Abstract
Background
The vital importance of imaging techniques in radiation oncology now extends beyond diagnostic evaluation and treatment planning. Recent technical advances have enabled the integration of various imaging modalities into the everyday practice of radiotherapy directly at the linear accelerator, improving the management of inter- and intrafractional variations.
Methods
We present the topic of image-guided radiotherapy (IGRT) on the basis of a selective review of the literature.
Results
IGRT can be performed with the aid of ultrasound, 2D X-ray devices, and computed tomography. It enables instant correction for positioning deviations and thereby improves the precision of daily radiotherapy fractions. It also enables immediate adjustment for changes in the position and filling status of the internal organs. Anatomical changes that take place over the course of radiotherapy, such as weight loss, tumor shrinkage, and the opening of atelectases, can be detected as they occur and accounted for in dosimetric calculations. There have not yet been any randomized controlled trials showing that IGRT causes fewer adverse effects or improves tumor control compared to conventional radiotherapy.
Conclusion
IGRT is more precise and thus potentially safer than conventional radiotherapy. It also enables the application of special radiotherapeutic techniques with narrow safety margins in the vicinity of radiosensitive organs. Proper patient selection for IGRT must take account of the goals of treatment and the planning characteristics, as well as the available technical and human resources. IGRT should be used for steep dose gradients near organs at risk, for highly conformal dose distributions in the gastrointestinal tract where adjustment for filling variations is needed, for high-precision dose escalation to avoid geographic miss, and for patients who cannot lie perfectly still because of pain or claustrophobia.
High-quality imaging by means of ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET)-CT is vital for diagnostic evaluation and treatment planning in radiation therapy. A local treatment such as radiotherapy depends crucially on the precision of the imaging procedure by which the target tissue is defined. Only by this means, together with precise tumor staging and accurate assessment of the constellation in the individual patient, can all tumor volumes be confidently identified and healthy tissue reliably spared. This is the basis for successful treatment with a minimal rate of complications.
Recent technical innovations have enabled integration of various imaging modalities directly into the linear accelerator. To date, cross-sectional imaging has been used for treatment planning. The subsequent treatment fractions are determined accordingly and the daily treatment settings are reproduced by markings on the skin or on fixation aids such as masks or vacuum mattresses. This can be checked by 2D X-ray or digital imaging, guaranteeing reproducibility within certain limits. Depending on the variation in positioning permitted by the chosen method of immobilization, positional deviations of several millimeters may occur (1, 2). In many treatment modalities this can be accounted for by the selection of adequate safety margins during the planning stage, thus assuring reliable coverage of the target volume.
Direct integration of imaging at the linear accelerator enables daily monitoring of patient positioning, tumor position, and alterations in patient anatomy, e.g., tumor shrinkage or the reinflation of an atelectasis (3). This permits immediate reaction to changes from the imaging findings at the planning stage (4). Any imprecision in positioning can be instantly corrected, and anatomic alterations that require modification of the irradiation procedure can be recognized as soon as they occur and the necessary measures taken. The options for radiation oncology have thus been expanded by image-guided radiotherapy (IGRT). Particularly during courses of treatment extending over a number of weeks, substantial changes can occur; with no modification of the original treatment plan, there may be pronounced deviations in dose distribution, tumor control, and the likelihood of adverse effects (5, 6).
This article describes the background, motivation, technical implementation, and application of IGRT. We carried out a selective review of the publications in PubMed using the search terms “IGRT,” “image guidance,” “image-guided radiotherapy,” “cone-beam CT, “in-room CT,” “fan-beam CT,” and “tomotherapy”.
Interfractional variations
Modern irradiation procedures permit individual dose gradients of just a few millimeters. This degree of precision enables computer calculation of highly precise radiation treatment plans on static planning images. In reality, however, it is not static CT scans that are being treated but patients with all the variables they bring. The alterations and deviations that can occur from one treatment session (fraction) to the next are described as interfractional variations.
