Abstract
Background
There are known racial disparities in the prevalence of anemia in adults with chronic kidney disease (CKD), but these differences have not been well described in children.
Study Design
Cohort study, cross-sectional analysis.
Setting and Participants
The Chronic Kidney Disease in Children (CKiD) Study is a multi-center prospective cohort study of children with mild-to-moderate CKD. This analysis included 429 children of African-American or white race.
Predictor
Race.
Outcomes & Measurements
This study examined the association of race with level of hemoglobin. Both multiple linear regression and generalized gamma modeling techniques were used to characterize the association between race and hemoglobin.
Results
79% of the cohort was white, 21% African-American. Neither median hemoglobin levels nor frequency of ESA use differed by race. In multivariate analysis, lower levels of iohexol-measured glomerular filtration rate (mGFR), African-American race, and glomerular disease (vs. non-glomerular disease) as the underlying cause of CKD were independently associated with decreased hemoglobin levels; independent of GFR and CKD diagnosis, African-American children displayed average hemoglobin levels 0.6 g/dL (95% confidence interval −0.9, −0.2 g/dL) lower than those of white children. Generalized gamma modeling demonstrated that the differences in hemoglobin observed by race become more pronounced when moving from high to low in the overall hemoglobin distribution.
Limitations
Cross-sectional analysis cannot establish causality, and data on iron stores were not available for all patients.
Conclusions
African-American children compared to white children demonstrate lower hemoglobin values in CKD, independent of the underlying cause of CKD. These racial differences in hemoglobin appear to increase at the lower end of the hemoglobin distribution in this population.
INDEX WORDS: kidney disease, hemoglobin, disparity, erythropoietin stimulating agent, glomerular filtration rate, generalized gamma
Introduction
Anemia is a common and treatable co-morbid condition in children with chronic kidney disease (CKD). The anemia of CKD is associated with increased risk for development and progression of left ventricular hypertrophy (LVH), and accelerated CKD progression.1, 2 In addition, anemia has been associated with lower health-related quality of life and increased risk of hospitalization in children with CKD.3, 4 Despite the widespread use of iron supplementation and erythropoietin stimulating agents (ESA), anemia remains common in both end stage renal disease (ESRD) and CKD populations.5
Racial disparities in hemoglobin (Hb) levels among both adults and children with ESRD are well established; black adults typically initiate dialysis with significantly lower Hb compared to whites, and African-American race has been shown to be a significant risk factor for anemia among children and teenagers on dialysis.6–8 Among adults with earlier stages of non-dialysis CKD, anemia is more prevalent in African-American compared to white patients.9, 10 In children with CKD, differences in Hb values and anemia prevalence by race have not been well described. Our objective in this analysis was to examine the associations between race and Hb level in children with CKD.
Methods
Study Population and Design
We analyzed baseline data collected on participants enrolled in the Chronic Kidney Disease in Children (CKiD) Study, an observational prospective cohort study of CKD in children conducted at 44 pediatric centers in North America.11 Eligibility criteria for enrollment in CKiD include: age 1–16 years, estimated glomerular filtration rate (GFR) 30 – 90 ml/min/1.73m2 by the Schwartz formula,12 no prior organ transplant, and informed consent by a parent or guardian.
Analysis was cross-sectional and restricted to individuals who self-reported their race as only African-American or only Caucasian (white). Additional inclusion criteria were: valid concomitant measurements of Hb and GFR, self-reported medication use, and known age, sex, ethnicity, and CKD diagnosis.
Measurements
Blood and urine samples are collected at the time of each baseline CKiD study visit. A complete blood count panel, including measurement of Hb, is performed locally. Albumin, urine creatinine and urine protein is performed by the CKiD central laboratory (University of Rochester, Rochester, NY). GFR is measured directly by iohexol plasma disappearance (mGFR), details of which have been published previously.13 Additional data used in this analysis include age, sex, height, weight, blood pressure, primary CKD diagnosis (glomerular or non-glomerular), medication use during the past 30 days, Tanner stage (Tanner stage 1 = pre-pubertal) and self-reported race, ethnicity, household income, and maternal education (12+ years vs. < 12 years). Age and sex specific height and body mass index (BMI) percentiles, as well as normalized scores for age and sex (z-scores), are calculated using standard growth charts for United States children. Blood pressure is measured by aneroid sphygmomanometer.14
Statistical Analyses
Hb levels, prevalence of Hb < 5th normative percentile for age and sex (based on NHANES III data in healthy children)15, and current ESA and iron supplement use were reported in African-American and white subjects. Continuous data were characterized with medians and 25th and 75th percentiles; categorical data were described by percentages and frequencies. Differences in the prevalence of categorical variables were tested using Fisher’s exact tests and of continuous variables using Wilcoxan rank sum tests.
