Abstract
In most modern populations, there are sex differentials in morbidity and mortality that favor women. This study addresses whether such female advantages existed to any appreciable degree in medieval Europe. The analyses presented here examine whether men and women with osteological stress markers faced the same risks of death in medieval London. The sample used for this study comes from the East Smithfield Black Death cemetery in London. The benefit of using this cemetery is that most, if not all, individuals interred in East Smithfield died from the same cause within a very short period of time. This allows for the analysis of the differences between men and women in the risks of mortality associated with osteological stress markers without the potential confounding effects of different causes of death. A sample of 299 adults (173 males, 126 females) from the East Smithfield cemetery was analyzed. The results indicate that the excess mortality associated with several osteological stress markers was higher for men than for women. This suggests that in this medieval population, previous physiological stress increased the risk of death for men during the Black Death to a greater extent than was true for women. Alternatively, the results might indicate that the Black Death discriminated less strongly between women with and without pre-existing health conditions than was true for men. These results are examined in light of previous analyses of East Smithfield and what is known about diet and sexually-mediated access to resources in medieval England.
Keywords: paleodemography, paleoepidemiology, selective mortality, Black Death
INTRODUCTION
In the majority of modern populations, women tend to experience lower age-specific mortality rates at most (if not all) ages and live longer than men (Heligman, 1983; Coale, 1991; Hill and Upchurch, 1995). For example, in 2008 the life expectancy at birth for U.S. females was nearly 6 years longer than that of males, and in many countries, the disparity in life expectancies was even greater. In that same year, male life expectancies at birth exceeded those of females only in a very small minority of countries for which data were available (https://www.cia.gov/library/publications/the-world-factbook/fields/2102.html). Mortality differentials favoring females occur because they appear to be more resistant to many diseases and generally more highly buffered against environmental stressors than males are (Stinson, 1985).
Females are often at lower risks than males of morbidity and mortality from many causes in modern populations, i.e. females often face lower probabilities than males of contracting or developing many different types of diseases and lower probabilities of dying from various causes. Many studies have found that males are more susceptible than females to a wide range of diseases caused by viruses, bacteria, parasites, and fungi (e.g. Hoff et al., 1979; Oliviera et al., 1981; Kirkwood et al., 1983; Brabin and Brabin, 1992; Acuna-Soto et al., 2000; Klein, 2000; Noymer and Garenne, 2000; Wells, 2000; Blessmann et al., 2002; Owens, 2002; Leone et al., 2004; Jansen et al., 2007; Noymer, 2008; Taylor et al., 2009). Males often suffer more severe symptoms or are at elevated risks of mortality from a variety of parasitic and infectious diseases, such as staph infection, trypanosomiasis, leptospirosis, and respiratory infections (Hoff et al., 1979; Laupland et al., 2003; Jansen et al., 2007; Falagas et al., 2008). However, males are not always at a disadvantage with respect to infectious and parasitic diseases. Many studies in human populations and experimental studies with animal models have found diseases that disproportionately affect females, such as malaria, leishmaniasis, listeriosis, and toxoplasmosis (Alexander, 1988; Roberts et al., 1995; Roberts et al., 2001; Pasche et al., 2005).
There are also important differences between men and women in terms of the morbidity and mortality associated with degenerative diseases. In modern populations, men suffer more severe symptoms or are at higher risks of mortality from such chronic diseases as cardiovascular diseases, respiratory tuberculosis, malignant neoplasms, renal disease, and cirrhosis of the liver (e.g. Lopez, 1984; Liu et al., 2003; Cerfolio et al., 2006; Kaminsky et al., 2006; Ng, 2007; Pilote et al., 2007; Kalra et al., 2008; Silbiger and Neugarten, 2008; Farinati et al., 2009; McGovern et al., 2009). However, some diseases disproportionately affect women. For example, there is a higher prevalence of diseases such as chronic obstructive pulmonary disease (COPD) and autoimmune disorders among women (Fairweather et al., 2008; Cote and Chapman, 2009), and women are more likely to suffer more severe symptoms and higher mortality from stroke and diabetes (Moriyama, 1984; Reeves et al., 2008; Turtzo and McCullough, 2008; Appelros et al., 2009).
Even though males might fare relatively well with respect to some causes of morbidity and mortality, overall it appears that women in modern populations are generally less frail than men, particularly given the near-universal pattern of longer life expectancy or lower age-specific mortality rates at most ages for women (Heligman, 1983). Frailty refers to an individual’s relative risk of death compared to other members of the population (Vaupel et al., 1979), or as Vaupel (1988: p. 277) describes it, “a set of susceptibilities and risk factors that alters their chances of death at different ages”. Differences in frailty among individuals within a population (i.e. heterogeneous frailty) exist because of differences in susceptibility to disease and death that may have genetic, biological, environmental, socioeconomic, or other causes (Wood et al., 1992; Aalen, 1994). The sex differentials in morbidity and mortality observed in modern populations have various causes. Some of the sex differences in favor of females might be genetically determined. For example, diseases which are caused by recessive X-linked genes, such as X-linked immunodeficiency syndromes, disproportionately affect males (Waldron, 1984). Some of the observed sex differences in infectious and parasitic disease patterns are attributed to sex hormones, which play an important role in the immune systems, as estrogens generally enhance immunocompetence, whereas androgens reduce it (Grossman, 1985; Ansar Ahmed et al., 1999; Klein, 2000; Roberts et al., 2001; Janele et al., 2006). Sex hormones also affect behaviors, such as aggression, which influence an individual’s risk of exposure to infectious disease (Klein, 2000). Sex hormones may also influence the risks of some degenerative diseases. For example, sex hormones may affect atherogenesis (plaque formation within arteries) and thereby influence risks of cardiovascular disease (Choi and McLaughlin, 2007). The sex differentials associated with some degenerative diseases are also the result of behavioral differences, such as higher rates of cigarette smoking and alcohol consumption among men which are linked to excess male mortality from such causes as coronary heart disease, certain cancers, and cirrhosis of the liver (Hetzel, 1984; Lopez, 1984).
The pervasiveness of morbidity and mortality differentials with respect to sex in modern populations raises the question of whether such differentials existed in the past. Researchers have examined mortality differentials in the past using documentary data, but such studies are generally limited to the past several hundred years. According to Bullough and Campbell (1980), men were believed to live longer than women in the ancient and Medieval world, but beginning in the 14th century, some documents indicate that women lived longer than men. Unfortunately, there are few empirical data to confirm this. Mortality data based on historical documents are available from northern Italy as early as the 14th century and from other European countries (e.g. England, Wales, and France) beginning in the 16th century, but relatively good data on ages at death for both sexes, which would allow for comparisons of both longevity and age-specific mortality rates between the sexes, do not generally appear until much later, during the 18th century (Russell, 1948; Hollingsworth and Hollingsworth, 1971; Herlihy, 1977; Wrigley and Schofield, 1981; Willigan and Lynch, 1982; Ell, 1985; Bonneuil, 1993; Gage, 2005). National data on mortality were not collected until the 18th century, beginning in Scandinavia and followed decades later by other European countries (Gage, 2005). There is some evidence from historical documents that a sex differential in favor of women existed in some populations by at least several hundred years ago. In 17th-century London, for example, mortality rates for women were apparently lower than those of men, despite reports from physicians that women suffered more than men from diseases and uniquely from complications associated with pregnancy and childbirth (Graunt, 1975). According to Coale (1991), mortality rates have been lower for women in European populations since at least the mid-19th century. At that time, women lived on average 2 to 3 years longer than men, and the disparity has increased since then such that women now live as many as 7 to 8 years longer, on average, than men in several European populations (Preston, 1977).
Investigators have attempted to address questions about mortality differentials in the past by examining life expectancy or mortality rates using skeletal data, with varying results. It is difficult to estimate sex-specific life expectancy from birth using skeletal data given the problems associated with determining sex for sub-adult skeletal material (Gage, 2000). Some researchers thus only estimate life expectancies at age 15 (or some other late teen/early adult age) or they estimate average ages at death among adults. Results from these studies have suggested that several different scenarios existed in past populations: longer life expectancies or higher average ages at death for females (e.g. among the prehistoric Pueblo and in 14th-century Japan: Bennett, 1973; Nagaoka et al., 2006), longer life expectancies or higher average ages at death for males (e.g. in several early agricultural populations and late medieval Croatia: Heligman, 1983; Šlaus, 2000), and crossovers such that life expectancy was higher in one sex at early ages but lower at older ages (e.g. among 18th-century Arikara and in medieval Sweden: Owsley and Bass, 1979; Högberg et al., 1987).
In addition to estimating life expectancies from skeletal samples, biological anthropologists can examine osteological stress markers which form in response to episodes of disease, malnutrition, or other physiological stressors to determine how sex affected risks of morbidity and mortality in past populations. Several studies which have looked at differences between men and women in the frequency of particular osteological stress markers have reported higher frequencies among men in past populations. For example, Ortner (1998), Larsen (1998), and Cassidy (1984) found higher frequencies of periostitis in men in prehistoric Native American samples. Analysis of an early Archaic population from Florida found higher frequencies of linear enamel hypoplasia in males than in females (Berbesque and Doran, 2008). According to Guatelli-Steinberg and Lukacs (1999), when significant differences in enamel hypoplasia exist in skeletal samples, there is generally a higher frequency in males than females. In an early medieval skeletal sample from Croatia, the frequency of periostitis in males was almost twice that of females (Šlaus, 2008). Such patterns of higher frequencies of stress markers in men, however, are not universal, and many studies have observed higher frequencies in women. Larsen (1997) summarizes several studies reporting higher frequencies or greater severity of periostitis among females in prehistoric Southwestern U.S. samples. Cucina et al. (2006) found a higher frequency of cribra orbitalia in females in a 2nd-3rd century A.D. Roman site, and according to Cohen and Bennett (1993) there is generally a higher frequency of both porotic hyperostosis and cribra orbitalia among women in prehistoric and historic skeletal samples. Šlaus (2000) found that the frequency of periostitis in women was twice that in men in a late medieval skeletal sample from Croatia, although the results were not statistically significant. Studies of maxillary sinusitis have found that the frequency of the pathology is significantly higher among women in various samples from Native American, English, and Nubian cemeteries (Roberts et al., 1998; Roberts, 2007). King et al. (2005) found a higher frequency of linear enamel hypoplasia among females in samples from 18th and 19th century London. There are also several studies which have failed to find differences in the frequencies of stress markers between men and women. A study of stress markers in a 1750–1500 B.C. Nubian site revealed no significant differences between males and females (Buzon and Judd, 2008). Storey (1998) did not find any differences in the frequency of childhood stress markers between men and women of the same social status among the Late Classic Maya of Copan. Buikstra and Cook (1981) and Powell (1988) found no sex differences in the frequency of tuberculosis in several Middle and Late Woodland and Mississippian sites. Møller-Christensen (1978) found no sex differences in the frequency of skeletal signs of leprosy in a medieval Danish site. Grauer et al. (1998) found in a sample from a 19th century Chicago poorhouse that frequencies of periostitis, linear enamel hypoplasia, and other lesions potentially indicative of infectious diseases did not differ significantly between the sexes. This is not an exhaustive survey of all the studies that have examined sex differences in osteological stress markers. However, the variation even in this brief overview demonstrates that no consistent pattern which would indicate sex differences in underlying frailty has yet been resolved using skeletal data.
