Abstract
Hepatic metabolism of methionine is the source of cysteine, the precursor of glutathione, the major intracellular antioxidant in the body. Methionine also is the immediate precursor of s-adenosylmethionine (SAM) the key methyl donor for phosphotidylcholine synthesis required for the export of VLDL triglycerides from the liver. We have examined the kinetics of methionine, its transmethylation and transsulfuration with estimates of whole body rate of protein turnover and urea synthesis in clinically stable biopsy confirmed subjects with non-alcoholic steatohepatitis (NASH). Subjects with NASH were more insulin resistant and had significantly higher plasma concentration of usCRP, TNF alpha and other inflammatory cytokines. There was no significant effect of insulin resistance and NASH on whole body rate of protein turnover (phenylalanine Ra) and on the rate of urea synthesis. The rates of methylation of homocysteine and transmethylation of methionine were significantly lower in NASH as compared with controls. There was no difference in the rate of transsulfuration of methionine between the two groups. Enteric mixed nutrient load resulted in a significant increase in all the measured parameters of methionine kinetics. Hetrozygosity for MTHFR (677C→T) did not impact methionine metabolism. We speculate that as a result of oxidant stress possibly due to high fatty acid oxidation, the activity of methionine adenosyltransferase is attenuated resulting in a lower rate of transmethylation of methionine and of SAM synthesis. These data are the first evidence for perturbed metabolism of methionine in NASH in humans and provide a rationale for the development of targeted intervention strategies.
Keywords: Methionine, Phenylalanine, Urea, Steatohepatitis, NASH, Insulin Resistance
Introduction
Nonalcoholic fatty liver disease (NAFLD) constitutes a spectrum ranging from simple triglyceride accumulation in the hepatocytes (hepatic steatosis) to steatosis with inflammation (steatohepatitis), fibrosis, and cirrhosis [1-3]. Based upon studies in humans and animal models, a pathophysiological metabolic paradigm for the progression of NAFLD from steatosis to steatohepatitis has been proposed [3-6]. These data suggest that hepatic steatosis is related to excessive delivery of fatty acids to the liver caused by an increased whole body rate of lipolysis due to systemic insulin resistance, coupled with increased hepatic de-novo lipogenesis and an attenuated export of hepatic triglycerides [7, 8]. In addition, due to high rate of fatty acid oxidation in the liver, there is increased oxidative stress leading to changes in mitochondrial function, depletion of ATP, DNA damage, lipid peroxidation, release of cytokines and consequently hepatic inflammation and fibrosis [9,10]. The increase in oxidative stress results in augmented consumption of the major intracellular antioxidant, glutathione, and in lower hepatic glutathione levels. In addition as a result of high fatty acid uptake by the liver coupled with higher de-novo lipogenesis, there is a need for higher rate of phosphotidylcholine synthesis for VLDL triglyceride export. The precursors of both glutathione and phosphotidylcholine i.e. cysteine for glutathione and methyl groups from SAM for phosphotidylcholine, are produced during the metabolism of methionine in the liver.
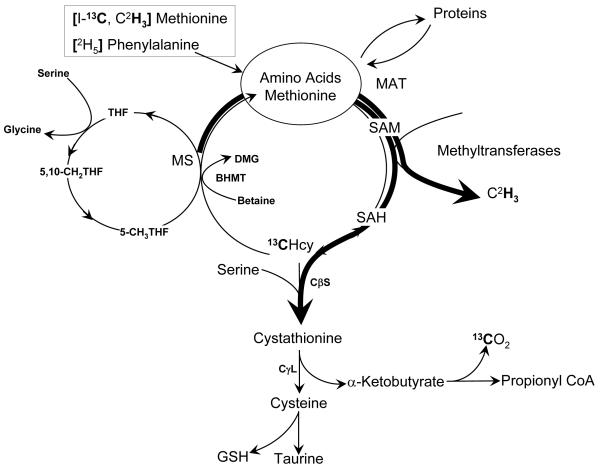
Methionine, an essential amino acid required for protein synthesis, is the source of methyl groups for a number of methylation reactions such as methylation of nucleic acids, proteins, biogenic amines, phospholipids, and for creatine synthesis etc. [15]. During its metabolism, methionine is converted to its active form s-adenosyl methionine (SAM) (Figure 1). Following SAM-dependent transmethylation, the product s-adenosyl homocysteine (SAH) is metabolized to adenosine and homocysteine. Homocysteine, either via transsulfuration pathway, is converted to cystathionine or is re-methylated back to methionine. The methyl group required for the methylation of homocysteine is obtained from either the folate dependent one carbon pool (5-methyl-tetrahydrofolate) or from (non vitamin-dependent) betaine in certain tissues. S-adenosyl methionine is also converted to SAH by glycine N-methyltransferase (GNMT), an enzyme abundant in the liver [12, 13]. The synthesis of SAM by s-adenosylmethionine synthase is regulated by hypoxia [14], glutathione [15], and availability of methionine [16], and modified by oxidant injury [17] and redox state [18] of the cell. Although the effect of ATP availability on SAM synthesis is not known, an increase in s-adenosylmethionine synthase activity in transfected Chinese hamster ovary cells caused a depletion of ATP [17]. Whether a limited availability of ATP as a result of mitochondrial dysfunction in vivo can cause decreased synthesis of SAM is not known. A decrease in synthesis of SAM in methionine adenosyl transferase 1A knockout mice predisposes the animal to hepatic injury and more susceptible to choline-deficient diet induced fatty liver [19]. Decreased SAM biosynthesis is also a consequence of all forms of chronic liver injury [20].
Figure 1.
We hypothesize that, as a result of oxidant insult and decreased availability of glutathione, there is attenuated activity of methionine adenosyltransferase which, in combination with lower ATP availability due to mitochondrial dysfunction, results in lower rate of synthesis of SAM. Consequently, the synthesis of phosphotidylcholine and VLDL export are reduced and leads to hepatic steatosis. The low thiol redox state results in unbalanced reactive oxygen species (ROS) production and propagates the hepatic injury. Although such a metabolic paradigm can be inferred from studies in animals, or from indirect evidence in humans, direct evaluation of methionine metabolism has not been done in patients with non-alcoholic steatohepatitis. In the present study we have quantified the whole body flux of methionine, its rate of transmethylation and transsulfuration, in biopsy confirmed subjects with NASH. In addition the effect of insulin resistance with NASH on whole body rate of protein turnover was examined using a phenylalanine tracer. Since hepatic urea synthesis requires energy, the impact of mitochondrial dysfunction, if any, in NASH on the rate of urea synthesis was also quantified.
