Abstract
Background
Cardiovascular diseases arise during 0,2% to 4% of all pregnancies in the industrialized world. In Germany, this type of complication, which is sometimes lethal, affects approximately 30 000 pregnant women per year.
Methods
We performed a simple literature search in the NCBI databases for publications that appeared from 2008 to 2010 and that contained the search terms “pregnancy” and one of the following: “valvular disease,” “endocarditis,” “coronary heart disease,” “cardiomyopathy,” “hypertension,” “anticoagulation.” We also took consideration of the relevant international medical society guidelines and of the new database of the Pharmakovigilanz- und Beratungszentrum für Embryonaltoxikologie in Berlin (Embryotox).
Results
There is a rising incidence, not only of hypertension during pregnancy, but also of valvular heart disease during pregnancy. Severe valvular stenosis, particularly mitral stenosis, raises the risk of pulmonary edema and should be treated before pregnancy, by valvuloplasty or surgically. Women with high-grade valvular insufficiency and restricted left-ventricular function are at risk of heart failure. For women with mechanical heart valves, the type of anticoagulation during pregnancy must be discussed on an individual basis. Coumarin derivatives are associated with an elevated risk of hemorrhage as well as coumarin embryopathy; recent studies have shown that the latter risk is low and dose-dependent. Spontaneous dissection of the coronary arteries is best treated by catheter intervention with the implantation of a bare metal stent.
Conclusion
Women of child-bearing age who are at risk for, or already have, cardiovascular disease should receive early counseling and treatment, not just from their family physician, but from an interdisciplinary team composed of gynecologists, cardiologists, and, if necessary, cardiac surgeons.
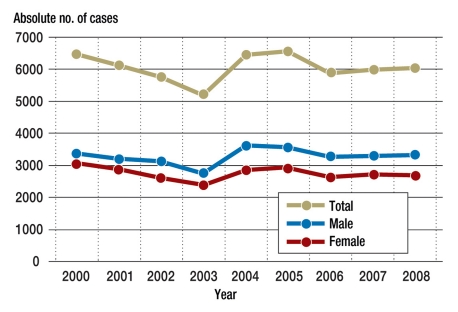
Cardiovascular diseases arise during 0.2% to 4% of all pregnancies in the industrialized world. In Germany this type of complication, which is sometimes life-threatening, affects almost 30 000 pregnant women per year. The number of fetuses and neonates harmed by complications during pregnancy, labor, and delivery has not changed significantly in the last eight years (Figure). Approximately 6000 fetuses and neonates are affected each year.
Figure.
Hospital diagnosis data for ICD-10: P00-P04, 2000–2008: approximately 6000 fetuses per year harmed as a result of complications during pregnancy, labor, and delivery, out of a total number of 349 862 male and 332 652 female live births in Germany in 2008.
Source: Federal Health Reporting, Federal Statistical Office, Robert Koch Institute, Berlin
Hypertensive disorders during pregnancy are one of the commonest causes of morbidity and mortality in mothers and babies in the industrialized world. Early identification of risk factors and Doppler ultrasound scans of the uterine artery during the first and second trimesters as a predictor of preeclampsia contribute to improved care for pregnant women (1).
The proportion of women of child-bearing age with congenital heart defects, surgically treated or otherwise, has increased substantially in recent decades due to improved surgical, anesthesiological, and cardiological care. As a result, congenital heart defects currently account for approximately 30% to 50% of all cardiac diseases during pregnancy (e1). The current figure for Germany is 120 000 patients, with an annual increase of around 5000 (2). In non-industrialized countries, 90% of all heart disorders in women of child-bearing age are of rheumatic origin. Worldwide, mitral stenosis is the most common valve defect responsible for maternal deaths with cardiac causes. It requires therapeutic intervention before or during pregnancy. Acquired heart valve defects account for 15% of cardiac complications in pregnant women in the industrialized world (3).
