Abstract
Mutation of integrin α7 (ITGA7) was previously identified in multiple human malignancies. Restoration of ITGA7 expression in prostate cancer and leiomyosarcoma cell lines suppressed tumor growth and cell motility both in vitro and in vivo. In this study, we showed that integrin-link kinase (ILK) binds with miniature chromosome maintenance 7 (MCM7), a DNA replication licensing protein. A 58 amino acid ILK binding motif was identified in the N-terminus of MCM7. Expression of ITGA7 induced phosphorylation of MCM7. Knocking down of ILK abrogated ITGA7 induced MCM7 phosphorylation. ANK, the dominant negative mutant of ILK, also blocked the phosphorylation of MCM7 induced by ITGA7. Phosphorylation of MCM7 reduced MCM7 chromatin association and inhibited cell growth. A MCM7 mutant that does not bind with ILK did not respond to ITGA7 stimulation, and behaved similar to a dominant MCM7 negative mutant and neutralized the effect of ITGA7. We conclude that ILK interaction with MCM7 and MCM7 phosphorylation may be a critical event in ITGA7 signaling pathway leading to tumor suppression.
Keywords: Tumor suppression, MCM7, integrin linked kinase, growth, prostate cancer
Introduction
As a major class of cell adhesion molecules in mammalian cells, twenty four members of integrins form various combination of hetero-dimers, and are involved in many cellular processes, including development, immune responses, leukocyte traffic, and hemostasis and cell proliferation (1). Recently, mutational analysis of ITGA7 (2) revealed ITGA7 mutations in prostate cancer (PC), hepatocellular carcinoma, soft tissue leiomyosarcoma (STL) and glioblastoma multiforme with frequencies ranging from 25% to 83%. Many of these mutations cause truncation, micro-deletion or frameshift of the protein. Interestingly, patients with ITGA7 mutations had higher rate of clinical relapse in both PC and hepatocellular carcinoma. Meta-analysis of previously published microarray data indicated that ITGA7 was down-regulated in non-metastatic prostate cancer and leiomyosarcoma, but the magnitude of the down-regulation was larger in metastatic cancers (2). PC and STL with focal or no ITGA7 expression were associated with less metastasis-free survival time. Forced expression of normal ITGA7 in PC and leiomyosarcoma cell lines suppressed tumor growth and cancer cell migration in vitro, while knocking down ITGA7 in lung cancer cell lines H358 and H1299 enhanced tumor cell growth and cell migration (2). A mouse model of PC3 and DU145 xenograft prostate tumors showed a dramatic reduction in tumor volume, metastatic rate and mortality rate when ITGA7 expression was restored (2). Integrin linked kinase (ILK) and Focal adhesion kinase (FAK) were speculated to play roles in ITGA7 signaling. However, the molecular mechanism of ITGA7-mediated tumor suppressor activity remains unclear. MCM7 is a critical component of DNA replication licensing complex (3). To ensure that DNA replication is initiated only once per cell division cycle, the synthesis of the MCM complex is temporally regulated. For example, MCM7 expression is shut down during S, G2 and early M phase to prevent re-initiation of DNA synthesis. Our previous study, along with others, indicated that MCM7 is amplified and overexpressed in prostate cancers that relapse (4, 5). Continuous expression of MCM7 in prostate cancer cell line Du145 resulted in higher level of DNA synthesis, cell proliferation and tumor invasiveness (4). Transgenic mouse model with a knock-in MCM7 expression under the control of a keratin promoter generated a strain of mouse prone to the development of multiple malignancies (6). It appears that high level of MCM7 expression is one of the determining factors for abnormal cell growth and tumorigenesis. In this study, we identified that ILK interacts with MCM7 in the nucleus. Expression of ITGA7 induced ILK mediated phosphorylation of MCM7, inhibited its DNA licensing activity and suppressed cell growth.
Materials and Methods
Constructions of pBD-MCM7 fusion plasmids
A mutagenic primer set (CAGCCA AGCTCAACATATGGCACTGAAGGACTACGCG and TTCATTTCAAGCAAAGTCGACGGAGTAAGTGCAGCATGGGAG) was designed to create two restriction sites (Nde1 and Sal1) for the full length of MCM7, so that the PCR product could be ligated into a pGBKT7 vector. A PCR reaction (Invitrogen, Carlsbad, CA) was performed on cDNA template of a donor prostate cDNA. The PCR product was restricted with Nde1 and Sal1, and ligated into a similarly restricted pGBKT7 vector. The fusion protein contained 719 amino acids from MCM7 and 219 amino acid from bait domain. The construct was transformed into One ShotTM competent cells (Invitrogen, Carlsbad, CA). The pBD-MCM7n (N-terminus) that contains MCM7 amino acids 2–248, pBD-MCM7c (C-terminus) that contains amino acids 428–720 and pBD-MCM7m (mid segment) that contains amino acids 248–428 were similarly constructed except primers for pBD-MCM7n are CAGCCAAGCTCAACATATGGCACTGAAGGACTACGCG/ATTTCCCACAGGCA CCTGATCACT, for pBD-MCM7c CAGCCAGCTCAACATATGATCCCTCGTAGTATCACGGTG/TTCATTTCAAGCAAAGTCGACGGAGTAAGTGCAGCATGGGAG, and for BD-MCM7m 5′-CAGCCA AGCTCAACATATGGCACTGAAGGACTACGCG-3′ and 5′-TTCATTTCAAGCAAAGT CGACGGAGTAAGTGCAGCATGGGAG-3.