Positioning
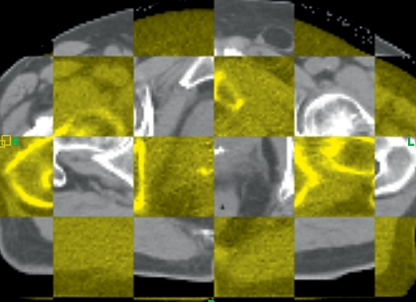
The first crucial factor is the positioning of the patient. The accuracy and reproducibility of positioning are strongly dependent on the anatomic region involved and on the positioning aids used. Various systems are employed to help place the patient in exactly the same position as for planning. Despite immobilization with the assistance of masks, shoulder fixators, arm positioning devices, or vacuum mattresses, variations of as much as 10 mm or more between individual fractions may occur (2, 7) (Figure 1). All of these methods depend on surface markings, but the actual position inside the body of, for instance, a lung tumor or an abdominal organ can change considerably (8).
Figure 1.
Example of positional deviation (superimposition of planning CT [gray] and position-monitoring CT [yellow]): altered position of the prostate. Image-guided radiotherapy accounts for the change in position.
IGRT enables the detection and immediate correction of such deviations and thus increases the precision of delivery. This is particularly important in the following circumstances:
Modern high-precision techniques with individual dose distribution
Escalation of dose in the target volume
Sparing of adjacent radiosensitive organs.
In these situations small deviations in positioning can lead to large variations in dose distribution.
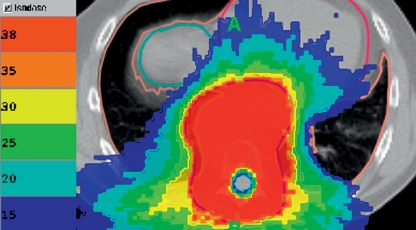
While conventional methods using a small number of conformal static fields showed limited susceptibility to such variations, in high-precision techniques these changes can result in underdosing of the target volume or overexposure of an organ that should be spared. Figure 2 illustrates this with a case of cord-sparing reirradiation. After previous irradiation of the spinal column a recurring plasmocytoma was treated conformally and the circumscribed spinal cord was spared. In such cases IGRT ensures precision of dose distribution (9).
Figure 2.
Cord-sparing reirradiation of recurrent plasmocytoma. Image-guided radiotherapy ensures precise application.
Organ mobility
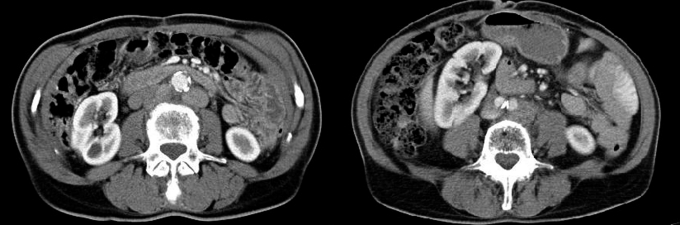
The mobility of internal organs is a further variable. Even with perfect positioning of skeletal structures, the position of the kidney, to name but one organ, can vary by up to several centimeters. In the case of ventral displacement, for example, radiotherapy of para-aortic lymph nodes can result in renal damage (Figure 3).
Figure 3.
Two contrast-enhanced CT scans of the abdomen show the positional variability of the right kidney. Imaging with the patient in exactly the position for treatment can avoid unintended exposure and damage of the kidney.
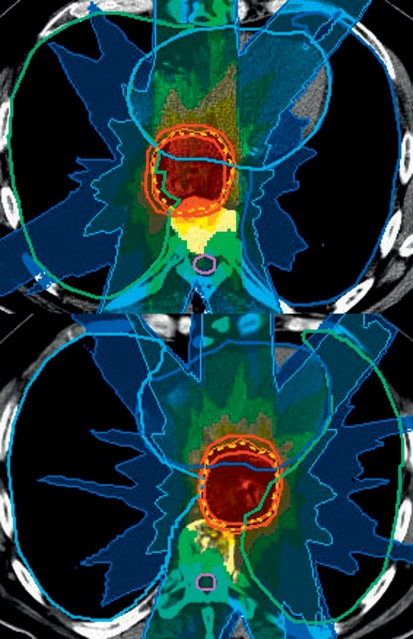
In the thorax, the esophagus can show enormous variations in position, as illustrated in Figure 4. A highly conformal treatment plan that does not involve targeting of the whole mediastinum is sensitive to this lateral displacement of the esophagus. Depending on the position determined by IGRT, irradiation can be planned accordingly and a geographic miss avoided (10).
Figure 4.
