Abstract
Introduction
This study examined the contribution of promoter hypermethylation to the pathogenesis of respiratory papillomatosis (RP), including recurrences (RRP) and progression to squamous cell carcinoma (SSC).
Materials and Methods
A retrospective cohort of 25 laryngeal papilloma cases included 21 RRP, two of which progressed to SCC. Aberrant methylation status was determined using the multi-gene (22 tumor suppressor genes) methylation-specific multiplex ligation-dependent probe amplification assay and confirmed using methylation specific PCR.
Results
Twenty genes had altered DNA methylation in 22 of 25 cases. Aberrant methylation of CDKN2B and TIMP3 was most frequent. Promoter hypermethylation of BRCA2, APC, CDKN2A and CDKN2B was detected in 2 RRP cases with subsequent progression to SCC. Of the 25 cases, 22 were positive for HPV-6, 2 for HPV-11 and 1 for HPV-16 and 33.
Conclusions
Consistent aberrant methylation of multiple tumor suppressor genes contributes to the pathogenesis of laryngeal papillomas. Persistent aberrant DNA methylation events in 2 RRP cases that progressed to cancer indicate an epigenetic monoclonal progression continuum to SCC.
Keywords: Laryngeal papillomas, recurrent papillomas, DNA methylation, Squamous cell carcinoma
INTRODUCTION
Papilloma is a benign exophytic neoplasm of epithelium on a connective tissue core1. Respiratory papillomatosis (RP) is a benign disease characterized by unregulated growth of wartlike neoplasms of the larynx, trachea, and bronchi with propensity for recurrences (RRP). In the larynx, the stratified squamous variety is the commonest form of papilloma 1. The histopathology is similar at all ages. Laryngeal papillomas usually run a benign but recurrent course. In the spontaneous transformation of RP or RRP to squamous cell carcinoma (SCC), a progression continuum to malignancy may not be histologically and clinically apparent, making these lesions difficult to diagnose early in the course of the transformation of the disease. Only a small percentage of RRP cases actually progress to malignancy 2, 3. Transformation of laryngeal papillomas to malignant neoplasms range from 1.25% to 42.9% 4, 5.
The human papilloma virus (HPV), which is associated with genital papillomas, has also been associated with laryngeal papillomas as an etiologic agent 6–8, particularly HPV types 6 and 119. Studies on HPV typing in benign laryngeal papillomas have demonstrated an association of HPV type 11 with a more aggressive course of the disease 10, 11. According to Lele et al 12, HPV-11 infection may be an early event in progression of RRP to carcinoma.
Epigenetics is the regulation of changes in gene expression by mechanisms that do not involve changes in DNA sequence. Establishment and maintenance of epigenetic control (gene silencing) has several aspects, which include promoter region hypermethylation, methyl-binding proteins, DNA methyltransferases, histone deacetylases and chromatin state. Aberrant methylation of CpG islands is a hallmark of human cancers and is found early during carcinogenesis 13. Epigenetic events of DNA hypermethylation contribute to RRP pathogenesis, some of which are initiating clonal alterations in the recurrence continuum 14. Aberrant methylation of CDKN2B and APC genes was most frequent, followed by CDKN2A, TIMP3, VHL, DAPK1, HIC1, and GSTP114.
Recurrent genomic aberrations are good indicators of genes that are causally associated with transformation, cancer development or progression. To asses the contribution of promoter methylation in RP tumorigenesis, we investigated an expanded retrospective cohort of 25 papilloma cases with an initial laryngeal papilloma biopsy between the years 1994 and 2004, with follow-up for subsequent transformation to carcinoma in situ, or SCC through August 2009. Aberrant promoter methylation of 22 unique methylation-prone tumor suppressor genes was evaluated using the high-throughput methylation-specific multiplex ligation dependent probe amplification (MS-MLPA) assay (41 gene probes, 35 unique genes, including control probes) and methylation specific PCR (MSP).
MATERIALS and METHODS
Patient Cohort
The laryngeal papilloma cohort of 25 subjects (21 Caucasian American {CA} and 4 African American {AA}), comprised 5 females and 20 males, all adult onset, ranging in age from 19 to 73 years and 1 female juvenile onset (1 year-old). Of the 25 cohort subjects, 4 were non-recurrent papillomas (RP) and 21 were RRP. Of the 21 RRP cases, DNA from multiple biopsies were available from 15 RRP for methylation assays. The number of recurrent biopsies ranged from 2 to 22 biopsies from the initial primary biopsy (follow-up through August 2009). The interval between biopsies for these subjects ranged from 23 days (shortest) to 102 months (longest).
When the primary RP biopsy was not available for DNA analysis, the first available RP became the reference biopsy. Of the 25 cases, there were 19 primary and 6 reference biopsies. Primary and reference biopsies included lesions with benign squamous papilloma (16 cases), mild dysplasia (5 cases), moderate dysplasia (1 case), and moderate/severe dysplasia (3 cases). Lesions with mixed moderate and severe dysplasia were classed separately from progression lesions of purely severe dysplasia, carcinoma in situ (CIS), and SCC.