A scatterplot of hemoglobin by mGFR (log-scale) and race was developed and overlaid with race-specific linear regression curves. Race specific uni- and multivariate modeling using linear ordinary least squares (OLS) regression was performed. Piecewise linear models allowing for effect modification of the Hb-mGFR relationship by race were tested. Potential covariates in the final model included race, log(mGFR), age, sex, CKD diagnosis, maternal education, BMI z-score, pubertal status, iron supplement use and ESA use. Model parameters were evaluated using a Wald Test. Statistical significance was evaluated at the α=0.05 level.
To further characterize differences in the distribution of Hb between racial groups we used percentile plots and generalized gamma models in a classic 2-sample problem – African-American vs. white subjects. Percentile plots are an extension of the classic boxplot, highlighting additional quantiles beyond the 25th, 50th, and 75th (we show the 5th, 10th, 90th and 95th as well as individual observations that sit outside of the 5th and 95th quantile values, respectively).16 The width of the percentile plot decreases in proportion to departures from the 50th percentile. Thus, in comparison to the width of the plot at the median (50th percentile), the plot is half as wide at the 25th and 75th percentiles, and 1/5th as wide at the 10th and 90th percentiles.
generalized gamma models draw upon the family of generalized gamma distributions that are characterized by three parameters: location (β), scale (σ), and shape (λ).17 This family includes commonly used distributions (e.g., lognormal characterized by λ = 0) and it is particularly useful for describing effects of exposures not just at the mean but at all percentiles (e.g., effects on the tails of the distribution). Differential effects of an exposure at different percentiles is accomplished by allowing the exposure not only to modify the location (β) but also the scale (σ) and/or shape parameters (λ). Using results from the generalized gamma models, we plotted race-specific probability density functions of Hb and calculated relative Hb percentiles. relative percentiles are simply the ratios of the pth percentile of Hb among black children to the pth percentile of Hb among white children for each value of p between 1 and 100. If the relative percentiles are equal to 1, this will correspond to the null hypothesis of no association with race; if they are < 1 this will indicate that African-American children have lower levels of Hb even in children with similar values of other covariates present in the model. An attractive feature of the model is that it allows for heterogeneity of the RP across different values of p. As such, it is possible that the lower half of the Hb values may show stronger differences by race than seen in the upper half of the race specific Hb distributions. To allow race to have differential effects at different percentiles, generalized gamma models with unique σ and λ estimates for each racial group were tested. To account for the effect of ESA therapy on Hb, individuals currently receiving an ESA had their Hb left-censored or considered to be equal to or less than the value measured but greater than zero. To achieve this, the model redistributes the Hb levels of treated individuals to values equal to or lower than their observed Hb, by looking at other subjects with similar covariates who are NOT on ESAs.18 Valid analyses allowing for left-censoring of hemoglobin by ESA use assume that ESA use is at random within racial groups and the measured covariates (i.e., two individuals with the same race and covariate values are equally likely to use ESA). Inclusion of parameters in the final model was based on the comparison of nested models using Akaike Information Criterion (AIC).19 Confidence intervals for the RP curves were calculated using the Delta Method.
All analysis was performed using SAS 9.2 (SAS Institute, Inc., www.sas.com). Figures were produced using S Plus 8.0 statistical software (TIBCO Software Inc., spotfire.tibco.com).
Results
As of January 2009, 565 children had completed baseline CKiD visits; 118 (21%) were African-American and 378 (67%) were Caucasian. Of these 496 children, 51 (10%) were excluded from analysis due to missing Hb, mGFR, medication use, or CKD diagnosis data. Of the 107 remaining African-American children, 16 reported a ‘multi-racial’ background and were excluded. This left 429 children who met eligibility criteria for the analysis. Of these, 79% (338) were white and 21% (91) African-American.
Demographic, clinical, and socioeconomic characteristics for African-American and white children are compared in Table 1. No differences in median age, gender distribution, or proportions of patients reporting Hispanic ethnicity were noted. There were no differences in the proportions of children who were pre-pubertal by race. African-American children compared to white children had higher median BMI percentile. Additionally, African-American children had higher prevalence of hypoalbuminemia (defined as albumin < 4 g/dL) compared to white children, although no difference in the prevalence of nephrotic-range proteinuria was observed. Although neither median Hb levels nor frequency of ESA or iron supplement use differed by race, the prevalence of Hb less than the 5th percentile for age and sex was higher in African-American children compared to white children (44% vs. 29%, p=0.01). Moreover, African-Americans had better kidney function as a group (mGFR 49 vs. 41 ml/min/1.73m2, p<0.001). African-American children were also more likely to have glomerular disease as the underlying cause of CKD compared to whites (37% vs. 17%, p<0.001).