Positional variability of the esophagus, depicted by means of in-room CT. Above: The original situation and the corresponding treatment plan. Below: In the case of lateral displacement an alternative plan can be employed. Image-guided radiotherapy prevents a geographic miss.
In radiotherapy of the prostate, variation in the filling status of the rectum can have a considerable effect on the prostate dose and on rectal exposure. In the case of high-risk prostate cancers (as defined by TNM stage, PSA value, and Gleason score), the desired therapeutic dose is as high as 80 Gy or even more—this is referred to as dose escalation. To minimize acute and chronic gastrointestinal toxicity, the rectum is spared by means of techniques such as intensity-modulated radiotherapy (IMRT). An excessively full rectum is displaced forward into the high-dose zone, possibly resulting in much higher exposure of the anterior rectal wall (Figure 5) (11, 12). With image guidance this can be recognized in time and the treatment can be carried out later under better conditions (13).
Figure 5.
Radiotherapy of the prostate: chessboard superimposition of planning CT (gray) and monitoring CT (yellow). Even with perfect matching of skin markings, variations in rectal filling can result in unintended overexposure of the rectum.
Anatomic variations during treatment
A change in density caused by alterations in the shape, volume and environment of a tumor can affect the tissue attenuation of X-ray beams. For example, the combination of radiogenic mucositis and chemotherapy-induced nausea during a long course of combined radiochemotherapy for an ENT tumor can lead to drastic weight loss. This may lead in turn to increased movement of the target structures towards the center of the body, resulting in increased exposure of the skin and salivary glands. Such phenomena should ideally be avoided by appropriate supportive measures and nutritional therapy to minimize treatment toxicity, but if weight loss still occurs it must be detected as early as possible. The imaging procedure used to check the patient’s position can also be exploited to calculate the current dose distribution. This gives the radiotherapist the opportunity to modify the irradiation plan and thus ensure optimal treatment over a period of several weeks (14– 16).
As well as weight loss, changes in tumor volume can also have a significant impact. Lymphomas, for instance, may decrease rapidly in size after the commencement of radiotherapy. Should such a lymphoma lie directly adjacent to a radiosensitive structure, tumor shrinkage may reduce attenuation of the X-ray beam and conceivably lead to increased exposure of the neighboring entity. Growth in tumor size during radiotherapy also has consequences. An increase in volume owing to an inflammatory reaction or bleeding may lead to expansion of the tumor out of the target area, so that it does not receive the envisaged dose. If true tumor progression occurs during a course of irradiation, consideration should be given to discontinuing treatment. Without image guidance in such a situation, the treatment would be continued and the patient subjected to ineffective therapy with inappropriate exposure to radiation. IGRT enables a timely change to another form of treatment. The monitoring of treatment progress in conventional radiotherapy serves principally to verify the target; soft tissue alterations may be overlooked. CT scanning is necessary if such changes are to be detected.
Alterations in the vicinity of a tumor, such as reinflation of an atelectasis or change in size of an effusion in the treatment of bronchial carcinoma, can be detected early and their impact on the planned irradiation dose calculated.
Intrafractional motion
When interfractional variations are recognized and either remedied or the treatment plan adapted to the altered anatomy, improved treatment precision is the result. Uncertainty remains, however, over what we call intrafractional variation, i.e., the sum of the changes that may occur during a session of radiotherapy. Because primarily movement phenomena are involved, this parameter is often simply referred to as intrafractional motion (17).
The first factor is movement by the patient. This must be prevented or minimized by clear explanation of the importance of staying still and by the use of suitable means of immobilization.
The second factor is respiratory motion, which particularly affects the position of lung and liver tumors.
While some tumors move only a few millimeters or not at all, displacements of up to 3 cm have been described (18). Movement of at-risk structures such as the kidneys or the stomach must also be accounted for. On the one hand such displacements can be reduced (to less than 1 cm) by means such as abdominal compression (19); on the other, registration of maximal amplitude of motion by time-resolved computed tomography (4D CT) enables selection of a safety margin that embraces the whole space within which the relevant structures may move.
Another strategy is to restrict irradiation to particular phases of respiration, so-called “gating” (20). By this means smaller safety margins can be selected and the exposure of neighboring tissues reduced somewhat. However, this technique requires not only initial registration of motion but also monitoring during the treatment itself. IGRT, with its continuous recording of tumor position, makes gating possible. There are also methods allowing the X-ray beam to follow the mobile tumor. This “tracking” of a tumor accelerates the treatment by shortening the inactive intervals. However, tracking involves highly complex techniques and apparatus in the form of mobile robot arms or rapid multileaf collimators.
