Abstract
In the search for new antiparasitic natural compounds from the medicinal plants from Cameroon, the new 22-hydroxyclerosterol, established as such on the basis of detailed chemical and spectroscopic analysis, was isolated from the hexane extract of the stem bark of Allexis cauliflora together with the known clerosterol. 22-Hydroxyclerosterol inhibited the growth of Trypanosoma brucei brucei cells with an ED50 value of 1.56 μM. The compound was also established as an uncompetitive inhibitor of the glycolytic enzyme PGI of T. brucei (Ki’= 3 ± 1 μM), an uncompetitive inhibitor of mammalian rabbit muscles’ enzyme PyK (Ki’= 26 ± 3 μM) and a mixed inhibitor of PyK of Leishmania mexicana (Ki’= 65 ± 10 μM; Ki= 24 ± 5 μM).
Keywords: Clerosterol, Enzyme inhibitor, Trypanocide, Trypanosoma brucei, Stigmastane sterols, Cytotoxicity, NMR, Structure elucidation, Natural products
Introduction
Allexis cauliflora, belonging to the family Violaceae, is commonly distributed from Congo to Cameroon [1]. Its stem bark is used in Cameroonian folk medicines to treat fever and syphilis [2]. Despite the availability of this ethnopharmacological information, there is no phytochemical report published on the genus Allexis to date. However, previous phytochemical studies on some species of other Violaceae genera indicated the occurrence of potentially useful medicinal secondary metabolites such as flavonoids and triterpenoids. They have been reported as matrix metalloproteinase inhibitors [3], topoisomerase inhibitors [4], antioxidants [5] and antiplasmodials [6]. Following an ethnopharmacological survey on medicinal plants used against parasitic diseases in Cameroon, the species A. cauliflora emerged as one of the most commonly used plants in the management of such diseases. As the result of that information, and as a continuation of our efforts to add value to medicinal plants of Cameroon, biological activity and the cytotoxicity profile of A. cauliflora were studied in an attempt to scientifically support its ethnomedical applications.
In this report, chemical constituents of the stem bark of this small tree are described and, specifically, analysed as potential inhibitors of both glycolytic enzymes and the growth of Trypanosoma brucei brucei bloodstream form (strain 427). The isolated compounds were also tested on MRC-5 mammalian cells (fibroblasts) in order to establish their toxicity profile.
Results and Discussion
Chemistry: structural elucidation of isolated compounds
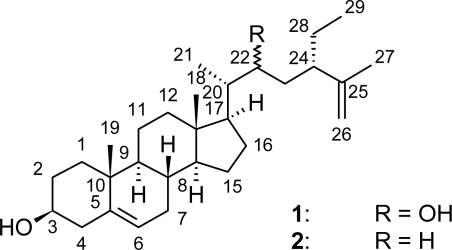
Chromatographic partitioning of the n-hexane extract (9.0 g) of dry stem bark (2.9 kg) of A. cauliflora on silica gel using n-hexane/ethyl acetate mixtures of increasing polarity, led to the isolation of the new 22-hydroxyclerosterol (1) and the known clerosterol (2). The structures of the two compounds (Figure 1) were established on the basis of detailed chemical and spectroscopic analyses and by comparison with previously reported data on clerosterols [7–10]. The known compound 2 was identified as clerosterol by combined analyses of HREIMS (m/z 412 [M+], NMR spectroscopic data (1H NMR, 13C NMR, 1H-1H COSY, HSQC and HMBC) and by comparing its spectroscopic and physical data with those available in the literature on clerosterol [7, 8].
Fig. 1.