Rather than examine frequencies of osteological stress markers to investigate sex differentials in morbidity and mortality in the past, the study presented here uses a hazards model to determine if the excess mortality associated with stress markers differs between the sexes in a medieval skeletal sample from London. Such differences would be informative about whether previous exposure to stressors affected risks of dying equally for men and women. The ultimate goal of this study is to examine differences in frailty between men and women in a medieval population, and the approach taken here is potentially more informative than comparing frequencies of stress markers because it does not assume that such markers indicate identical levels of frailty for all individuals in a sample. Rather, this study allows for (but does not require) variation between the sexes with respect to the excess mortality associated with osteological stress markers. According to Cohen and Bennett (1993: p. 284), there may be inherent differences between males and females in the body’s ability to buffer stress or the ways in which stress leaves visible signs on the skeleton. If females were less frail than males in past populations, they might have developed osteological stress markers but nonetheless been more resistant to a variety of causes of death than males. In other words, the effects of pre-existing health conditions on risk of dying might have been stronger and thus increased risks of mortality to a greater extent among men than among women. If this was the case, the excess mortality associated with osteological stress markers should be higher among men than women in skeletal samples; i.e. stress markers should be associated with more highly elevated risks of death in males than females.
This study examines sex differences in the risk of mortality associated with osteological stress markers using a sample from the East Smithfield cemetery in London. The East Smithfield cemetery was established in the 14th century for the exclusive purpose of burying victims of the Black Death in London (Hawkins, 1990). Given that all individuals in East Smithfield died during the Black Death, most (if not all) individuals in the East Smithfield cemetery died from the same cause. With the East Smithfield sample it is therefore possible to control for cause of death to a greater extent than is possible with normal (i.e. non-epidemic) mortality samples. This allows for the analysis of the differences between men and women in the risks of mortality associated with osteological stress markers without the potential confounding effects of different causes of death.
Previous studies have examined the East Smithfield cemetery to determine the selectivity of the Black Death with respect to sex and pre-existing health conditions (or frailty) (DeWitte and Wood, 2008; DeWitte, 2009). Such studies were done in part to evaluate the assumption that because Black Death mortality was very high (i.e. 30 to 50 percent of affected populations died during the epidemic), the epidemic killed people indiscriminately, unlike normal causes of mortality, which tend to be selective. Selective mortality, or selectivity, refers to the fact that most normal causes of mortality generally target (and thus select out of the population) those individuals at the highest risks of death in the population rather than killing all individuals at the same rate (Wood et al., 1992). Skeletal samples, which are obviously samples of dead individuals, are typically not representative of the original living population because of selective mortality. Individuals who die at a given age, and thus comprise a skeletal sample, are usually those with the highest frailty within the population and thus are those most likely to die and be selected out of the population (Wood et al., 1992). Previously, it was not clear whether the Black Death was selective or if everyone exposed to the disease was at equal risk of dying. Some contemporary chronicles of the Black Death include statements that suggest that factors such as age, sex, and social status had no effect on risk of death, but rather, all individuals were equally likely to succumb to the disease (e.g. see excerpts from and translations of chronicles in Horrox, 1994; Cohn, 2002). DeWitte and Wood (2008) used a multi-state model of health and mortality (Usher, 2000), which was also used for the current study and is described below in Materials and Methods, to determine whether the Black Death was selective with respect to frailty. They examined the relationship between osteological stress markers and risk of death in East Smithfield to determine if pre-existing health conditions affected risks of death during the Black Death. Estimates of excess mortality associated with osteological stress markers within the East Smithfield sample were compared to those estimated for a pre-Black Death normal mortality sample from Denmark to evaluate whether the patterns of selectivity during the Black Death cemetery were similar to those that existed under conditions of normal mortality. Analysis of the normal mortality Danish sample was necessary to establish that the presence of stress markers really was indicative of poor health under normal mortality conditions. All of the stress markers included in the study were associated with excess mortality in the normal mortality Danish cemeteries. These results confirmed earlier findings that these particular stress markers are informative about health condition or frailty; i.e. individuals with stress markers in Denmark were in poor health and at higher risks of mortality than their peers without stress markers. Results from the East Smithfield Black Death cemetery suggest that the Black Death was in fact selective with respect to pre-existing health, such that individuals who were in relatively poor health before the epidemic were more likely than their healthier peers to die during the Black Death. Comparison of the results from the Black Death cemetery to those from a normal mortality sample revealed a consistent pattern of higher estimated values of the risk of death associated with stress markers within the normal mortality sampled compared to East Smithfield. These differences suggest that though the Black Death was selective with respect to pre-existing health, it was perhaps not as strongly selective as normal mortality, presumably because many more otherwise healthy individuals were victims of the Black Death than would have died under conditions of normal, medieval mortality.
Another previous study of the East Smithfield cemetery examined the effect of sex on risk of death during the Black Death (DeWitte, 2009). For the previous study, a sample from East Smithfield was used to model sex as a covariate acting upon the parameters of the Gompertz-Makeham model of adult mortality; no attempt was made to include children given the difficulty of determining sex for subadults. The estimated value of the parameter representing the effect of the sex covariate did not significantly differ from zero, which suggests that there was no substantial difference in the risk of death between men and women in London during the Black Death. The results from that study suggested that Black Death mortality was not selective with respect to sex among adults; i.e. men and women were at approximately equal risks of dying during the epidemic. However, in that previous study, sex was modeled as a covariate affecting the entire Gompertz-Makeham model; i.e. sex was modeled as proportional to the entire hazard, independent of age (DeWitte, 2009). It is possible that differences in risk of death existed between men and women at different adult ages, but such age patterns were not captured using the proportional hazard assumption. As discussed below, the results from this previous study might inform the interpretation of the results from the current study.
Given the results from the DeWitte and Wood (2008) study, i.e. that the Black Death was selective with respect to frailty, the current study examines whether the strength of that selectivity was the same for both sexes or if there were differences between men and women. This study addresses the question of whether previous exposure to stressors had the same effect on risk of death for men and women. The previous studies described above did not address potential sex differences in the excess mortality associated with osteological stress markers. However, it is possible that such differences exist. As mentioned above, there may be differences between males and females with respect to the body’s ability to buffer stress or the ways in which physiological stress leaves visible signs on the skeleton (Cohen and Bennett, 1993). It is possible that there are important sex differences in the relationship between osteological stress markers and mortality in past populations and that stress markers are therefore not equally informative about health condition, or frailty, in men and women in those populations. To evaluate differences in the strength of Black Death selectivity with respect to previous physiological stress, this study examines differences between men and women in the risks of death associated with osteological stress markers in the East Smithfield cemetery. Such differences might be informative about underlying differences in frailty between men and women in the medieval population of London.
MATERIALS AND METHODS
Skeletal sample
East Smithfield Black Death Cemetery
The skeletal material for this study comes from the East Smithfield Black Death cemetery in London, a site in northeast London located near the Tower of London. The East Smithfield cemetery is an exclusively Black Death cemetery that is one of only a few excavated cemeteries with both documentary and archaeological evidence linking it to the 14th-century epidemic. The East Smithfield cemetery was established in late 1348 or early 1349 to contain thousands of individuals killed during the Black Death in London, and it was used until the epidemic ended in 1350 (Hawkins, 1990). The cemetery was excavated as part of the larger Royal Mint site by the Museum of London Department of Greater London Archaeology (now the Museum of London Archaeology Service: http://www.molas.org.uk) from 1986–1988. The Royal Mint site also includes the Cistercian Abbey of St. Mary Graces (circa 1350 – 1538), a victualling yard for the Royal Navy (circa 1560 – 1785), and remains of the Royal Mint itself (circa 1800s-1960s) (Grainger and Hawkins, 1988). Excavation of the East Smithfield cemetery revealed several hundred skeletons, and approximately 600 individuals were recovered and are currently curated at the Museum of London. The East Smithfield cemetery provides a unique sample of the population of mid-14th century London. Individuals of all ages and both sexes are represented in the cemetery. Importantly, for the purposes of this study, all individuals in the cemetery died within a short period of time and most, if not all, were victims of medieval plague. As the goal of this study is to evaluate sex differences in the risk of mortality associated with osteological stress markers, it is beneficial to be able to control, as much as possible, for cause of death, as different causes of death might vary in their selectivity with respect to pre-existing health conditions (DeWitte and Wood, 2008). The East Smithfield cemetery provides an almost ideal sample for an evaluation of how pre-existing health conditions differentially affected male and female risks of succumbing to a single cause of death.
For this study, a sample of 299 adults (173 males, 126 females) was selected from the East Smithfield cemetery and analyzed by the author at the Museum of London Centre for Human Bioarchaeology. This sample comprises all of the excavated adults from East Smithfield who were preserved well enough to provide sufficient data on age and sex and the presence of certain osteological stress markers. Because of the difficulties associated with determining sex in juveniles, this study only examines patterns of mortality among adults.