For construction of the glutathione-s-transferase (GST)-MCM7n fusion protein, a mutagenic primer set (TGGGATCCCACTGAAGGACTACGCGCTAGA / CGCTCG AGATTTCCCACAGGCACCTGATCACT) was designed to create a BamH1 and Xho1 restriction site within the MCM7 coding region that encodes a 233 amino acid region of the 5’ terminal of MCM7. A PCR was performed using these primers under the following conditions: 94°C for 1 min followed by 35 cycles of 94°C for 30 s, 68°C for 3 min and a final 10-min extension step at 68°C. The PCR product was subsequently ligated into a pCR2.1 TA cloning vector. The DNA was transformed into Escherichia coli. DNA from the selected transformants was restricted with BamH1 and Xho1, and ligated into a similarly restricted pGEX-5T vector in frame. A series of deletions, including 5’ or 3’ deletions of pGST-MCM7n were performed using the primer sets described previously (7) (also see figure 1 for amino acid sequences in the constructs). The procedures for generating these mutants were similar to those described for pGST-MCM7n. The pGST-MCM7n and their mutants were transformed into E. coli BL21 cells for recombinant protein production.
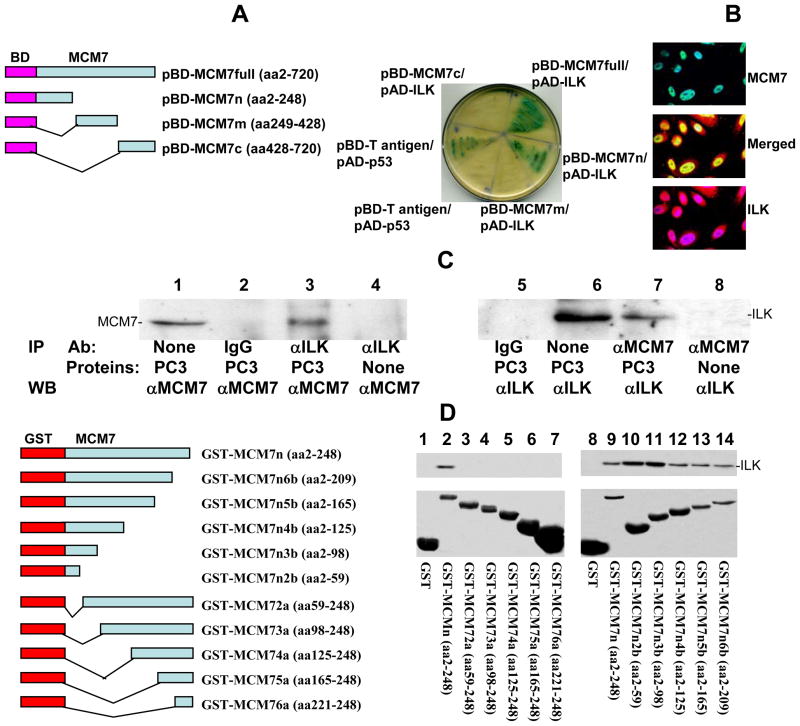
Figure 1. MCM7 binds ILK.
(A) MCM7 binds ILK in Yeast Two Hybrid co-transfection. Left: Schematic diagram of Yeast Two hybrid pGBKT7-MCM7 constructs. Fragments of MCM7 were ligated in frame with the DNA binding (BD) domain of GAL4 protein. Right: Growth and α-galactosidase activity of Yeast AH109 cells co-transfected with the indicated vectors in SD-4 (-leu/-trp/-ade/-his) medium. (B) Co-localization of MCM7 and ILK in PC3 cells. PC3 cells were immunostained with antibodies specific for MCM7 (mouse) and ILK (goat). Immunofluorescence staining was then performed using FITC conjugated antibodies against mouse (MCM7) or Rhodamine-conjugated antibodies against goat (ILK). (C) Co-immunoprecipitation of ILK and MCM7 from PC3 cells. Protein extracts from PC3 cells were immunoprecipitated with the indicated antibodies, electrophoresed in 8% SDS-PAGE and immunoblotted with either anti-MCM7 or ILK antibodies. (D) In vitro binding analysis of GST-MCM7 fusion proteins with ILK. Left: Schematic diagram of GST-MCM7 fusion protein and deletion constructs. Right: Upper panel: Immunoblots of GST-MCM7 fusion proteins pull-down ILK. Lower panel: Coomassie staining of GST-MCM7 fusion proteins.
To construct the small interfering RNA (siRNA) vectors for ILK and a scrambled control sequence, oligonucleotides corresponding to ILK or scramble was previously described (2, 8) (5’-CACCGTGACGAAGCTCAACGAGAACGAATTCTCGTTGAGCTTCGTCA-3’/5’-AAAATGACGAAGCTCAACGAGAATTCGTTCTCGTTGAGCTTCGTCAC-3’), or scrambled siRNA (5’-CACCGTAATGTATTGGAACGCATATTTTGATATCCGAATATGCGTTCCAATAC ATTA-3’/5’-AAAATAATGTATTGGAACGCATATTCGGATATCAAAATATGCGTTCCAATAC ATTA-3’) were annealed and ligated into a pENTRTM/U6 vector. The ligated products were transfected into E. coli and plated on kanamycin plates (50 μg/mL). Six colonies per transfection were picked and sequenced for the presence of inserts. The selected clones (5 μg/106 cells) were then transfected into cultured cells to generate pENTR-siILK-transfected or pENTR-siScr transfected PITT1, PITT2, H358 or H1299 cells.
Yeast transformation and library screening
The Yeast competent cell preparation was described previously (9). One hundred microliters of freshly prepared competent AH109 cells were mixed with plasmid DNA (0.25 –0.50 μg) plus 0.5 μg DNA from prostate Yeast Two Hybrid cDNA library constructed in pACT2 in 0.5 ml of PEG/LiAc, incubated at 30°C for 30 min. Following this initial incubation with plasmid DNA, the cell solution was combined with 20 μl of dimethyl sulfoxide (DMSO) and subjected to 15-min incubation at 42°C. The cells were pelleted, re-suspended in 1 ml YPD medium and shaken at 30°C for 40 min. The transformed cells were then pelleted, re-suspended in 0.5 ml 0.9% NaCl and plated onto the appropriate SD agar plate. The transformants were first plated on low and medium stringency plates of SD-Leu/-Trp and SD-Leu/-Trp/-His, respectively. The grown colonies were subjected to the colony-lift filter β-galactosidase assay as described previously (9) and allowed to grow further in the high stringency plate (SD-Ade/-His/-Leu/-Trp).