Methods
Methionine, phenylalanine and urea kinetics were quantified in 15 subjects with NASH and compared with 19 healthy age-matched controls (Table 1). Subjects with NASH were recruited from the metabolic clinics at MetroHealth Medical Center and at the Cleveland Clinic. NASH was confirmed by liver biopsy according to the criteria of Kleiner et al [21]. All subjects in this study were evaluated by the investigators and were abstinent from alcohol for at least 6 months. Their possible remote consumption of alcohol was less than that suggested to cause liver injury. Control subjects were recruited by advertisement. All healthy controls underwent a detailed history and clinical examination. A hepatic ultrasound study was performed by the co-investigator (SD) in order to exclude hepatic steatosis. MTHFR (5, 10, methylene-tetrahydrofolate reductase) polymorphism 677C→T was determined in all subjects. The study protocol was approved by the Institutional Review Boards at Cleveland Clinic and MetroHealth Medical Center. Written informed consent was obtained from all subjects after fully explaining the procedure.
Table 1.
Study Population
|
NASH (N = 15; M/F = 8/7) |
Controls (N = 19, M/F = 8/11) |
p | |
|---|---|---|---|
| Age (years) | 43.4 [11.7] (20-63) |
42.2 [12.8] (24-69) |
NS |
| Weight (kg) | 104.3 [19.6] (74.3 - 135.1) |
76.6 [18.5] (48.7 - 127.0) |
<0.001 |
| BMI (kg/m2) | 34.0 [6.2] (24.7 – 44.0) |
26.8 [5.2] (19.6 – 36.9) |
0.001 |
| BSA (m2) | 2.2 [0.2] | 1.9 [0.2] | 0.001 |
| HOMA | 2.8 [2.3, 3.9] | 0.7 [0.4, 1.0] | <0.001 |
| ALT (U/L) | 54.0 [39.0, 91.0] | 16.0 [14.0, 22.0] | <0.001 |
| TG (mg/dl) | 116.0 [91.0, 207.0] | 68.0 [55.0, 104.0] | 0.015 |
| 8-OHdG (mg/ml) | 42.8 [19.5] (22.5 – 127.0) |
51.4 [20.5] (22.2 – 111.7) |
ns |
Data are mean [SD] or median [quartiles], (x) range; BMI: body mass index.BSA: body surface area. HOMA: homeostatic model of insulin resistance. ALT: plasma alanine aminotransferase. TG: plasma triglycerides. 8-OHdG: 8-hydroxy-2-deoxyguanosine.
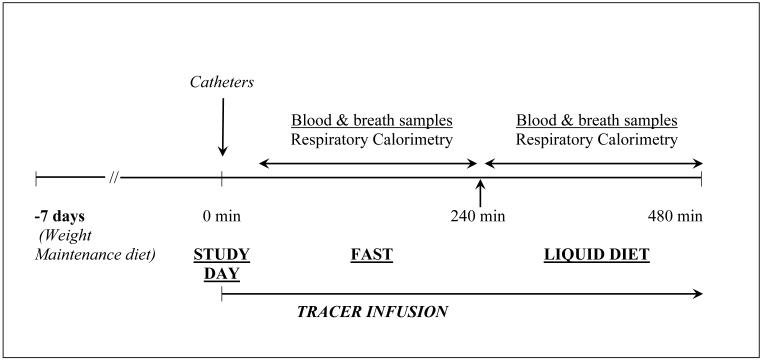
Study subjects were kept on their usual diet except they were advised to take a minimum of 70g protein per day for seven days prior to the tracer isotope study. Dietary compliance was monitored by the clinical nutritionist. Tracer kinetic studies were performed in the Clinical Research Unit (CRU) of the Cleveland Clinic (NIH, CTSA, Case Western Reserve University). Subjects reported to the CRU on the morning of the tracer study after an overnight fast of ~10h. Following a physical examination including height and weight, the subject rested in a bed for the duration of the study. Two indwelling intravenous cannulae were placed, one in each superficial vein on the dorsum of the hand, one for the infusion of the isotopic tracer and the other for obtaining blood samples. The sample site was kept warm (by a thermostatically controlled heating pad) to obtain arterialized blood samples, and kept patent by infusing isotonic saline solution. The study design is displayed in Fig.2.
Figure 2.
L-[1-13C]methionine, L-[C2H3]methionine, L-ring [2H5]phenylalanine, [13C]sodium bicarbonate and [15N2]urea were purchased from Cambridge Laboratories (Andover, MA) or from Isotec Inc. (St. Louis, MO). The tracer had been tested for purity, pyrogenicity and sterility by the manufacturer. A weighed amount of the tracer was dissolved in sterile 0.45% saline solution, passed through a 0.22 micron filter, and administered intravenously as prime-constant rate infusion. The doses of respective isotopic tracer were as follows: L-[1-13C]methionine prime: 3 μmoles.kg−1, infusion rate 2 μmoles.kg−1.h−1; L-[C2H3]methionine prime: 0.8 μmoles.kg−1, infusion rate 1 μmole.kg−1.h−1; L-ring [2H5]phenylalanine prime: 1.6 μmoles.kg−1; infusion rate 2 μmoles.kg−1.h−1; [15N2]urea prime 33 μmoles.kg−1, infusion rate 3 μmoles.kg−1.h−1. A priming dose of [13C] sodium bicarbonate (5 mg) was given to achieve an early steady state enrichment in the bicarbonate/carbon dioxide pool. The actual rate of infusion was confirmed by gravimetrically measuring the rate of flow using the same tubing and equipment at the end of each study.
After a basal period (180 minutes), the response to a mixed-nutrient load was examined by giving 45 ml oral Boost® High Protein drink (Nestlé USA, Glendale, CA) every 30 minutes for the next 180 minutes. Each 45ml of Boost® contains ~45 kcal and 2.8 gm protein.
Arterialized blood samples (6 ml) were obtained at 30-minute intervals throughout the study. Blood samples were centrifuged immediately in cold (4°C) and the separated plasma was frozen at −70°C until analysis. Breath samples for the measurement of 13C enrichment in the expired carbon dioxide were obtained at 30-minute intervals, as described previously [22]. Rate of production of carbon dioxide (VCO2) and oxygen consumption (VO2) were measured using an open hood system (Viasys Encore, Cardinal Health).