Cardiomyopathy, arrhythmia and coronary heart disease are considerably rarer diseases but can also lead to complications during pregnancy. There are new therapeutic approaches for peripartum cardiomyopathy (PPCM). Specific treatment involving bromocriptine (to inhibit prolactin byproducts) has shown positive results so far in pilot studies. Bromocriptine combined with an anticoagulant, due to the increased risk of thrombosis, is therefore currently being investigated in a prospective study in the treatment of peripartum cardiomyopathy (4).
This review article concentrates on heart valve disorders, endocarditis, and coronary heart disease. Anticoagulants are often indicated for these clinical conditions, to minimize the risks to mother and infant. Hypertension, arrhythmia, and peripartum cardiomyopathy have already been explored in detail in earlier Deutsches Ärzteblatt articles (1, 5) (e2).
For almost all medical issues during pregnancy, there are very few prospective randomized trials. Treatment decisions are essentially based on observational studies and case descriptions. Many recommendations are based on evidence of only grade C, expert opinion.
Methods
We gathered data from a simple literature search in the databases of the National Center for Biotechnology Information (NCBI), using the search terms “pregnancy” and one of the following: “valvular disease,” “endocarditis,” “coronary heart disease,” “cardiomyopathy,” “hypertension,” “anticoagulation.” 196 German- and English-language publications dating from between 2008 and 2010 were analyzed, in addition to the guidelines of medical associations on new aspects of diagnosis and treatment (3, 6, 7). We also consulted the Embryotox database (www.embryotox.de, in German) of the Pharmakovigilanz- und Beratungszentrum für Embryonaltoxikologie in Berlin for pharmacological data.
Cardiovascular adaptation, diagnosis, and risk stratification
Physiological changes during pregnancy put a strain on the heart and can mimic heart disorders. The heart rate rises by between 10 and 30 beats per minute, and cardiac output increases by 30% to 50% by the 32nd week of pregnancy. Vasodilation as well as reversible enlargement of the heart of up to 30% develop (e3). Systolic blood pressure falls during the first half of pregnancy and returns to previous levels towards the end of pregnancy. During labor and vaginal delivery, maternal oxygen consumption rises up to threefold. Blood pressure spikes of up to 200 mm Hg are possible during the stage of expulsion. This leads to increased blood clotting and an increase in blood serum lipids (8). ECG alterations such as negative T waves can also occur with no pathological correlate. As CK and CK-MB are also expressed in the uterus and the placenta, their diagnostic value is limited. Cardiac troponins can be used to diagnose ischemia (9).
In addition to basic examinations, exercise ECG, echocardiography, and stress echocardiography without the use medications are extremely important in diagnosing ischemia. Stress of up to 70% of maximum heart rate is recommended (3). Procedures involving nuclear medicine are contraindicated; MRI scans should be used instead. Radiological guidelines for MRI use require risk/benefit analysis during the first trimester, as residual risks to the fetus have not yet been completely ruled out (6). Cardiac catheter examinations are only justified if absolutely essential. If they are performed, access via the arteria radialis is preferred.
One tool to assess the risks of pregnancy-related diseases in the mother is the Siu risk score (10). Contraindications to pregnancy according to the score are stated in the Box(3).
Box. Cardiovascular diseases that make pregnancy unadvisable*.
High-grade pulmonary hypertension of any origin (≥50% of systemic pressure)
Severe left or right ventricular dysfunction (LV ejection fraction <40%)
Patients with severe heart failure (NYHA class III/IV)
Severe left heart obstruction (aortic stenosis with average pressure gradient >50 mm Hg according to Doppler ultrasound, high-grade [recurrent] aortic isthmus stenosis)
Severe mitral stenosis (average pressure gradient >10 mm Hg, valve aperture area <1 cm2)
Marfan syndrome with ectasia of the aorta ascendens (≥45 mm)
Cyanotic heart disease (especially with oxygen saturation <80%)
*modified according to (3)
Delivery
Vaginal delivery is always recommended when the patient is hemodynamically stable at the end of pregnancy. A natural birth is possible even when there is manifest coronary heart disease. Peridural anesthesia and assisted vaginal delivery should be used to minimize the duration of labor. The use of drugs to induce labor should be avoided whenever possible.
