of ductal structures per portal vein. Thus, we have revealed a previously unappreciated role for Notch signaling in the maintenance of an intact communicating IHBD network and describe a model of evolving cholestasis.
Resin casting and microCT analysis have allowed us to define a role for Notch signaling in the maintenance of intact communicating IHBDs. Activation of Notch1 within BHPCs results in an increase in peripheral branch formation. Although Notch2 is the predominant receptor required for bile duct development (Geisler et al., 2008), we have previously shown that loss of Notch2 can be partially compensated for by an increase in Notch1 (Sparks et al., 2010). Interestingly, we have also shown that Notch1 is not only sufficient to promote tubulogenesis, but is also sufficient to maintain bile ducts within a periportal competence zone. This is in contrast to the study of Tchorz et al., in which the activation of Notch2 was found to be sufficient to promote tubulogenesis, but was not sufficient to maintain tubular structures outside the portal environment (Tchorz et al., 2009). Therefore, we use Notch1 activation on the basis of its ability to maintain bile ducts with age. In this study, we show that constitutive activation of Notch1 in BHPCs results in a slight overall increase in cast volume compared with control at all ages. We speculate that the changes in volume would be significantly greater should we have the ability to resolve small branches (<20 μm) within the context of the whole left lobe resin cast. Furthermore, our data indicate a consistent increase in intermediate branches (20–220 μm) and main branches (240–520 μm) within the IHBD resin cast, which was confirmed by individual cast branch diameter analysis at P60, P90 and P120 (Figs 3, 4; supplementary material Fig. S4).
By contrast, loss of Notch signaling in BHPCs resulted in a loss of cast volume with age, as a result of a reduced frequency of intermediate and main branches (Fig. 2B, Figs 3, 4; supplementary material Fig. S4). Reduced frequency of intermediate branches in RBP KO resin casts was due in part to branch loss, as demonstrated by a reduction in segment number with age, but we cannot rule out the contribution of branch shortening to the phenotype (Fig. 5A,B). Conversely, the reduced frequency of main branches was due to shortening without branch loss (Fig. 3C,D). We infer this because the stereotypical main branch structure did not change in RBP KO resin casts compared with control (data not shown). Therefore, RBP KO resin casts demonstrate progressive reduction with age and thus an inability to maintain an intact IHBD network.
To determine the underlying physiology of 3D branch loss, we analyzed the number of bile ducts per portal vein. We found that the number of bile ducts per portal vein was reduced in RBP KO mice at P60 compared with control, but there was no further reduction at P120 (Fig. 6A). The initial reduction in bile ducts per portal vein can be attributed to the reduced number of ductal cells specified early in development when RBP-J is lost from BHPCs (Zong et al., 2009; Sparks et al., 2010). These results do not preclude that loss of entire portal tracts might contribute to the 3D phenotype. Yet we have demonstrated that this is not the case because the number of PV/mm is maintained from P60 to P120 in RBP KO mice. However, we were surprised to discover a reduction in PV/mm in RBP KO mice at P60 and P120 compared with age-matched controls. This phenomenon has not been described in AGS patient biopsies, but has not been investigated to our knowledge. Reduced PV/mm could be due to a feedback requirement of the biliary system for proper venous development or an inability of the portal vein to expand with the increased liver:body weight ratio observed in RBP KO mice (supplementary material Fig. S3A). At P15, the increased liver:body weight ratio and reduction in PV/mm was already observed (data not shown), suggesting that this is an early developmental defect; however, the mechanism of this reduction remains to be understood. Together, our 3D and 2D results demonstrate a failure to maintain an intact 3D biliary system without concomitant loss of ductal structures.
A reduction of intact structure with age in RBP KO mice without 2D ductal loss suggests an obstruction or physical collapse within the IHBD. In the periphery of RBP KO casts, the resin changes from a completely filled structure to fine tenuous branches (Fig. 1D). This fill pattern is indicative of a blockage at the point of transition between complete structure and tenuous branches, where the resin can only pass in fine, high velocity streams. An alternative explanation for the apparently contradictory 2D and 3D observations is non-productive compensatory sprouting. This phenomenon has been noted in tumor angiogenesis after Delta-like-4 blockade (Noguera-Troise et al., 2006). If this were the case, we would expect to see an increase in ductal structures with progressive 3D loss; however, in our model the number of bile ducts per portal vein was unchanged with age. Therefore, this model is not as likely to explain our results. Instead, we hypothesize that loss of intact structure is due to a progressive obstruction of the intact IHBD, which prevents resin from completely filling the distal tips.