DNA Extraction
Whole 5 micron formalin-fixed tissue sections or microdissected papilloma tissue, and subsequent transformation to severe dysplasia, CIS, or SCC lesions (2 cases), were processed for DNA extraction as previously described 15, 16.
HPV Detection
HPV status was identified using the Linear Array HPV Genotyping kit (Roche, Indianapolis, IN) in all cases. PCR using HPV type primers for HPV 6, 11, 16, 31 and 33 especially designed to amplify less than 120 base pair DNA fragment lengths (Table 1) was also used to detect HPV status in some cases.
Table 1.
HPV PCR Primers
| GenBank Accession # | Start to end (bp) | Forward & Reverse (5′ to 3′) | PCR Length | Annealing Temp | Regions |
|---|---|---|---|---|---|
| AF335604.1: HPV6-137 | 1080 to 1217 | F: ACATGCGTCATGTGGAAGAG | 137bp | 52°C | L1 |
| R: AGGCGATAACCCAAAGTTCC | |||||
| M14119.1: HPV 11-182 | 578 to 760 | F: CCTGCAGCCTCCTGACCCTGT | 182bp | 60°C | E7 |
| R: CTCCGTCTGTGCACTCCACAA | |||||
| M14119.1: HPV 11-230 | 291 to 521 | F: TGCAGCGTGTGCCTGTTGCTT | 230bp | 60°C | E6 |
| R: AGCAACGACCCTTCCACTGGT | |||||
| NC001526.1: HPV 16-101 | 497 to 597 | F: TGGACCGGTCGATGTATGT | 101bp | 54°C | E6 & E7 |
| R: CATATATTCATGCAATGTAGGTGTA | |||||
| NC001526.1: HPV 16-173 | 425 to 597 | F: AAGCCACTGTGTCCTGAAGAA | 173bp | 54°C | E6 & E7 |
| R: CATATATTCATGCAATGTAGGTGTA | |||||
| NC001526.1: HPV 16-258 | 382 to 640 | F: AATACAACAAACCGTTGTGTGATT | 258bp | 58°C | E6 & E7 |
| R: CAGTAGAGATCAGTTGTCTCTGGTTGC | |||||
| J04353.1: HPV 31-124 | 3861 to 3962 | F: TTTGCTTTGCTTTTGTGTGCTAC | 124bp | 52°C | E5 |
| R: TGGAGAGGTTGCAATAACCCATA | |||||
| M12732.1: HPV 33-149 | 424 to 572 | F: TGTCAAAGACCTTTGTGTCCTC | 149bp | 54°C | E6 |
| R: GGCGTTTTTACACGTCACAG |
The Methylation-Specific Multiplex Ligation Dependent Probe Amplification (MS-MLPA) Assay
The Multiplex Ligation-Dependent Probe Amplification assay allows for the relative quantification of approximately 41 different DNA sequences in a single reaction requiring only 20 ng of human DNA. The standard use of the technique to observe quantitative changes in copy number (MLPA) 16, 17 and adaptation of MLPA to detect aberrant methylation (MS-MLPA) has been detailed elsewhere 14, 16, 18.
Bisulfite Modification and Methylation-Specific Polymerase (MSP) Chain Reaction Assay
Genomic DNA (100ng) from formalin-fixed paraffin embedded papilloma tissue and control universal methylated DNA (Chamicon International, Inc) and control unmethylated DNA (normal genomic DNA) were modified using the EZ DNA methylation gold kit (Zymo Research, Orange, CA) during which methylated DNA is protected andunmethylated cytosine is converted to uracil18. The modified DNA served as a template using primers specific for the methylated or modified unmethylated sequences (Table 2). MSP amplification was performed using 3ul of bisulfite modified DNA in a final volume of 25ul PCR mix containing 1X PCR buffer, 2.5mM dNTP, 1mM MgCl2 and 1U Amp gold Taq DNA polymerase, 0.5uM primer followed by 38 cycles at 95°C 45 seconds, 62°C 45 seconds, 72°C 1min18. The resultant PCR products were separated on 2% agarose gel stained with ethidium bromide and visualized under UV illumination.
Table 2.
Methylation and Unmethylation MSP Primer Sequences for Laryngeal Papillomas
| Gene | Methylation Specific Primers | Unmethylation Specific Primers | Product Size | |
|---|---|---|---|---|
| BRCA2 | Forward | 5′-GACGGTTGGGATGTTTGATAAGG | 5′-AGGGTGGTTTGGGATTTTTAAGG | M - 337bp |
| Reverse | 5′-AATCTATCCCCTCACGCTTCTCC | 5′-TCACACTTCTCCCAACAACAACC | U - 250bp | |
| APC | Forward | 5′-TATTGCGGAGTGCGGGTC | 5′-GTGTTTTATTGTGGAGTGTGGGTT | M - 97bp |
| Reverse | 5′-TCGAAGAACTCCCGACGA | 5′-CCAATCAACAAACTCCCAACAA | U - 108bp | |
| GSTP1 | Forward | 5′-TTCGGGGTGTAGCGGTCGTC | 5′-GATGTTTGGGGTGTAGTGGTTGTT | M - 91bp |
| Reverse | 5′-GCCCCAATACTAAATCACGACG | 5′-CCACCCCAATACTAAATCACAACA | U - 97bp | |
| CDKN2AARF | Forward | 5′-TATCGAGTTTTTTGTGTTTAGTCC | 5′-TATTGAGTTTTTTGTGTTTAGTTT | M - 112bp |
| Reverse | 5′-AACGACCAACAAAAAAAAAAAACG | 5′-CAACAAAAAAAAAACAACCAAC | U - 124bp | |
| CDKN2B† | Forward | 5′-GAAGGTGCGATAGTTTTTGGAAGTCGGCGC | 5′-TGGAGAAGGTGTGATAGTTTTTGGAAGTTGGTGT | M - 160bp |
| Reverse | 5′-GACGATCTAAATTCCAACCCCGATCCGCCG | 5′-CATCAACAATCTAAATTCCAACCCCAATCCACCA | U - 169bp |
M = methylated product
U = unmethylated product
Nygren, A.O., et al., Methylation-specific MLPA (MS-MLPA): simultaneous detection of CpG methylation and copy number changes of up to 40 sequences. Nucleic Acids Res, 2005. 33(14):e128.