Patients with advanced heart failure (NYHA class III/IV) and hemodynamic instability, particularly if there is severe aortic stenosis and in patients with Marfan syndrome, should undergo primary abdominal Cesarean section, in the 38th week of pregnancy if possible. When selecting anesthesia, a decrease in peripheral vascular resistance should be avoided if there are stenotic heart defects.
Acquired heart valve defects
In Germany, 1798 women aged between 15 and 45 years were treated in a hospital for mitral and/or aortic defects in 2008 (11). Due to increasing numbers in recent decades of valvular heart defects with hemodynamic effects, surgically treated or otherwise, problems during pregnancy can be expected to occur more often. Acquired heart valve defects are responsible for 15% of cardiac complications in pregnant women in the industrialized world.
Table 1 provides an overview of the complications typical of each defect. Patients with high-grade stenotic malformations are particularly at risk due to hemodynamic alterations during pregnancy and childbirth. During labor, the rapid rise in cardiac output, heart rate, and blood pressure may lead to pulmonary edema, particularly if the mother suffers from mitral stenosis. In patients with obstruction of the left ventricle, the main risks are of diastolic heart failure and arrhythmia (12). In patients with valve insufficiency and restricted left ventricular function, this can lead to heart failure. Postpartum, the risk of this is higher, due to the increased venous return flow.
Table 1. Maternal and neonatal complications in acquired valve defects*.
| Defect | Severity | NYHA class | Maternal complications | Neonatal complications | Recommendations | Recommendation of evidence |
| Mitral insufficiency | ||||||
| Mild | I–II | Rare: CA | None | Monitoring; med. only for symptomatic CA | I–C | |
| Moderate | I–II | Slightly increased: CA, rare: HF | None | Med. for CA | IIa–C | |
| Severe | III–IV | Significantly increased: CA, HF, PE, EC | Increased: 1, 2, 3 | Pregnancy before surgery contraindicated | I–C | |
| Mitral stenosis | ||||||
| Mild | I | Rare: CA | None | Monitoring; med. only for symptomatic CA, beta-blockers | I–C | |
| Moderate | II | Increased: CA, TE, PE, EC | Increased: 1, 2, 3 | For atrial fibrillation: anticoagulants, beta-blockers, digitalis, verapamil to control heart rate, valvuloplasty ideally before pregnancy, and if symptomatic also during pregnancy | IIa–C | |
| Severe | II–IV | Greatly increased: CA, PE, EC | Increased: 1, 2, 3 | No pregnancy before valvuloplasty; in pregnant women with symptoms valvuloplasty can be performed | I–C | |
| Aortic insufficiency | ||||||
| Mild | I–II | None | None | Monitoring | I–C | |
| Moderate | I–II | Rare: CA, HF | Rare: 1, 2 | Med. for CA and HF | IIa–C | |
| Severe | III–IV | Increased: CA, HF, EC | Increased: 1, 2 | No pregnancy before surgery | I–C | |
| Aortic stenosis | ||||||
| Mild | I | Rare: CA | None | Monitoring; med. only for symptomatic CA | I–C | |
| Moderate | II | Increased: HF, CA | Increased: 1, 2, 3 | Med. for CA and HF | IIa–C | |
| Severe | III–IV | Greatly increased: HF, CA, sudden cardiac death | Increased: 1, 2, 3 | No pregnancy before intervention (valvuloplasty/surgery); bed rest during 3rd trimester of pregnancy; C section | I–C | |
EC: endocarditis; HF: heart failure; PE: pulmonary edema; med: medication; CA: cardiac arrhythmias, TE: thromboembolism; Neonatal complications: 1 = intrauterine growth retardation: 2 = low birth weight: 3 = stillbirth
*modified according to (3)
Patients with heart valve defects should receive detailed cardiological counseling on the risks of pregnancy before becoming pregnant. An interdisciplinary treatment plan should be drawn up in case they become pregnant.
Mitral stenosis (MS)
Asymptomatic women with valve aperture area >1.5 cm2 usually tolerate pregnancy well. If heart rate or venous return flow increases postpartum, beta-1 selective beta-blockers and diuretics can be administered, initially at low doses (3).