TABLE 1.
Study Population Demographic, Clinical, and SES[ND1] Characteristics by Race
| CKD Characteristica | African-American | White | p-valueb |
|---|---|---|---|
| N= | 91 | 338 | |
| Age, yrs | 11 [8,15] | 11 [8,14] | 0.7 |
| Male, % | 70% (64) | 62% (209) | 0.1 |
| Hispanic Ethnicity, % | 8% (7) | 15% (49) | 0.1 |
| Pre-pubertal, %c | |||
| Among Males | 56% (33/59) | 62% (125/201) | 0.4 |
| Among Females | 52% (14/27) | 55% (69/125) | 0.8 |
| Height %ile | 34 [8,60] | 22 [7,51] | 0.05 |
| BMI, kg/m2 | 20 [17,24] | 18 [16,21] | 0.02 |
| BMI %ile | 84 [38,96] | 61 [33,85] | 0.002 |
| mGFR, ml/min/1.73 m2 | 49 [37,62] | 41 [32,52] | 0.001 |
| Duration of CKD, yrs | 5 [2,9] | 7 [3,11] | 0.02 |
| Glomerular CKD | 37% (34) | 17% (57) | <0.001 |
| Nephrotic-range proteinuria, %d | 18% (16) | 12% (39) | 0.2 |
| Hypoalbuminemia, %e | 22% (20) | 11% (38) | 0.01 |
| Uncontrolled Hypertension, %f | 28% (25) | 21% (69) | 0.15 |
| Hemoglobin, g/dL | 12.3 [11.2,13.4] | 12.6 [11.7,13.6] | 0.09 |
| Hemoglobin < 5th %ile, %g | 44% (40) | 29% (99) | 0.01 |
| Currently on Iron Supplement, % | 27% (25) | 30% (100) | 0.8 |
| Currently on ESA, % | 14% (13) | 15% (52) | 0.9 |
| SES Characteristics | |||
| Annual Household Income ≤ $36,000 | 61% (54) | 32% (108) | <0.001 |
| Maternal Education ≥ 12 years, % | 82% (75) | 89% (301) | 0.1 |
Note: Coversion factors for units: GFR in mL/min/1.73 m^2 to mL/s/1.73m^2, x0.01667; serum albumin in g/dL to g/L, x10; hemoglobin in g/dL to g/L, x10.
N = 429. Medians [25th and 75th percentiles] for continuous variables; percent (n) for categorical variables. Missing data: Height %ile for 8 patients; Weight %ile, 1; BMI %ile, 19; Uncontrolled Hypertension, 10; Albumin, 2; Household Income, 8; Maternal Education, 9; Single Parent Household, 2; Tanner staging, 17.
Wilcoxon Rank Sum test for continuous variables; Fisher’s Exact Test for categorical variables
Pre-pubertal defined as having a Tanner Stage =1
Nephrotic-range proteinuria defined by protein-creatinine ratio ≥ 2.
Hypoalbuminemia defined by Albumin <4 g/dL
Uncontrolled Hypertension defined by SBP or DBP ≥ 95th %ile for age, sex, and height.
Hemoglobin < 5th %ile for age and sex
Abbreviations: ESA, erythropoietin-stimulating agent; SES = socioeconomic status; BMI, body mass index; Hb, hemoglobin; CKD, chronic kidney disease
Compared to white children, African-Americans were disproportionately more likely to come from households with annual income of less than $36,000 (61% vs. 32%, p<0.001). There was no significant difference in maternal education by race.
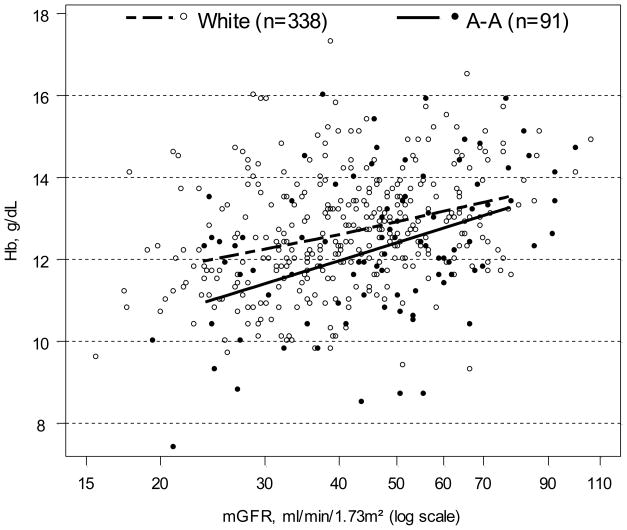
Figure 1 displays a scatterplot of Hb by mGFR and race, overlaid with race-specific least-squares linear regression curves. This figure illustrates that Hb decreases as mGFR decreases in all children, but that Hb values in African-American children are on average lower than those in white children across the spectrum of mGFR.