RESULTS
Promoter hypermethylation by MS-MLPA or by MSP was recorded in 22 of 25 cases. Twenty of 22 tumor suppressor genes in the multi-gene panel had altered DNA methylation in at least one RP biopsy. Aberrant methylation of TIMP3 and CDKN2B genes was most frequent, occurring in 13 of 22 and 11 of 22 cases, respectively, followed by CDKN2A, APC and VHL genes in 9 of 22 cases, and TP73, GSTP1, HIC1, MLH1 and DAPK1 genes in 5 of 22 cases.
Of the 21 RRP cases, multiple biopsies were examined for aberrant methylation in 15 cases. Identical abnormally methylated genes were found in recurrent biopsies of 5 of 15 RRP cases and an aberrantly methylated CDKN2B gene linked all 5 cases (cases 4, 7, 11, 12, 13) 14. MSP confirmed aberrant methylation of CDKN2B in RRP cases 4, 7, 11 and 12 in multiple recurrent biopsies (MSP for Case 13 was not performed).
Progression to SCC occurred in RRP cases 1 and 5 (Table 3). In RRP Case 1, the papillomas in biopsies 1 through 3 were located on both the left and right vocal folds. Subsequent dysplastic papillomas were located on both left and right true as well as false vocal cords (biopsies 4–6, Table 3). For Case 5, the laryngeal subsite for the reference biopsy and the subsequent recurrent lesions was the right true vocal cord.
Table 3.
Epigenetically Linked Progressive Laryngeal Cases
| RRP | Lesion Type | Biospy | Time Interval | BRCA2 | APC | CDKN2A | CDKN2B | HPV (Roche) | HPV (PCR) |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Squamous Papilloma | 1 | Primary | M* | M† | U* | U* | 16 | 16 |
| Squamous Papilloma with severe dysplasia | 2 | 4 months | M† | M† | U* | U | 16 | ||
| Primary SCC, Block 1 tumor | 3T | 6 months | M† | U* | U* | U | 16 & 33 | ||
| Primary SCC, Block 1 dysplastic papilloma | 3P | 6months | M† | M† | U* | U | 16 | ||
| Recurrent Severe Dysplasia | 4 | 50 months | M† | M* | M† | U* | neg | neg | |
| Carcinoma in Situ | 5 | 51 months | M† | M† | M* | U* | 6 | neg | |
| Recurrent SCC | 6 | 53 months | M† | M† | M* | U, NR by MSP | not informative | neg | |
| Case 5 | Squamous Papilloma with moderate to severe dysplasia | 1 | Reference | M† | M* | M* | U* | neg | 11 |
| Squamous Papilloma with mild to moderate dysplasia | 2 | 2 months | M† | M† | M* | M | neg | neg | |
| Primary SCC | 3 | 9 months | M† | M† | M* | M | neg | neg | |
| Carcinoma in Situ | 4 | 11 months | M† | M* | M† | U* | neg | neg |
RRP = Recurrent Respiratory Papilloma
SCC = Squamous Cell Carcinoma
M = methylation detected by MS-MLPA only
M† = methylation detected by MSP only
M* = MS-MLPA methylation confirmed by MSP
U = unmethylated by MS-MLPA only
U* = unmethylated by MS-MLPA and MSP
NR = no reaction by MSP because of insufficient DNA
neg = negative for HPV
In RRP Case 1, aberrant methylation of BRCA2 and APC, identified in the primary biopsy, was also present in the recurrent severe dysplasia, CIS, and recurrent SCC (Table 3). MSP confirmed MS-MLPA methylation of BRCA2 (biopsy 1), APC (biopsy 4), GSTP1 (biopsy 6), and CDKN2A (biopsies 5 and 6). MSP and MS-MLPA were concordant for lack of methylation APC, GSTP1, and CDKN2A, and CDKN2B (Table 3).