Age and Sex Estimation
Age estimation
Ages were estimated using the method of transition analysis (Boldsen et al., 2002). This age-estimation method uses statistical methods to avoid the problem of age mimicry associated with traditional methods (age mimicry refers to estimated ages that are biased toward the age distribution of a known-age reference sample) (Bocquet-Appel and Masset, 1982; Boldsen et al., 2002). For transition analysis, the conditional probability that a skeleton exhibits a certain age indicator stage given the individual’s known age is estimated from a known-age reference sample. This conditional probability is then combined with a prior distribution of ages at death (either a uniform prior or a prior distribution based on documentary information) using Bayes’ theorem to determine the posterior probability that a skeleton died at a certain age given that it displays particular age indicator stages. For this study, transition analysis was applied to skeletal age indicators present on the pubic symphysis and iliac auricular surface and to cranial suture closure as described by Boldsen et al. (2002), and the ADBOU (Anthropological Database, Odense University) Age Estimation software was used to determine individual ages-at-death. The ADBOU program uses a conditional probability estimated from the Smithsonian Institution’s Terry Collection that an individual with certain age indicators will be a given age. The program also uses a prior age-at-death distribution based on data from 17th-century Danish rural parish records to provide estimates for the age-at-death distribution in the target sample. Using Bayes’ theorem, the ADBOU program combines the informative prior and the conditional probability to estimate the highest posterior point estimate of age for each individual in the sample.
Sex determination
Sex was determined from dimorphic features of the skull and pelvis using the standards described in Buikstra and Ubelaker (1994). The following features of the skull were scored: glabella/supraorbital ridge, supraorbital margin, mastoid process, external occipital protruberance/nuchal crest, and the mental eminence. The following features of the pelvis were also evaluated for sex determination: the ventral arc of the pubis, subpubic concavity, ischiopubic ramus ridge, and the greater sciatic notch. Researchers have demonstrated that the accuracy of these individual traits, or combinations thereof, for the purposes of sex determination ranges from 68 to over 96 percent (Phenice, 1969; Sutherland and Suchey, 1991; Graw et al., 1999; Ubelaker and Volk, 2002; Rogers, 2005; Walker, 2005; Williams and Rogers, 2006). Multiple skeletal indicators of sex were used for this study given that including more than one indicator improves the accuracy of sex determination (Meindl et al., 1985; Rogers, 2005; Williams and Rogers, 2006; Walker, 2008).
Osteological Stress Markers
Osteological Stress Markers
Using the model described below, this study analyzes the risk of death associated with the following non-specific osteological markers of physiological stress: linear enamel hypoplasia, porotic hyperostosis, cribra orbitalia, and tibial periostitis. Periostitis is part of an inflammatory response to trauma or infection, and it results in excess bone formation. It can occur at any age (i.e. it is not generally restricted to childhood as are the other stress markers used in this study) (Larsen, 1997; Ortner, 2003). For the current study, periostitis was scored on the tibia because paleopathological studies have repeatedly demonstrated that the tibia is commonly affected by periostitis and because the tibia is a robust bone that is therefore often well-preserved in skeletal samples (Eisenberg, 1991; Milner, 1991; Larsen, 1997; Roberts and Manchester, 2005). Only the anterior and medial surfaces of the diaphysis of the tibia were scored for periostitis, as the posterior surface of the tibia is often covered by muscle markings that can interfere with identification of subtle pathologies. Periostitis was identified macroscopically and was scored as present if the there was at least one distinct patch of woven or sclerotic bone (or a combination of the two) laid down on the surface of the diaphysis.
Porotic hyperostosisis and cribra orbitalia are lesions on the cranial vault bones and orbital roofs, respectively, that are characterized by a porous appearance of the outer table of the affected bone that is often associated with expansion of the underlying diploic bone (Mensforth et al., 1978; Ortner, 2003). Both porotic hyperostosis and cribra orbitalia are usually associated with anemia or other causes during childhood that result in an expansion of the bone marrow and thus an expansion of the surrounding diplöic bone. Both stress markers can be retained into adulthood (Walker et al., 2009). For the current study, the cranial vault was scored for porotic hyperostosis, and the roofs of both orbits were scored for cribra orbitalia. Both stress markers were identified macroscopically. Porotic hyperostosis and cribra orbitalia were scored as present if at least one square centimeter of porosity was visible.
Linear enamel hypoplasia is a tooth enamel defect caused by the disruption of enamel formation as a result of infection or malnutrition (Huss-Ashmore et al., 1982; Dahlberg, 1991; Roberts and Manchester, 2005). Enamel hypoplasia occurs only while the teeth are developing during childhood, but it is not subject to remodeling and thus can be retained well into adulthood (Roberts and Manchester, 2005). Linear enamel hypoplasias appear as horizontal lines of varying width on the surface of the affected tooth. For the current study, linear enamel hypoplasias were identified macroscopically on the buccal surface of the mandibular canines. The mandibular canine has a relatively long developmental time-span (approximately 10 years) and is highly sensitive to physiological stress (Goodman et al., 1980; Huss-Ashmore et al., 1982; Santos and Coimbra, 1999). Only permanent dentition with very little or no wear were scored. Linear enamel hypoplasia were scored as “present” if one or more depressions on the surface of the tooth were palpable and were visible to the naked eye under good lighting.
These osteological stress markers are used in this study only as non-specific indicators of general health. It should be emphasized that the Black Death killed too quickly to leave visible signs on the skeleton, so osteological stress markers in the East Smithfield sample were not caused by the Black Death itself. For this study, the stress markers indicate an individual’s general level of health (i.e. pre-existing health condition or previous exposure to physiological stressors) before ultimately dying during the Black Death.
Model
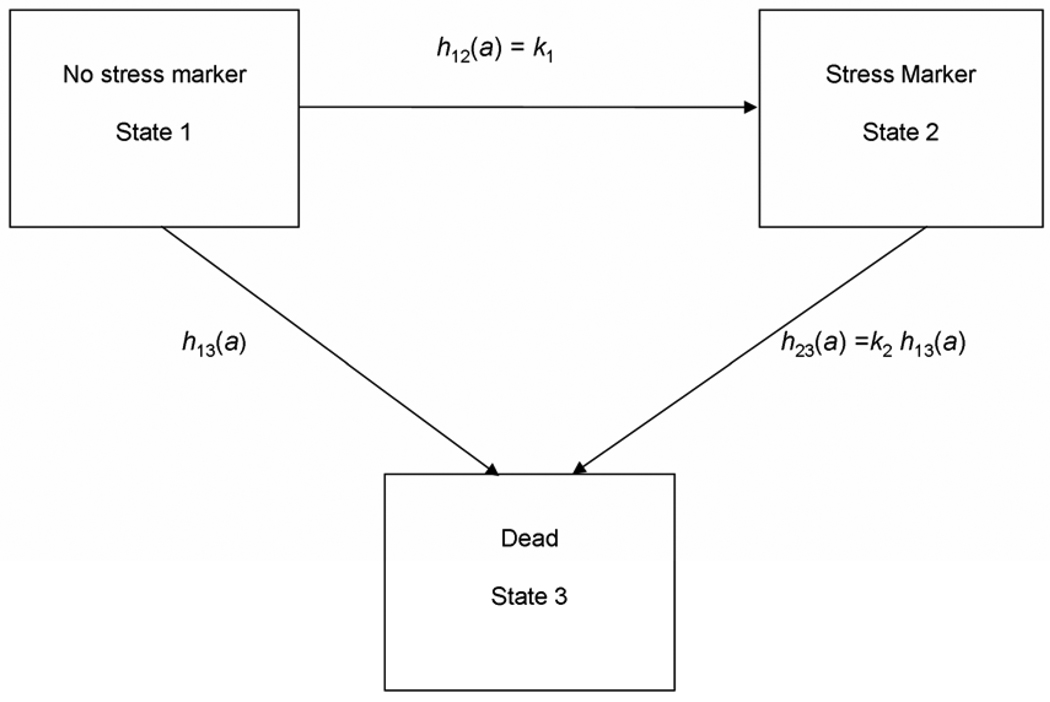
The risk of death associated with osteological stress markers among adults in the East Smithfield cemetery was assessed using a multistate model of health and mortality that was developed for paleodemography by Usher (2000) and was previously used to examine selective mortality in the East Smithfield cemetery by the author (DeWitte and Wood, 2008). The model, shown in Figure 1, has three, non-overlapping states: State 1 includes all those individuals in the sample without any osteological stress markers that are visible to the naked eye, State 2 includes those with visible osteological stress markers, and State 3 is death. Everyone in the skeletal sample used for this study is, of course, dead and thus observed in State 3, so States 1 and 2 represent the possible living states the individuals could have been in right before they died. In the Usher model, individuals can move from State 1 to State 2 (i.e. suffer some physiological stress and thus develop an osteological stress marker), and individuals can die from either of the two living states (i.e. with or without stress markers). The transitions from either of the two living states to death are determined by age-specific hazard rates. The model allows for (but does not require) variation in the hazard rates between each of the two living states and death; i.e. the hazard of making the transition from State 2 to death can be higher or lower than, or the same as the hazard of making the transition from State 1 to death. Thus, the model can be used to estimate the differential risk of death associated with the living states. The model allows one to address the question of whether certain osteological stress markers really are associated with increased risks of death – i.e. whether individuals with osteological stress markers are at elevated risks of dying compared to their peers without such stress markers (Ortner, 1991; Wood et al., 1992). Even though everyone in a cemetery sample is observed in State 3, data on age-at-death and the presence of osteological stress markers can be used to estimate all of the parameters of the model described below.
Figure 1.
Three-state model of morbidity and mortality. Individuals are born into State 1, and transitions between states i and j occur at age-specific hazard rates hij(a), where a is age in years. The transition between States 1 and 3 follows the baseline Gompertz-Makeham hazard function h13(α)=α1+α2 exp(βα), and the proportional term associated with a particular stress marker, k2, acts to modify this baseline rate for those who die from State 2. The hazard of developing a detectable stress marker is set equal to the constant. k1 (Redrawn from Usher 2000.)