In patients with moderate and severe MS (valve aperture area <1.5 cm2), balloon valvuloplasty should be performed before pregnancy, regardless of clinical symptoms, as there is a risk of pulmonary edema, often accompanied by tachyarrhythmia (3). Balloon valvuloplasty is the treatment of choice and even during pregnancy can be performed with low complication rates. Maternal and fetal mortality following valvuloplasty is <1% and morbidity is also low, at 2% to 4%. The most common complication is mitral insufficiency (e4).
If there is severe calcification of the valve, a closed commissurotomy can be considered. Maternal mortality is <2%, and fetal mortality between 2% and 8%. With open commissurotomy or valve replacement, fetal mortality is between 10% and 30%.
Biological prostheses are favored for valve replacements, although they do have the following disadvantages:
High degeneration rate: between 10% and 30%
Reoperation rates between 30% and 50% after 10 years, with
Perioperative mortality between 3% and 10% (13).
Artificial prostheses require anticoagulants even during pregnancy. This requires intensive risk analysis, due to the associated problems of coumarin embryopathy and hemorrhaging complications (14).
Aortic stenosis
In the industrialized world, most aortic valve stenoses in women of child-bearing age are congenital bicuspid aortic valves. In non-industrialized countries, rheumatic origin is the most common.
While patients with mild and moderate aortic stenosis often tolerate pregnancy with no complications, severe aortic stenosis (valve aperture area <1.0 cm2 or <0.6 cm2/m2 body surface; average Doppler ultrasound pressure gradient >50 mm Hg) is associated with a significantly increased risk to the fetus and risk to the mother of heart failure and cardiac arrhythmias (12). Symptomatic patients with severe aortic stenosis and asymptomatic women with restricted left ventricular function or a pathological stress ECG should be advised against becoming pregnant and should ideally undergo valvuloplasty or surgery before a planned pregnancy (3). If valvuloplasty cannot be performed, heart valve replacement should be considered. A Ross operation (replacement of the aortic valve using an autologous pulmonary valve and implantation of a homograft or biological prosthesis in the pulmonary position) has the advantage, for women who wish to have children, that no anticoagulants are required even if the patient later becomes pregnant. However, because of the high reoperation risk in adults this is a controversial procedure (15).
Cardiologists should closely monitor asymptomatic women with severe aortic stenosis not diagnosed until pregnancy, normal left ventricular function, and a normal stress ECG as part of their care. Symptomatic treatment involves diuretics. In the event of heart failure, beta-1 selective beta-blockers are also used, and reduced physical activity is recommended. For patients who do not respond to drug treatment and for whom early delivery is impossible, a valvuloplasty or valve surgery must be performed during pregnancy (3).
Aortic insufficiency and mitral insufficiency
Due to their reduced peripheral vascular resistance, patients with only moderate to severe valve insufficiency tolerate pregnancy well provided their left ventricular function is normal. Patients whose main symptom is shortness of breath respond well to restricted salt intake, diuretics and digitalis. Precautionary use of nifedipine or hydralazine is possible if patients exhibit hypertension. Patients with NYHA class III/IV or restricted left ventricular function are at significantly higher risk and should undergo heart surgery, preferably reconstructive, before pregnancy.
Mechanical heart valve prostheses and anticoagulants
Hemodynamically, patients with mechanical heart valves tolerate pregnancy well provided their left ventricular function is not restricted and they do not suffer from pulmonary hypertension.
The necessary oral anticoagulants are associated with an increased risk of hemorrhage and a risk of coumarin embryopathy; recent studies have shown that the latter risk is low (16, 17) and dose-dependent (18, 19). No embryopathy is observed with warfarin doses <5 mg, equivalent to <3 mg phenprocoumon. At higher doses, the rate was 8% (18, 19).