Figure 1.
Scatterplot of hemoglobin (Hb) vs. log-transformed iohexol-measured glomerular filtration rate (mGFR) by race. White children (N=338) are depicted with open circles; African-American (A-A) children (N=91) are depicted with solid circles. Race -specific linear regression lines of Hb on log(mGFR) are overlayed. Regression line for white children is a dashed line,; for A-A children is a solid line.
The results of uni- and multivariate linear regression models of Hb on race as well as age, sex, BMI, pubertal status, mGFR, CKD diagnosis, maternal education, current ESA use, and current iron supplement use are presented in Table 2. In the univariate (unadjusted) models, decreasing mGFR, African-American race, glomerular disease as the underlying cause of CKD, current ESA use, and current iron supplement use were all associated with decreased Hb, while higher maternal education and higher normalized BMI (z-score) were associated with increased Hb. Male sex was associated with higher Hb amongst the peri- and post-pubertal children. Among male subjects only, being pre-pubertal vs. peri- or post-pubertal was associated with lower Hb. Univariately, annual household income was not associated with significant difference in Hb (not shown in Table 2). After adjusting for all risk factors reported in Table 2, average Hb levels for African-American children were estimated to be 0.6 g/dL (95% CI: −0.9, −0.2) less than white subjects with similar anthropometric, clinical and SES characteristics. In addition, lower BMI for age and sex, lower mGFR, glomerular diagnosis, and use of iron supplements continued to be independently associated with lower Hb. For all children, a 20% decrease in mGFR was associated with an average decrease in Hb of 0.2 to 0.4 g/dL. Iron supplement use was also associated with Hb levels on average 0.5 g/dL lower than in children not receiving iron. As in the univariate analysis, an interaction (effect modification) was observed between sex and pubertal status. Among peri- and post-pubertal children, average Hb levels were estimated 0.7 g/dL lower in females than males. Among males only, pre-pubertal status was independently associated with lower Hb.
Table 2.
Linear Regression of Hemoglobin on mGFR, African-American Race, and Covariates
| Change in Hb, g/dL | ||||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| estimate (95% CI) | p-value | estimate (95% CI) | p-value | |
| Age, per 2 year increase | 0.0 (−0.1, 0.1) | 0.7 | −0.0 (−0.1, 0.1) | 0.7 |
| Male v. female sexa | ||||
| pre-pubertal | −0.1 (−0.5, 0.3) | 0.6 | −0.2 (−0.5, 0.2) | 0.4 |
| peri-/post-pubertal | 0.7 (0.2, 1.1) | 0.004 | 0.7 (0.2, 1.1) | 0.002 |
| African-American race | −0.4 (−0.7, −0.0) | 0.03 | −0.6 (−0.9, −0.2) | <0.001 |
| BMI z-score, per 1 SD increase | 0.2 (0.1, 0.3) | <0.001 | 0.2 (0.1, 0.3) | 0.002 |
| Pre-pubertala | ||||
| Among males | −0.5 (−0.9, −0.1) | 0.01 | −0.7 (−1.1, −0.2) | 0.009 |
| Among females | 0.3 (−0.2, 0.8) | 0.2 | 0.2 (−0.4, 0.8) | 0.5 |
| mGFR, per 20% decrease | −0.3 (−0.4, −0.2) | <0.001 | −0.3 (−0.4, −0.2) | <0.001 |
| Glomerular CKD | −0.4 (−0.8, −0.1) | 0.01 | −0.5 (−0.9, −0.1) | 0.006 |
| Maternal Education ≥ 12 years | 0.5 (0.0, 0.9) | 0.04 | 0.4 (−0.1, 0.8) | 0.1 |
| ESA use | −0.7 (−1.1, −0.3) | <0.001 | 0.1 (−0.3, 0.5) | 0.7 |
| Iron Supplement use | −0.7 (−1.0, −0.4) | <0.001 | −0.5 (−0.8, −0.2) | 0.003 |
Note: N = 429. Conversion factors for units: hemoglobin in g/dL to g/L, x10.
heterogeneity modeled using a sex × pubertal status interaction term: univariate, p=0.01; adjusted, p=0.004.