The majority (96%) of AGS patients present with chronic cholestasis, which is thought to be the result of a lack of formed bile duct structures. However, a subset of these patients demonstrates an inability to transport bile, based on failure to excrete tracer upon hepatobiliary scintigraphy (Emerick et al., 1999). The underlying cause of this failed bile transport is not understood. It is interesting to speculate that some patients might have irreversible cholestasis due to lack of bile duct structures, whereas others might have evolving cholestasis as a result of bile duct obstruction. One mechanism by which progressive obstruction can occur is through chronic inflammation. It has been reported in a mouse model of biliary atresia that inflammatory cells can downregulate hepatobiliary transporters, leading to improper bile formation and thus to obstruction of the IHBD (Yang et al., 2009). In liver-specific Notch-deficient mouse models, chronic inflammation has been previously described (Geisler et al., 2008; Sparks et al., 2010). Thus, we postulate that increased inflammation might lead to improper bile formation and thus blockage of the IHBD.
In this study, we describe a mouse model in which progressive obstruction of the IHBD results in a reduction of intact IHBD, without concomitant loss of ductal structures. Although we do not observe progressive ductal paucity as we originally anticipated, we have instead defined a functional paucity, and thus a model in which to study evolving cholestasis. It is important to note that cell-autonomous deletion of Notch signaling within the BHPCs, as demonstrated here, might not reflect the AGS genetic situation. AGS patients have a deficiency of a single copy of either Jagged1 or Notch2 within every cell (liver epithelial and non-epithelial), whereas, in our mouse model, both alleles of RBP-J are lost within only liver epithelial cells. Nonetheless, the effects of evolving cholestasis, as described in our mouse model, can be seen in patients with AGS. For example, liver transplantation is required in 21–31% of patients, and is often due to complications of chronic cholestasis (Kamath et al., 2010). Distinguishing predictors of liver transplantation in AGS patients have not been well defined. Thus, our mouse model of evolving cholestasis provides the potential for future translational research that is directed at identifying factors that predict complications of chronic cholestasis that would lead to liver transplantation in AGS patients.
METHODS
Mouse lines
CD1 mice carrying the Albumin-Cre transgene (Alb-Cre) (Postic and Magnuson, 2000) were crossed with the conditional deletion allele for RBP-J (RBP-Jflox/flox) (Han et al., 2002), the conditional activation allele for Notch1 (ROSANotch1) (Murtaugh et al., 2003) and the ROSA26-YFP (R26R-YFP) allele (Srinivas et al., 2001). Genotyping was performed by PCR analysis as previously described (Han et al., 2002; Murtaugh et al., 2003). Control genotypes are: Alb-Cre or RBP-Jflox/flox or ROSANotch1. Husbandry and experimental procedures were performed with prior approval of the Vanderbilt Institutional Animal Care and Use Committee. Infection with Helicobacter hepaticus was ruled out by testing fecal samples for bacterial DNA by PCR.
Resin casting
Mice were sacrificed and 200–400 μl of a resin/catalyst mixture was injected into the common bile duct, and the liver was excised and macerated as previously described (Sparks et al., 2010). Casts were imaged using a Leica MZ 16 FA stereoscope and QImaging RETIGA 4000R camera.
MicroCT
Whole left lobe corrosion casts were scanned by microCT (model μCT40; Scanco Medical AG, Brüttisellen, Switzerland). Resin casts from control, RBP KO and NICD mice at P60 (n=4, 5 and 3, respectively), P90 (n=4, 6 and 3, respectively) and P120 (n=4, 4 and 3, respectively) were analyzed. Images were acquired with a 20 μm voxel size at 45 kV, 114 μA, 500 projections per 180° rotation, 300 ms integration, and 2× frame averaging to reduce noise. An optimized density threshold and Gaussian noise filter were applied to the 3D stack of images to segment the resin cast from air and generate a 3D reconstruction of the entire IHBD network. Analysis of ductal architecture was performed with the manufacturer’s software. The methods used to calculate the structural properties of the IHBD are used extensively to study analogous properties of trabecular bone (Muller, 2009), but have equal applicability here.