Continued oral administration of anticoagulants until the 36th week of pregnancy and a subsequent switch to unfractionated heparin until delivery is the safest treatment for the mother (14). Anticoagulation using low-molecular weight heparin in the first trimester is only justified when regular monitoring of anti-factor Xa activity (1.0–1.2 U/L) is guaranteed and high doses of phenprocoumon are required (3, 7). If anticoagulation is inadequate during pregnancy, the mother is at high risk of valve thrombosis and thromboembolism. An overview of current anticoagulation medication is provided in Table 2 (14).
Table 2. Anticoagulants during pregnancy.
| Drug | Teratogenic in humans | Fetotoxicity | Relative dose during lactation | Recommendation |
| Heparin, unfractionated | No | No | Does not pass into BM | Drug of choice except with MHVR |
| Low-molecular weight heparin | No | No | Does not pass into BM | Drug of choice; not to be used with MHVR (no approval) |
| Phenprocoumon | Coumarin embryopathy | CNS hemorrhage | Max. 10%, no abnormal findings in breastfed children | Stop/replace no more than 8 weeks after LMP; except with MHVR |
| Low-dose ASA | No | No | No abnormal findings in breastfed children | Can be used |
| Clopidogrel | No | No | Insufficient data available | Second-line drug |
| Ticlopidine | Insufficient data available | Insufficient data available | Insufficient data available | Refrain from using, as insufficient data available |
| Danaparoid | Well tolerated so far | Well tolerated so far | No anti-Xa activity in BM | Alternative to heparins, e.g. with HIT |
| Desirudin | No | No | Insufficient data available | Second-line drug |
| Argatroban | Insufficient data available | Tolerated (isolated cases) | Insufficient data available | Refrain from using, as insufficient data available |
| Fondaparinux | Insufficient data available | Tolerated (isolated cases) | Insufficient data available | Second-line drug |
BM: breastmilk; MHVR: mechanical heart valve replacement; LMP: last menstrual period; ASA, acetylsalicylic acid; HIT, heparin-induced thrombocytopenia
Endocarditis
Endocarditis rarely occurs during pregnancy: its incidence is approximately 0.006% (e5) and 0.5% when there is known history of heart disease (e6). Maternal mortality is 33%, and fetal mortality 29% (7). Common life-threatening complications are severe valve insufficiency with subsequent heart failure or an embolic event. In a pregnant woman with fever of unknown origin and heart murmur, differential diagnosis of endocarditis should be considered.
Many antibiotics have not been sufficiently researched in pregnant women. However, most are not suspected of causing prenatal toxicity. Penicillins, cephalosporins, and macrolides are preferred. Second-line drugs include cotrimoxazole and, until the 15th week of pregnancy, doxycycline, in addition to the gyrase inhibitors ciprofloxacin and norfloxacin (e7). Due to their ototoxicity, systemic treatment with aminoglycosides should only be used when absolutely essential.
Heart valve replacement during pregnancy is associated with increased maternal mortality. Its rates of prenatal and perinatal child mortality are 25% to 30% (20). However, for severe, acute, treatment-resistant valve insufficiency, obstruction of a duct or shunt, or treatment-resistant staphylococcal endocarditis, risk benefit analysis may favor heart surgery.
Due to a paradigm shift regarding prophylactic antibiotic treatment for endocarditis, it is now indicated only for high-risk patients, similar to the indications for non-pregnant patients. Prophylactic antibiotic treatment should be administered to women who undergo dental surgery during pregnancy following endocarditis and following heart valve replacement, for example. Prophylaxis during delivery is not recommended according to the guidelines of the European Society of Cardiology (7).
For antibiotic prophylaxis of rheumatic fever, the recommendations are the same as for non-pregnant patients: continuous prophylaxis for at least five years or until the age of 21 years. This affects mainly young women who are not from Western Europe or North America.
Coronary heart disease and myocardial infarction
According to data of the MONICA/KORA myocardial infarction registry (Augsburg) from 1987 and 2007, the number of men and women in Germany dying of myocardial infarction is falling. Heart attacks are becoming more common among young women, however (21).
Nevertheless, clinical manifestations of coronary heart disease occur only rarely in pregnant women. As a result, very few cases of myocardial infarction during pregnancy or childbirth have been reported (22). In these cases the reported mortality rates were high, at 20% to 37% for the mother and 17% for the infant (e8, e9).