Fasting only studies: Analysis of the enrichment data (see results) showed an increase in 13C enrichment of plasma homocysteine following an enteral mixed nutrient load, in the presence of decreasing 13C enrichment of plasma methionine. In order to confirm that the increase in 13C enrichment of homocysteine during feeding (180-360 minutes) was a true effect of feeding and not related to a lack of tracer equilibrium, we studied four subjects with NASH and eight healthy controls, only in the fasting state for six hours. The tracer isotopic infusions were the same as above.
Analytical procedures
The clinical biochemical analyses were performed in the Cleveland Clinic Reference Laboratory. The concentration of total homocysteine, glutathione, total cysteine and amino acids in the plasma and in the infusates were measured by HPLC [23,24].
All of the GC-mass spectrometry analytical methods used have been reported from our laboratory previously [25,26]. Urea and amino acids in the plasma were separated using mixed-bed ion exchange chromatography. [15N2] enrichment of urea was measured as reported previously [27]. A heptafluorobutyryl-n-propyl ester derivative of methionine was prepared as described by Davis et al [28]. Negative chemical ionization was used to monitor m/z ratio of 367 (m0), 368 (m1), 369 (m2), 370 (m3) and 371 (m4) in order to quantify unlabeled and labeled methionine. The m/z 367 (m1) represented the enrichment of L-[1-13C]methionine, and the mass 370 (m3) represented the enrichment of L-[C2H3]methionine. Multiple linear regression analyses were performed to calculate the relative enrichments and correction for natural abundance of m1 ([1-13C]tracer) and m3 ([C2H3]methyl) methionine by using an in-house-developed software (Isomet, developed by J. Kim). The 13C enrichment of homocysteine in the plasma was measured as described by Davis et al [28]. The enrichment of plasma phenylalanine was measured as described [26]. Enrichment of 13C in the carbon dioxide was quantified by isotope ratio mass-spectrometry (Metabolic Solutions, Nashua, NH).
Calculations
The whole body rate of appearance (Ra) of various amino acids was calculated by tracer dilution during isotopic steady state as described [29]. The rates of transmethylation and transsulfuration were calculated as described by Storch et al [30] and MacCoss et al [31], and detailed previously [25]. The Ra of methionine, measured by [1-13C]methionine tracer (QC), represents methionine entering the circulation from proteolysis and that entering from exogenous sources (food). During the conversion of methionine to homocysteine, and back to methionine, the 13C on carboxyl carbon is retained [Fig.1]. In contrast, the 2H labeled methyl group is lost during the conversion of methionine to homocysteine, and replaced by an unlabeled methyl group during the formation of methionine from homocysteine (re-methylation). Therefore the Ra, measured by the dilution of the methyl tracer (QM) is the sum of methionine released from protein breakdown and the methionine that is exogenously (food) administered, plus the amount that is produced by methylation of homocysteine. Therefore the difference between QM and QC is a measure of the rate of remethylation: RM = QM – QC. The rate of transsulfuration was assumed to be equal to the rate of oxidation of methionine and was estimated from the rate of appearance of 13C in the expired carbon dioxide [32]. It is assumed that during the formation of cysteine, an equimolar quantity of α-ketobutyrate is formed from cystathionine and is oxidized in the tricarboxylic acid cycle.
We did not correct the kinetic data for the intracellular enrichment of methionine, as measured by the enrichment of homocysteine in the plasma because of uncertainty regarding which intracellular pool such data represent. Therefore the reported estimates of methionine kinetics (Ra, transmethylation and transsulfuration) are lower than actual. As shown in the results and reported previously [25], feeding resulted in an increase in 13C enrichment of homocysteine, probably a consequence of suppression of intracellular protein breakdown. We did not adjust for possible retention of carbon dioxide. The use of a 20% tracer retention in the bicarbonate pool would only increase our estimates of transsulfuration by such magnitude.
Statistical analysis
All data are reported as mean ± SD, or median and 25 and 75th percentile. Descriptive statistics were computed for all variables. These included means, standard deviations and percentiles for continuous variables and frequencies for categorical factors. A univariable analysis was performed to assess differences between subjects with NASH and controls; t-tests and Wilcoxon rank sum tests were used for continuous variables and chi-square or Fisher’s Exact tests for categorical factors. In addition, in order to assess association of subject group with phenylalanine and methionine kinetics while adjusting for BMI, analysis of covariance (ANCOVA) was performed. Kinetic data during the fasting state were compared to those after feeding using paired t-tests. Correlations between various parameters were evaluated using Spearman’s correlation coefficients. For BMI, HOMA and CRP, linear regression analysis was done to assess interactions between said clinical characteristics and subject group. A p<0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 software (The SAS Institute; Cary, NC).
Results
We studied clinically and biochemically characterized and biopsy-proven subjects with non-alcoholic steatohepatitis (Table 1). The liver biopsy of all subjects with NASH showed evidence of cellular ballooning. Hepatic fibrosis was seen in five subjects. The NASH activity score was 5.6±1.4. The subjects with NASH were obese, had higher BMI, higher body surface area, elevated plasma ALT, triglycerides, had higher fasting insulin levels, and higher measure of insulin resistance (HOMA), when compared with controls. The plasma concentration of 8-hydroxy-2-deoxyguanosine, a measure of oxidative damage, was not different between the groups. The plasma concentrations of ultrasensitive CRP and TNFα were significantly higher in NASH (Table 2). In addition, several other inflammatory cytokines were significantly higher in subjects with NASH as compared with controls. The plasma concentration of folate, B12 and thyroid stimulating hormone (TSH) in both healthy controls and NASH were within normal range (data not shown). There were ten heterozygotes and no homozygote for MTHFR(677C→T) polymorphism amongst subjects with NASH and there were eight heterozygotes and one homozygote amongst controls.
Table 2.