Validation of protein interactions in AH109
Plasmid DNA samples from positive clones were isolated from yeast, transformed into E. coli., and selected with ampicillin (100 μg/ml) to obtain genes interacting with the bait-domain fusion protein. The purified AD/library plasmid DNA was then co-transformed with pBD-MCM7 into AH109 yeast cells and grown in a SD-Ade/-His/-Leu/-Trp high stringency medium. α-galactosidase activity was assayed on cells grown in this medium.
Immunoprecipitation
Protein extracts of PC3 cells were incubated with MCM7 or ILK antibodies for 16 h, then with protein G Sepharose for 3 h. The complex was washed 5 times with RIPA buffer, and the bound proteins were eluted with SDS-PAGE sample buffer. The bound ILK or MCM7 was electrophoresed in 8% SDS-PAGE and immunoblotted with anti-ILK or MCM7 antibodies.
GST fusion proteins pull down to examine ILK/MCM7 binding
The cells were grown in 100 ml of LB medium supplemented with ampicillin (100 μg/ml) overnight and induced by IPTG (final concentration of 1 mM) for 3 h. The cells were then pelleted, re-suspended in 1× phosphate-buffered saline (PBS), and sonicated for 2 min. The proteins were solubilized in 1% triton X-100. The supernatant was collected after centrifugation at 15,000 g for 5 min. The GST and GST- MCM7n fusion protein were purified through a Glutathione Sepharose 4B column (Amersham Bioscience). The PC3 protein extracts were pre-cleared with the column for 15 min at 4°C. The flow-through was collected after spinning at 3000 g for 1 minute. The pre-cleared cell lysates were then incubated with GST fusion protein packed Glutathione Sepharose 4B at 4°C for 2 h. The column was spun at 3000 g at room temperature for 1 min, and further washed twice with PBS. The proteins were eluted from the column with 40 μl of SDS-PAGE gel sample loading dye. SDS-PAGE and Western blot analyses were subsequently conducted.
Bromo-Deoxyuridine labeling analysis
To label PITT1 and PITT2 cells – both are PC3 cells stably transfected with pCNDA4-ITGA7/pCDNA6, and are inducible for ITGA7 expression with tetracycline (2), 10 μl of BrdU solution (1 mM BrdU in 1× PBS) was added directly to each ml of tissue culture media. The treated cells were then incubated for 3 hours at 37°C. Cells were then resuspended with 100 μl of BD Cytofix/Cytoperm Buffer per sample (BD-Pharmagen) and incubated for 30 minutes at room temperature. The cells were then pelleted and washed with 1 ml of 1× BD Perm/Wash Buffer. The cells were then incubated with Cytoperm Plus Buffer for 10 minutes on ice. The permeation procedure was repeated twice. The cells were resuspended with 100 μl of diluted DNase (diluted to 300 μg/ml in DPBS) per tube, (ie, 30 μg of DNase to each tube), and incubated for 1 hour at 37°C. The cells were washed with 1× BD Perm/Wash Buffer, and incubated with 50 μl of BD Perm/Wash Buffer containing diluted fluorescent anti-BrdU and propidium iodide for 20 minutes at room temperature. The incubation was washed with 1 ml of 1× BD Perm/Wash Buffer. The results of the stains were analyzed in LSC-II flowcytometer.
Immunofluorescence staining
PITT1 cells were cultured with 5 μg/ml tetracycline on chamber slides for 24 h. The slides were washed with PBS for 3 times. The cells were fixed with 4% paraformaldehyde for 1 h at room temperature as described previously (2). After washing the slides with PBS twice, the cells were blocked with 10% donkey serum with 0.4% Triton X-100. The cells were then incubated with mouse monoclonal antibody against MCM7 and rabbit antisera against ILK (Santa Cruz Inc, CA) at room temperature for 1 h. The slides were washed with PBS twice. Secondary antibodies from donkey against mouse (fluorescein conjugated) and against rabbit (rhodamine conjugated) were added and incubated at room temperature for 1 h. The slides were then washed with PBS twice before addition of 4'-6-Diamidino-2-phenylindole (DAPI). After additional washes with PBS, slides were mounted with immuno-mounting buffer. Immunofluorescence staining was examined under confocal microscope.
Chromatin association assay
PITT1 or PITT2 cells were cultured to 75% confluence, synchronized in serum-free medium for 24 hours, and then treated with tetracycline for 24 or 48 hours. These cells were washed with PBS and trypsinized. These cells were resuspended in 1 ml of Buffer A (110 mM KC2H3O2, 15mM NaC2H3O2, 2 mM MgC2H3O2, 0.5 mM EGTA, 20 mM HEPES pH 7.3). The cell suspension was treated to the final concentration of 2 mM DTT and 50 μg/ml digitonin. The cells were incubated at 4°C for 10 min in a rotator. Nuclei were pelleted by centrifugation at 1500 g for 10 min. They were resuspended in hypotonic buffer (Buffer B: 1 mM HEPES pH 7.5, 0.5 mM EDTA supplemented with 0.5% NP-40). The nuclear suspensions were then incubated at 4°C for 15 min in a rotator and laid on top of a 10 ml sucrose cushion (100 mM sucrose, 0.5 mM Tris HCl, pH 8.5) and centrifuged at 3500 g for 15 min at 4°C. The chromatin pellets were suspended in 0.25 mM EDTA pH 8.0, and sonicated 10 s for three times each sample. The chromatin suspensions were centrifuged twice at high speed for 10 min at 4°C, and the supernatants were retained.
Colony Formation assay
Colony formation assay was similar to those previously described (10). Five thousand cells were cultured in 60-mm dishes. Triplicate experiments were performed for each cell clones. Medium containing 10% fetal bovine serum (InVitrogen, Inc, Carlsbad, CA for figure 4 and Mediatech.com, Herndon, VA for figure 5) was changed every 4 days. On the 10th day, the plates were stained with 1% crystal violet, and colonies with diameter of more than 2 mm were counted.