Risk factors and pathophysiology
The most common risk factors among women are nicotine consumption and diabetes mellitus (e10). Others include the following:
Hyperlipidemia
Arterial hypertension (particularly preeclampsia/eclampsia)
Obesity
Family history
Hyperhomocysteinemia (3).
Alongside common plaque rupture in a coronary vessel altered due to arteriosclerosis, angina pectoris symptoms and myocardial infarction also occur in angiographically normal coronary vessels. These are due to local coronary thromboses and continuous coronary spasms (sometimes caused by bromocriptine, oxytocin and prostaglandin). One particular form of coronary heart disease not caused by arteriosclerosis is spontaneous dissection of the coronary arteries, which occurs mainly in the third trimester and up to three months postpartum (23). Pathophysiologically, there is infiltration of eosinophils into the adventitia (8). Coronary abnormalities and vasculitis can also lead to myocardial ischemia during pregnancy and childbirth (3).
Treatment
Beta-1selective beta-blockers are the drugs of choice for coronary heart disease. Nitrates can also be used in symptomatic patients. Thrombocyte aggregation inhibition using acetylsalicylic acid (ASA) as secondary prevention is controversial, due to the increased risk of hemorrhage (22). However, it should be administered following coronary intervention (PCI) with stent implantation and following myocardial infarction. There are insufficient data available on clopidogrel. ACE inhibitors are contraindicated after the first trimester. Statins should not be prescribed, as they have not yet been proved safe (24).
Management of acute coronary syndrome (ACS) and myocardial infarction require close cooperation between cardiologists and gynecologists. Coronary angiography with possible PCI is preferable to thrombolysis, particularly when spontaneous dissection of the coronary arteries is suspected. Bare stents should be used where possible. During the first trimester extremely meticulous risk/benefit analysis must be carried out, due to the potential harm to the fetus (25). No teratogenic effects of thrombolysis have been described, as thrombolytic drugs do not generally cross the placental barrier. However, there is an increased risk of hemorrhage.
Conclusion
Women of child-bearing age with known heart valve defects, evidence of heart disease, or risk factors for coronary heart disease should be treated early. In addition to their family physicians, they should also be counseled and treated by an interdisciplinary team of gynecologists, pediatric and adult cardiologists, and, if necessary, cardiac surgeons, in order to minimize maternal and fetal mortality. As individual doctors will be faced with such patients only rarely, guidelines are particularly important.
Key Messages.
Cardiovascular diseases are the most common cause of maternal death during pregnancy in the Western industrialized world. Women of child-bearing age with heart disease or cardiovascular risk factors should therefore be counseled and treated early by an interdisciplinary team of gynecologists, cardiologists, and, if necessary, cardiac surgeons.
Patients with severe stenotic heart valve disorders should undergo catheterization or surgery before becoming pregnant.
Oral anticoagulants must be discussed on an individual basis for mechanical heart valve replacement. They are associated with an increased risk of hemorrhage and coumarin embryopathy. Recent studies have shown the risk to be low and dose-dependent.
Prophylaxis for endocarditis is only indicated for high-risk patients. Prophylaxis during delivery is not recommended according to the most recent guidelines of the European Society of Cardiology.
Spontaneous dissection of the coronary arteries, which is most common in the third trimester and up to three months postpartum, should ideally be treated using catheterization and implantation of a bare stent.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
Footnotes
Conflict of interest statement
Dr. Kruck received fees for talks from Sanofi-Aventis, Daiichi Sankyo, Berlin-Chemie, Astra Zeneca, and Pfizer. The other authors declare that no conflict of interest exists.