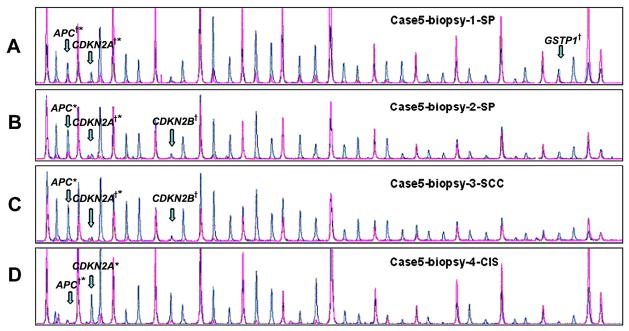
In RRP Case 5, aberrant methylation of BRAC2, APC and CDKN2A in the reference papilloma biopsy and CDKN2B in biopsy 2 were also identified in the subsequent progression lesions (Table 3, Figure 1). MSP confirmed MS-MLPA methylation of APC (biopsies 1 and 4) and CDKN2A (biopsies 1–3). MSP also confirmed absence of methylation for CDKN2B (biopsies 1 and 4) and GSTP1 (biopsies 2–4) detected by MS-MLPA (Figure 2).
Figure 1.
Case 5 results of MS-MLPA. Note methylation of APC by MS-MLPA with confirmation by MSP (APC†*) in biopsies 1 and 4. MSP alone detected APC methylation in biopsies 2 and 3 (APC*). Note methylation of CDKN2A by MS-MLPA with confirmation by MSP (CDKN2A†*) in biopsies 1, 2 and 3. MSP alone detected CDKN2A methylation in biopsy 4 (CDKN2A*). Note methylation of CDKN2B in biopsies 2 and 3 and GSTP1 in biopsy 1 by MS-MLPA only (CDKN2B†, GSTP1†). (SP - squamous papilloma, CIS - carcinoma in situ, SCC - squamous cell cancer).
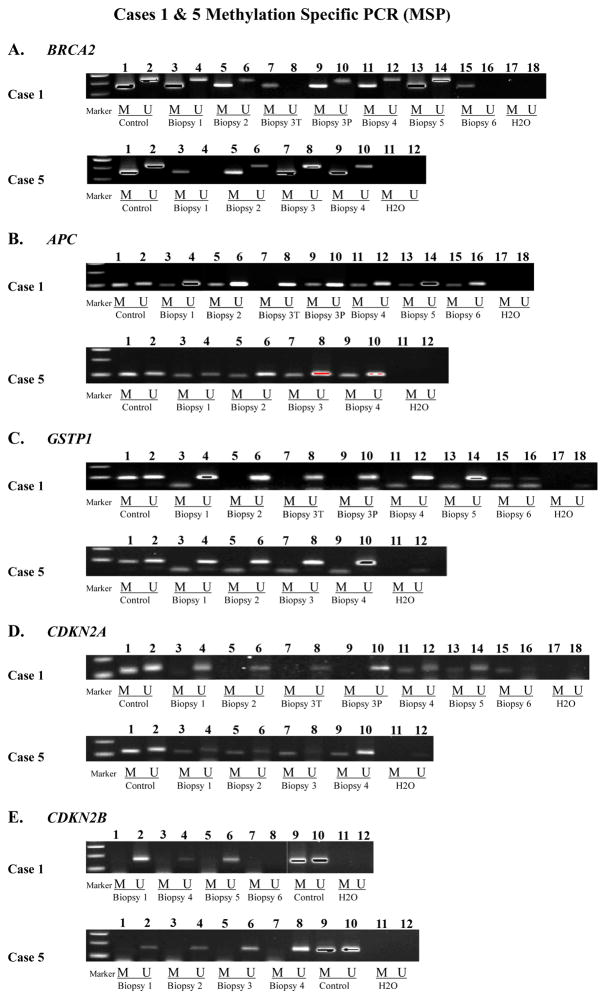
Figure 2.
Methylation Specific PCR (MSP) for BRCA2, APC, GSTP1, CDKN2A and CDKN2B.
Figure 2A (BRCA2): Lanes 1 & 2: universal methylated and unmethylated controls; Case 1: Lanes 3-16 span biopsies 1-6. Note presence of methylated product in all biopsies. Lanes 17 & 18: negative control. Case 5: Lanes 3-10 span biopsies 1-4. Note presence of methylated product in all biopsies. Lanes 11 & 12: negative control.
Figure 2B (APC): Lanes 1 & 2: universal methylated and unmethylated controls; Case 1: Lanes 3-16 span biopsies 1-6. Note presence of methylated product in biopsies 1, 2, 3P, 4, 5 and 6 (Table 3). Note absence of methylated product in biopsy 3T. Lanes 17 & 18: negative control. Case 5: Lanes 3-10 span biopsies 1-4. Note presence of methylated product in all biopsies. Lanes 11 & 12: negative control.
Figure 2C (GSTP1): Lanes 1 & 2: universal methylated and unmethylated controls; Case 1: Lanes 3-16 span biopsies 1-6. Note presence of methylated product in biopsy 6. Note absence of methylated product in biopsies 1-5. Lanes 17 & 18: negative control. Case 5: Lanes 3-10 span biopsies 1-4. Note absence of methylated product in all biopsies. Lanes 11 & 12: negative control.