Both gating and tracking are costly and elaborate procedures. They are necessary in only a small proportion of patients and are employed if conventional methods would be associated with excessive exposure of at-risk organs.
Technical implementation
To account for the above-mentioned uncertainty factors, the changes we have described can now be visualized directly at the linear accelerator and the necessary corrections can be made immediately (21). There are various technical solutions that permit integration of the individual imaging modalities.
Soft tissue structures such as the prostate can be depicted by ultrasound and correlated with the CT planning scan with the aid of a stereotactic coordinate system (22). Positioning can also be checked by means of digital radiography in two projections, but this form of imaging is largely restricted to bony structures. The method therefore lends itself primarily to the depiction of small masses in or immediately adjacent to bone, e.g., osseous metastases or skull base tumors. Soft-tissue structures can be visualized better with volumetric imaging by means of CT, using either a separate CT scanner in the treatment room or new models of linear accelerators (23– 25, e1, e2). Figure 6 shows a CT scanner integrated into the linear accelerator.
Figure 6.
Accelerator with integrated cone-beam CT (accelerator as radiation source)
Integration into clinical radiotherapy
IGRT is a complex modality. It requires special technical facilities and represents a challenge in terms of financial and personnel resources. The current standard consists in reducing the variations that occur to a minimum and accounting for them when selecting safety margins. Treatment can also be monitored by employing suitable targeting procedures such as stereotaxy and obtaining control images in the form of 2D X-rays or CT scans. Daily image guidance directly at the linear accelerator with immediate correction is much more expensive with regard to both capital and maintenance costs and places much higher demands on human resources in terms of examination time, staff training, and quality assurance.
There have been no randomized controlled trials to compare toxicity and efficacy. Nevertheless, IGRT is an important method in radio-oncology, facilitating the safe and accurate use of modern techniques such as intensity-modulated radiotherapy or particle therapy (3). Critically, the methods described form just one link in the chain extending from diagnosis via imaging, immobilization, and planning through to daily precise administration of radiotherapy doses. Only when all these elements are in harmony can the full potential of radiotherapy be realized.
It is also important to remember that by no means every patient needs image guidance. In many cases the variations we have discussed can be included in the calculations in advance and adequate safety margins selected to ensure safe administration of the intended tumor dose. IGRT should be utilized in situations where this is not possible owing to tumor type, tumor site, the intended dose, or the presence of neighboring at-risk structures. This is the case for tumors in the immediate vicinity of the spinal cord, brain stem, optic nerve, or parotid gland, especially if dose escalation is desired or particular attention has to be paid to organ sparing in the case of reirradiation. Moreover, IGRT is advantageous in the treatment of abdominal or pelvic tumors, because variations in gastrointestinal filling can cause organ displacement (e3); a good example is provided by high-dose radiotherapy of prostate cancer. The medical societies have not yet issued any guidelines on the indications for IGRT. In particular, there are no randomized controlled trials showing any advantage of IGRT with regard to the spectrum of adverse effects or the likelihood of controlling the tumor.
Furthermore, it should be noted that the supplementary imaging involved in IGRT exposes the patient to more radiation. The extra dose amounts to between 0.1% and 3% of the treatment dose, depending on the technique used, so IGRT should not be employed without good reason (e4). If, however, this additional dose enables narrower safety margins and guarantees precise administration of the therapeutic radiation at the correct site, the overall exposure will be lower and the risk to the patient smaller. A reduction of safety margins can reduce the exposure of adjacent structures and thus lower the likelihood of adverse effects.
IGRT raises both capital expenditure and maintenance costs. Moreover, the increased time required to administer each fraction—ca. 5 min—decreases patient throughput. On the other hand, IGRT is assumed to increase safety and precision and reduce the probability of adverse effects. Furthermore, precise application of high-precision techniques may make it more likely that radiotherapy will be successful. These assumptions remain to be proved. Finally, improved tolerability has to be weighed against increased costs.