For this study, the baseline risk of death from State 1, h13(a), was specified as a three-parameter Gompertz-Makeham model:
In this model, ai is the age of the ith skeleton in years, α1 is the constant age-independent risk of mortality that everyone within the population faces (i.e. the chance of dying from accidents and other causes that are unrelated to aging), and α2eβa is the exponentially increasing senescent risk of mortality, where α2 is the overall level of mortality among adults and β is the rate at which this risk increases with age (Gage, 1988). The Gompertz-Makeham function is a parsimonious model of adult mortality that fits the general human pattern of relatively low mortality during the young adult ages and an increasing risk of death with senescence (Wood et al., 2002). The hazard of moving from State 1 to State 2, i.e. of developing stress markers, h12(a), was estimated as a constant k1. For this study, an age-specific hazard of moving from State 1 to State 2 was not included in the model because for most osteological stress markers, one does not know the age at which an individual became ill or suffered some other physiological stress sufficient to cause a stress marker, nor does one know precisely how long it took for a stress marker to develop. For simplicity, in this study, the age of onset of stress markers was modeled as an exponential random variable. The hazard of dying from State 2, h23(a), was modeled as proportional to the baseline age-specific risk of dying from State 1. Under the proportional hazards specification, k2, is a proportional term on the Gompertz-Makeham function and is thus independent of age. The k2 parameter value indicates the proportional difference in risk of death between individuals with and without stress markers. Estimated k2 values significantly greater than one indicate that individuals with stress markers were at higher risks of dying compared to peers without stress markers. Estimated k2 values significantly lower than one indicate that individuals with stress markers face decreased risks of death compared to their peers. Estimated k2 values equal to one indicate that individuals with and without stress markers were at approximately the same risk of death.
For this study, the Usher model was fit to data on age, sex, and the presence of stress markers. To evaluate sex differences in the excess mortality associated with osteological stress markers within the East Smithfield sample, and thus whether previous exposure to stressors had the same effect on risk of death for men and women, sex was modeled as a covariate affecting the k2 parameter in the Usher model. In this analysis, females were coded as 0, and males were coded as 1. A significant positive or negative estimate for the parameter representing the effect of the sex covariate would suggest that the excess mortality associated with stress markers was higher or lower, respectively, for men compared to women. The model was fit separately to data on the presence of each of the four following stress markers: porotic hyperostosis, cribra orbitalia, linear enamel hypoplasia, and tibial periostitis. Maximum likelihood analysis was used to estimate the parameters for this study, and estimation was done with Holman’s mle program (2005). Likelihood ratio tests (LRT) were used to evaluate the fit of the full model, which included all the parameters of the Usher model described above plus the parameter representing the effect of the sex covariate, compared to the reduced model which did not include sex as a covariate. The LRT therefore tests the null hypothesis that the risk of death associated with stress markers was the same for men and women. The LRT was computed as follows: LRT = −2[ln(Lreduced) – ln(Lfull)], where LRT approximates a χ2 distribution with df=1 (the degrees of freedom are equal to the number of additional parameters in the full model). It should be noted that the errors associated with age estimates were not taken into account when estimating the Usher model. Therefore, the estimated values of the k2 parameter and the parameter representing the effect of the sex covariate should be viewed as general, qualitative measures of the excess mortality associated with stress markers and the sex differences thereof.
A hazards model was used in this study rather than the more traditional life-table approach to avoid the potential problems associated with paleodemographic life tables. Life tables, which display age-specific mortality rates (and often other, related measures), are an important tool in demographic research, and there is a long history of their use in paleodemography (Wood et al., 2002). The paleodemographic use of life tables is based on the idea that the distribution of ages-at-death within a skeletal sample is equivalent to the cohort age-at-death column in a life table (Milner et al., 2008). However, this is true only when the population that gave rise to a cemetery sample was stationary (i.e. closed to migration, an intrinsic rate of increase equal to zero, and age-specific mortality and fertility rates that do not change over time), and the assumption that a past population was stationary is not necessarily valid (Milner et al., 2008). Furthermore, life tables are considered by many researchers to be an inefficient way to deal with paleodemographic age-at-death data (Buikstra, 1997; Hoppa and Vaupel, 2002; Konigsberg and Frankenberg, 2002; Wood et al., 2002; Milner et al., 2008). Life tables require the estimation of one parameter (the central mortality rate) for every age interval used, and there is no way to do this reliably without huge samples and knowledge of the original population at risk, both of which are often unavailable for most paleodemographic investigations (Wood et al., 2002). Some paleodemographers have tried to avoid these and other problems associated with life tables by using a model life table approach, i.e. the application of a theoretical life table that matches the age-at-death distribution observed in a cemetery sample (Weiss, 1973; Wood et al., 2002). Unfortunately, it is not always clear which model life table is most appropriate to use in paleodemographic studies (Gage, 1988; Milner et al., 2008). Many researchers argue that rather than using life tables, some form of parametric or semi-parametric hazards analysis is the most powerful way to derive information from the small samples typical of paleodemography (Gage, 1988; Konigsberg and Frankenberg, 1992; Buikstra, 1997; Hoppa and Vaupel, 2002; Konigsberg and Frankenberg, 2002; Wood et al., 2002). According to Holman et al. (2002), most human age-at-death distributions can be described using five or fewer parameters, and parsimonious parametric models provide a useful alternative to life tables in paleodemographic studies.
RESULTS
The maximum likelihood estimates of k2, the excess mortality associated with the osteological stress markers used in this study, and the estimated values of the parameter representing the effect of the sex covariate, along with the standard errors associated with these estimates and the results from the likelihood ratio tests, are shown in Table 1. These results indicate that all stress markers are associated with increased risks of death in the East Smithfield sample, as all the estimated k2 values are greater than one. These results are not surprising given similar findings from a previous study of East Smithfield, which thereby suggested that the Black Death was selective with respect to frailty (DeWitte and Wood, 2008). For the current study, though the estimated values of k2 suggest that exposure to stressors prior to the Black Death increased the risk of death for both men and women during the epidemic, the consistently positive estimated values of the parameter representing the effect of the sex covariate suggests that the excess mortality associated with stress markers was higher for men than for women.
Table 1.
Maximum likelihood estimates of k2 and the effect of the sex covariate with associated standard errors, and the results of the likelihood ratio test of the null hypothesis H0: effect of sex covariate = 0. LEH = linear enamel hypoplasia.
| Stress Marker | k̂2 (s.e.) | Sex (s.e.) | LRT |
|---|---|---|---|
| Tibial periostitis | 1.74 (0.30) | 0.47 (0.28) | 50.8 |
| Porotic Hyperostosis | 1.72 (0.27) | 1.71 (0.33) | 15.6 |
| Cribra Orbitalia | 1.90 (0.60) | 0.84 (0.36) | 5.45 |
| LEH mandibular canine | 2.90 (1.39) | 1.3 (0.54) | 12.3 |
It should be noted that the k2 estimates and the estimated values of the parameter representing the effect of the sex covariate have relatively large standard errors associated with them as shown in Table 1, and these standard errors are probably underestimated. As mentioned above, to estimate the parameters of the model used in this study, point estimates of age were used without their corresponding errors. Because the errors associated with ages were not incorporated when the model was fit, the reported standard errors might be underestimated to an unknown degree, and the standard error estimates should thus be viewed with caution. Nonetheless, there is a consistent pattern, as the k2 estimates for all stress markers are greater than one and the estimated values of the parameter representing the effect of the sex covariate are positive. The consistent pattern suggests that these stress markers are indicative of higher frailty and that the risk of death associated with such markers were higher for men. However, the numerical value of estimates of the k2 parameter and the parameter representing the effect of the sex covariate should not necessarily be taken at face value given the uncertainty associated with the standard error estimates. The results of the likelihood ratio tests further suggest that the excess mortality associated with stress markers was higher for men than women in the East Smithfield sample, as they indicate that including the sex covariate improved the fit of the model with respect to all of the stress markers considered.
DISCUSSION
The estimated values of the excess mortality associated with osteological stress markers from this study suggest that exposure to physiological stressors prior to the Black Death increased the risk of death for both men and women during the 14th-century epidemic. Men and women with osteological stress markers were more likely than their peers without such markers to die during the Black Death. As mentioned above, these results are not particularly surprising, given similar results from a previous study of East Smithfield (DeWitte and Wood, 2008). However, even though it appears that prior exposure to stressors elevated risks of death for both sexes during the Black Death, the estimated values for the parameter representing the effect of the sex covariate suggest that the excess mortality associated with the stress markers considered in this study was higher for men than for women. These differences between men and women might suggest that the effect of pre-existing health condition on risk of dying during the Black Death was stronger among men than among women and that males were, on average, frailer than women in the population of medieval London. That is, pre-existing health conditions made all individuals more vulnerable to death during the Black Death, but the risk for men relative to their healthier peers might have been even greater than that among women. Women in medieval London with a history of physiological stress might have been better able to resist dying from the Black Death, perhaps because of superior immune competence. An alternative explanation of the observed data is that, rather than suggesting sex differences in frailty in general, these results might suggest that the Black Death was not quite as strongly selective with respect to frailty among women and killed more otherwise healthy women than healthy men.
The possible interpretation that women were less frail than men in medieval London is consistent with the widespread perspective that female buffering or female advantages in morbidity and mortality are biological norms for humans and other species. Numerous studies have revealed female morbidity and mortality advantages in humans and in many other animal species (Promislow, 1992; Klein, 2000; Moore and Wilson, 2002). As described above, in most modern human populations, women tend to experience lower age-specific mortality rates at most ages and live longer than men. There are exceptions, and in some countries (e.g. in South Asia), life expectancy at birth is lower for women and age-specific mortality is higher for females at many ages (Bhatia, 1984). However, fetal and neonatal mortality rates are higher for males in those countries (Chen et al., 1981; Bhatia, 1984). Furthermore, studies of the effects of stress on prenatal growth generally show that males are more vulnerable to stress in utero than are females (Stinson, 1985). The sex differentials that occur during the pre- or neonatal period are the result of endogenous factors (i.e. rather than being the result of preferential treatment or heterogeneity in exposure to risk factors) and thus, according to some researchers, reflect the “innate frailty of the male” (Bhatia, 1984: pg. 167), or the “higher biological risk of male children” (Chen et al., 1981: pg. 57). When post-natal morbidity and mortality is lower in males than females, researchers often attribute the differential to preferential treatment of males (Chen et al., 1981; Stinson, 1985). Differentials favoring males are also often attributed to the complications associated with pregnancy and childbirth that ultimately act to balance the natural female advantages (see Lopez and Ruzicka, 1984; Gage, 1994).