Chemical structures of compounds 1 and 2
The new compound 1 (mp: 166–168 °C) gave a positive reaction to the Liebermann-Buchard reagent, suggesting its sterol nature. The 13C NMR spectra of compound 1 displayed signals of 29 carbon atoms that were similar to those of compound 2. A detailed comparison of the 13C NMR chemical shift values of the two compounds (Table 1) indicated that they had the same 3β-hydroxy-Δ5 sterol ring system and they differed only in the functionality of their side chains. A striking difference between compounds 1 and 2 was observed in the 13C chemical shifts for carbon numbered C-22 of the side chain. The signal of this side chain carbon (C-22) was not only dramatically shifted from δ 33.7 in the spectrum of 2 to a lower field (δ 70.7) in the spectrum of 1 but, as indicated by DEPT spectra, also changed from a methylene in compound 2 to a methine in compound 1. Furthermore, HMBC and HSQC spectra revealed that the proton at C-22 in compound 1 (δ 3.6), attached to a carbon atom bearing an oxygen, belong to the side chain as evidenced by a network of correlations between the carbon C-22 and the carbon atoms at δ 12.5 (C-21), 45.7 (C-24) and 52.9 (C-17) through three bonds and with those at δ 33.1 (C-23) and 42.4 (C-20) through two bonds, respectively. Additionally, the difference between compounds 1 and 2 was obtained from the mass spectra which revealed that the molecular mass of 1 (m/z 428 [M+]) was greater by 16 units than that of 2, thus confirming the presence of an extra oxygen atom in compound 1. Based on these evidences, the structure of compound 1 was established as 22-hydroxyclerosterol, a new derivative of clerosterol (Figure 1). Further heterocorrelation across signals in the ring system and the side chain were fully consistent with the proposed structure. Complete 1H and 13C NMR data of 22-hydroxyclerosterol (1) together with HMBC (2JCH, 3JCH) correlations are presented in Table 1.
Tab. 1.
13C NMR Chemical Shifts of compounds 1 and 2 (CDCl3, 125 MHz, TMS).
| # | Compound 1 | Compound 2 | |
|---|---|---|---|
|
| |||
| δc | HMBC of attached H | δc | |
| 1 | 37.3 | 37.3 | |
| 2 | 31.7 | 31.7 | |
| 3 | 71.8 | C-1, C-2, C-4 | 71.8 |
| 4 | 42.3 | 42.3 | |
| 5 | 140.8 | 140.8 | |
| 6 | 121.6 | C-4, C-7, C-10 | 121.7 |
| 7 | 31.9 | 31.9 | |
| 8 | 31.9 | 31.9 | |
| 9 | 50.2 | 51.1 | |
| 10 | 36.5 | 36.5 | |
| 11 | 21.1 | 21.1 | |
| 12 | 39.8 | 39.8 | |
| 13 | 42.7 | 42.3 | |
| 14 | 56.4 | 56.8 | |
| 15 | 24.4 | 28.2 | |
| 16 | 27.5 | 29.4 | |
| 17 | 52.9 | 56.1 | |
| 18 | 11.8 | C-12, C-13, C-14, C-17 | 11.8 |
| 19 | 19.4 | C-1, C-5, C-10, C-11 | 19.4 |
| 20 | 42.4 | 35.5 | |
| 21 | 12.5 | C-17, C-20, C-22 | 18.7 |
| 22 | 70.7 | C-17, C-20, C-21, C-23, C-24 | 33.7 |
| 23 | 33.1 | 24.3 | |
| 24 | 45.7 | 49.5 | |
| 25 | 147.0 | 147.6 | |
| 26 | 112.6 | C-24, C-27 | 111.4 |
| 27 | 17.7 | C-24, C-25, C-26 | 17.8 |
| 28 | 27.0 | 26.5 | |
| 29 | 12.1 | C-24, C-28 | 12.1 |
Biological assays
In vitro assays of compounds 1 and 2 on Trypanosoma brucei cells
The isolated compounds 1 and 2 were tested on Trypanosoma brucei brucei bloodstream form cells cultured in vitro as well as on some glycolytic enzymes in order to establish their trypanocidal activity. The results obtained indicated that compound 1, with an ED50 value of 1.56 μM on the growth of Trypanosoma brucei brucei, was more active on T. brucei cells than previously studied sterols such as saringerol and 24-hydroxyperoxy-24-vinylcholesterol displaying IC50 values ranging from 3.2 to 7.8 μM [11]. Compound 1 was 86 fold more potent than compound 2 (ED50 134.34 μM) which is known to exhibit weak antitrypanosomal activity and to be non toxic to mammalian cells [11].
The significant trypanocidal activity of compound 1 in comparison with that of compound 2 (Table 2) raised a question on its toxicity profile. In this context and for a selectivity concern, compound 1 was tested on growth of the mammalian fibroblast cell line. The result obtained indicated that compound 1 inhibited both mammalian cells and growth of the T. brucei cells at quite similar concentrations (ED50 1.12 μM and ED50 1.56 μM, respectively). This cytotoxicity could be explained by the presence of the hydroxyl group at C-22 in its side chain since compound 2, lacking the hydroxyl functionality in its side chain, is non toxic on mammalian cells.
Tab. 2.