Abbreviations: ESA, erythropoietin-stimulating agent; mGFR, measured glomerular filtration rate; BMI, body mass index; Hb, hemoglobin; CKD, chronic kidney disease; SD, standard deviation, CI, confidence interval.
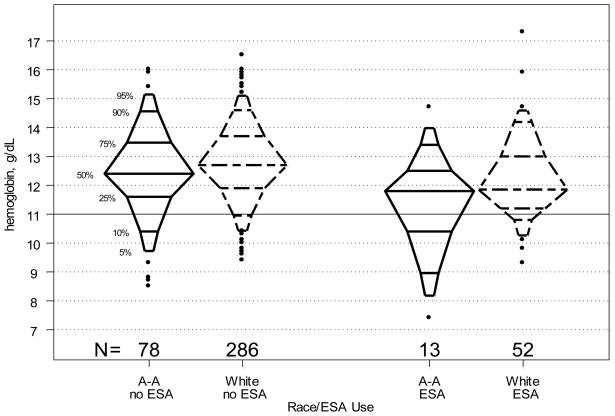
The linear regression models reported above (Figure 1 and Table 2), while informative, describe only the differences in mean Hb levels by race. However, the mean difference between the two groups does not fully characterize the differences in Hb distribution by race. Figure 2 shows race and ESA use-specific Hb distributions using percentile plots. The plots show that for the lower ¾ of the distributions, the relative Hb percentile levels of blacks are consistently lower than those of whites, independent of ESA use. Moreover, the discrepancy in Hb level at any given percentile increases as percentile decreases, suggesting that the greatest difference in Hb between the two racial groups may be found in the lower tails of the distributions.
Figure 2.
Percentile plots displaying the distribution of hemoglobin (Hb) levels for children not using (n=364) versus using (n=91) erythropoietin stimulating agent (ESA) by race. Plots for African-American (A-A) children are shown with solid lines; plots for White children are shown with dashed lines.
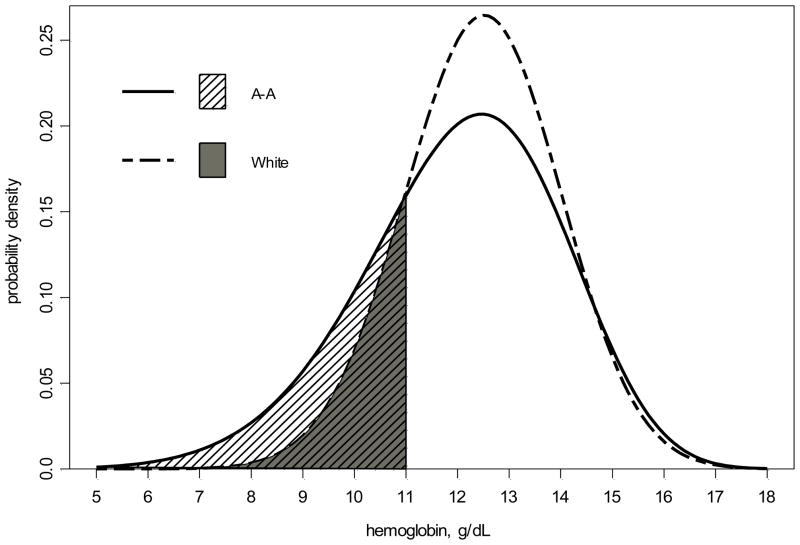
Figure 3 compares the Hbs for African-American and white children, respectively. These s are derived from fitting the data with race-specific, unadjusted generalized gamma models and left-censoring the Hb levels for children currently treated with ESAs. While little difference is seen in the upper tails of the two curves, differences between the two racial groups in the modeled distributions of Hb are apparent in the lower range. As such, 28% of African-American and 16% of white children were estimated to have ESA-free Hb levels below 11 g/dL.
Figure 3.
Univariate, race-specific generalized gamma derived probability density curves of hemoglobin (Hb) with left-censoring for current erythropoietin stimulating agent (ESA) users. The Hb distribution for African-American children [GG(2.55, 0.14, 0.86)] is shown with a solid line; Hb distribution for white children [GG(2.54, 0.12, 0.41)] is shown with a dashed line. The shaded areas show that 28% of African American children (striped area) compared to only 16% of white children (solid grey area) are estimated to have a Hb <11 g/dL.