If, as the results of this study might suggest, women were less frail than men in medieval London, could this be potentially informative about female access to resources in medieval London? This is important because nutritional status can affect immune functioning. If females do not have adequate nutrition, they might be immunocompromised despite any innate advantages associated with being female. Higher levels of malnutrition among females compared to males have been observed in many studies within modern populations (e.g. Chen et al., 1981; Bhatia, 1984; Khadi et al., 1996; Esimai et al., 2001; Zhou et al., 2005; Sakisaka et al., 2006; Dey and Chaudhuri, 2008). According to Chen et al. (1981), because of preference for sons in rural Bangladesh, malnutrition is much more common in female children and is at least partly due to unbalanced provisioning of children within families. The health effects of such disparities are exacerbated because male children are also much more likely to receive medical care, and they therefore are more likely to recover from infectious diseases. Kehoe and Giletti (1981) and Rosenberg (1980) summarize several studies that have demonstrated cultural dietary rules that prevent females from obtaining adequate high quality foods in modern stratified societies, such as restrictions on female consumption of meat, certain vegetables, or dairy products. According to Ortner (1998), in many developing countries, men have priority access to higher quality and larger quantities of food than women, and he argues that such differential access to food has probably existed for a long time in human populations. Unequal distribution of food and other resources between the sexes might have important consequences for observed patterns of frailty. Indeed, many of the examples of preferential treatment of males occur (or occurred in the past) in areas in which males experience longer life expectancies and lower age-specific mortality rates.
Given that malnutrition increases vulnerability to infectious disease (Scrimshaw, 1987; Ortner, 1998), did sex determine access to food in medieval England in ways that might have influenced differences in frailty? In many parts of medieval Europe, anything a woman owned became the legal property of her husband, so sons were generally preferred over daughters because of the desire to keep heritable properties within the family (Bennett, 1987; Wiesner, 2000). This preference might have resulted in reduced care for or provisioning of daughters. However, the data from historical records on differential treatment of sons and daughters are ambiguous (Bennett, 1987). In early medieval religious communities, women were given smaller rations of food because of their smaller size (Pearson, 1997), and among the laity and in religious communities, women were encouraged to fast as a means of reducing sexual urges (Bynum, 1987). According to Pearson (1997), the nutritional needs of early medieval women, particularly given the strains of pregnancy, childbirth, and lactation, were probably not adequately met for many women, given what is known about the foods available at that time. Some historical records indicate that women did not live as long as men in the early Middle Ages, and their apparent reduced longevity might have been the result of iron-deficiency anemia caused by a lack of sufficient iron in the diet coupled with iron depletion through menstruation, pregnancy, and lactation (Bullough and Cameron, 1980). Pre-menopausal women would have been at elevated risks of anemia because of their higher iron requirements compared to men, and anemia might have rendered women more susceptible to morbidity and mortality from a variety of other causes. According to Bullough and Campbell (1980), several historical sources indicate that woman were living longer than men beginning in the 14th century, and they argue that the differential in favor of women was the result of dietary changes, particularly an increase in the amount of protein and iron available in the diet. Following the adoption of the three-field system of agriculture in the 9th century, more protein-rich plant foods, such as peas and broadbeans, were included in the typical diet, and at the same time, more meat was also apparently being consumed (Bullough and Cameron, 1980). These dietary changes might have benefitted women more than men because of an increase in iron-rich foods in the diet which lowered the prevalence of anemia among women. The results of the current study might provide additional support for this argument.
Studies of dietary differences between medieval men and women have been done using stable isotope analysis of skeletal material. Such studies are somewhat limited, however, as they are not capable of revealing everything that was consumed by individuals in the past. Reconstruction of diets, based on strontium and barium analyses, in an early medieval site in Poland does not reveal any differences between males and females with respect to the consumption of seafood and milk products (Szostek et al., 2009). At the site of St. Andrew, Fishergate in York, northern England, dated to the 13th-16th centuries, nitrogen and carbon isotope ratios were generally lower among females than males, suggesting that women consumed less marine foods than men (Muldner and Richards, 2007). However, analysis of males and females from the same household and status revealed no significant differences between the sexes. The lower ratios for women within the site might reflect different migratory patterns or differences between members of the local monastic order and the laity rather than differential access to food based on sex. Mays (1997) found no differences in isotopes indicative of marine resources between males and females in two 13–16th century sites in northern England (Hartpoole and Newcastle). Studies of sites dating to the 11–14th centuries in Orkney revealed significant difference between the sexes, with more marine resources consumed by men, perhaps reflecting differences in activities (e.g. greater involvement of men in fishing or seal hunting) (Barrett and Richards, 2004; Richards et al., 2006).
The existing historical and stable isotope data provide useful information about medieval dietary patterns, but they do not clarify whether men and women in medieval London were equally well (or poorly) nourished or if one sex had preferential access to food. If the results of this study really do indicate lower frailty among women in medieval London, they, in turn, suggest that women in general were not more highly malnourished than men. If women in medieval London had lower average frailty than men, this might have been the result of a lack of disparities in access to food resources. Approximately equal access to food perhaps enabled these women to achieve their biological potential of lower frailty relative to men. Alternatively, men might have eaten more and better foods than women, but given that medieval diets in general might not have been very high in nutritional quality (Dyer, 1983), preferential access might not have made any appreciable difference with respect to immune functioning and frailty.
The alternative explanation for the results from this study must be also considered. As mentioned above, rather than demonstrating lower frailty in women compared to men, the values for the parameter representing the effect of the sex covariate shown in Table 1 might indicate that the Black Death discriminated less strongly between women with and without pre-existing health conditions than was true for men. There is excess mortality associated with osteological stress markers for both men and women, so the results should not be interpreted as indicating that the Black Death killed women totally irrespective of pre-existing health condition. However, the lower excess mortality associated with stress markers among women compared to men as indicated by the estimated values of the parameter representing the effect of the sex covariate might indicate that the Black Death killed more otherwise healthy women than healthy men. This interpretation of the results in turn suggests that the Black Death disproportionately affected women. Alternatively, if the results indicate that men in medieval London were frailer than women, a reasonable inference is that men in general were at a higher risk of death than women during the Black Death. Thus, the two alternative explanations for the results observed in this study lead to very different inferences about the sex pattern of Black Death mortality. There are numerous modern examples of diseases that differentially affect either women or men, some of which are described above, so either scenario is plausible.
Is either inference about the sex pattern of Black Death mortality consistent with existing evidence regarding sex differences in medieval plague mortality? Several contemporary chroniclers believed that more women than men were killed by the 14th-century Black Death, while others claimed that the epidemic killed indiscriminately (Cohn, 2002). Previous investigations of the sex patterns of Black Death mortality using skeletal remains from the East Smithfield cemetery suggest that neither sex was at a disproportionately elevated risk of dying during the Black Death relative to the patterns observed under conditions of normal mortality (Waldron, 2001; DeWitte, 2009). However, some have interpreted the higher number of males within East Smithfield as indicating female advantages during the Black Death (Waldron, 2006). Burial records and several historical accounts indicate that in some of the outbreaks of medieval plague that followed the 1347–1351 epidemic, men were disproportionately affected. However, several other chroniclers also noted that mortality was the same for men and women in some of these subsequent outbreaks (Cohn, 2002). For example, burial records for the London parish of St. Botolph’s without Bishopsgate reveal an excess of male deaths in the 1603 and 1625 epidemics (Hollingsworth and Hollingsworth, 1971). But this pattern was neither consistent across all parishes in London nor across the 1593, 1603, and 1625 outbreaks (Finlay, 1979). Bradley (1977) did not find a difference in mortality between men and women during the 1665–6 plague in Eyam, England, and Schofield (1977) similarly found no evidence that either sex was disproportionately affected in the town of Colyton, England that same year. Thus, the existing evidence regarding mortality during the Black Death and subsequent outbreaks of plague does not clearly indicate that one sex was disproportionately affected. This evidence therefore does not strongly support the conclusion that the results of this study indicate higher male frailty in medieval England nor that the results suggest the Black Death was less strongly selective for frailty among women. Future studies of the differences in the risks of death associated with osteological stress markers might help determine which of these two possible interpretations of the data from this study is more likely. For example, finding that the differences with respect to the risks of death associated with stress markers between men and women in non-catastrophic cemeteries are similar to those observed in East Smithfield might further support the idea that women were less frail than men in past populations.
CONCLUSION
The results of this study suggest differences between men and women with respect to the excess mortality associated with osteological stress markers. These results suggest that in medieval London, a difference existed in the average frailty of men and women, such that exposure to physiologic stressors increased risks of death to a greater extent among men than women. If this is true, it might mean that the modern pattern of excess female longevity has underlying causes that are not unique to modern populations and that that the morbidity and the mortality advantages experienced by women in modern populations also existed in the past. An alternative explanation for the results of this study is that the Black Death was selective with respect to frailty among both men and women, but that it discriminated less strongly among women. If this was the case, the lower risk of mortality associated with stress markers among women compared to men was the result of the Black Death killing a greater proportion of healthy women than healthy men, rather than reflecting lower average frailty among women. Additional studies of the sex differences in the risk of mortality associated with stress markers in a variety of cemetery samples might yield data that further support the hypothesis that women were less frail on average than men in past populations.
ACKNOWLEDGEMENTS
I would like to thank Christopher Ruff, the Associate Editor, and two anonymous reviewers for their very careful reading of and helpful comments on this manuscript. I am very grateful to Bill White, Jelena Bekvalac, and Rebecca Redfern at the Museum of London Centre for Human Bioarchaeology for providing access to the East Smithfield skeletons and for generously providing the physical facilities for this work. I also thank Dr. Eric Jones for his helpful comments and continued support. Funding was provided by the American Association of University Women, the National Science Foundation (grant number BCS-0406252), the Wenner-Gren Foundation for Anthropological Research (grant number 7142), the American-Scandinavian Foundation, the University at Albany Center for Social and Demographic Analysis, and the University at Albany Research Foundation.
Grant Sponsors: The American Association of University Women, National Science Foundation (grant number BCS-0406252), the Wenner-Gren Foundation for Anthropological Research (grant number 7142), the American-Scandinavian Foundation, University at Albany Center for Social and Demographic Analysis (CSDA), and the University at Albany Research Foundation.