References
- 1.Rath W, Fischer T. The diagnosis and treatment of hypertensive disorders of pregnancy: new findings for antenatal and inpatient care. Dtsch Arztebl Int. 2009;106:733–738. doi: 10.3238/artebl.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaemmerer H, Hess J. Adult patients with congenital heart abnormalities: present and future. Dtsch Med Wochenschr. 2005;130:97–101. doi: 10.1055/s-2005-837381. [DOI] [PubMed] [Google Scholar]
- 3.Regitz-Zagrosek V, Gohlke-Bärwolf C, Geibel-Zehender A, Haass M, Kaemmerer H, Kruck I, Nienaber C. Heart diseases in pregnancy. Clin Res Cardiol. 2008;97:630–665. doi: 10.1007/s00392-008-0685-2. [DOI] [PubMed] [Google Scholar]
- 4.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Trappe HJ. Antiarrhythmische Therapie in der Schwangerschaft. Dtsch Arztebl. 2006;103(Heft 30):A 2036–A 2040. [Google Scholar]
- 6.Chen MM, Coakley FV, Kaimal A, Laros RK., Jr Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol. 2008;112:333–340. doi: 10.1097/AOG.0b013e318180a505. [DOI] [PubMed] [Google Scholar]
- 7.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 8.Kamran M, Guptan A, Bogal M. Spontaneous coronary artery dissection: case series and review. J Invasive Cardiol. 2008;20:553–559. [PubMed] [Google Scholar]
- 9.Shivvers SA, Wians FH, Jr, Keffer JH, Ramin SM. Maternal cardiac troponin I levels during normal labor and delivery. Am J Obstet Gynecol. 1999;180 doi: 10.1016/s0002-9378(99)70161-4. [DOI] [PubMed] [Google Scholar]
- 10.Siu SC, Sermer M, Harrison DA, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. 1997;96:2789–2794. doi: 10.1161/01.cir.96.9.2789. [DOI] [PubMed] [Google Scholar]
- 11.Gesundheitsberichterstattung. Krankenhausstatistik-Diagnosedaten ICD10 angeborene und erworbene Aorten -und Mitralklappenerkrankungen bei Frauen. Statistisches Bundesamt. Robert Koch-Institut Berlin: 2008. [Google Scholar]
- 12.Yap SC, Drenthen W, Pieper PG, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Int J Cardiol. 2008;126:240–246. doi: 10.1016/j.ijcard.2007.03.134. [DOI] [PubMed] [Google Scholar]
- 13.Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984-1996. Am J Obstet Gynecol. 1998;179:1643–1653. doi: 10.1016/s0002-9378(98)70039-0. [DOI] [PubMed] [Google Scholar]
- 14.Gohlke-Bärwolf C, Pildner von Steinburg S, Kaemmerer H, Regitz-Zagrosek V. Antikoagulation und Gerinnungsstörungen in der Schwangerschaft. Internist. 2008;49:779–787. doi: 10.1007/s00108-008-2071-6. [DOI] [PubMed] [Google Scholar]
- 15.Klieverik LM, Takkenberg JJ, Bekkers JA, Roos-Hesselink JW, Witsenburg M, Bogers AJ. The Ross operation: a Trojan horse? Eur Heart J. 2007;28:1993–2000. doi: 10.1093/eurheartj/ehl550. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer C, Hannemann D, Meister R, et al. Vitamin K antagonists and pregnancy outcome. A multi-centre prospective study. Thromb Haemost. 2006;95:949–957. doi: 10.1160/TH06-02-0108. [DOI] [PubMed] [Google Scholar]
- 17.van Driel D, Wesseling J, Sauer PJ, Touwen BC, van der Veer E, Heymans HS. Teratogen update: fetal effects after in utero exposure to coumarins overview of cases, follow-up findings, and pathogenesis. Teratology. 2002;66:127–140. doi: 10.1002/tera.10054. [DOI] [PubMed] [Google Scholar]
- 18.Vitale N, De Feo M, De Santo LS, Pollice A, Tedesco N, Cotrufo M. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol. 1999;33:1637–1641. doi: 10.1016/s0735-1097(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 19.Cotrufo M, De Feo M, De Santo LS, et al. Risk of warfarin during pregnancy with mechanical valve prostheses. Obstet Gynecol. 2002;99:35–40. doi: 10.1016/s0029-7844(01)01658-1. [DOI] [PubMed] [Google Scholar]