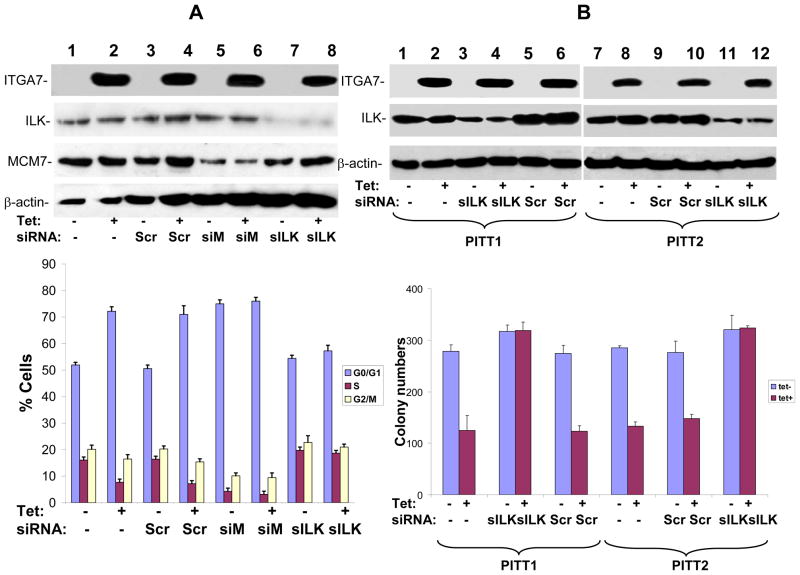
Figure 4. ILK is essential for ITGA7 mediated cell growth inhibition and tumor suppressor activity.
(A) BrdU labeling and cell cycle analysis of ITGA7 stimulated PITT1 cells. PITT1 cells were transfected with siRNA specific for MCM7 (siM), ILK (siILK) or scramble control (Scr). These cells were synchronized in serum free medium, and stimulated with tetracycline to induce ITGA7. The DNA synthesis was monitored through BrdU labeling and propridium iodide staining, and analyzed by FACS. Blue represents G0/G1 phase, purple S phase, yellow G2/M phase. (B) Colony formation analysis of ITGA7 stimulated PITT1 and PITT2 cells. PITT1 and PITT2 cells were transfected with siRNA specific for ILK (siILK) or scramble control (Scr), and stimulated with tetracycline to induce ITGA7. Colony formation assays were subsequently performed on these treated cells.
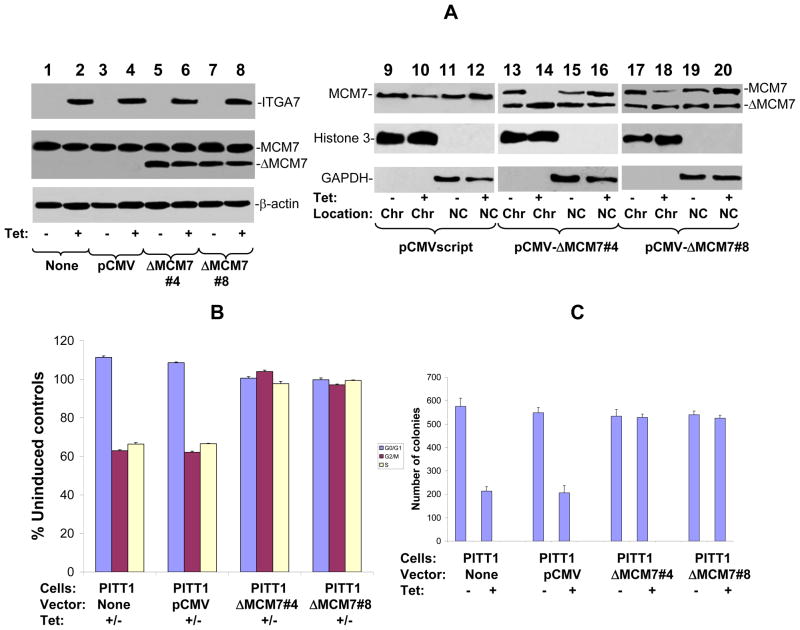
Figure 5. Tumor suppressor activity of ITGA7 is dependent on ILK/MCM7 interaction.
(A) MCM7 mutant that does not bind ILK fails to dissociate from chromatin upon ITGA7 stimulation. MCM7 mutant that contains 58 amino acid ILK binding motif deletion in its N-terminus was transfected into PITT1 cells. Two colonies (#4 and #8) were selected for analysis. Chromatin association analyses were subsequently performed to evaluate its chromatin binding activity. (B) BrdU Labeling and cell cycle analyses of wild type or mutant MCM7 in PITT1 cells. Blue represents G0/G1 phase, purple S phse, and yellow G2/M phase. (C) Colony formation analysis of wild type and mutant MCM7 expressing PITT1 cells.
Results
To investigate what proteins regulate the function of MCM7 and how such interaction results in enhanced invasion in prostate cancer cells, we performed a Yeast two hybrid screening using GAL4 DNA binding domain-MCM7 fusion proteins, utilizing MATCHMAKER system 3 from CLONETECH, INC. Four BD-MCM7s were constructed (Figure 1A). All were demonstrated with proper expression in the yeast (data not shown). Using pBD-MCM7, we identified 41 positive colonies after three rounds of metabolic screening of a prostate Yeast two hybrid cDNA library. These colonies were subsequently isolated. After several restriction enzyme digestions, several redundant clones were eliminated. Twenty-five unique clones were identified and sequenced. One of these clones contained a cDNA encoding ILK.