Total resin cast volume was calculated directly from the 3D reconstructions using total segmented voxel volume. Branch thickness was calculated by direct thickness determination, as described previously (Hildebrand and Rüegsegger, 1997). Briefly, a sphere is fitted within each point of the cast reconstruction, the diameter of the sphere is recorded, and the mean of all spheres represents mean branch thickness. Use of distance ridge calculation allows for inclusion of local neighboring voxels; although this method does not eliminate redundant points, it ensures that all points are included in the analysis. This calculation has no structural assumption.
The total number of segments, independent of the total analyzed volume, was calculated using the plate-model of Parfitt (Parfitt et al., 1983) to provide segment number per total analyzed volume (1/mm3). To determine the total number of segments, segment number per total analyzed volume was multiplied by the total volume (mm3).
Immunohistochemistry
Liver tissue was fixed for 4–6 hours at 4°C in 4% paraformaldehyde. Frozen embedded tissue was sectioned at 10 μm. Paraffin-embedded tissue was sectioned at 6 μm. For GFP staining, frozen embedded tissue was incubated in chicken-anti-GFP (1:500; Abcam, Cambridge, MA), diluted in 1% BSA, overnight at 4°C. Sections were then incubated with anti-chicken-Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA). For CK19 staining and CK19/GFP co-immunofluorescence, paraffin-embedded tissue was subjected to 100 mM Tris, pH 10, treatment overnight at 60°C. For CK19 staining alone, sections were incubated with rat-anti-CK19 (TROMA III, 1:1000; Developmental Studies Hybridoma Bank, Iowa City, IA), diluted in 1% BSA, overnight at 4°C. Sections were then incubated with anti-rat-Biotin-SP followed by incubation with R.T.U. Vectastain Elite Universal ABC kit (Vector, Burlingame, CA). Slides were developed using the substrate DAB (Vector) and counterstained with Mayer’s hematoxylin or Nuclear Fast Red (Newcomer Supply, Middleton, WI). For CK19/GFP co-immunofluorescence, slides were incubated with rat-anti-CK19 (1:250) and rabbit-anti-GFP (1:500; Novus Biologicals, Littleton, CO), diluted in 1% BSA, overnight at 4°C. Sections were then incubated with anti-rat-Cy2 and anti-rabbit-Cy3 (1:300; Jackson ImmunoResearch). Images were acquired using an Axioplan2 microscope and QImaging RETIGA EXi camera.
TRANSLATIONAL IMPACT.
Clinical issue
Alagille syndrome (AGS) is a pleiotropic developmental disorder that affects multiple organs, including the liver, heart, skeleton, kidneys and eyes. Liver defects include bile duct paucity and chronic cholestasis (defective flow of bile from the liver). Although there is a strong genetic link between AGS and altered Notch signaling (observed in >94% of patients), the clinical presentation varies widely. The underlying etiology and predictors of the variant clinical presentation are not well understood. In particular, the mechanism by which Notch signaling leads to bile duct paucity remains unclear. Studies of mouse models carrying liver-specific inactivation of the Notch signaling pathway have suggested a role for Notch signaling in bile duct development. However, serial biopsies from AGS patients have suggested that bile duct paucity is instead due to an inability to maintain formed ducts. Until now, a continuing requirement for Notch signaling following the initial development of bile ducts has not been investigated.
Results
In this study, the authors examine conditional mouse strains using three-dimensional resin casting and microCT analysis to show that loss of Notch signaling in liver progenitor cells leads to a postnatal loss of intact communicating ductal structure with age. They further demonstrate that this quantified three-dimensional loss of communicating ductal structure is not concomitant with ductal loss, which suggests a progressive obstruction of the ductal system in the absence of intact Notch signaling. Furthermore, the overexpression of Notch signaling in liver progenitor cells leads to an increase in the intact communicating ductal structure, and this is maintained with age.
Implications and future directions
Although cholestasis in AGS patients is attributed to a lack of bile ducts, a subset of patients fail to excrete biliary tracer upon hepatobiliary scintigraphy, suggesting an obstruction of the bile ducts. However, this patient population has not been studied in depth. This paper defines a mouse model of evolving postnatal cholestasis that might help to shed light on the mechanisms underlying AGS in this subset of patients. Furthermore, 21–31% of AGS patients require liver transplantation, which is often a result of chronic cholestasis. Therefore, this mouse model will be useful for directing translational research that aims to identify predictive factors for complications of chronic cholestasis leading to liver transplantation in AGS patients.