Cytotoxicity of isolated compounds (ED50, μM)
| Compound | Trypanosoma brucei (strain 427) | Mammalian cells (MRC-5 fibroblasts) |
|---|---|---|
| 1 | 1.56 | 1.12 |
| 2 | 134.34 | no effect |
Inhibition of three glycolytic enzymes of T. brucei by isolated compounds
Following the significant activity (ED50= 1.56 μM) of compound 1 on the growth of T. brucei cells, and in an attempt to establish its mechanism of action, it was deemed necessary to test isolated compounds as potential inhibitors of some glycolytic enzymes (Aldolase, PGK, PFK, PGI, GAPDH and PyK).
A global analysis of obtained results (Table 3) showed that compound 1 was selective of the parasite enzyme. It inhibited GAPDH and PGI of T. brucei with an IC50 value of 30 μM.
Tab. 3.
Enzymatic inhibition (IC50, μM)
| PGI | GAPDH | PyK | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Compound | T. brucei | Rabbit muscle | T. brucei | Rabbit muscle | L. mexicana | Rabbit muscle | |
| 1 | 30 ± 10 | n.i. | 30 ± 10 | n.i. | 30 ± 5 | 80 ± 10 | |
| 2 | 45 ± 10 | n.i. | n.i. | n.i. | n.i. | n.i. | |
n.i. … no inhibition observed at a concentration of 50 μM.
Meanwhile, compound 2 was only active on T. brucei PGI and selective of the enzyme with an IC50 value of 45 μM. It has also been shown that 1 was more active on rabbit muscle pyruvate kinase (PyK) than on its isolated homologue from Leishmania mexicana.
Kinetic studies showed that compound 1 was an uncompetitive inhibitor of the phospoglycerate isomerase (PGI) enzyme. Compound 1 inhibited the enzyme (Ki’= 3 ± 1 μM) by binding the fructose-6-phosphate (F6P)/PGI complex with an affinity 76-fold more important than that of the natural substrate (F6P) to the enzyme (PGI). Moreover, it was also shown to be an uncompetitive inhibitor of PyK of rabbit muscle (Ki’= 26 ± 3 μM) and a mixed inhibitor of Pyk of Leishmania mexicana (Ki’= 65 ± 10 μM; Ki= 24 ± 5 μM); whereas compound 2 was successfully shown to be a mixed inhibitor of PGI of T. brucei (Ki= 10 ± 2 μM; Ki’= 68 ± 5 μM).
In conclusion, the isolation and structural elucidation of clerosterol (2) and its new isomer 22-hydroxyclerosterol (1) from stem bark of Allexis cauliflora have been achieved using chemical and spectroscopic methods. Compound 1 has been shown to be more toxic than compound 2 on T. brucei cells. Obtained results for clerosterol are in agreement with those observed by Hoet et al. in 2007 [12].
Experimental
General experimental procedures
Melting points were determined on a Büchi Melting point apparatus B-540 and are uncorrected. NMR spectra (1H and 13C) were recorded using a Bruker DRX 500 spectrometer in CDCl3, with TMS as internal standard at room temperature. Chemical shifts are given in δ and coupling constants (J) in Hz. The EIMS spectra were obtained at 70 eV in a VG Autospec apparatus. HRMS spectra were recorded on a MasSpec sector field mass spectrometer (Micromass Ltd., Manchester, UK) with a direct insertion probe Column chromatography was performed using silica gel (0.063–0.2 mm/70–230 mesh) from Merck. Visualization of the compounds on the chromatographic plates was made under ultraviolet light, exposure to iodine vapor or spraying with 50% diluted sulphuric acid in water, followed by gentle heating.
Plant material
The stem bark of A. cauliflora (Violaceae) were collected at Ebolowa on the Elephants Hill, South Province of Cameroon, in July 2007, and identified by Mr. Nana of the Cameroonian National Herbarium (Yaoundé) where a Voucher specimen (HNC 18374) has been deposited.
Extraction and isolation
Air-dried pieces (2.9 kg) of stem bark of A. cauliflora were powdered and macerated with n-hexane (10 l, 72 h) at room temperature. The residue obtained upon evaporation of solvent to dryness afforded 9 g (0.31%) of crude n-hexane extract.
The major part of the n-hexane extract (8.5 g) was then fractionated over an open silica gel column using n-hexane/EtOAc mixtures of increasing polarities as eluents. The n-hexane/EtOAc (19:1) fraction afforded 104.8 mg (1.23%) of the known clerosterol (2). Further elution with increasing EtOAc concentration afforded 241.4 mg (2.84%) of the new 22-hydroxyclerosterol (1) at n-hexane/EtOAc (9:1). The chemical structures (Figure 1) of isolated compounds were determined by means of spectroscopic methods (1H-NMR, 13C-NMR, 1H-1H COSY, HSQC and HMBC) and by comparing spectroscopic and physical data with those of the literature [7–10].