Adipokines and Markers of Inflammation
|
NASH (N = 15) |
Controls (N = 19) |
Wilcoxon Rank Sum | |
|---|---|---|---|
| IL-1B (pg/ml) | 1.4 [1.7] | 0.3 [0.7] | <0.01 |
| IL-6 (pg/ml) | 7.8 [12.4] | 46.4 [142.0] | <0.05 |
| IL-8 (pg/ml) | 2.9 [2.3] | 1.3 [0.4] | <0.01 |
| Leptin (mmol/h) | 51.0 [45.8] | 24.5 [16.0] | 0.02 |
| TNF-α (pg/ml) | 4.2 [2.0] | 2.6 [0.9] | 0.05 |
| MCP-1 (pg/ml) | 137.4 [44.9] | 122.3 [52.8] | ns |
| HGF (pg/ml) | 1285.6 [661.5] | 731.7 [350.2] | <0.02 |
| NGF (pg/ml) | 33.1 [67.5] | 6.4 [1.4] | <0.01 |
| usCRP (mg/dl) | 4.2 [3.0, 9.6] | 0.7 [0.3, 1.4] | 0.001 |
Data are mean [SD] or median [quartile]; IL-1B: Interleukin 1, beta; IL-6: Interleukin 6; IL-8: Interleukin 8; TNF-α: tumor necrosis factor, alpha; MCP-1: monocytes chemoattractant protein-1; HGF: hepatocyte growth factor; NGF: nerve growth factor; usCRP: Ultra sensitive C-reactive protein
The plasma concentrations of total cysteine, homocysteine and glutathione during fasting were not different between the controls and NASH subjects (Table 3). In response to enteral protein load, there was a significant decrease in total cysteine and increase in total homocysteine in the plasma in control subjects. In subjects with NASH, there was a significant increase in plasma homocysteine, and no change in plasma total cysteine concentration in response to protein feeding. There was no change in plasma glutathione in either group. The respiratory calorimetry data are displayed in Table 4. There was no significant difference between the groups for the rate of oxygen consumption, rate of CO2 production, or the respiratory exchange ratio during fasting or during the fed state.
Table 3.
Plasma concentration of total glutathione, cysteine and homocysteine
| Cysteine (μmoles.L−1) | Homocysteine (μmoles.L−1) | Glutathione (μmoles.L−1) | ||||
|---|---|---|---|---|---|---|
| Fast | Fed | Fast | Fed | Fast | Fed | |
| NASH [N = 11] | 404.1 ± 60.8 | 403.5 ± 56.2 | 8.8 ± 3.2 | 10.5 ± 3.8** | 4.7 ± 1.0 | 5.8 ± 3.0 |
| Controls [N = 11] | 381.8 ± 47.0 | 366.7 ± 43.2* | 7.9 ± 0.9 | 8.9 ± 1.4§ | 5.5 ± 2.3 | 4.7 ± 1.4 |
All data are mean ± SD;
compared with fast, paired t, p=0.014;
p=0.003;
p=0.001
Table 4.
Respiratory Calorimetry
| VO2 mmoles.kg−1.h−1 | VCO2 mmoles.kg−1.h−1 | RER | ||||
|---|---|---|---|---|---|---|
| Fast | Fed | Fast | Fed | Fast | Fed | |
| NASH [N = 15] | 7.06 ± 0.74 | 7.54 ± 0.95 | 5.59 ± 0.66 | 6.12 ± 0.84 | 0.80 ± 0.04 | 0.81 ± 0.03 |
| Controls [N = 19] | 7.31 ± 1.97 | 8.14 ± 0.90 | 6.14 ± 0.63 | 6.61 ± 0.65 | 0.81 ± 0.06 | 0.82 ± 0.09 |
All data are mean ± SD
VO2: rate of oxygen consumption
VCO2: rate of production of carbon dioxide
RER: respiratory exchange ratio
Phenylalanine kinetics
The rate of appearance (Ra) of phenylalanine was calculated by tracer dilution during isotopic steady state. The data on subjects studied both during fast and in response to feeding are displayed in Table 5.There was no significant difference in weight specific Ra of phenylalanine between the controls and NASH during fasting [NASH(15):30.9±5.1 μmol/kg.h,Controls(18): 32.9±5.7 p=ns] or during feeding (Table 5), suggesting an unchanged rate of whole body protein breakdown. Total Ra of phenylalanine was significantly higher in NASH subjects, reflecting their higher body weight. However, Ra phenylalanine, adjusted for BMI, was not different between controls and NASH (analysis of covariance). There was a significant negative correlation between plasma insulin (rho − 0.47, p=0.042), HOMA (rho − 0.48, p=0.038) and phenylalanine Ra, during fasting, in the control subjects only. A positive correlation between plasma bilirubin levels and phenylalanine Ra during fast, was seen in both controls (rho 0.59, p=0.008) and in subjects with NASH (rho 0.62, p=0.044). In response to the mixed nutrient load there was a significant increase in phenylalanine Ra in both NASH and healthy controls (Table 5).
Table 5.
Rate of Appearance of Phenylalanine
| (μmol/kg.h) |
(mmol/h) |
|||
|---|---|---|---|---|
| Fast | Fed | Fast | Fed | |
| NASH [N = 11] | 30.0 ± 4.5 | 35.9 ± 6.8= | 3.1 ± 0.6* | 3.6 ± 0.6§= |
| Controls [N = 10] | 32.4 ± 5.6 | 38.7 ± 6.6= | 2.4 ± 0.6 | 2.9 ± 0.7= |
| Rate of Appearance of Urea | ||||
|---|---|---|---|---|
| (μmol/kg.h) |
(mmol/h) |
|||
| Fast | Fed | Fast | Fed | |
| NASH [N = 11] | 208.6 ± 63.8** | 201.0 ± 54.6§§ | 21.6 ± 8.4 | 20.6 ± 7.2 |
| Controls [N = 10] | 333.2 ± 92.6 | 297.9 ± 76.8= | 24.9 ± 8.5 | 22.2 ± 7.2= |
Data are mean ± SD
compared with controls p=0.002,
p=0.01,
p=0.016,
p=0.004,
compared with fast, p=0.001
Urea synthesis
The rates of urea synthesis in subjects studied both during fasting and during the fed state are displayed in Table 5. The weight-specific rate of urea synthesis was significantly lower in subjects with NASH, both during fasting and in response to feeding. In response to the feed, the rate of urea synthesis decreased (Δ 35.3 ± 23.6 μmoles.kg−1.h−1) in all control subjects (p<0.001). The response of NASH subjects was variable, decrease in 7 subjects and unchanged or increase in 4 subjects. The rate of urea synthesis, adjusted for BMI, was not different in controls and subjects with NASH. There was a significant negative correlation between urea Ra and usCRP both during fasting and following feed in the NASH group (Fast: rho −0.69,p=0.004; Fed: rho −0.78, p=0.005). Additionally urea Ra was also negatively correlated with the HOMA score during fasting in the NASH group (rho −0.5, p=0.05).