Figure 2D (CDKN2A): Lanes 1 & 2: universal methylated and unmethylated controls; Case 1: Lanes 3-16 span biopsies 1-6. Note presence of methylated product in biopsies 4, 5 and 6. Note absence of methylated product in biopsies 1-3P. Lanes 17 & 18: negative control. Case 5: Lanes 3-10 span biopsies 1-4. Note presence of methylated product in all biopsies. Lanes 11 & 12: negative control.
Figure 2E (CDKN2B): Case 1: Lanes 1-8 span biopsies 1, 4-6. Note absence of methylated product in biopsies 1, 4 and 5. No reaction in biopsy 6 due to insufficient DNA. MSP was not performed on biopsies 2, 3T and 3P. Lanes 9 & 10: universal methylated and unmethylated controls. Lanes 11 & 12: negative control. Case 5: Lanes 1-8 span biopsies 1-4. Note absence of methylated product in all biopsies. Lanes 9 & 10: universal methylated and unmethylated controls. Lanes 11 & 12: negative control.
HPV was identified in all 25 cases by either the Roche Linear Array and/or by PCR. Among the 21 HPV positive RRP cases, 18 were HPV-6, 1 was HPV-16 and 33 (Case 1), and 2 were HPV-11 positive (Cases 5 and 7). The remaining 4 RP were positive for HPV-6. Case 1 was positive for HPV-16 and 33 in the primary SCC but was not detected in the recurrent SCC lesion. HPV-6 status in Case 1, biopsy 5, detected by the Roche Linear Array was not confirmed by PCR. Case 5 biopsies were negative for HPV by the Roche Linear Array. However, HPV-11 by PCR, using two different primer sets, identified HPV-11 only in the first (reference) biopsy and confirmed lack of HPV for biopsies 2 through 4 (Table 3).
DISCUSSION
Recurrent genomic aberrations are good indicators of genes that are causally associated with cancer development, transformation or progression. Our previous studies 14, 16 have demonstrated that epigenetic events of DNA hypermethylation underlie the pathogenesis of benign sinonasal and recurrent laryngeal papillomas, establishing a monoclonal origin for RRP. Our current findings reiterate consistent DNA hypermethylation events in a larger cohort of laryngeal papillomas and trace a progression continuum to SCC. The results further support a monoclonal progression for malignant transformation in 2 RRP cases.
Spontaneous transformation of RP to laryngeal squamous cell carcinoma (LSCC) is not easily characterized by a histologic progression through dysplasia over time, making these lesions difficult to diagnose histologically and clinically early on in the course of the transformation of the disease. Several studies have attempted to identify markers that canpredict which patients with RP are ata higher risk for more frequently recurring aggressive diseaseor malignant transformation. However, results in both benign laryngeal lesions (papillomatosis) and malignant lesions havenot been definitive 12, 19–22. Currently, there are no biomarkers of aggressive RP that predict benign recurrence and transformation to malignancy from RP.
In this study, TIMP3 was the most frequently methylated gene (13/22 cases), followed by CDKN2B, CDKN2A, APC, VHL, TP73, GSTP1, HIC1, MLH1 and DAPK 1. TIMP3 induces apoptosis 23, inhibits angiogenesis 24, impedes cell migration 25, and is a physiological regulator of inflammation 26. Promoter methylation of TIMP3 has been observed in many tumor types 27, 28 and is involved in the genesis of esophageal adenocarcinoma notably during progression from dysplasia to carcinoma 29, 30.
CDKN2B and CDKN2A were hypermethylated in 11 of 22 and 9 of 22 cases, respectively. Genetic alterations in CDKN2A and CDKN2B genes, which map to 9p21, have been linked to malignant progression in HNSCC 31–33. Inactivation of the CDKN2B (p15) and CDKN2A (p14 and p16) genes at the genomic and epigenetic level is a frequent event in human oral SCCs and in HNSCC 17, 34, 35. One study reported aberrant methylation of CDKN2B (p15) and CDKN2A (p16) in more than 50% of the oral squamous cell carcinomas (OSCC) 36. The presence of aberrant methylation of p15 and p16 in precancerous oral tissues 35 implicates methylation of p15 and p16 as early events in the pathogenesis of oral lesions. In undifferentiated nasopharyngeal carcinoma (NPC), preferential methylation of CDKN2B has been shown to be a useful tumor marker for NPC 37. In Case 5, aberrant methylation of CDKN2A in the reference biopsy and CDKN2B in biopsy 2 and subsequent transformation biopsies occurs as an early event and provides evidence of a monoclonal progression continuum to SCC.
Hypermethylation of the APC gene was detected in multiple biopsies in 8/15 RRP cases and 1 RP. APC (adenomatosis polyposis coli) is a tumor suppressor gene originally implicated in colon cancer. Genetic and epigenetic alterations in this gene have since been recognized in other malignancies including OSCC, gastric cancers and esophageal adenocarcinomas. Uesugi et al. 38 previously reported mutations and/or deletions of APC in primary OSCC and suggested that loss of APC function contributes to carcinogenesis in the oral region. APC inactivation as a result of promoter hypermethylation occurred in 25% of OSCC cell 38. Hypermethylation of APC, observed in the initial and subsequent biopsies in RRP cases 1 and 5 is an early event and supports a monoclonal progression continuum to SCC.