To validate the yeast two-hybrid screening results, pAD-ILK and pBD-MCM7 were co-transfected into Yeast AH109 cells, grown in high stringency medium, and tested for α-galactosidase activity. Both pBD-MCM7 (full length) and pBD-MCM7n (N-terminus) showed positive galactosidase activity, while the C-terminus and the mid-segment of MCM7 were negative, suggesting that the ILK binding activity is mediated by a region located in the N-terminus of MCM7 (Figure 1A). To verify the interaction, an in vivo MCM7-ILK binding analysis was performed in protein extracts of PC3 cells. As shown in Figure 1C, co-immunoprecipitation of MCM7 and ILK was readily apparent in either MCM7 or ILK immunoprecipitated complex. To visualize whether MCM7 and ILK co-localize, PC3 cells were subjected to double immunofluorescence staining using antibodies against MCM7 and ILK. As demonstrated in figure 1B, MCM7 and a significant amount of ILK colocalized in the nucleus. This also suggests that ILK is a nuclear protein. Similar colocalization results were obtained with Du145 cells (data not shown).
To validate the interaction between MCM7 N-terminus and ILK in vitro, a fragment of 247 amino acids from the N-terminus of MCM7 was constructed into pGEX-5T to create a GST-MCM7n fusion protein. The results of the binding assays indicate that GST-MCM7n binds with ILK in a cell free system (figure 1D). A series of deletion mutants of GST-MCM7n were constructed to identify the motifs that were required to interact with ILK. A stretch of 58 amino acids located in N-terminus (2–59) of MCM7 was found crucial for MCM7 binding with ILK, because the fusion proteins with deletions in this sequence did not bind ILK, while all proteins containing this sequence bound ILK (figure 1D).
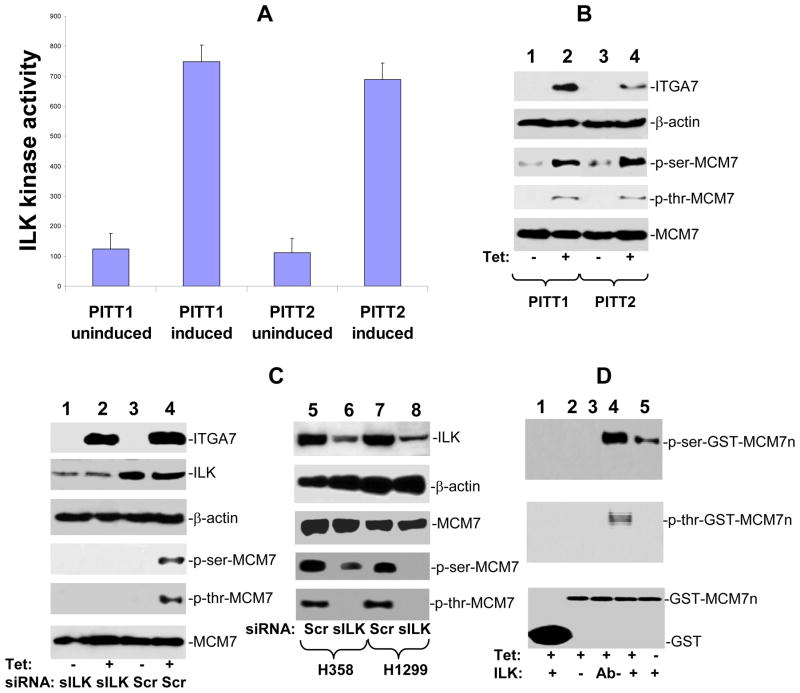
ILK is one of the pivotal molecules that mediates the signaling of several integrins (11, 12). To investigate whether ILK-MCM7 interaction mediates the signaling process of ITGA7, a newly defined tumor suppressor gene (2), we performed an analysis to examine whether ILK dependent phosphorylation is stimulated by ITGA7. As shown in figure 2A, induction of ITGA7 expression in PITT1 and PITT2 cells (pCDNA4-ITGA7/pCDNA6 transformed PC3 cells) increased the phosphorylation activity of immunoprecipitated ILK on basic myelin protein. Subsequently, an immunoblot analysis was performed on phosphorylated MCM7 of PITT1 and PITT2 cells treated with or without tetracycline. Induction of ITGA7 expression significantly increased both serine and threonine phosphorylations of MCM7 protein (figure 2B). To investigate whether ILK is required for ITGA7-induced phosphorylation of MCM7, PITT1 cells were treated with siRNA specific for ILK and induced with tetracycline. A dramatic reduction of MCM7 phosphorylation was identified at both serine and threonine residues (figure 2C). To investigate whether MCM7 phosphorylation in cells that contain endogenous wild type ITGA7 is similarly ILK dependent, we knocked down ILK expression in H358 and H1299 cells where no mutation of ITGA7 is found (2). The results showed a dramatic reduction of MCM7 phosphorylation when cells were treated with siRNA specific for ILK (figure 2C), suggesting a universal nature of ILK-MCM7 signaling. In vitro induction of phosphorylation of MCM7 N-terminus was observed when ILK immunoprecipitates were incubated with GST-MCM7n fusion proteins, while cell extract depleted of ILK were found negative of MCM7 phosphorylation upon ITGA7 induction (figure 2D).
Figure 2. ITGA7 induces MCM7 phosphorylation is ILK dependent.
(A) ITGA7 induces basic myelin phosphorylation by ILK immunoprecipitates. PITT1 or PITT2 cells were induced with or without 5 μg/ml tetracycline. ILK was immunoprecipitated. Basic myelin phosphorylation by ILK was performed through ELISA assay. (B) Induction of MCM7 phosphorylation by ITGA7 in PITT1 and PITT2 cells. PITT1 or PITT2 cells were induced with or without 5 μg/ml tetracycline. MCM7 was immunoprecipitated, electrophoresed in 8% SDS-PAGE and immunoblotted with antibodies against phospho-serine, phospho-threonine and MCM7. The top two panels are protein extracts immunoblotted with anti-ITGA7 and β-actin antibodies. (C) ILK is required for MCM7 phosphorylation in vivo and in vitro. Left panel: Knocking down of ILK abrogates MCM7 phosphorylation induced by ITGA7. PITT1 cells were transfected with siRNA specific for ILK (lanes 1–2) or scramble control (lanes 3–4), and induced with or without tetracycline. The MCM7 immunoprecipitates were immunbloted with the indicated antibodies. The top two panels are protein extracts immunoblotted with anti-ITGA7 and β-actin antibodies. Right panel: Decrease of MCM7 phosphorylation in H358 and H1299 cells with siRNA specific for ILK. Top two panels: Protein extracts from H358 and H1299 cells treated with scramble siRNA (Scr) or ILK siRNA (sILK) were SDS-PAGED and immunoblotted with antibodies specific for ILK and β-actin. Bottom three panels: Immunoprecipitates of MCM7 from H358 and H1299 cells were immunoblotted with antibodies specific for MCM7, phospho-serine, and phospho-threonine. (D) GST-MCM7 N-terminus phosphorylated by ILK immunoprecipitates. Kinase assays were performed on ILK immunoprecipitates using GST or GST-MCM7n as substrate. GST and GST-MCM7n were electrophoresed in 10% SDS-PAGE and immunoblotted with antibodies specific for phospho-serine or phospho-threonine. Ab- denotes protein extract pre-cleared by ILK antibody column before immunoprecipitation.