Methionine kinetics
As with phenylalanine Ra, the rate of appearance of methionine measured using [1-13C]methionine tracer was not different between NASH and controls both during fasting and in the fed state (Table 6). In contrast, the weight specific methionine Ra, measured using [C2H3]methionine tracer was significantly lower during fasting in subjects with NASH. Although the fractional rate of transsulfuration of methionine measured by the appearance of 13C in expired CO2 was higher during fasting in NASH (NASH: 14.2 ± 3.9%; Controls: 10.5 ± 4.1%, p=0.047), the actual rate of transsulfuration was not different between the two groups (Table 6). The rate of methylation of homocysteine (Rm: the difference between methionine Ra quantified by the two tracers) and the rate of transmethylation of methionine (Tm) was significantly lower (p<0.03) during fasting in subjects with NASH as compared with controls. As anticipated, in response to feeding, there was a significant increase in all the measured parameters of methionine kinetics: Ra, Tm, Ts, Rm, in both controls and in subjects with NASH.
Table 6.
Methionine Kinetics in NASH
| NASH |
Controls |
|||
|---|---|---|---|---|
| Fast (15) | Fed (11) | Fast (19) | Fed (11) | |
| Methionine Ra [1-13C] tracer | 14.7 ± 3.1 | 16.7 ± 4.0 | 16.1 ± 2.4 | 18.4 ± 2.2 |
| Methionine Ra [C2H3] tracer | 24.8 [20.6,29.2]* | 28.1 [24.4,31.2] | 29.7 [25.0,37.4] | 35.6 [29.8,44.1] |
| Ts | 2.0 ± 0.9 | 4.8 ± 1.0 | 1.8 ± 0.6 | 4.4 ± 1.6 |
| Rm | 10.9 ± 4.6* | 13.7 ± 6.3 | 15.0 ± 5.5 | 18.3 ± 7.5 |
| Tm | 12.4 ± 4.4* | 13.7 [12.7,19.0] | 16.7 ± 5.9 | 22.5 [14.5,29.6] |
Data are mean ± SD or median [quartiles], micromoles.kg−1.h−1
Ra: rate of appearance
( )=n
NASH vs. Controls: p<0.03
Ts – Transsulfuration
Rm – Remethylation
Tm – Transmethylation
[13C] enrichment of homocysteine
We measured the [13C] enrichment of homocysteine in order to estimate the enrichment of intracellular pool of methionine. During fasting, the ratio of [13C] enrichment of plasma homocysteine and methionine was not different in the two groups (NASH: 0.36 ± 0.06; Controls: 0.32 ± 0.07, p=0.16). In response to mixed nutrient feeding, there was an increase in [13C] enrichment of homocysteine in all subjects, suggesting a suppression of intracellular protein breakdown. In contrast, due to the entry of unlabeled methionine into the circulation, there was a significant decrease in [13C] and [C2H3] enrichment of plasma methionine. The net effect was a significant increase in the ratio of [13C] enrichment of plasma homocysteine and methionine [NASH: 0.59 ± 0.07; controls: 0.53 ± 0.07, p=0.045].
MTHFR (677 C→T) heterozygosity had no impact on methionine metabolism, neither in controls nor in subjects with NASH (data not shown).
Discussion
Our data show that in subjects with NASH, in association with insulin resistance, the whole body rate of protein turnover as well as the rate of urea synthesis, during fasting, were unchanged when compared with healthy controls. The rate of transmethylation of methionine was lower in NASH when compared with controls. There was no significant change in the absolute rate of transsulfuration of homocysteine although the fractional rate of transsulfuration was higher in NASH. These data should be examined in the context of insulin resistance and of insulin resistance plus steatohepatitis.
Phenylalanine Kinetics
The weight specific rate of appearance of phenylalanine was not significantly different between the controls and subjects with NASH, both in the basal state and in response to enteral nutrient (protein) administration. The total phenylalanine Ra was significantly higher in NASH. However, when adjusted for BMI, all differences became statistically not significant. In addition, a subgroup analysis of subjects matched for body weight did not show any significant difference in phenylalanine Ra during fasting between controls and NASH [controls (11) 2.6 ± 0.6 mmole per hour; NASH (10) 2.9 ± 0.6, p = 0.23]. These data suggest that insulin resistance in NASH, as measured by HOMA, does not appear to have any significant impact on the whole body rate of protein breakdown during fasting. These data are similar to other studies where no difference in whole body rate of protein turnover was observed between lean and obese, insulin resistant subjects with and without glucose intolerance [33-36]. This is in contrast to the acutely induced insulin resistance by intravenous fatty acid infusion, where a decrease in the rate of appearance of phenylalanine across the leg was observed [37]. The fatty acid induced decrease in proteolysis may be a direct inhibitory effect of fatty acids on protein breakdown [38, 39]. The negative correlation between plasma insulin levels and phenylalanine Ra is consistent with the effect of insulin on whole body rate of protein breakdown [40]. The lack of such relationship in NASH maybe the result of confounding effect of the observed inflammatory response (Table 2), since the inflammatory cytokines have been shown to impact skeletal muscle protein metabolism( 41-43). An interesting observation in our study was the significant correlation between plasma bilirubin levels and phenylalanine Ra in both controls and NASH. Although the exact reason for this observation cannot be discerned from the present data, the correlation may reflect the contribution of red blood cell (hemoglobin) turnover to phenylalanine Ra. Only studies in subjects with high red blood cell turnover will confirm this hypothesis.
Urea synthesis
The weight specific rate of urea synthesis was significantly less in the NASH both during fasting and during the fed state. However, total rate of urea synthesis and the rate of urea synthesis adjusted for BMI were not significantly different amongst the two groups, suggesting no impact of steatohepatitis on urea synthesis. These data are similar to those reported by us previously [22]. In response to feeding, as anticipated, there was a decrease in urea Ra in healthy subjects, probably mediated by an increase in insulin and decrease in glucagon levels [44-46]. In contrast, we did not observe a consistent suppression of urea synthesis in NASH, suggesting an impaired hepatic insulin action in NASH. This is in agreement with the significant negative correlation between urea Ra and HOMA in this group. The resistance to insulin action on urea synthesis is in contrast to our previous data where urea Ra decreased in response to change in hepatic redox induced by increase in hepatic fatty acid oxidation [22], suggesting a discordance between hormonal and redox regulation of urea synthesis in NASH. The negative correlation between usCRP and urea Ra in NASH may be related to the inflammation induced insulin resistance and consequent impaired action of insulin on urea synthesis.