BRCA2 (Breast cancer 2, early onset) is a tumor suppressor gene whose mutations are strongly associated with an elevated risk of breast and ovarian cancers 39. Mutations in BRCA2 gene are associated with an increased risk of prostate, pancreas, stomach, melanoma, lung, and bladder cancers 40. Chromosome instability may be caused by failure in the repair of DNA double-strand breaks (DSB) 41 and BRCA2 is involved in maintaining genome stability. Aberrant promoter hypermethylation of BRCA2 was detected in 42% of non-small cell lung cancer (NSCLC) compared to absent or low methylation in their matched normal lung tissue 42. In this study, aberrant methylation of BRCA2 in the initial and subsequent transformation biopsies in RRP cases 1 and 5, similar to APC, occurred early with retention in the progression continuum.
The study cohort, drawn from a multi-ethnic primary care patient population with nearly 40% AAs, revealed a nearly 5:1 predominance of CA with RP. CA race predilection for RP is supported by the Moore et al. 21 study, which reported a 4:1 CA predominance for cohort subjects drawn from a mostly tertiary care patient population setting. We found a preponderance of male RP patients as compared to female RP (20 males: 5 females). This is a deviation from previous reports that indicate approximately equal gender distribution for RP 21, 43.
In RP, human papillomavirus types 6 and 11 account for 80–90% of RP 44. In our cohort, types 6 and 11 account for 96% of the cases with 22 cases positive for HPV-6 and 2 cases positive for HPV-11. HPV-11 appears to confer a more aggressive neoplastic phenotype than HPV-6 and is associated more often with atypia and frequent recurrence45. Of the two RRP cases in this cohort positive for HPV-11, only Case 5 progressed to SCC. Though the majority of RP harbor low risk HPV 6 and 11, high-risk HPV types 16 and 18 have been reported and multiple HPV types were detected in 11.8% of RP 21. RRP Case 1 with multiple HPV types (HPV-16 and 33 positive) progressed to SCC. High-risk HPV DNA alone may be sufficient to initiate tumorigeneisis in the absence of traditional risk factors such as tobacco or alcohol use 21. Oncogenic HPV, particularly HPV-16, has been established as a causative agent for 25% of head and neck squamous cell carcinoma (HNSCC) 20 and the development of laryngeal carcinoma is associated with HPV infection 19, 20.
MSP for the most part confirmed promoter hypermethylation detected by MS-MLPA. MSP did not confirm MS-MLPA methylation of CDKN2B observed in Case 1 and Case 5 biopsies. While a distinct advantage of MS-MLPA is the ability to examine aberrant promoter methylation in multiple cancer genes in a single assay run, multiplex PCR of a large number of gene probes (22 unique genes) inherently encounters competitive amplification and detection algorithms may miss hypermethylation events that do not reach the threshold for detection 34. In contrast, MSP examines only one gene at a time 18 and therefore, is more sensitive than MS-MLPA 18 and is underscored by aberrant methylation of BRCA2 in Case 1 and Case 5 biopsies by MSP alone. In cases where MSP did not confirm MS-MLPA methylation, background noise presenting as spurious peaks may be a contributing factor. Spurious peaks (background noise) may be attributed to challenges posed to DNA from formalin-fixed tissue, the quality of which is dependent on tissue fixation variables. Regardless, MS-MLPA profiling of multiple genes for aberrantly methylated promoter regions is a valuable screening tool to determine frequency and pattern of promoter methylation in neoplasia. These epigenetic signatures, upon subsequent validation as diagnostic or prognostic biomarkers, can become reduced to a more definitive candidate gene panel of only a few key genes. The latter would be amenable to higher detection sensitivities using a targeted 3 or 4 MS-MLPA gene probe panel or by MSP alone.
Malignant transformation rates of benign laryngeal papillomas can range from 1.25% to 42.9%4, 5 and larger benign RP cohorts will be key to providing more accurate progression rates. Though this study is limited in its sample size (25 patients) and the number of cases that progressed to SCC (2 cases), it closely mirrors other larger study cohorts with similar transformation rates 21. In the two cases with progression to SCC, promoter methylation occurred as an early event and persisted in initial and subsequent biopsies for Cases 1 and 5 with progression to cancer supporting an epigenetic monoclonal progression continuum to SCC.
The high frequency of DNA hypermethylation events in this study supports the utilization of gene silencing mechanisms as one of the driving forces behind the growth of laryngeal papillomas, reiterating DNA hypermethylation events as hallmarks of RP pathogenesis, some of which are initiating clonal alterations in the recurrence continuum in some RRP cases 14. Aberrant methylation of BRCA2, APC, CDKN2A and CDKN2B, confirmed by MSP and detected in the initial and all subsequent transformation biopsies in RRP Cases 1 and 5, appears to be an early event in the pathogenesis of laryngeal papillomatosis tracing a monoclonal progression continuum to SCC.
Epigenetic alterations identified in precancerous lesions with biomarker potential would have high clinical significance in risk assessment and early detection, and may also serve as molecular targets for chemopreventive interventions. Because promoter hypermethylation is potentially reversible, the molecules that regulate methylation status of DNA are considered promising targets for new cancer therapies.
Acknowledgments
Supported by R01 NIH DE 15990
Drs. Stephen and Worsham had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study was supported by R01 NIH DE 15990 (Dr. Worsham).