Conclusion
Image-guided radiotherapy can make conventional radiotherapy procedures safer by virtue of increased precision of delivery. Moreover, it facilitates the precise application of specialized irradiation techniques with narrow safety margins to radiosensitive organs. Painstaking selection of patients is crucial, giving proper consideration to the treatment goal, the nature of the treatment plan, and responsible use of technical resources. IGRT should be used in the following circumstances:
Steep dose gradients to at-risk structures in the immediate vicinity
Highly conformal dose distributions in the gastrointestinal tract to detect filling variations
Dose escalation by means of high-precision techniques to decrease the danger of geographic miss
Patients who cannot lie perfectly still because of pain or claustrophobia.
Key Messages.
The term “image-guided radiotherapy” (IGRT) describes direct integration of imaging into the linear accelerator.
Imprecision can arise even in properly immobilized patients; we distinguish between interfractional and intrafractional variation.
IGRT increases the precision of radiotherapy by instant correction of deviations.
Image guidance is not always needed; it is used for mobile tumors, tumors immediately adjacent to at-risk organs, and in difficult cases of reirradiation.
There have been no randomized controlled trials of the toxicity and efficacy of IGRT compared with conventional radiotherapy
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Kupelian PA, Lee C, Langen KM, et al. Evaluation of image-guidance strategies in the treatment of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1151–1157. doi: 10.1016/j.ijrobp.2007.07.2371. [DOI] [PubMed] [Google Scholar]
- 2.Sterzing F, Kalz J, Sroka-Perez G, et al. Megavoltage CT in helical tomotherapy - clinical advantages and limitations of special physical characteristics. Technol Cancer Res Treat. 2009;8:343–352. doi: 10.1177/153303460900800504. [DOI] [PubMed] [Google Scholar]
- 3.Allison RR, Gay HA, Mota HC, Sibata CH. Image-guided radiation therapy: current and future directions. Future Oncol. 2006;2:477–492. doi: 10.2217/14796694.2.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Dawson LA, Sharpe MB. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol. 2006;7:848–858. doi: 10.1016/S1470-2045(06)70904-4. [DOI] [PubMed] [Google Scholar]
- 5.Xing L, Thorndyke B, Schreibmann E, et al. Overview of image-guided radiation therapy. Med Dosim. 2006;31:91–112. doi: 10.1016/j.meddos.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Morin O, Aubin M, Bucci MK, Chuang CF, Pouliot J. Dose-guided radiation therapy with megavoltage cone-beam CT. Br J Radiol. 2006;79(Spec No 1):87–98. doi: 10.1259/bjr/60612178. [DOI] [PubMed] [Google Scholar]
- 7.Zeidan OA, Langen KM, Meeks SL, et al. Evaluation of image-guidance protocols in the treatment of head and neck cancers. Int J Radiat Oncol Biol Phys. 2007;67:670–677. doi: 10.1016/j.ijrobp.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Stoiber EM, Lechsel G, Giske K, et al. Quantitative assessment of image-guided radiotherapy for paraspinal tumors. Int J Radiat Oncol Biol Phys. 2009;75:933–940. doi: 10.1016/j.ijrobp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Sterzing F, Herfarth K, Debus J. IGRT with helical tomotherapy—effort and benefit in clinical routine. Strahlenther Onkol. 2007;183(Spec No 2):35–37. doi: 10.1007/s00066-007-2014-5. [DOI] [PubMed] [Google Scholar]
- 10.Jensen AD, Grehn C, Nikoghosyan A, et al. Catch me if you can—the use of image guidance in the radiotherapy of an unusual case of esophageal cancer. Strahlenther Onkol. 2009;185:469–473. doi: 10.1007/s00066-009-1935-6. [DOI] [PubMed] [Google Scholar]
- 11.Guckenberger M, Pohl F, Baier K, Meyer J, Vordermark D, Flentje M. Adverse effect of a distended rectum in intensity-modulated radiotherapy (IMRT) treatment planning of prostate cancer. Radiother Oncol. 2006;79:59–64. doi: 10.1016/j.radonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kalz J, Sterzing F, Schubert K, Sroka-Perez G, Debus J, Herfarth K. Dosimetric comparison of image guidance by megavoltage computed tomography versus bone alignment for prostate cancer radiotherapy. Strahlenther Onkol. 2009;185:241–247. doi: 10.1007/s00066-009-1913-z. [DOI] [PubMed] [Google Scholar]