Methionine metabolism in NASH
As reported by us previously [22], there was no significant difference in the plasma concentration of cysteine, homocysteine and glutathione, during fasting amongst healthy controls and subjects with NASH (Table 3). The data on association between insulin resistance and plasma homocysteine levels in humans are conflicting. Acute hyperinsulinemia in healthy humans caused a decrease in plasma homocysteine levels possibly due to acute suppression of proteolysis by insulin [47]. In contrast, streptozotocin induced lack of insulin in the rat, by increasing the transsulfuration flux, resulted in a significant (30%) decrease in plasma homocysteine levels [48]. Treatment with insulin resulted in an increase in tHcy levels. A significant negative correlation was seen between plasma insulin levels [49], measures of insulin resistance [50,51], and plasma homocysteine concentration in healthy human subjects. In contrast other studies in human have shown a positive [52,53] or no [54] significant correlation between total Hcy levels and measures of insulin resistance. The positive correlation has been attributed to the confounding effect of associated low grade inflammation (high CRP and IL-6) in these subjects [53]. Thus the observed lack of significant difference in plasma homocysteine levels in our subjects may also be related to the associated inflammation in subjects with NASH. In response to a mixed nutrient load, there was a significant increase in plasma tHcy levels in both groups. The increase in plasma homocysteine is consistent with the increase in transmethylation flux following a protein load (Table 6).
The mechanism for the decrease in plasma cysteine concentration following a protein load in the healthy controls is unclear. The lack of any change in the plasma glutathione levels following a nutrient load (Table 3) is in contrast to the data showing an increase in plasma glutathione in NASH in response to an intravenous intralipid infusion [22].These differences i.e. no response to enteral mixed nutrient and parenteral fatty acid load, reflect the difference in the magnitude of oxidative stress induced by the two interventions.
During fasting there was no significant effect of insulin resistance and NASH on the whole body rate of appearance of methionine (measured by [1-13C]methionine tracer). These data are similar to those for phenylalanine (Table 5) and add credence to these analyses. The whole body (primarily the liver) rate of remethylation of homocysteine and of transmethylation of methionine was significantly lower in subjects with NASH. The possible mechanisms of this decrease include (i) a lower availability of methyl groups, not likely since the NASH subjects were all folate sufficient and had plasma folate levels in the normal range. (ii) A lower methylation demand, also not likely since the major reactions that require methyl groups, i.e. creatine synthesis and VLDL export, are not known to be attenuated in NASH. Creatine synthesis is unlikely to be changed in the presence of unchanged skeletal muscle mass and VLDL triglyceride export is expected to be higher in subjects with obesity and insulin resistance. (iii) The lower rate of transmethylation is not likely related to impaired insulin action (insulin resistance), since both lack of insulin and insulin resistance in animal models cause an increase in the expression and activity of betaine homocysteine methyl transferase (BHMT) and therefore increase the remethylation of homocysteine [55,56]. In contrast in human subjects with type 1 diabetes, withdrawal of insulin was associated with lower rates of methylation of homocysteine and insulin treatment normalized the transmethylation rate [57]. The reason for this discrepancy between animal and human data is not clear but may be related to the effect of counterregulatory hormones in type 1 diabetes, and to acute (12h) withdrawal of insulin in humans [57], and to chronic lack of insulin in the animal model [55,56]. (iv)We propose that the lower rate of transmethylation in subjects with NASH is related to oxidant injury [4-7] and change in the redox state of the hepatocytes. The synthesis of S-adenosyl methionine by s-adenosyl methionine synthase is regulated by glutathione [58] and hypoxia [59], and modified by oxidant injury and redox state of the cell [17,18]. A decrease in synthesis of SAM as a result of methionine adenosyl transferase 1A deletion in mice predisposes the animal to hepatic injury and makes them more susceptible to choline-deficient diet induced fatty liver [19].We speculate that as a consequence of high rate of hepatic fatty acid oxidation [22], there is a high rate of production of ROS and decreased availability of glutathione, resulting in lower activity of methionine adenosyl transferase and a lower rate of transmethylation.
Although the fractional rate of transsulfuration was higher in NASH, the total rate was not different from that in the control. The enzymes of the transsulfuration pathway, cystathionine beta synthase and cystathionine gamma lyase, are downregulated by insulin and upregulated by glucagon and glucocorticoids and by lack of insulin [45,52]. We had hypothesized that in the presence of insulin resistance, and in the presence of higher demands for glutathione in NASH, the flux through this pathway would be increased. The unchanged rate of transsulfuration may simply reflect a compensated state, in order to meet the heightened demands for glutathione, in the presence of a lower rate of flux through the methionine cycle.
In summary, data from our studies for the first time show a lower rate of transmethylation of methionine in insulin resistant subjects with nonalcoholic steatohepatitis. These studies point to the potential mechanism for hepatocellular injury in NASH and suggest a rationale for the use of SAM [59] and antioxidant therapy [60] for therapeutic interventions. . It will be interesting to evaluate whether anti-oxidant therapy in NASH patients would increase the transmethylation of methionine and increase the rate of VLDL expression, or whether SAM supplementation would increase VLDL secretion.
Acknowledgments
This work was supported by National Institute of Health grants DK079937 to SCK and CTSA IULI RR024989 to Case Western Reserve University and by the Cleveland Clinic institutional support to SCK.
The authors thank Mrs. Joyce Nolan and Manoa Hui for their support. The expert support of the clinical and laboratory staff of the Clinical research Unit at the Cleveland Clinic is gratefully appreciated.