LITERATURE CITED
- Aalen OO. Effects of frailty in survival analysis. Stat Methods Med Res. 1994;3(3):227–243. doi: 10.1177/096228029400300303. [DOI] [PubMed] [Google Scholar]
- Acuna-Soto R, Maguire JH, Wirth DF. Gender distribution in asymptomatic and invasive amebiasis. Am J Gastroenterol. 2000;95(5):1277–1283. doi: 10.1111/j.1572-0241.2000.01525.x. [DOI] [PubMed] [Google Scholar]
- Alexander J. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology. 1988;96(Pt 2):297–302. doi: 10.1017/s0031182000058303. [DOI] [PubMed] [Google Scholar]
- Ansar Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environ Health Perspect. 1999;107 Suppl 5:681–686. doi: 10.1289/ehp.99107s5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Barrett JH, Richards MP. Identity, Gender, Religion and Economy: New Isotope and Radiocarbon Evidence for Marine Resource Intensification in Early Historic Orkney, Scotland, UK. European Journal of Archaeology. 2004;7(3):249–271. [Google Scholar]
- Bennett JM. Women in the medieval English countryside : gender and household in Brigstock before the plague. xv. New York: Oxford University Press; 1987. p. 322. [Google Scholar]
- Bennett KA. On the estimation of some demographic characteristics on a prehistoric population from the American Southwest. Am J Phys Anthropol. 1973;39(2):223–231. doi: 10.1002/ajpa.1330390212. [DOI] [PubMed] [Google Scholar]
- Berbesque JC, Doran GH. Brief Communication: Physiological stress in the Florida Archaic - Enamel hypoplasia and patterns of developmental insult in early North American hunter-gatherers. Am J Phys Anthropol. 2008;136(3):351–356. doi: 10.1002/ajpa.20816. [DOI] [PubMed] [Google Scholar]
- Bhatia S. Traditional practices affecting female health and survival: evidence from countries of South Asia. In: Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1984. pp. 165–191. [Google Scholar]
- Blessmann J, Van Linh P, Nu PA, Thi HD, Muller-Myhsok B, Buss H, Tannich E. Epidemiology of amebiasis in a region of high incidence of amebic liver abscess in central Vietnam. Am J Trop Med Hyg. 2002;66(5):578–583. doi: 10.4269/ajtmh.2002.66.578. [DOI] [PubMed] [Google Scholar]
- Bocquet-Appel JP, Masset C. Farewell to paleodemography. J Hum Evol. 1982;11:321–333. [Google Scholar]
- Boldsen JL, Milner GR, Konigsberg LW, Wood JW. Transition analysis: A new method for estimating age from skeletons. In: Hoppa RD, Vaupel JW, editors. Paleodemography: Age distributions from skeletal samples. Cambridge: Cambridge University Press; 2002. pp. 73–106. [Google Scholar]
- Bonneuil N. The trend method applied to English data. In: Reher DS, Schofield R, editors. Old and new methods in Historical Demography. Oxford: Clarendon; 1993. pp. 57–65. [Google Scholar]
- Brabin L, Brabin BJ. Parasitic infections in women and their consequences. Adv Parasitol. 1992;31:1–60. doi: 10.1016/s0065-308x(08)60020-2. [DOI] [PubMed] [Google Scholar]
- Bradley L. Some Medical Aspects of Plague. In The Plague reconsidered: a new look at its origins and effects in 16th and 17th century England. Matlock, Derbyshire.: Local Population Studies in association with the S. S. R. C. Cambridge Group for the History of Population and Social Structure. 1977:11–24. [Google Scholar]
- Buikstra JE. Paleodemography: Context and promise. In: Paine RR, editor. Integrating archaeological demography : multidisciplinary approaches to prehistoric population. Carbondale, Ill: Center for Archaeological Investigations. Southern Illinois University at Carbondale; 1997. [Google Scholar]
- Buikstra JE, Cook DC. Pre-Columbian tuberculosis in West-Central Illinois: prehistoric disease in biocultural perspective. Northwestern University Archaeological Program Scientific Papers No. 5. In: Buikstra JE, editor. Prehistoric tuberculosis in the Americas. Evanston, IL: Northwestern University Archaeological Program; 1981. pp. 115–139. [Google Scholar]
- Buikstra JE, Ubelaker DH, editors. Standards for data collection from human skeletal remains: Proceedings of a seminar at the Field Museum of Natural History (Arkansas Archaeology Research Series 44) Fayetteville, Ark: Arkansas Archeological Survey Press; 1994. [Google Scholar]
- Bullough V, Cameron C. Female Longevity and Diet in the Middle Ages. Speculum. 1980;55(2):317–325. doi: 10.2307/2847291. [DOI] [PubMed] [Google Scholar]
- Buzon MR, Judd MA. Investigating health at Kerma: Sacrificial versus nonsacrificial individuals. Am J Phys Anthropol. 2008;136(1):93–99. doi: 10.1002/ajpa.20781. [DOI] [PubMed] [Google Scholar]
- Bynum CW. Holy feast and holy fast : the religious significance of food to medieval women. xvi. Berkeley: University of California Press; 1987. p. 444. [430] of plates p. [Google Scholar]
- Cassidy CM. Skeletal evidence for prehistoric subsistence adaptation in the Central Ohio River Valley. In: Cohen MN, Armelagos GJ, editors. Paleopathology at the origins of agriculture. New York: Academic Press; 1984. pp. 307–345. [Google Scholar]
- Cerfolio RJ, Bryant AS, Scott E, Sharma M, Robert F, Spencer SA, Garver RI. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130(6):1796–1802. doi: 10.1378/chest.130.6.1796. [DOI] [PubMed] [Google Scholar]
- Chen LC, Huq E, D'Souza S. Sex Bias in the Family Allocation of Food and Health Care in Rural Bangladesh. Population and Development Review. 1981;7(1):55–70. [Google Scholar]
- Choi BG, McLaughlin MA. Why men's hearts break: cardiovascular effects of sex steroids. Endocrinol Metab Clin North Am. 2007;36(2):365–377. doi: 10.1016/j.ecl.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Coale AJ. Excess Female Mortality and the Balance of the Sexes in the Population: An Estimate of the Number of "Missing Females". Population and Development Review. 1991;17(3):517–523. [Google Scholar]
- Cohen MN, Bennett S. Skeletal evidence for sex roles and gender hierarchies in prehistory. In: Miller BD, editor. Sex and gender hierarchies. Cambridge: Cambridge University Press; 1993. pp. 273–296. [Google Scholar]
- Cohn SK. The Black Death transformed: disease and culture in early Renaissance Europe. London: Arnold; 2002. [Google Scholar]
- Cote CG, Chapman KR. Diagnosis and treatment considerations for women with COPD. Int J Clin Pract. 2009;63(3):486–493. doi: 10.1111/j.1742-1241.2008.01987.x. [DOI] [PubMed] [Google Scholar]
- Cucina A, Vargiu R, Mancinelli D, Ricci R, Santandrea E, Catalano P, Coppa A. The necropolis of Vallerano (Rome, 2nd-3rd century AD): an anthropological perspective on the ancient Romans in the Suburbium. International Journal of Osteoarchaeology. 2006;16(2):104–117. [Google Scholar]
- Dahlberg AA. Interpretations of general problems in amelogenesis. In: Ortner DJ, Aufderheide AC, editors. Human paleopathology: current syntheses and future options. Washington, DC: Smithsonian Institution Press; 1991. pp. 269–272. [Google Scholar]
- DeWitte SN. The effect of sex on risk of mortality during the Black Death in London, A.D. 1349–1350. Am J Phys Anthropol. 2009;139:222–234. doi: 10.1002/ajpa.20974. [DOI] [PubMed] [Google Scholar]
- DeWitte SN, Wood JW. Selectivity of the Black Death with Respect to Preexisting Health. Proc Natl Acad Sci U S A. 2008;105(5):1436–1441. doi: 10.1073/pnas.0705460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey I, Chaudhuri RN. Gender inequality in nutritional status among under five children in a village in Hooghly district, West Bengal. Indian J Public Health. 2008;52(4):218–220. [PubMed] [Google Scholar]
- Dyer C. English diet in the later middle ages. In: Aston TH, Coss PR, Dyer C, Thirsk J, editors. Social relations and ideas: essays in honour of RH Hilton. Cambridge: Cambridge University Press; 1983. pp. 191–216. [Google Scholar]
- Eisenberg LE. Mississippian cultural terminations in Middle Tennessee: what the bioarcheological evidence can tell us. In: Powell ML, Bridges PS, Mires AM, editors. What mean these bones. Tuscaloosa University of Alabama Press; 1991. pp. 70–88. [Google Scholar]
- Ell SR. Iron in Two Seventeenth-Century Plague Epidemics. Journal of Interdisciplinary History. 1985;15(3):445–457. [PubMed] [Google Scholar]
- Esimai OA, Ojofeitimi EO, Oyebowale OM. Sociocultural practices influencing under five nutritional status in an urban community in Osun State, Nigeria. Nutr Health. 2001;15(1):41–46. doi: 10.1177/026010600101500105. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173(3):600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Vardakas KZ, Mourtzoukou EG. Sex differences in the incidence and severity of respiratory tract infections. Respir Med. 2008;102(4):627. doi: 10.1016/j.rmed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Farinati F, Sergio A, Giacomin A, Di Nolfo MA, Poggio PD, Benvegnu L, Rapaccini G, Zoli M, Borzio F, Giannini EG others. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2009 doi: 10.1097/MEG.0b013e32831a86f8. [DOI] [PubMed] [Google Scholar]
- Finlay R. Population and metropolis: the demography of London. Cambridge: Cambridge University Press; 1979. pp. 1580–1650. [Google Scholar]
- Gage TB. Mathematical hazard models of mortality: an alternative to model life tables. Am J Phys Anthropol. 1988;76(4):429–441. doi: 10.1002/ajpa.1330760403. [DOI] [PubMed] [Google Scholar]
- Gage TB. Population Variation in Cause of Death: Level, Gender, and Period Effects. Demography. 1994;31(2):271–296. [PubMed] [Google Scholar]
- Gage TB. Demography. In: Stinson S, Bogin B, Huss-ashmore R, O'Rourke D, editors. Human Biology: An Evolutionary and Biocultural Perspective. New York: Wiley-Liss; 2000. pp. 507–552. [Google Scholar]
- Gage TB. Are modern environments really bad for us?: revisiting the demographic and epidemiologic transitions. Am J Phys Anthropol Suppl. 2005;41:96–117. doi: 10.1002/ajpa.20353. [DOI] [PubMed] [Google Scholar]
- Goodman AH, Armelagos GJ, Rose JC. Enamel hypoplasias as indicators of stress in three prehistoric populations from Illinois. Hum Biol. 1980;52(3):515–528. [PubMed] [Google Scholar]