Footnotes
Conflict of Interest
All authors have no conflicts of interest.
References
- 1.Capper JW, Bailey CM, Michaels L. Squamous papillomas of the larynx in adults. A review of 63 cases. Clin Otolaryngol Allied Sci. 1983;8(2):109–119. doi: 10.1111/j.1365-2273.1983.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 2.Doyle DJ, Henderson LA, LeJeune FE, Jr, Miller RH. Changes in human papillomavirus typing of recurrent respiratory papillomatosis progressing to malignant neoplasm. Arch Otolaryngol Head Neck Surg. 1994;120(11):1273–1276. doi: 10.1001/archotol.1994.01880350079014. [DOI] [PubMed] [Google Scholar]
- 3.Dedo HH, Yu KC. CO(2) laser treatment in 244 patients with respiratory papillomas. Laryngoscope. 2001;111(9):1639–1644. doi: 10.1097/00005537-200109000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Go C, Schwartz MR, Donovan DT. Molecular transformation of recurrent respiratory papillomatosis: viral typing and p53 overexpression. Ann Otol Rhinol Laryngol. 2003;112(4):298–302. doi: 10.1177/000348940311200402. [DOI] [PubMed] [Google Scholar]
- 5.Kossak-Glowczewska M. Spontaneous neoplastic transformation of laryngeal papilloma in adults. Otolaryngol Pol. 1991;45(3):186–194. [PubMed] [Google Scholar]
- 6.Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A. 1982;79(17):5425–5429. doi: 10.1073/pnas.79.17.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbott M, Cossart YE, Kan A, Konopka M, Chan R, Rose BR. Human papillomavirus and host variables as predictors of clinical course in patients with juvenile-onset recurrent respiratory papillomatosis. J Clin Microbiol. 1997;35(12):3098–3103. doi: 10.1128/jcm.35.12.3098-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80(2):560–563. doi: 10.1073/pnas.80.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penaloza-Plascencia M, Montoya-Fuentes H, Flores-Martinez SE, Fierro-Velasco FJ, Penaloza-Gonzalez JM, Sanchez-Corona J. Molecular identification of 7 human papillomavirus types in recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2000;126(9):1119–1123. doi: 10.1001/archotol.126.9.1119. [DOI] [PubMed] [Google Scholar]
- 10.Hartley C, Hamilton J, Birzgalis AR, Farrington WT. Recurrent respiratory papillomatosis--the Manchester experience, 1974–1992. J Laryngol Otol. 1994;108(3):226–229. [PubMed] [Google Scholar]
- 11.Lie ES, Karlsen F, Holm R. Presence of human papillomavirus in squamous cell laryngeal carcinomas. A study of thirty-nine cases using polymerase chain reaction and in situ hybridization. Acta Otolaryngol. 1996;116(6):900–905. doi: 10.3109/00016489609137949. [DOI] [PubMed] [Google Scholar]
- 12.Lele SM, Pou AM, Ventura K, Gatalica Z, Payne D. Molecular events in the progression of recurrent respiratory papillomatosis to carcinoma. Arch Pathol Lab Med. 2002;126(10):1184–1188. doi: 10.5858/2002-126-1184-MEITPO. [DOI] [PubMed] [Google Scholar]
- 13.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 14.Stephen JK, Vaught LE, Chen KM, et al. An epigenetically derived monoclonal origin for recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg. 2007;133(7):684–692. doi: 10.1001/archotol.133.7.684. [DOI] [PubMed] [Google Scholar]
- 15.Raju ULM, Sethi S, Qureshi H, Wolman SR, Worsham MJ. Molecular Classification of Breast Carcinoma In Situ. Current Genomics. 2006;7(8):523–532. doi: 10.2174/138920206779315719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephen JK, Vaught LE, Chen KM, et al. Epigenetic events underlie the pathogenesis of sinonasal papillomas. Mod Pathol. 2007;20(10):1019–1027. doi: 10.1038/modpathol.3800944. [DOI] [PubMed] [Google Scholar]
- 17.Worsham MJ, Pals G, Schouten JP, et al. Delineating genetic pathways of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129(7):702–708. doi: 10.1001/archotol.129.7.702. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Sawhney R, Khan M, et al. Methylation of multiple genes as diagnostic and therapeutic markers in primary Head and Neck Squamous Cell Carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133(11):1131–1138. doi: 10.1001/archotol.133.11.1131. [DOI] [PubMed] [Google Scholar]
- 19.Uobe K, Masuno K, Fang YR, et al. Detection of HPV in Japanese and Chinese oral carcinomas by in situ PCR. Oral Oncol. 2001;37(2):146–152. doi: 10.1016/s1368-8375(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 20.Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):622–635. doi: 10.1067/moe.2001.115392. [DOI] [PubMed] [Google Scholar]
- 21.Moore CE, Wiatrak BJ, McClatchey KD, et al. High-risk human papillomavirus types and squamous cell carcinoma in patients with respiratory papillomas. Otolaryngol Head Neck Surg. 1999;120(5):698–705. doi: 10.1053/hn.1999.v120.a91773. [DOI] [PubMed] [Google Scholar]