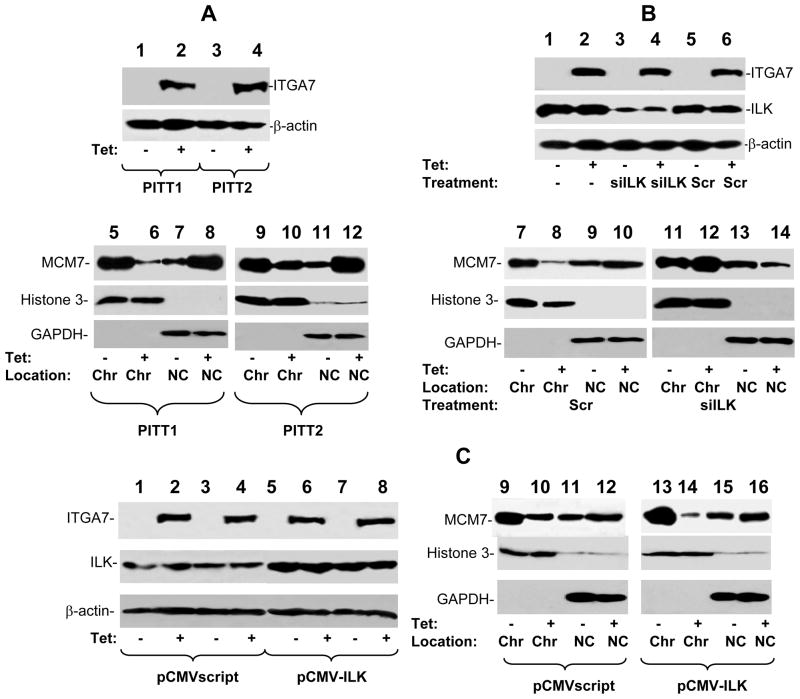
MCM7 is a critical component of DNA licensing complex. To investigate the functional significance of ILK-MCM7 interaction in ITGA7 signal transduction, we performed chromatin association analysis to examine whether activation of ITGA7 has impact on MCM7 DNA replication licensing. As shown in figure 3A, expression ITGA7 decreased MCM7 chromatin association by 70% in PITT1 cells and 50% in PITT2 cells, suggesting that activation of ILK by ITGA7 decreases the licensing activity of MCM7. Treatment of siRNA specific for ILK largely reversed the ITGA7 induced reduction of MCM7-chromatin association (figure 3B). Forced expression of ILK, on the other hand, exaggerated the inhibition of MCM7-chromatin association (figure 3C).
Figure 3. ITGA7 inhibits MCM7-chromatin association.
(A) ITGA7 decreases MCM7 association with chromatin. PITT1 or PITT2 cells were treated with or without tetracycline. Chromatin was then purified, electrophoresed on 8% SDS-PAGE and immunoblotted with antibodies against MCM7. Antibodies against Histone 3 and GAPDH were used to examine the purity of the chromatin fraction. Chr-chromatin; NC-non-chromatin. (B) Knocking down of ILK reverse ITGA7 effect on MCM7-chromatin association. PITT1 cells were transfected with siRNA specific for ILK or scramble control, and treated with or without tetracycline. Chromatin association analyses of MCM7 were performed as in (A). (C) Over-expression of ILK exaggerates the inhibition of MCM-chromatin association. PITT1 cells were transfected with pCMV-ILK or pCMVscript, and treated with or without tetracycline. Chromatin association analyses of MCM7 were performed as in (A).
To investigate whether ILK-MCM7 interaction mediates ITGA7 tumor suppressor activity, we examined DNA synthesis of PITT1 cells when either MCM7 or ILK was knocked down using siRNA specific for ILK or MCM7. Upon induction of ITGA7 expression, there was a 30% reduction of BrdU labeling in PITT1 cells and a 40% drop of cells entering S phase (figure 4A). There was also concomitant 50% decrease of colony formation (figure 4B and supplemental figure 1). Knocking down of ILK largely reversed these cell growth inhibition effects, while knocking down of MCM7 produced cell growth arrest even at the absence of ITGA7.