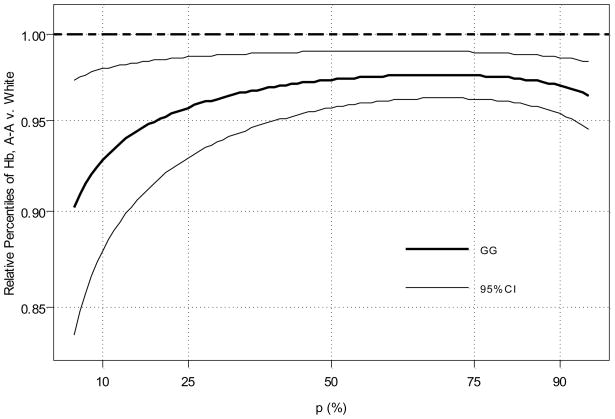
A multivariate generalized gamma model allowing race to modify the scale parameter only and covariates to modify the location parameter provided an appropriate fit to the data. Covariates included in the final model included: sex, BMI z-score, pubertal status, mGFR, CKD diagnosis, and iron supplement use. Using the parameters estimated from such a model, adjusted (i.e., for individuals with same covariates) Hb relative percentiles were calculated as the ratio of the African-American percentile value to the white value at each percentile level (Figure 4). relative percentiles close to 1 suggest no difference in Hb level between the two racial groups at a given percentile, while departures from 1 indicate discrepancies between groups. The estimated relative percentiles are shown with the thick, solid curve and the 95% CI is shown with thin lines. This relative percentile curve suggests that independent of sex, BMI, pubertal status, GFR, CKD diagnosis, and iron use, black subjects have lower Hb levels at any given percentile of the Hb distribution, but that this difference becomes more pronounced as one moves from high to low in the distribution on Hb (right to left in Figure 4).
Figure 4.
Relative percentile plot of hemoglobin (Hb) for 81 African-American (A-A) vs. 404 white children, based on multivariate generalized gamma modeling with left-censoring for current erythropoietin stimulating agent (ESA) users. Covariates in the generalized gamma model include: sex, body mass index z-score, pubertal status, iohexol-measured glomerular filtration rate, glomerular cause of CKD, and current use of iron supplements.
Discussion
Our analysis shows that among children with pre-dialysis CKD, African-American race is associated with lower Hb levels. Compared to white children, African-Americans demonstrated lower Hb values, on average 0.6 g/dL lower than their white counterparts, independent of mGFR or cause of kidney disease. In addition, fewer African-American children overall achieved Hb values > 5th percentile for age and sex as recommended by the KDOQI guidelines for anemia in CKD.15 This racial disparity in Hb values increases at the lower end of the Hb distribution. These differences were observed despite similar ESA and iron supplement use across racial groups. However, no difference in the magnitude of the association between Hb and mGFR was noted by race.
There is evidence that normal Hb values differ by race and ethnicity even amongst healthy children. An analysis of non-iron deficient African-American children aged 2–18 years carried out in the NHANES III database found that African-American children have significantly lower mean Hb levels, in every gender- and age-group, compared to white children.20 The presence of iron deficiency, sickle cell anemia or α-thalassemia among African-American children does not appear to explain these observed differences. In a study of African-American and white adults, whites on average exhibited higher mean Hb levels than African-Americans (difference of means: 0.72 g/dL in women, 0.58 g/dL in men).21 When those with iron-deficiency were excluded, the gap narrowed to 0.6 g/dL in women and was unchanged in men. However, further exclusion of patients with sickle cell trait did not change point estimates, and the frequency of the α-thalassemia trait among African-Americans was found to account for only about one-third of the difference in Hb levels between African-Americans and whites. The remainder of the Hb difference was due to other, as-yet undefined mechanisms.
The magnitude of the average Hb difference seen by race in our analysis (0.6 g/dL) does appear to parallel differences noted among healthy children.20, 21 Reasons for this difference in the healthy population have been widely discussed in the literature, with speculation that socioeconomic factors (including access to an iron-rich diet) may play a role.22, 23 We did observe differences in markers of socioeconomic status including household income by race in this cohort. Also, the higher BMI values noted among the African-American children in the cohort is of interest, given some evidence that increased BMI may be associated with functional iron deficiency, which may in turn contribute to anemia.24 However, adjustment for socioeconomic factors and BMI in the multivariate model failed to eliminate the observed difference in Hb by race. Regardless of racial differences in Hb values in healthy children, the current KDOQI clinical practice recommendations for anemia in CKD do not suggest that Hb targets should be race dependent. Further studies of the clinical significance of this Hb difference will be necessary before race-specific guidelines could be considered.