(3β,24S)-Stigmasta-5,25-diene-3,22-diol (22-Hydroxyclerosterol, 1)
White powder, M.p.: 166–168°C. 1H-NMR (500 MHZ, CDCl3): δ 0.69 (3H, s H-18), 0.83 (3H, t, J = 7.3Hz, H-29), 0.93 (3H, d, J = 6.8Hz, H-21), 0.93 (1H, H-9) 0.97 (1H, H-14), 1.01 (3H, s, H-19), 1.07 (1H, H-1b), 1.07 (1H, H-17), 1.09 (1H, H-15b), 1.17 (1H, H-12b), 1.28 (1H, H-23a), 1.30 (1H, H-16b), 1.33 (1H, H-23b), 1.36 (2H, H-28), 1.46 (1H, H-11b), 1.5 (1H, H-2b), 1.5 (1H, H-7b), 1.5 (1H, H-8), 1.50 (1H, H-11a), 1.58 (1H, H-15a), 1.61 (3H, s, H-27), 1.65 (1H, H-16a), 1.66 (1H, H-20), 1.84 (1H, H-2a), 1.85 (1H, H-1a), 1.97 (1H, H-7a), 2.01 (1H, H-12a), 2.21 (1H, H-24), 2.23 (1H, ddd, J = 12.9, 5.2, 2.1 Hz, H-4b), 2.29 (1H, H-4a), 3.52 (1H, m, H-34), 3.62 (1H, ddd, J = 9.8, 3.2, 2.8 Hz, H-22), 4.77 (1H, H-26a), 4.83 (1H, H-26b), 5.35 (1H, dis. t, J = 5.2 Hz, H-6). HREIMS: m/z 428.366308 [M]+, calcd for C29H48O2: 428.365431.
(3β,24S)-Stigmasta-5,25-dien-3-ol (Clerosterol, 2)
White powder, M.p.: 120–122°C (lit. [7] 120–121°C). 1H NMR (500 MHz, CDCl3 selected signals): δ 0.67 (3H, s H-18), t, J = 7.3 Hz, H-29), 0.91 (3H, d, J = 6.8 Hz, H-21), 1.01 (3H, s, H-19), 1.56 (3H, s, H-27), 3.52 (1H, m, H-3), 4.64, 1H, H-26a), 4.73 (1H, H-26b), 5.35 (1H, H-6). For 13C NMR, see Table 1. HREIMS: m/z 412.368935 [M]+, calculated for C29H48O: 412.370517.
Biology essay procedure
Cell culture
Trypanosoma brucei brucei (strain 427) bloodstream forms were cultured in HMI-9 medium containing 10% heat-inactivated [12]. The mammalian fibroblast cell line MRC-5 was grown in DMEM (Gibco) supplemented with 10% heat-inactivated FBS and extra glutamine. Cells were grown at 37 °C in humidified incubators in an atmosphere of 5% CO2 [13].
Antitrypanosomal and cytotoxicity assays
The assay performed, the long incubation low inoculation test (LITIT), was previously described by Räz et al., 1997 [14] with minor modifications by Baltz et al., 1985 [15]. To evaluate the selectivity of the in vitro antitrypanosomal activity, in vitro cytotoxicity tests were also performed. The same general method was used in order to facilitate the comparison.
The compounds were initially dissolved in DMSO at a concentration of 20 mg/ml and diluted with the medium. Series of dilution were done in 96-well plates; each dilution was tested in duplicate. Cells in suspension were thereafter added to each well at a density such as, after 72 h of incubation, in control wells the culture reached the end of logarithmic growth phase (T. brucei), or adhesive cells have formed a confluent monocellular film (mammal cells MRC-5). The highest concentration of DMSO was 0.5%. After 72 h of incubation, Alamar Blue, a marker of cell viability, was added in each well and fluorescence was quantified after a total incubation time of 76 h at an excitation wavelength of 530 nm and at an emission wavelength of 590 nm. ED50 values were calculated by linear interpolation. Assays with commercial drugs were also carried out in order to have reference values. All results represent average values obtained in at least three independent experiments, each experiment comprising a duplicate set of tests.
Acknowledgments
This work was financially supported by grants from European Commission-FP6 and International Foundation for Science (IFS). We thank Sybille Lorenz for mass spectroscopic analysis and Emily Wheeler for editorial assistance.