- Grainger I, Hawkins D. Excavations at the Royal Mint site 1986–1988. The London Archaeologist. 1988;5:429–436. [Google Scholar]
- Grauer AL, McNamara EM, Houdek DV. A history of their own: patterns of death in a nineteenth-century poorhouse. In: Grauer AL, Stuart-Macadam P, editors. Sex and gender in paleopathological perspective. Cambridge: Cambridge University Press; 1998. pp. 149–164. [Google Scholar]
- Graunt J. Natural and political observations mentioned in a following index and made upon the bills of mortality. New York: Arno Press; 1975. [Google Scholar]
- Graw M, Czarnetzki A, Haffner HT. The form of the supraorbital margin as a criterion in identification of sex from the skull: investigations based on modern human skulls. Am J Phys Anthropol. 1999;108(1):91–96. doi: 10.1002/(SICI)1096-8644(199901)108:1<91::AID-AJPA5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227(4684):257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- Guatelli-Steinberg D, Lukacs JR. Interpreting sex differences in enamel hypoplasia in human and non-human primates: Developmental, environmental, and cultural considerations. Am J Phys Anthropol. 1999;110(S29):73–126. doi: 10.1002/(sici)1096-8644(1999)110:29+<73::aid-ajpa4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hawkins D. Black Death and the new London cemeteries of 1348. Antiquity. 1990;64:637–642. [Google Scholar]
- Heligman L. Patterns of sex differentials in mortality in less developed countries. In: Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1983. pp. 7–32. [Google Scholar]
- Herlihy D. Population patterns in the past. New York: Academic Press; 1977. Deaths, marriages, births, and the Tuscan economy (ca. 1300–1550) pp. 135–164. [Google Scholar]
- Hetzel BS. Life style factors in sex differentials in mortality in developed countries. In: Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1984. pp. 247–277. [Google Scholar]
- Hill K, Upchurch DM. Gender Differences in Child Health: Evidence from the Demographic and Health Surveys. Population and Development Review. 1995;21(1):127–151. [Google Scholar]
- Hoff R, Mott KE, Silva JF, Menezes V, Hoff JN, Barrett TV, Sherlock I. Prevalence of parasitaemia and seroreactivity to Trypanosoma cruzi in a rural population of northeast Brazil. Am J Trop Med Hyg. 1979;28:461–466. doi: 10.4269/ajtmh.1979.28.461. [DOI] [PubMed] [Google Scholar]
- Högberg U, Iregren E, Siven C-H, Diener L. Maternal deaths in medieval sweden: an osteological and life table analysis. J Biosoc Sci. 1987;19(04):495–503. doi: 10.1017/s0021932000017120. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MF, Hollingsworth TH. Plague mortality rates by age and sex in the parish of St. Botolph's without Bishopsgate, London, 1603. Population Studies. 1971;25:131–146. doi: 10.1080/00324728.1971.10405789. [DOI] [PubMed] [Google Scholar]
- Holman DJ. Version 2.1 ed. Seattle, WA: 2005. mle: A programming language for building likelihood models. [Google Scholar]
- Holman DJ, Wood JW, O’Connor KA. Estimating age-at-death distributions from skeletal samples. In: Hoppa RD, Vaupel JW, editors. Paleodemography: Age distributions from skeletal samples. Cambridge: Cambridge University Press; 2002. pp. 193–201. [Google Scholar]
- Hoppa RD, Vaupel JW. Paleodemography : age distribution from skeletal samples. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Horrox R. The Black Death. Manchester: Manchester University Press; 1994. [Google Scholar]
- Huss-Ashmore R, Goodman AH, Armelagos GJ. Nutritional inference from paleopathology. Advances in Archaeological Method and Theory. 1982;5:395–473. [Google Scholar]
- Janele D, Lang T, Capellino S, Cutolo M, Da Silva JA, Straub RH. Effects of testosterone, 17beta-estradiol, and downstream estrogens on cytokine secretion from human leukocytes in the presence and absence of cortisol. Ann N Y Acad Sci. 2006;1069:168–182. doi: 10.1196/annals.1351.015. [DOI] [PubMed] [Google Scholar]
- Jansen A, Stark K, Schneider T, Schoneberg I. Sex differences in clinical leptospirosis in Germany: 1997–2005. Clin Infect Dis. 2007;44(9):e69–e72. doi: 10.1086/513431. [DOI] [PubMed] [Google Scholar]
- Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14(39):5945–5961. doi: 10.3748/wjg.14.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Z, Wang SC, Petronis A. Complex disease, gender and epigenetics. Ann Med. 2006;38(8):530–544. doi: 10.1080/07853890600989211. [DOI] [PubMed] [Google Scholar]
- Kehoe AB, Giletti DH. Women's Preponderance in Possession Cults: The Calcium-Deficiency Hypothesis Extended. AA. 1981;83(3):549–561. [Google Scholar]
- Khadi PB, Khateeb J, Patil MS. Development of rural girl children -- a bias. Indian J Matern Child Health. 1996;7(1):24–27. [PubMed] [Google Scholar]
- King T, Humphrey LT, Hillson S. Linear enamel hypoplasias as indicators of systemic physiological stress: Evidence from two known age-at-death and sex populations from postmedieval London. Am J Phys Anthropol. 2005;128(3):547–559. doi: 10.1002/ajpa.20232. [DOI] [PubMed] [Google Scholar]
- Kirkwood B, Smith P, Marshall T, Prost A. Relationships between mortality, visual acuity and microfilarial load in the area of the Onchocerciasis Control Programme. Trans R Soc Trop Med Hyg. 1983;77(6):862–868. doi: 10.1016/0035-9203(83)90308-5. [DOI] [PubMed] [Google Scholar]
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24(6):627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Konigsberg LW, Frankenberg SR. Estimation of age structure in anthropological demography. Am J Phys Anthropol. 1992;89(2):235–256. doi: 10.1002/ajpa.1330890208. [DOI] [PubMed] [Google Scholar]
- Konigsberg LW, Frankenberg SR. Deconstructing death in paleodemography. Am J Phys Anthropol. 2002;117(4):297–309. doi: 10.1002/ajpa.10039. [DOI] [PubMed] [Google Scholar]
- Larsen CS. Bioarchaeology: interpereting behavior from the human skeleton. New York: Cambridge University Press; 1997. [Google Scholar]
- Larsen CS. Gender, health, and activity in foragers and farmers in the American southeast: implications for social organization in the Georgia Bight. In: Grauer AL, Stuart-Macadam P, editors. Sex and gender in paleopathological perspective. Cambridge: Cambridge University Press; 1998. pp. 165–187. [Google Scholar]
- Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;187(9):1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- Leone M, Honstettre A, Lepidi H, Capo C, Bayard F, Raoult D, Mege JL. Effect of sex on Coxiella burnetii infection: protective role of 17beta-estradiol. J Infect Dis. 2004;189(2):339–345. doi: 10.1086/380798. [DOI] [PubMed] [Google Scholar]
- Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24(3):313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- Lopez AD. The sex mortality differntial in developed countries. In: Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1984. pp. 53–120. [Google Scholar]
- Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1984. [Google Scholar]
- Mays SA. Carbon Stable Isotope Ratios in Mediaeval and Later Human Skeletons From Northern England. Journal of Archaeological Science. 1997;24(6):561–567. [Google Scholar]
- McGovern SL, Liao Z, Bucci MK, McAleer MF, Jeter MD, Chang JY, O'Reilly MS, Cox JD, Allen PK, Komaki R. Is sex associated with the outcome of patients treated with radiation for nonsmall cell lung cancer? Cancer 9999(9999):NA. 2009 doi: 10.1002/cncr.24361. [DOI] [PubMed] [Google Scholar]
- Meindl RS, Lovejoy CO, Mensforth RP, Carlos LD. Accuracy and direction of error in the sexing of the skeleton: Implications for paleodemography. Am J Phys Anthropol. 1985;68(1):79–85. doi: 10.1002/ajpa.1330680108. [DOI] [PubMed] [Google Scholar]
- Mensforth RP, Lovejoy CO, Lallo JW, Armelagos GJ. The role of constitutional factors, diet, and infectious disease in the etiology of porotic hyperostosis and periosteal reactions in prehistoric infants and children. Med Anthropol. 1978;2:1–58. doi: 10.1080/01459740.1978.9986939. [DOI] [PubMed] [Google Scholar]
- Milner GR. Health and cultural change in the Late Prehistoric American Botton, Illinois. In: Powell ML, Bridges PS, Mires AM, editors. What mean these bones? Tuscloosa: University of Alabama Press; 1991. pp. 52–69. [Google Scholar]
- Milner GR, Wood JW, Boldsen JL. Paleodemography. In: Katzenberg M, Saunders S, editors. Biological Anthropology of the Human. Skeleton: Wiley-Liss; 2008. pp. 561–600. [Google Scholar]
- Møller-Christensen V. Leprosy changes of the skull. Odense, Denmark: Odense University Press; 1978. [Google Scholar]
- Moore SL, Wilson K. Parasites as a Viability Cost of Sexual Selection in Natural Populations of Mammals. Science. 2002;297:2015–2018. doi: 10.1126/science.1074196. [DOI] [PubMed] [Google Scholar]
- Moriyama IM. The determinants of sex differentials in mortality. In: Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1984. pp. 279–295. [Google Scholar]
- Muldner G, Richards MP. Diet and diversity at later medieval Fishergate: the isotopic evidence. Am J Phys Anthropol. 2007;134(2):162–174. doi: 10.1002/ajpa.20647. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Hirata K, Yokota E, Matsu'ura S. Paleodemography of a medieval population in Japan: analysis of human skeletal remains from the Yuigahama-minami site. Am J Phys Anthropol. 2006;131(1):1–14. doi: 10.1002/ajpa.20402. [DOI] [PubMed] [Google Scholar]
- Ng MK. New perspectives on Mars and Venus: unravelling the role of androgens in gender differences in cardiovascular biology and disease. Heart Lung Circ. 2007;16(3):185–192. doi: 10.1016/j.hlc.2007.02.108. [DOI] [PubMed] [Google Scholar]
- Noymer A. The 1918–19 influenza pandemic affected tuberculosis in the United States: reconsidering Bradshaw, Smith, and Blanchard. Biodemography Soc Biol. 2008;54(2):125–133. doi: 10.1080/19485565.2008.9989137. discussion 134–140. [DOI] [PubMed] [Google Scholar]