In our analysis, African-American children were more likely to have glomerular disease as the underlying cause of CKD compared to whites. African-American children also experienced a higher prevalence of hypoalbuminemia. This raises the question of whether differences in underlying diagnosis may explain the observed racial differences in Hb. However, after adjusting for mGFR, age, sex, and glomerular causes of CKD, African-American race continued to be associated with an approximate 0.6 g/dL decrease in Hb. This suggests that African-American race and glomerular causes of CKD may both independently lead to lower Hb values in children with CKD.
A substantial body of literature suggests that disparity in anemia prevalence by race does seem to widen as patients, both adults and children, approach ESRD and initiate renal replacement therapy. The utilization of generalized gamma modeling techniques in our analysis has allowed us to demonstrate that in fact, although African-American patients overall have lower average Hb values than whites as suggested by our linear regression analyses, this difference is accentuated in the lower tail of the Hb distribution. In other words, while African-American children as a group exhibit lower Hb levels than white children, the greatest racial disparity occurs amongst those children who are the sickest (i.e., those with the lowest Hb levels). Although our linear regression analysis did not demonstrate a quantitative difference by race in the Hb - mGFR relationship, we can speculate that specific factors promoting anemia in children with progressive CKD, including erythropoietin deficiency, inflammation, and hyperparathyroidism, may have differential effects in children of different races. African-American children may start off with slightly lower Hb levels which may or may not have clinical implications; as these children become more anemic in the context of CKD progression, the racial disparity in Hb levels widens. Of course, this cannot be fully demonstrated on the basis of cross-sectional data alone, but will also be more clearly delineated as longitudinal analysis of the cohort progresses.
Among adult CKD patients, non-Hispanic African-American adults have been shown to initiate dialysis with lower hematocrit levels and be more likely to commence dialysis as ESA naïve.9 This does not appear to be the case in this population of 429 children with CKD: there were no differences in the proportions of African-American and white children treated with iron supplements or ESAs. It should be noted, however, that all CKiD subjects are under the care of pediatric nephrologists. In the general population of children with CKD, access to subspecialty care – or lack thereof - at earlier CKD stages, and later diagnosis of CKD, may be in part responsible for a significant portion of the more pronounced racial disparities in anemia and associated morbidities observed in patients with ESRD.
There are several limitations to this study. As this is a cross-sectional study, no conclusions can be made regarding the temporal relationship between changes in kidney function and changes in Hb and how race may modify this relationship. CKiD longitudinal data will serve as a basis for such analysis in the near future. Secondly, we did not have data on the history of iron supplement and ESA use in these children, only use during the past 30 days. Moreover, ESA and iron use in this cohort may occur among those subjects who are most anemic. This phenomenon, known as selection by indication, makes it difficult to evaluate the role therapy may have in the Hb-GFR-race paradigm.25 Third, CKiD recruits its subjects from nephrology clinics. As a result, the profile of CKiD subjects at baseline regarding Hb, GFR, anemia therapy and race may not be generalizable to all children with CKD in the U.S. Additionally, measures of iron stores were not available for all patients at the time of this analysis; however, as a sensitivity analysis in the sub-cohort 91 patients (71 white and 20 African-American) who did have ferritin and TSAT values available, there were no significant differences by race in the proportions of patients with either serum ferritin < 100 ng/mL or transferrin saturation < 20% (data not shown).
This study is unique in that it is the first examination of race and Hb within a prospective cohort of children with CKD in whom a precise and accurate measurement of GFR has been made using the plasma disappearance of iohexol. It is also the largest prospective cohort study of children with CKD in existence, and uses standardized data collection methods across centers.
In conclusion, among children with pre-dialysis CKD, Hb is associated with race. African-American children demonstrated lower Hb values and a higher frequency of Hb values below the 5th percentile for age and sex compared to white children, despite no significant overall differences in either ESA or iron prescription. African-American children do demonstrate a higher prevalence of glomerular disease as the underlying cause of kidney disease, but the association between race and Hb is independent of this. The average difference in Hb by race seen in children early stage CKD, who are under the care of pediatric nephrologists, appears to parallel observed differences by race seen in otherwise healthy children. The racial differences seen in Hb do appear to increase at the lower end of the Hb distribution in this population.
Acknowledgments
The authors wish to thank Dr Alvaro Munoz for his valuable assistance in designing the statistical analysis.
Support: Dr Atkinson was supported by grant funding from Amgen Inc., The Thrasher Research Fund, The National Kidney Foundation of Maryland, and The National Kidney Foundation.