Footnotes
This article is available from: http://dx.doi.org/10.3797/scipharm.1012-10
Authors’ Statement
Competing Interests
The authors declare no conflict of interest.
References
- [1].Hutchinson J, Dalziel JM. Crown Agents for oversea Governments and Administrations. 1,2nd Edition. Vol. 1. Millbank; London, S.W: 1954. p. 98. [Google Scholar]
- [2].Stewart M, Bartholomew B, Currie F, Abbiw DK, Latif L, Sarker SD, Nash RJ. Pyranoisoflavones from Rinorea welwitschii. Fitoterapia. 2000;71:595–597. doi: 10.1016/S0367-326X(00)00210-0. [DOI] [PubMed] [Google Scholar]
- [3].Moon HI, Lee J, Zee OP, Chung JH. A glycosidic isoflavonoid from Viola hondoensis W. Becker et H. Boissieu (Violaceae), and its effect on the expression of matrix metalloproteinase-1 caused by ultraviolet irradiation in cultured human skin fibroblasts. Biol Pharm Bull. 2005;28:1123–1125. doi: 10.1248/bpb.28.1123. [DOI] [PubMed] [Google Scholar]
- [4].Ma J, Jones SH, Marshall R, Wu X, Hecht SM. DNA Topoisomerase I inhibitors from Rinorea anguifera. Bioorg Med Chem Lett. 2005;15:813–816. doi: 10.1016/j.bmcl.2004.10.095. [DOI] [PubMed] [Google Scholar]
- [5].Vukics V, Kery A, Bonn GK, Guttman A. Major flavonoid components of heartsease (Viola tricolor L.) and their antioxidant activities. Anal Bioanal Chem. 2008;390:1917–1925. doi: 10.1007/s00216-008-1885-3. [DOI] [PubMed] [Google Scholar]
- [6].Moon HI, Jung JC, Lee J. Antiplasmodial activity of triterpenoid isolated from whole plants of Viola genus from South Korea. Parasitol Res. 2007;100:641–644. doi: 10.1007/s00436-006-0279-8. [DOI] [PubMed] [Google Scholar]
- [7].Goswami P, Kotoky J, Chen Z-N, Lu Y. A sterol glycoside from leaves of Clerodendron colebrookianum. Phytochemistry. 1996;41:279–281. doi: 10.1016/0031-9422(95)00557-9. [DOI] [Google Scholar]
- [8].Yang H, Wang J, Hou A, Guo Y, Lin Z, Sun H. New steroids from Clerodendrum clebrookianum. Fitoterapia. 2000;71:641–648. doi: 10.1016/S0367-326X(00)00223-9. [DOI] [PubMed] [Google Scholar]
- [9].Ahmad VU, Aliya R, Perveen S, Shameel M. A sterol glycoside from marine green algae. Codium iyengarii. Phytochemistry. 1992;31:1429–1431. doi: 10.1016/0031-9422(92)80311-2. [DOI] [Google Scholar]
- [10].Ahmad VU, Aliya R, Perveen S, Shameel M. Sterol from marine green algae Codium decorticatum. Phytochemistry. 1993;33:1189–1192. doi: 10.1016/0031-9422(93)85047-U. [DOI] [Google Scholar]
- [11].Hoet S, Pieters L, Muccioli GG, Habib-Jiwan J-L, Opperdoes FR, Quetin-Leclercq J. Antitrypanosomal activity of triterpenoids and sterols from the leaves of Strychnos spinosa and reated compounds. J Nat Prod. 2007;70:1360–1363. doi: 10.1021/np070038q. [DOI] [PubMed] [Google Scholar]
- [12].Brun R, Lun ZR. Drug sensitivity of Chinese Trypanosoma evansi and Trypanosoma equiperdum isolates. Vet Parasitol. 1994;52:37–46. doi: 10.1016/0304-4017(94)90033-7. [DOI] [PubMed] [Google Scholar]
- [13].Hirumi H, Hirumi K. Axenic culture of African trypanosome bloodstream forms. Parasitol Today. 1994;10:80–84. doi: 10.1016/0169-4758(94)90402-2. [DOI] [PubMed] [Google Scholar]
- [14].Räz B, Iten M, Grether-Bühler Y, Kaminsky R, Brun R. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Tropica. 1997;68:139–147. doi: 10.1016/S0001-706X(97)00079-X. [DOI] [PubMed] [Google Scholar]
- [15].Baltz T, Baltz D, Giroud C, Crockett J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985;4:1273–1277. doi: 10.1002/j.1460-2075.1985.tb03772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]