References
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Day CP, James OFW. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne CD, Olufad R, Bruce KD, Cagampang FR, Ahmed MH. Metabolic disturbance in non-alcoholic fatty liver disease. Clin. Sci. 2009;116:539–564. doi: 10.1042/CS20080253. [DOI] [PubMed] [Google Scholar]
- 6.Varela-rey M, Embade N, Ariz U, Lu SL, Mato JM, Martinez-Chantar ML. Non-alcoholic steatohepatitis and animal models: understanding the human disease Int. J. Biochem. Cell Biol. 2009;41:969–976. doi: 10.1016/j.biocel.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G194–G198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- 8.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G852–G8588. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 9.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, Varela N, Contreras J, Lazarte R, Csendes A, Rojas J, Maluenda F, Burdiles P, Diaz JC, Smok G, Thielemann L, Poniachik J. Oxidative stress related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein JD. Methionine metabolism in mammals. J. Nutr. Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 12.Luka Z, Cerone R, Phillips JA, III, Mudd SH, Wagner C. Mutations in human glycine N-methyltransferase give insights into its role in methionine metabolism. Hum. Genet. 2002;220:68–74. doi: 10.1007/s00439-001-0648-4. [DOI] [PubMed] [Google Scholar]
- 13.Rowling MJ, McMullen MH, Chipman DC, Chalinske KL. Hepatic glycine N-methyltransferase is up-regulated by excess dietary methionine in rats. J. Nutr. 2002;132:2545–2550. doi: 10.1093/jn/132.9.2545. [DOI] [PubMed] [Google Scholar]
- 14.Avila MA, Carretero MV, Rodriguez EN, Mato JM. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology. 1998;114:364–371. doi: 10.1016/s0016-5085(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 15.Pajares MA, Durán C, Corrales F, Pliego MM, Mato JM. Modulation of rat liver s-adenosylmethionine synthetase activity by glutathione. J. Biol. Chem. 1992;267:17698–17605. [PubMed] [Google Scholar]
- 16.Martínez-Chantar ML, Latasa MU, Varela-Rey M, García-Trevijano ER, Mato JM, Avila MA. L-methionine availability regulates expression of the methionine adenosyltransferase 2A gene in human hepatocarcinoma cells. J. Biol. Chem. 2003;278:19885–19890. doi: 10.1074/jbc.M211554200. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Góngora E, Pastorino JG, Alvarez L, Pajares MA, García C, Viña JR, Mato JM, Farber JL. Increased sensitivity to oxidative injury in Chinese hamster ovary cells stably transfected with rat liver S-adenosylmethionine synthetase cDNA. Biochem. J. 1996;319:767–773. doi: 10.1042/bj3190767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu SC, Huang Z-Z, Yang H, Mato JM, Vaila MA, Tsukamoto H. Changes in methionine adenosyltransferase and s-adenosylmethionine homeostasis in alcoholic rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;729:G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- 19.Lu SC, Alvarez L, Huang Z-Z, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. PNAS. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mato JM, Lu SC. Role of s-adenosyl-l-methionine in liver health and disease. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanval AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Dasarathy S, Kasumov T, Edmison JM, Gruca LL, Bennett C, Duenas C, Marczewski S, McCullough AJ, Hanson RW, Kalhan SC. Glycine and urea kinetics in nonalcoholic steatohepatitis in human: effect of intralipid infusion. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;297:G567–G575. doi: 10.1152/ajpgi.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia AJ, Apitz-Castro R. Plasma total homocysteine quantification: an improvement of the classical high-performance liquid chromatographic method with fluorescence detection of the thiol-SBD derivatives. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2002;779:359–363. doi: 10.1016/s1570-0232(02)00401-4. [DOI] [PubMed] [Google Scholar]
- 24.Turnell DC, Cooper JD. Rapid assay for amino acids in serum or urine by precolumn derivatization and reversed-phase liquid chromatography. Clin. Chem. 1982;28:527–531. [PubMed] [Google Scholar]
- 25.Dasarathy J, Gruca LL, Bennett C, Parimi PS, Duenas C, Marczewski S, Fierro J, Kalhan SC. Methionine metabolism in human pregnancy. Am. J. Clin. Nutr. 2010;91:357–365. doi: 10.3945/ajcn.2009.28457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parimi PS, Devapatla S, Gruca L, Amini SB, Hanson RW, Kalhan SC. Effect of enteral glutamine or glycine on whole body nitrogen kinetics in very low birth weight infants. Am. J. Clin. Nutr. 2004;79:402–409. doi: 10.1093/ajcn/79.3.402. [DOI] [PubMed] [Google Scholar]
- 27.Tserng K-Y, Kalhan SC. Gas chromatograph-mass spectrometric determination of [15N]urea in plasma and application to urea metabolism study. Ann. Chem. 1982;54:489–481. doi: 10.1021/ac00240a031. [DOI] [PubMed] [Google Scholar]
- 28.Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., III Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am. J. Physiol. Endocrinol. Metab. 2004;286:E272–E279. doi: 10.1152/ajpendo.00351.2003. [DOI] [PubMed] [Google Scholar]
- 29.Tserng K-Y, Kalhan SC. Calculation of substrate turnover rate in stable isotope tracer studies. Am. J. Physiol. 1983;245:E308–E311. doi: 10.1152/ajpendo.1983.245.3.E308. [DOI] [PubMed] [Google Scholar]
- 30.Storch KJ, Wagner DA, Burke JF, Young VR. Quantitative study in vivo of methionine cycle in humans using [methyl-2H3]- and [1-13C]methionine. Am. J. Physiol. 1988;255:E322–E331. doi: 10.1152/ajpendo.1988.255.3.E322. [DOI] [PubMed] [Google Scholar]
- 31.MacCoss MJ, Fukagawa NK, Matthews DE. Measurement of intracellular sulfur amino acid metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 2001;280:E947–E955. doi: 10.1152/ajpendo.2001.280.6.E947. [DOI] [PubMed] [Google Scholar]
- 32.Young VR, Wagner DA, Burini R, Storch KJ. Methionine kinetics and balance at the 1985 FAO/WHO/UNU intake requirement in adult men studied with L-[2H3-methyl-1-13C]methionine as a tracer. Am. J. Clin. Nutr. 1991;54:377–385. doi: 10.1093/ajcn/54.2.377. [DOI] [PubMed] [Google Scholar]
- 33.Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63. doi: 10.2337/db07-0887. [DOI] [PubMed] [Google Scholar]