- 22.Rady PL, Schnadig VJ, Weiss RL, Hughes TK, Tyring SK. Malignant transformation of recurrent respiratory papillomatosis associated with integrated human papillomavirus type 11 DNA and mutation of p53. Laryngoscope. 1998;108(5):735–740. doi: 10.1097/00005537-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101(6):1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi JH, Ebrahem Q, Moore N, et al. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9(4):407–415. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 25.van der Laan WH, Quax PH, Seemayer CA, et al. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003;10(3):234–242. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]
- 26.Mohammed FF, Smookler DS, Taylor SE, et al. Abnormal TNF activity in Timp3−/− mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36(9):969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 27.Momparler RL, Bovenzi V. DNA methylation and cancer. J Cell Physiol. 2000;183(2):145–154. doi: 10.1002/(SICI)1097-4652(200005)183:2<145::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile of human cancer. Cancer Res. 2001;61(8):3225–3229. [PubMed] [Google Scholar]
- 29.Bian YS, Osterheld MC, Fontolliet C, Bosman FT, Benhattar J. p16 inactivation by methylation of the CDKN2A promoter occurs early during neoplastic progression in Barrett’s esophagus. Gastroenterology. 2002;122(4):1113–1121. doi: 10.1053/gast.2002.32370. [DOI] [PubMed] [Google Scholar]
- 30.Eads CA, Lord RV, Wickramasinghe K, et al. Epigenetic patterns in the progression of esophageal adenocarcinoma. Cancer Res. 2001;61(8):3410–3418. [PubMed] [Google Scholar]
- 31.Worsham MJ, Chen KM, Tiwari N, et al. Fine-mapping loss of gene architecture at the CDKN2B (p15INK4b), CDKN2A (p14ARF, p16INK4a), and MTAP genes in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(4):409–415. doi: 10.1001/archotol.132.4.409. [DOI] [PubMed] [Google Scholar]
- 32.Danahey DG, Tobin EJ, Schuller DE, Bier-Laning CM, Weghorst CM, Lang JC. p16 mutation frequency and clinical correlation in head and neck cancer. Acta Otolaryngol. 1999;119(2):285–288. doi: 10.1080/00016489950181837. [DOI] [PubMed] [Google Scholar]
- 33.Lydiatt WM, Davidson BJ, Schantz SP, Caruana S, Chaganti RS. 9p21 deletion correlates with recurrence in head and neck cancer. Head Neck. 1998;20(2):113–118. doi: 10.1002/(sici)1097-0347(199803)20:2<113::aid-hed3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Worsham MJ, Chen KM, Meduri V, et al. Epigenetic events of disease progression in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(6):668–677. doi: 10.1001/archotol.132.6.668. [DOI] [PubMed] [Google Scholar]
- 35.Shintani S, Nakahara Y, Mihara M, Ueyama Y, Matsumura T. Inactivation of the p14(ARF), p15(INK4B) and p16(INK4A) genes is a frequent event in human oral squamous cell carcinomas. Oral Oncol. 2001;37(6):498–504. doi: 10.1016/s1368-8375(00)00142-1. [DOI] [PubMed] [Google Scholar]
- 36.Yeh KT, Chang JG, Lin TH, et al. Epigenetic changes of tumor suppressor genes, P15, P16, VHL and P53 in oral cancer. Oncol Rep. 2003;10(3):659–663. [PubMed] [Google Scholar]
- 37.Wong TS, Tang KC, Kwong DL, et al. Differential gene methylation in undifferentiated nasopharyngeal carcinoma. Int J Oncol. 2003;22(4):869–874. [PubMed] [Google Scholar]
- 38.Uesugi H, Uzawa K, Kawasaki K, et al. Status of reduced expression and hypermethylation of the APC tumor suppressor gene in human oral squamous cell carcinoma. Int J Mol Med. 2005;15(4):597–602. [PubMed] [Google Scholar]
- 39.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62(3):676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancer risks in BRCA2 mutation carriers. The Breast Cancer Linkage Consortium. J Natl Cancer Inst. 1999;91(15):1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 41.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2(3):196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 42.Lee MN, Tseng RC, Hsu HS, et al. Epigenetic inactivation of the chromosomal stability control genes BRCA1, BRCA2, and XRCC5 in non-small cell lung cancer. Clin Cancer Res. 2007;13(3):832–838. doi: 10.1158/1078-0432.CCR-05-2694. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg BM, Abramson AL. Laryngeal papillomas. Clin Dermatol. 1985;3(4):130–138. doi: 10.1016/0738-081x(85)90057-4. [DOI] [PubMed] [Google Scholar]
- 44.Duggan MA, Lim M, Gill MJ, Inoue M. HPV DNA typing of adult-onset respiratory papillomatosis. Laryngoscope. 1990;100(6):639–642. doi: 10.1288/00005537-199006000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Rabah R, Sakr W, Thomas R, Lancaster WD, Gregoire L. Human papillomavirus type, proliferative activity, and p53: potential markers of aggressive papillomatosis. Arch Pathol Lab Med. 2000;124(5):721–724. doi: 10.5858/2000-124-0721-HPTPAA. [DOI] [PubMed] [Google Scholar]