Quantitative PCR
Genomic DNA was prepared from whole liver tissue isolated at E14, E15 and E16. 25 ng of total DNA was used for q-PCR analysis using an ABI Prism 7900 sequence detection system. RBP-J locus recombination was measured from at least three independent samples per genotype per time point run in triplicate. Primers detecting RBP-J genomic recombination: for 5′-GAGATAGAC-CTTGGTTTGTTG-3′; rev 5′-CCACTGTTGTGAACTGGCGT-3′. Reference primers were upstream of the floxed sites within the RBP-J locus: for 5′-GCCACTCCATGTCCAAAAGA-3′; rev 5′-GCAAGTTATAGCTCAGAACAGCAA-3′.
Number of bile ducts per portal vein and PV/mm calculations
Mice of each genotype (control and RBP KO) were stained with CK19. A total of nine cross-sections from the left or medial lobe for each mouse were analyzed for independent CK19+ lumenal structures per portal vein in the periphery. A portal vein is defined as having a bile duct associated. The number of portal veins was divided by total peripheral tissue area as calculated by ImageJ software (Abramoff et al., 2004).
Statistical analysis
Data was subjected to a two-tailed Student’s t-test and a P-value of ≤0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank Tasuko Honjo, Mark Magnuson, Frank Constantini and Doug Melton for providing mice; Anna Means for technical advice; Kevin Song for genotyping mice; Xin Sun and Chris Bjornson for primer sequences; Guoqiang Gu, Mark Magnuson and Mario Strazzabosco for helpful comments; and Hernan Correa, Lynette Gillis and Charles Vanderpool for clinical consultation. These studies were supported by grants from the NIH to S.S.H. ( RO1-DK078640), the Vanderbilt Digestive Disease Research Center ( P30-DK058404) providing Core Services, and the Vanderbilt Institute for Clinical and Translational Research, Clinical and Translational Science Award from the NCRR/NIH ( UL1 RR024975).
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
E.E.S. and S.S.H. formulated and designed experiments; D.S.P. and T.E.P. provided expertise and guidance with microCT analysis; E.E.S. and K.A.H. performed experiments and analyzed data; E.E.S. and S.S.H. prepared and edited the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.005793/-/DC1
REFERENCES
- Abramoff M., Magelhaes P., Ram S. (2004). Image processing with ImageJ. Biophotonics Int. 11, 36–42 [Google Scholar]
- Chiba S. (2006). Notch signaling in stem cell systems. Stem Cells 24, 2437–2447 [DOI] [PubMed] [Google Scholar]
- Emerick K. M., Rand E. B., Goldmuntz E., Krantz I. D., Spinner N. B., Piccoli D. A. (1999). Features of Alagille syndrome in 92 patients: frequency and relation to prognosis. Hepatology 29, 822–829 [DOI] [PubMed] [Google Scholar]
- Geisler F., Nagl F., Mazur P. K., Lee M., Zimber-Strobl U., Strobl L. J., Radtke F., Schmid R. M., Siveke J. T. (2008). Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology 48, 607–616 [DOI] [PubMed] [Google Scholar]
- Glaser S. S., Gaudio E., Rao A., Pierce L. M., Onori P., Franchitto A., Francis H. L., Dostal D. E., Venter J. K., DeMorrow S., et al. (2009). Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab. Invest. 89, 456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadchouel M. (1992). Paucity of interlobular bile ducts. Semin. Diagn. Pathol. 9, 24–30 [PubMed] [Google Scholar]
- Han H., Tanigaki K., Yamamoto N., Kuroda K., Yoshimoto M., Nakahata T., Ikuta K., Honjo T. (2002). Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 [DOI] [PubMed] [Google Scholar]
- Hildebrand T., Rüegsegger P. (1997). A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 185, 67–75 [Google Scholar]
- Kamath B. M., Schwarz K. B., Hadzic N. (2010). Alagille syndrome and liver transplantation. J. Pediatr. Gastroenterol. Nutr. 50, 11–15 [DOI] [PubMed] [Google Scholar]