- 34.Guillet C, Delcourt I, Rance M, Giraudet C, Walrand S, Bedu M, Duche P, Boirie Y. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J. Clin. Endocrinol. Metab. 2009;94:3044–3050. doi: 10.1210/jc.2008-2216. [DOI] [PubMed] [Google Scholar]
- 35.Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am. J. Clin. Nutr. 2005;82:355–365. doi: 10.1093/ajcn.82.2.355. [DOI] [PubMed] [Google Scholar]
- 36.Patterson BW, Horowitz JF, Wu G, Watford M, Coppack SW, Klein S. Regional muscle and adipose tissue amino acid metabolism in lean and obese women. Am. J. Physiol. Endocrinol. Metab. 2002;282:E931–E936. doi: 10.1152/ajpendo.00359.2001. [DOI] [PubMed] [Google Scholar]
- 37.Katsanos CS, Aarsland A, Cree MG, Wolfe RR. Muscle protein synthesis and balance responsiveness to essential amino acid s ingestion in the presence of elevated plasma free fatty acid concentrations. J. Clin. Endocrinol. Metab. 2009;94:2984–2990. doi: 10.1210/jc.2008-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalhan SC. Fatty acids, insulin resistance, and protein metabolism. J. Clin. Endocrinol. Metab. 2009;94:2725–2727. doi: 10.1210/jc.2009-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamel FG. Preliminary report: inhibition of cellular proteasome activity by free fatty acids. Metabolism. 2009;58:1047–1049. doi: 10.1016/j.metabol.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 40.Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Mathews DE, Bier DM, Young VR. Insulin-mediated reduction of whole body protein breakdown. J. Clin. Invest. 1985;76:2306–2311. doi: 10.1172/JCI112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balage M, Averous J, Remond D, Bos C, Pujos-Guillot E, Papet I, Mosoni L, Combaret L, Dardevet D. Presence of low grade inflammation impaired postprandial stimulation of muscle protein synthesis in old rats. J. Nutr. Biochem. 2010;21:325–331. doi: 10.1016/j.jnutbio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J. Physiol.Endocrinol Metab. 2005;288:E883–E891. doi: 10.1152/ajpendo.00353.2004. [DOI] [PubMed] [Google Scholar]
- 43.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr Opin in Clin Nutr Care. 2005;8:255–263. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 44.McLean P, Novello F. Influence of pancreatic hormones on enzymes concerned with urea synthesis in rat liver. Biochem. J. 1965;94:410–421. doi: 10.1042/bj0940410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almdal TP, Holst JJ, Heindorff H, Vilstrup H. Glucagon immunoneutralization in diabetic rats normalizes urea synthesis and decreases nitrogen wasting. Diabetes. 1992;41:12–16. doi: 10.2337/diab.41.1.12. [DOI] [PubMed] [Google Scholar]
- 46.Wohl P, Krušinová E, Klementová M, Wohl P, Kratochvílová S, Pilikánová T. Urinary urea nitrogen excretion during the hyperinsulinemic euglycemic clamp in type 1 diabetic patients and healthy subjects. Physiol. Res. 2008;57:247–252. doi: 10.33549/physiolres.931138. [DOI] [PubMed] [Google Scholar]
- 47.Nagai Y, Takamura T, Nohara E, Yamashita H, Kobayashi K-I. Acute hyperinsulinemia reduces plasma concentrations of homocysteine in healthy men. Diabetes Care. 1999;22:1004. doi: 10.2337/diacare.22.6.1004b. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs RL, House JD, Brosnan ME, Brosnan JT. Effects of streptozotocin-induced diabetes and of insulin treatment on homocysteine metabolism in the rat. Diabetes. 1998;47:1967–1970. doi: 10.2337/diabetes.47.12.1967. [DOI] [PubMed] [Google Scholar]
- 49.Bar-On H, Kidron M, Friedlander Y, Ben-Yehuda A, Selhub J, Rosenberg IH, Friedman G. Plasma total homocysteine levels in subjects with hyperinsulinemia. J. Int. Med. 2000;247:287–294. doi: 10.1046/j.1365-2796.2000.00592.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosolová H, Šimon JH, Mayer O, Jr., Racek J, Dierzé T, Jacobsen DW. Unexpected inverse relationship between insulin resistance and serum homocysteine in healthy subjects. Physiol. Res. 2002;51:93–98. [PubMed] [Google Scholar]
- 51.Fonesca VA, Fink LM, Kern PA. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis. 2003;167:105–109. doi: 10.1016/s0021-9150(02)00386-6. [DOI] [PubMed] [Google Scholar]
- 52.Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, D’Agostino RB, Wilson PWF. Fasting plasma homocysteine levels in the insulin resistance syndrome. Diabetes Care. 2001;24:1403–1410. doi: 10.2337/diacare.24.8.1403. [DOI] [PubMed] [Google Scholar]
- 53.Martos R, Valle M, Morales R, Cañete R, Gavilan MI, Sánchez-Margalet V. Hyperhomocysteinemia correlates with insulin resistance and low-grade systemic inflammation in obese prepubertal children. Metab. Clin. Exp. 2006;55:72–77. doi: 10.1016/j.metabol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Godsland IF, Rosankiewicz JR, Proudler AJ, Johnston DG. Plasma total homocysteine concentrations are unrelated to insulin sensitivity and components of the metabolic syndrome in healthy men. J. Clin. Endocrinol. Metab. 2001;86:719–723. doi: 10.1210/jcem.86.2.7213. [DOI] [PubMed] [Google Scholar]
- 55.Ratnam S, Wijekoon EP, Hall B, Garrow TA, Brosnan ME, Brosnan JT. Effects of diabetes and insulin on betaine-homocysteine S-methyltransferase expression in rat liver. Am. J. Physiol. Endocrinol. Metab. 2006;290:E933–E939. doi: 10.1152/ajpendo.00498.2005. [DOI] [PubMed] [Google Scholar]
- 56.Nieman KM, Rowling MJ, Garrow TA, Schalinske KL. Modulation of methyl group metabolism by streptozotocin-induced diabetes and all-trans-retinoic acid. J. Biol. Chem. 2004;279:45708–45712. doi: 10.1074/jbc.M408664200. [DOI] [PubMed] [Google Scholar]
- 57.Abu-Lebdeh HS, Barazzoni R, Meek SE, Bigelow ML, Persson X-MT, Nair KS. Effects of insulin deprivation and treatment on homocysteine metabolism in people with type 1 diabetes. J. Clin. Endocrinol. Metab. 2006;91:3344–3348. doi: 10.1210/jc.2006-0018. [DOI] [PubMed] [Google Scholar]
- 58.Pajares MA, Durán C, Corrales F, Pliego MM, Mato JM. Modulation of rat liver s-adenosylmethionine synthetase activity by glutathione. J. Biol. Chem. 1992;267:17698–17605. [PubMed] [Google Scholar]
- 59.Avila MA, Carretero MV, Rodriguez EN, Mato JM. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology. 1998;114:364–371. doi: 10.1016/s0016-5085(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 60.Lieber CS. S-adenosyl-L-methionine: its role in the treatment of liver disorders. Am. J. Clin. Nutr. 2002;76:1183S–1187S. doi: 10.1093/ajcn/76/5.1183S. [DOI] [PubMed] [Google Scholar]
- 61.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]