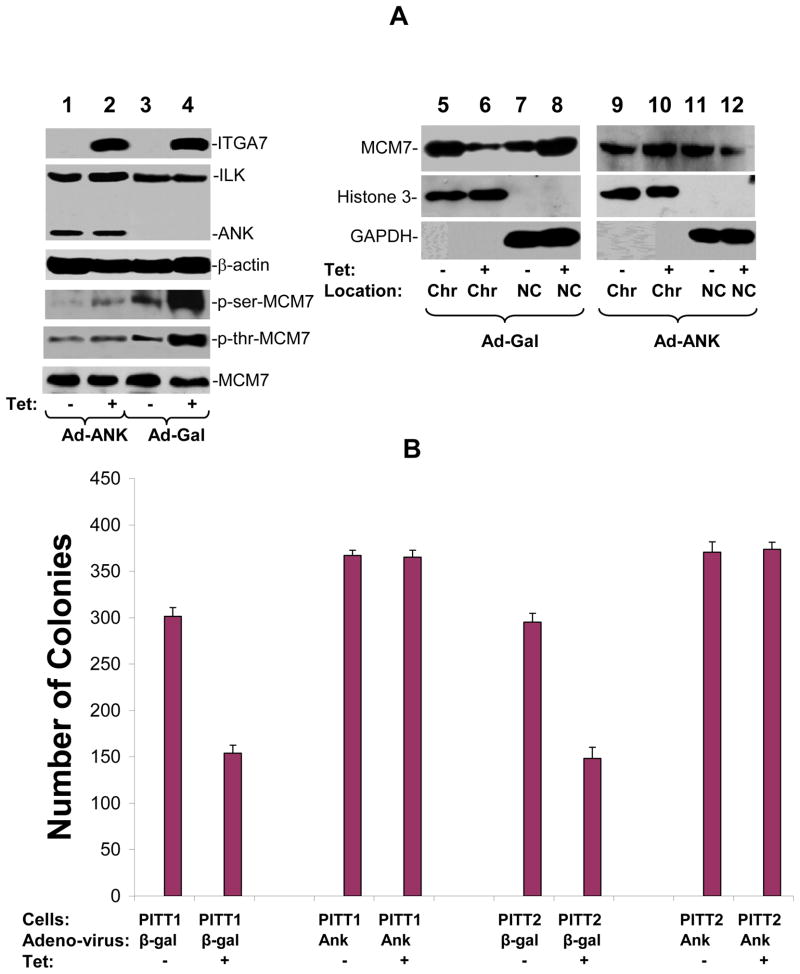
To investigate whether ILK-MCM7 interaction is responsible for the decreased chromatin association activity of MCM7 mediated by ITGA7, a MCM7 mutant with 58 amino acids ILK interaction motifs (amino acids 2–59) deleted was inserted into pCMVscript to create pCMV-ΔMCM7. This vector was subsequently transfected into PITT1 cells. Two clones (ΔMCM7#4 and ΔMCM7#8) with stably expressing mutant MCM7 were selected for further analyses. As shown in figure 5A, mutant MCM7 did not respond to ITGA7 stimulation, even though its wild type counterpart redistributed to non-chromatin fraction when ITGA7 expression is stimulated, supporting that ILK interaction is necessary for ITGA7 mediated reduction of MCM7 chromatin association. In addition, when PITT1 cells were infected with Ad-ANK, a dominant negative ILK mutant (13, 14), phosphorylation of both serine and threonine residues of MCM7 were dramatically decreased upon the induction of ITGA7 (figure 6A). This is accompanied with a reversal of ITGA7 effect on MCM7 chromatin association. These results suggest that ILK dependent phosphorylation of MCM7 might be necessary for ITGA7-MCM7 signaling activity. Consistent with the analyses of MCM7-chromatin association, MCM7 mutant that does not interact with ILK largely blocked the ITGA7 mediated DNA synthesis suppression effect (figure 5B) and abrogated the inhibition of colony formation (figure 5C and supplemental figure 2), behaving similar to a dominant negative mutant. Similarly, ANK, the dominant negative ILK mutant, largely resisted the tumor suppressor effect of ITGA7 by reversing the ITGA7 induced suppression of colony formation (figure 6B). Overall, these results support that interaction of ILK/MCM7 and induction of the ILK mediated phosphorylation of MCM7 are critical for ITGA7 tumor suppression activity.
Figure 6. ANK inhibits ILK dependent MCM7 phosphorylation and colony formation inhibition induced by ITGA7.
(A) ANK inhibits phosphorylation of MCM7 by ILK and reverses MCM7-chromatin association inhibition by ITGA7. PITT1 cells were infected with Ad-Gal or Ad-ANK, and stimulated with tetracycline to induce ITGA7 expression. MCM7 was then immunoprecipitated and blotted with anti-phospho-serine or anti-phospho-threonine antibodies. Chromatin association analyses were similarly performed as in figure 3. (B) ANK reverses ITGA7 tumor suppressor activity. PITT1 and PITT2 cells were infected with either Ad-GAL or Ad-ANK, and treated with or without tetracycline. Colony formation analyses were performed on these cells.
Discussion
The signaling pathway of ITGA7 tumor suppression remains unclear. Previous study showed that restoration of ITGA7 expression in human malignant cells activates the expression of CDKN3 and RACGAP1(2). This study suggests that inactivation of MCM7 DNA licensing activity through ILK/MCM7 interaction and phosphorylation is a critical pathway for ITGA7 tumor suppression signaling. Several lines of evidence support that ILK interacts MCM7 and mediates its phosphorylation: First, ILK and MCM7 was co-immunoprecipitated using either antibodies against MCM7 or ILK in PC3 cells. The binding activity was also supported by Yeast Two hybrid nutrient and α-galactosidase activity. Second, ILK and MCM7 was found co-localized in nucleus. Third, in vitro binding analysis using GST-MCM7 fusion proteins found that ILK bound to a 58 amino sequence in the N-terminus of MCM7. Fourth, ITGA induction of MCM7 phosphorylation only occurred when ILK was present. Knocking down of ILK eliminated MCM7 phosphorylation induced by ITGA7. ANK, the dominant negative mutant of ILK, reversed the ITGA7 effect on MCM7 phosphorylation. Fifth, phosphorylation of MCM7 by ILK immunoprecipitates was also identified in vitro with bacterial expressed MCM7 fragment. The ratio of serine/threonine phosphorylation of MCM7 N-terminus appears matching those observed in vivo.
Our study clearly shows that ILK is required for the ITGA7 induced phosphorylation of MCM7 and the phosphorylation of MCM7 negatively regulates the DNA licensing activity. The repressor effect of ILK on MCM7 DNA licensing activity is supported by several experiments: Knocking down of ILK or dominant negative mutant ILK abrogated the ITGA7 effect. Mutant MCM7 that does not interact with ILK did not de-associate from chromatin as the endogenous wild type does. The de-association of MCM7 from chromatin was reflected by lower level of DNA synthesis and cell growth, a finding consistent with MCM7 de-licensing of DNA synthesis. As a result, interaction between ILK and MCM7 appears critical for ITGA7 mediated tumor suppressor activity.