- Libbrecht L., Spinner N. B., Moore E. C., Cassiman D., Van Damme-Lombaerts R., Roskams T. (2005). Peripheral bile duct paucity and cholestasis in the liver of a patient with Alagille syndrome: further evidence supporting a lack of postnatal bile duct branching and elongation. Am. J. Surg. Pathol. 29, 820–826 [DOI] [PubMed] [Google Scholar]
- Loomes K. M., Russo P., Ryan M., Nelson A., Underkoffler L., Glover C., Fu H., Gridley T., Kaestner K. H., Oakey R. J. (2007). Bile duct proliferation in liver-specific Jag1 conditional knockout mice: effects of gene dosage. Hepatology 45, 323–330 [DOI] [PubMed] [Google Scholar]
- Lozier J., McCright B., Gridley T. (2008). Notch signaling regulates bile duct morphogenesis in mice. PLoS ONE 3, e1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniell R., Warthen D. M., Sanchez-Lara P. A., Pai A., Krantz I. D., Piccoli D. A., Spinner N. B. (2006). NOTCH2 mutations cause Alagille syndrome, a heterogeneous disorder of the notch signaling pathway. Am. J. Hum. Genet. 79, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L. (2004). Mouse knockout models of biliary epithelial cell formation and disease. In The Pathophysiology of Biliary Epithelia (ed. Alpini G., Alvaro D., Marzioni M., LeSage G., LaRusso N.). Georgetown, TX: Landes Bioscience [Google Scholar]
- Muller R. (2009). Hierarchical microimaging of bone structure and function. Nat. Rev. Rheumatol. 5, 373–381 [DOI] [PubMed] [Google Scholar]
- Murtaugh L. C., Stanger B. Z., Kwan K. M., Melton D. A. (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. USA 100, 14920–14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguera-Troise I., Daly C., Papadopoulos N. J., Coetzee S., Boland P., Gale N. W., Lin H. C., Yancopoulos G. D., Thurston G. (2006). Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032–1037 [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Mathews C. H., Villanueva A. R., Kleerekoper M., Frame B., Rao D. S. (1983). Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J. Clin. Invest. 72, 1396–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault J. (1981). Paucity of interlobular bile ducts: getting to know it better. Dig. Dis. Sci. 26, 481–484 [DOI] [PubMed] [Google Scholar]
- Perrien D. S., Akel N. S., Edwards P. K., Carver A. A., Bendre M. S., Swain F. L., Skinner R. A., Hogue W. R., Nicks K. M., Pierson T. M., et al. (2007). Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology 148, 1654–1665 [DOI] [PubMed] [Google Scholar]
- Postic C., Magnuson M. A. (2000). DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis 26, 149–150 [DOI] [PubMed] [Google Scholar]
- Sparks E. E., Huppert K. A., Brown M. A., Washington M. K., Huppert S. S. (2010). Notch signaling regulates formation of the three-dimensional architecture of intrahepatic bile ducts in mice. Hepatology 51, 1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchorz J. S., Kinter J., Muller M., Tornillo L., Heim M. H., Bettler B. (2009). Notch2 signaling promotes biliary epithelial cell fate specification and tubulogenesis during bile duct development in mice. Hepatology 50, 871–879 [DOI] [PubMed] [Google Scholar]
- Warthen D. M., Moore E. C., Kamath B. M., Morrissette J. J., Sanchez P., Piccoli D. A., Krantz I. D., Spinner N. B. (2006). Jagged1 (JAG1) mutations in Alagille syndrome: increasing the mutation detection rate. Hum. Mutat. 27, 436–443 [DOI] [PubMed] [Google Scholar]
- Yang H., Plosch T., Lisman T., Gouw A. S., Porte R. J., Verkade H. J., Hulscher J. B. (2009). Inflammation mediated down-regulation of hepatobiliary transporters contributes to intrahepatic cholestasis and liver damage in murine biliary atresia. Pediatr. Res. 66, 380–385 [DOI] [PubMed] [Google Scholar]
- Zong Y., Panikkar A., Xu J., Antoniou A., Raynaud P., Lemaigre F., Stanger B. Z. (2009). Notch signaling controls liver development by regulating biliary differentiation. Development 136, 1727–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.