Footnotes
Financial disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mitsnefes MM, Kimball TR, Kartal J, et al. Progression of left ventricular hypertrophy in children with early chronic kidney disease: 2-year follow-up study. J Pediatr. 2006;149(5):671–675. doi: 10.1016/j.jpeds.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Furth SL, Cole SR, Fadrowski JJ, et al. The association of anemia and hypoalbuminemia with accelerated decline in GFR among adolescents with chronic kidney disease. Pediatr Nephrol. 2007;22(2):265–271. doi: 10.1007/s00467-006-0313-1. [DOI] [PubMed] [Google Scholar]
- 3.Staples AO, Wong CS, Smith JM, et al. Anemia and risk of hospitalization in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(1):48–56. doi: 10.2215/CJN.05301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerson A, Hwang W, Fiorenza J, et al. Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis. 2004;44(6):1017–1023. doi: 10.1053/j.ajkd.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Rossert J, Gassmann-Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant. 2007;22(3):794–800. doi: 10.1093/ndt/gfl716. [DOI] [PubMed] [Google Scholar]
- 6.Collins AJ, Foley R, Herzog C, et al. Excerpts from the United States Renal Data System 2007 annual data report. Am J Kidney Dis. 2008;51(1 Suppl 1):S1–320. doi: 10.1053/j.ajkd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson MA, Neu AM, Fivush BA, Frankenfield DL. Disparate outcomes in pediatric peritoneal dialysis patients by gender/race in the End-Stage Renal Disease Clinical Performance Measures project. Pediatr Nephrol. 2008;23(8):1331–1338. doi: 10.1007/s00467-008-0832-z. [DOI] [PubMed] [Google Scholar]
- 8.Frankenfield DL, Atkinson MA, Fivush BA, Neu AM. Outcomes for adolescent Hispanic hemodialysis patients: findings from the ESRD Clinical Performance Measures Project. Am J Kidney Dis. 2006;47(5):870–878. doi: 10.1053/j.ajkd.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Lea JP, Norris K, Agodoa L. The role of anemia management in improving outcomes for African-Americans with chronic kidney disease. Am J Nephrol. 2008;28(5):732–743. doi: 10.1159/000127981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFarlane SI, Chen SC, Whaley-Connell AT, et al. Prevalence and associations of anemia of CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008;51(4 Suppl 2):S46–55. doi: 10.1053/j.ajkd.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol. 2006;1(5):1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34(3):571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69(11):2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 14.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 15.National Kidney Foundation. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50(3):471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Esty W, Banfield J. The Box-Percentile Plot. Journal of Statistical Software. 2003;8(17):1–14. [Google Scholar]
- 17.Cox C, Chu H, Schneider MF, Munoz A. Parametric survival analysis and taxonomy of hazard functions for the generalized gamma distribution. Stat Med. 2007;26(23):4352–4374. doi: 10.1002/sim.2836. [DOI] [PubMed] [Google Scholar]
- 18.Lau B, Gange SJ. Methods for the analysis of continuous biomarker assay data with increased sensitivity. Epidemiology. 2004;15(6):724–732. doi: 10.1097/01.ede.0000142154.86749.e4. [DOI] [PubMed] [Google Scholar]
- 19.Akaike H. A New Look at the Statistical Model Identification. IEEE Transaction on Automatic Control. 1974;AC-19:716–723. [Google Scholar]
- 20.Robins EB, Blum S. Hematologic reference values for African American children and adolescents. Am J Hematol. 2007;82(7):611–614. doi: 10.1002/ajh.20848. [DOI] [PubMed] [Google Scholar]
- 21.Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106(2):740–745. doi: 10.1182/blood-2005-02-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan WH, Habicht JP. The non-iron-deficiency-related difference in hemoglobin concentration distribution between blacks and whites and between men and women. Am J Epidemiol. 1991;134(12):1410–1416. doi: 10.1093/oxfordjournals.aje.a116046. [DOI] [PubMed] [Google Scholar]
- 23.Jackson RT. Separate hemoglobin standards for blacks and whites: a critical review of the case for separate and unequal hemoglobin standards. Med Hypotheses. 1990;32(3):181–189. doi: 10.1016/0306-9877(90)90121-t. [DOI] [PubMed] [Google Scholar]
- 24.Sharma AP, McKenna AM, Lepage N, Nieuwenhuys E, Filler G. Relationships among serum iron, inflammation, and body mass index in children. Adv Pediatr. 2009;56(1):135–144. doi: 10.1016/j.yapd.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Ahdieh L, Gange SJ, Greenblatt R, et al. Selection by indication of potent antiretroviral therapy use in a large cohort of women infected with human immunodeficiency virus. Am J Epidemiol. 2000;152(10):923–933. doi: 10.1093/aje/152.10.923. [DOI] [PubMed] [Google Scholar]