- 13.El-Bassiouni M, Davis JB, El-Attar I, Studer GM, Lutolf UM, Ciernik IF. Target motion variability and on-line positioning accuracy during external-beam radiation therapy of prostate cancer with an endorectal balloon device. Strahlenther Onkol. 2006;182:531–536. doi: 10.1007/s00066-006-1581-1. [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Langen KM, Lu W, et al. Assessment of parotid gland dose changes during head and neck cancer radiotherapy using daily megavoltage computed tomography and deformable image registration. Int J Radiat Oncol Biol Phys. 2008;71:1563–1571. doi: 10.1016/j.ijrobp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Langen KM, Meeks SL, Poole DO, et al. The use of megavoltage CT (MVCT) images for dose recomputations. Phys Med Biol. 2005;50:4259–4276. doi: 10.1088/0031-9155/50/18/002. [DOI] [PubMed] [Google Scholar]
- 16.Morin O, Chen J, Aubin M, et al. Dose calculation using megavoltage cone-beam CT. Int J Radiat Oncol Biol Phys. 2007;67:1201–1210. doi: 10.1016/j.ijrobp.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 17.Huntzinger C, Munro P, Johnson S, et al. Dynamic targeting image-guided radiotherapy. Med Dosim. 2006;31:113–125. doi: 10.1016/j.meddos.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Hof H, Rhein B, Haering P, Kopp-Schneider A, Debus J, Herfarth K. 4D-CT-based target volume definition in stereotactic radiotherapy of lung tumours: comparison with a conventional technique using individual margins. Radiother Oncol. 2009;93:419–423. doi: 10.1016/j.radonc.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 19.Hof H, Herfarth K, Debus J. Stereotaktische Bestrahlung von Lungentumoren. 2004;44:484–490. doi: 10.1007/s00117-004-1040-x. [DOI] [PubMed] [Google Scholar]
- 20.Guckenberger M, Krieger T, Richter A, et al. Potential of image-guidance, gating and real-time tracking to improve accuracy in pulmonary stereotactic body radiotherapy. Radiother Oncol. 2009;91:288–295. doi: 10.1016/j.radonc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Jaffray D, Kupelian P, Djemil T, Macklis RM. Review of image-guided radiation therapy. Expert Rev Anticancer Ther. 2007;7:89–103. doi: 10.1586/14737140.7.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Boda-Heggemann J, Kohler FM, Kupper B, et al. Accuracy of ultrasound-based (BAT) prostate-repositioning: a three-dimensional on-line fiducial-based assessment with cone-beam computed tomography. Int J Radiat Oncol Biol Phys. 2008;70:1247–1255. doi: 10.1016/j.ijrobp.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Thieke C, Malsch U, Schlegel W, et al. Kilovoltage CT using a linac-CT scanner combination. Br J Radiol. 2006;79(Spec No 1):79–86. doi: 10.1259/bjr/88849490. [DOI] [PubMed] [Google Scholar]
- 24.Pouliot J. Megavoltage imaging, megavoltage cone beam CT and dose-guided radiation therapy. Front Radiat Ther Oncol. 2007;40:132–142. doi: 10.1159/000106032. [DOI] [PubMed] [Google Scholar]
- 25.Morin O, Gillis A, Chen J, et al. Megavoltage cone-beam CT: system description and clinical applications. Med Dosim. 2006;31:51–61. doi: 10.1016/j.meddos.2005.12.009. [DOI] [PubMed] [Google Scholar]
- e1.Mackie TR, Balog J, Ruchala K, et al. Tomotherapy. Semin Radiat Oncol. 1999;9:108–117. doi: 10.1016/s1053-4296(99)80058-7. [DOI] [PubMed] [Google Scholar]
- e2.Mackie TR, Holmes T, Swerdloff S, et al. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709–1719. doi: 10.1118/1.596958. [DOI] [PubMed] [Google Scholar]
- e3.Perkins CL, Fox T, Elder E, Kooby DA, Staley CA, 3rd, Landry J. Image-guided radiation therapy (IGRT) in gastrointestinal tumors. Jop. 2006;7:372–381. [PubMed] [Google Scholar]
- e4.Ding GX, Coffey CW. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys. 2009;73:610–617. doi: 10.1016/j.ijrobp.2008.10.006. [DOI] [PubMed] [Google Scholar]