ILK was originally identified as kinase interacting with integrin β1 (ITGB1) (15). The interaction location between ILK and ITGB1 is located in the cytoplasm(15). In fact, most of the analyses so far have been focusing on the function of ILK in the cytoplasm. Interestingly, the interaction of ILK and MCM7 occurs in the nucleus, not in ILK’s usual location. By mediating a tumor suppressor activity, one may argue that the ILK translocating to nucleus carries out distinct physiological function than the one in the cytoplasm. Indeed, this finding is consistent with a recent finding that nuclear ILK contains transcription repression activity, and knocking-out of ILK in the liver produces higher level of cell proliferation and organomegaly (16–19). MCM7 is widely considered a critical DNA licensing factor in both yeast and mammalian cells. Recent findings suggest that MCM7 functions as a transcription factor and an oncogene in malignant tumor cells (4, 6, 7). Amplification of MCM7 had been identified in several human malignancies including prostate cancer and gastric carcinoma (4, 20). Reducing chromatin association of MCM7 by ITGA7 appears to critically contribute to the tumor suppressor activity observed in ITGA7 expressing cells.
This study also suggests for the first time that MCM7 is a target of tumor suppressor pathways, and provides a novel example of inactivation of a DNA replication licensing factor by a tumor suppressor. Previous studies indicate that MCM7 expression and function are tightly coupled to oncogene and hormonal factor such as c-myc and androgen receptor (4, 21). Aberrant activation or over-expression MCM7 not only serves as an autonomous signal for DNA synthesis and cell growth, but also increases the expression of its embedded miR-106b cluster (22) which in turn inhibits several critical tumor suppressor genes. As a result, intervention of MCM7 expression or function may hold the promise for controlling the behavior of clinical malignancies.
Acknowledgments
This work was supported by grants from National Cancer Institute (RO1 CA098249 to JHL), Department of Defense (W81 XWH-09-1-0376 to JHL), and American Cancer Society (RSG-08-137-01-CNE to YPY).
References
- 1.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Ren B, Yu YP, Tseng GC, et al. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst. 2007;99:868–80. doi: 10.1093/jnci/djk199. [DOI] [PubMed] [Google Scholar]
- 3.Blow JJ, Hodgson B. Replication licensing--defining the proliferative state? Trends Cell Biol. 2002;12:72–8. doi: 10.1016/s0962-8924(01)02203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren B, Yu G, Tseng GC, et al. MCM7 amplification and overexpression are associated with prostate cancer progression. Oncogene. 2006;25:1090–8. doi: 10.1038/sj.onc.1209134. [DOI] [PubMed] [Google Scholar]
- 5.Levesque MH, El-Alfy M, Berger L, Labrie F, Labrie C. Evaluation of AIbZIP and Cdc47 as markers for human prostatic diseases. Urology. 2007;69:196–201. doi: 10.1016/j.urology.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Honeycutt KA, Chen Z, Koster MI, et al. Deregulated minichromosomal maintenance protein MCM7 contributes to oncogene driven tumorigenesis. Oncogene. 2006;25:4027–32. doi: 10.1038/sj.onc.1209435. [DOI] [PubMed] [Google Scholar]
- 7.Shi YK, Yu YP, Zhu ZH, et al. MCM7 Interacts with Androgen Receptor. Am J Pathol. 2008;173:1758–67. doi: 10.2353/ajpath.2008.080363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu BC, Li MX, Zhang JD, et al. Inhibition of integrin-linked kinase via a siRNA expression plasmid attenuates connective tissue growth factor-induced human proximal tubular epithelial cells to mesenchymal transition. American journal of nephrology. 2008;28:143–51. doi: 10.1159/000110019. [DOI] [PubMed] [Google Scholar]
- 9.Yu YP, Luo JH. Myopodin-mediated suppression of prostate cancer cell migration involves interaction with zyxin. Cancer Research. 2006;66:7414–9. doi: 10.1158/0008-5472.CAN-06-0227. [DOI] [PubMed] [Google Scholar]
- 10.Jing L, Liu L, Yu YP, et al. Expression of myopodin induces suppression of tumor growth and metastasis. Am J Pathol. 2004;164:1799–806. doi: 10.1016/S0002-9440(10)63738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 13.Guo L, Sanders PW, Woods A, Wu C. The distribution and regulation of integrin-linked kinase in normal and diabetic kidneys. Am J Pathol. 2001;159:1735–42. doi: 10.1016/S0002-9440(10)63020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L, Wu C. Regulation of fibronectin matrix deposition and cell proliferation by the PINCH-ILK-CH-ILKBP complex. Faseb J. 2002;16:1298–300. doi: 10.1096/fj.02-0089fje. [DOI] [PubMed] [Google Scholar]
- 15.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 16.Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc Natl Acad Sci U S A. 2007;104:6782–7. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donthamsetty S, Bowen W, Mars W, et al. Liver Specific Ablation of Integrin Linked Kinase (ILK) in Mice Results in Enhanced and Prolonged Cell Proliferation and Hepatomegaly after Phenobarbital Administration. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apte U, Gkretsi V, Bowen WC, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844–51. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gkretsi V, Apte U, Mars WM, et al. Liver-specific ablation of integrin-linked kinase in mice results in abnormal histology, enhanced cell proliferation, and hepatomegaly. Hepatology. 2008;48:1932–41. doi: 10.1002/hep.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kan T, Sato F, Ito T, et al. The miR-106b-25 polycistron, activated by genomic amplification, functions as an oncogene by suppressing p21 and Bim. Gastroenterology. 2009;136:1689–700. doi: 10.1053/j.gastro.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koppen A, Ait-Aissa R, Koster J, et al. Direct regulation of the minichromosome maintenance complex by MYCN in neuroblastoma. Eur J Cancer. 2007;43:2413–22. doi: 10.1016/j.ejca.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 2008;68:6162–70. doi: 10.1158/0008-5472.CAN-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]