- 20.Hall R, Olivier GJ, Rossouw D, Grove AF, Doubell D. Pregnancy outcome in women with prosthetic heart valves. J Obstet Gynaecol. 2001;21:149–153. doi: 10.1080/01443610020026047. [DOI] [PubMed] [Google Scholar]
- 21.Gesundheitsberichterstattung Statistisches Bundesamt. Letalität des Myokardinfarkt. Robert Koch -Institut Berlin: 1987-2007. MONICA/KORA Herzinfarktregister Augsburg. [Google Scholar]
- 22.Wilson AM, Boyle AJ, Fox P. Management of ischaemic heart disease in women of child-bearing age. Intern Med J. 2004;34:694–697. doi: 10.1111/j.1445-5994.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- 23.Bac DJ, Lotgering FK, Verkaaik AP, Deckers JW. Spontaneous coronary artery dissection during pregnancy and post partum. Eur Heart J. 1995;16:136–138. doi: 10.1093/eurheartj/16.1.136. [DOI] [PubMed] [Google Scholar]
- 24.Edison RJ, Muenke M. Central nervous system and limb anomalies in case reports of first-trimester statin exposure. N Engl J Med. 2004;350:1579–1582. doi: 10.1056/NEJM200404083501524. [DOI] [PubMed] [Google Scholar]
- 25.Johnson LW, Moore RJ, Balter S. Review of radiation safety in the cardiac catheterization laboratory. Cathet Cardiovasc Diagn. 1992;25:186–194. doi: 10.1002/ccd.1810250304. [DOI] [PubMed] [Google Scholar]
- e1.Kaemmerer H, Hess J. Congenital heart disease. Transition from adolescence to adulthood. Internist. 2009;50:1221-1222–1224-1227. doi: 10.1007/s00108-009-2400-4. [DOI] [PubMed] [Google Scholar]
- e2.Hilfiker-Kleiner D, Schieffer E, Meyer GP, Podewski E, Drexler H. Postpartum cardiomyopathy: a cardiac emergency for gynecologists, general practitioners, internists, pulmonologists, and cardiologists. Dtsch Arztebl Int. 2008;105:751–756. doi: 10.3238/arztebl.2008.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Geva T, Mauer MB, Striker L, Kirshon B, Pivarnik JM. Effects of physiologic load of pregnancy on left ventricular contractility and remodeling. Am Heart J. 1997;133:53–59. doi: 10.1016/s0002-8703(97)70247-3. [DOI] [PubMed] [Google Scholar]
- e4.Sivadasanpillai H, Srinivasan A, Sivasubramoniam S, et al. Long-term outcome of patients undergoing balloon mitral valvotomy in pregnancy. Am J Cardiol. 2005;95:1504–1506. doi: 10.1016/j.amjcard.2005.02.025. [DOI] [PubMed] [Google Scholar]
- e5.Montoya ME, Karnath BM, Ahmad M. Endocarditis during pregnancy. South Med J. 2003;96:1156–1157. doi: 10.1097/01.SMJ.0000054503.18393.1E. [DOI] [PubMed] [Google Scholar]
- e6.Avila WS, Rossi EG, Ramires JA, et al. Pregnancy in patients with heart disease: experience with 1 000 cases. Clin Cardiol. 2003;26:135–142. doi: 10.1002/clc.4960260308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Schaefer C, Spielmann H, Vetter K. München: Urban & Fischer; 2006. Arzneiverordnung in Schwangerschaft und Stillzeit. [Google Scholar]
- e8.Hartel D, Sorges E, Carlsson J, Romer V, Tebbe U. Myocardial infarction and thromboembolism during pregnancy. Herz. 2003;28:175–184. doi: 10.1007/s00059-003-2453-4. [DOI] [PubMed] [Google Scholar]
- e9.Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol. 2008;52:171–180. doi: 10.1016/j.jacc.2008.03.049. [DOI] [PubMed] [Google Scholar]
- e10.Hennekens CH. Risk factors for coronary heart disease in women. Cardiol Clin. 1998;16:1–8. doi: 10.1016/s0733-8651(05)70378-7. [DOI] [PubMed] [Google Scholar]