- Noymer A, Garenne M. The 1918 influenza epidemic's effects on sex differentials in mortalit in the United States. Population and Development Review. 2000;26(3):565–581. doi: 10.1111/j.1728-4457.2000.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliviera JSM, Mello de Oliveira JA, Frederiques U, Jr, Lima Filho EC. Apical aneurism of Chagas' heart disease. Br Heart J. 1981;46:432–437. doi: 10.1136/hrt.46.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner DJ. Theoretical and methodological issues in paleopathology. In: Ortner DJ, Aufderheide AC, editors. Human paleopathology: current syntheses and future options. Washington, DC: Smithsonian Institution Press; 1991. pp. 5–11. [Google Scholar]
- Ortner DJ. Male-female immune reactivity and its implications for interpreting evidence in human skeletal paleopathology. In: Grauer AL, Stuart-Macadam P, editors. Sex and gender in paleopathological perspective. Cambridge: Cambridge University Press; 1998. pp. 79–92. [Google Scholar]
- Ortner DJ. Identification of pathological conditions in human skeletal remains. Amsterdam: Academic Press; 2003. [Google Scholar]
- Owens IP. Ecology and evolution. Sex differences in mortality rate. Science. 2002;297(5589):2008–2009. doi: 10.1126/science.1076813. [DOI] [PubMed] [Google Scholar]
- Owsley DW, Bass WM. A demographic analysis of skeletons from the Larson site (39WW2) Walworth County, South Dakota: Vital statistics. Am J Phys Anthropol. 1979;51(2):145–154. [Google Scholar]
- Pasche B, Kalaydjiev S, Franz TJ, Kremmer E, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Lengeling A, Busch DH. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun. 2005;73(9):5952–5960. doi: 10.1128/IAI.73.9.5952-5960.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KL. Nutrition and the Early-Medieval Diet. Speculum. 1997;72(1):1–32. [Google Scholar]
- Phenice TW. A newly developed visual method of sexing the os pubis. Am J Phys Anthropol. 1969;30(2):297–301. doi: 10.1002/ajpa.1330300214. [DOI] [PubMed] [Google Scholar]
- Pilote L, Dasgupta K, Guru V, Humphries KH, McGrath J, Norris C, Rabi D, Tremblay J, Alamian A, Barnett T others. A comprehensive view of sex-specific issues related to cardiovascular disease. CMAJ. 2007;176(6):S1–S44. doi: 10.1503/cmaj.051455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ML. Status and health in prehistory: a case study of the Moundville Chiefdom. Washington: Smithsonian Institution Press; 1988. [Google Scholar]
- Preston SH. Mortality Trends. Annual Review of Sociology. 1977;3:163–178. [Google Scholar]
- Promislow DEL. Costs of Sexual Selection in Natural Populations of Mammals. Proceedings: Biological Sciences. 1992;247(1320):203–210. [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MP, Fuller BT, Molleson TI. Stable isotope palaeodietary study of humans and fauna from the multi-period (Iron Age, Viking and Late Medieval) site of Newark Bay, Orkney. Journal of Archaeological Science. 2006;33(1):122–131. [Google Scholar]
- Roberts CA. A bioarcheological study of maxillary sinusitis. Am J Phys Anthropol. 2007;133(2):792–807. doi: 10.1002/ajpa.20601. [DOI] [PubMed] [Google Scholar]
- Roberts CA, Lewis ME, Boocock P. Infectious disease, sex, and gender: the complexity of it all. In: Grauer AL, Stuart-Macadam P, editors. Sex and gender in paleopathological perspective. Cambridge: Cambridge University Press; 1998. pp. 93–113. [Google Scholar]
- Roberts CA, Manchester K. The archaeology of disease. Ithaca, N.Y: Cornell University Press; 2005. [Google Scholar]
- Roberts CW, Cruickshank SM, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun. 1995;63(7):2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14(3):476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TL. Determining the sex of human remains through cranial morphology. J Forensic Sci. 2005;50(3):493–500. [PubMed] [Google Scholar]
- Rosenberg EM. Demographic effects of sex-differential nutrition. In: Jerome NW, Kandel RF, Pelto GH, editors. Nutritional anthropology : contemporary approaches to diet & culture. Pleasantville, N.Y: Redgrave Pub. Co.; 1980. pp. 181–203. [Google Scholar]
- Russell JC. British medieval population. Albuquerque: University of New Mexico; 1948. [Google Scholar]
- Sakisaka K, Wakai S, Kuroiwa C, Cuadra Flores L, Kai I, Mercedes Aragon M, Hanada K. Nutritional status and associated factors in children aged 0–23 months in Granada, Nicaragua. Public Health. 2006;120(5):400–411. doi: 10.1016/j.puhe.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Santos RV, Coimbra CE., Jr Hardships of contact: enamel hypoplasias in Tupi-Monde Amerindians from the Brazilian Amazonia. Am J Phys Anthropol. 1999;109(1):111–127. doi: 10.1002/(SICI)1096-8644(199905)109:1<111::AID-AJPA9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Schofield J. The Plague reconsidered: a new look at its origins and effects in 16th and 17th century. England. Matlock, Derbyshire: Local Population Studies in association with the S. S. R. C. Cambridge Group for the History of Population and Social Structure; 1977. An Anatomy of an Epidemic: Coylton, November 1645 to November 1646; pp. 95–126. [Google Scholar]
- Scrimshaw NS. The phenomenon of famine. Annu Rev Nutr. 1987;7(1):1–22. doi: 10.1146/annurev.nu.07.070187.000245. [DOI] [PubMed] [Google Scholar]
- Silbiger S, Neugarten J. Gender and human chronic renal disease. Gend Med. 2008;5 Suppl A:S3–S10. doi: 10.1016/j.genm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Šlaus M. Biocultural analysis of sex differences in mortality profiles and stress levels in the Late Medieval population from Nova Rača, Croatia. Am J Phys Anthropol. 2000;111:193–209. doi: 10.1002/(SICI)1096-8644(200002)111:2<193::AID-AJPA6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Šlaus M. Osteological and dental markers of health in the transition from the Late Antique to the Early Medieval period in Croatia. Am J Phys Anthropol. 2008;136:455–469. doi: 10.1002/ajpa.20829. [DOI] [PubMed] [Google Scholar]
- Stinson S. Sex differences in environmental sensitivity during growth and development. Am J Phys Anthropol. 1985;28(S6):123–147. [Google Scholar]
- Storey R. The mothers and daughters of a patrilineal civilization: the health of females among Late Classic Maya of Copan, Honduras. In: Grauer AL, Stuart-Macadam P, editors. Sex and gender in paleopathological perspective. Cambridge: Cambridge University Press; 1998. pp. 133–148. [Google Scholar]
- Sutherland LD, Suchey JM. Use of the ventral arc in pubic sex determination. J Forensic Sci. 1991;36(2):501–511. [PubMed] [Google Scholar]
- Szostek K, Glab H, Pudlo A. The use of strontium and barium analyses for the reconstruction of the diet of the early medieval coastal population of Gdansk (Poland) A preliminary study. Homo. 2009 doi: 10.1016/j.jchb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Taylor BC, Yuan JM, Shamliyan TA, Shaukat A, Kane RL, Wilt TJ. Clinical outcomes in adults with chronic hepatitis B in association with patient and viral characteristics: A systematic review of evidence. Hepatology. 2009;49(5 Suppl):S85–S95. doi: 10.1002/hep.22929. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, McCullough LD. Sex Differences in Stroke. Cerebrovasc Dis. 2008;26:462–474. doi: 10.1159/000155983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubelaker DH, Volk CG. A test of the phenice method for the estimation of sex. J Forensic Sci. 2002;47(1):19–24. [PubMed] [Google Scholar]
- Usher BM. A multistate model of health and mortality for paleodemography : Tirup cemetery [Dissertation] Pennsylvania State University; 2000. [Google Scholar]
- Vaupel JW. Inherited frailty and longevity. Demography. 1988;25(2):277–287. [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Waldron HA. Are plague pits of particular use to palaeoepidemiologists? Int J Epidemiol. 2001;30(1):104–108. doi: 10.1093/ije/30.1.104. [DOI] [PubMed] [Google Scholar]
- Waldron I. The role of genetic and biological factors in sex differences in mortality. In: Lopez AD, Ruzicka LT, editors. Sex differentials in mortality: trends, determinants, and consequences. Canberra: Dept. of Demography, Australian National University; 1984. pp. 141–164. [Google Scholar]
- Waldron T. Nutrition and the Skeleton. In: Woolgar CM, Serjeantson D, Waldron T, editors. Food in medieval England: diet and nutrition. Oxford: Oxford University Press; 2006. pp. 254–266. [Google Scholar]
- Walker PL. Greater sciatic notch morphology: Sex, age, and population differences. Am J Phys Anthropol. 2005;127(4):385–391. doi: 10.1002/ajpa.10422. [DOI] [PubMed] [Google Scholar]
- Walker PL. Sexing skulls using discriminant function analysis of visually assessed traits. Am J Phys Anthropol. 2008;136(1):39–50. doi: 10.1002/ajpa.20776. [DOI] [PubMed] [Google Scholar]
- Walker PL, Bathurst RR, Richman R, Gjerdrum T, Andrushko VA. The causes of porotic hyperostosis and cribra orbitalia: A reappraisal of the iron-deficiency-anemia hypothesis. Am J Phys Anthropol. 2009;139(2):109–125. doi: 10.1002/ajpa.21031. [DOI] [PubMed] [Google Scholar]
- Weiss KM. Demographic Models for Anthropology. Washington, D.C: Society for American Archaeology; 1973. [Google Scholar]
- Wells JC. Natural selection and sex differences in morbidity and mortality in early life. J Theor Biol. 2000;202(1):65–76. doi: 10.1006/jtbi.1999.1044. [DOI] [PubMed] [Google Scholar]
- Wiesner ME. Women and gender in early modern. Europe. New York: Cambridge University Press; 2000. [Google Scholar]
- Williams BA, Rogers TL. Evaluating the Accuracy and Precision of Cranial Morphological Traits for Sex Determination. J Forensic Sci. 2006;51(4):729–735. doi: 10.1111/j.1556-4029.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Willigan JD, Lynch KA. Sources and methods of historical demography. New York, N.Y: Academic Press; 1982. [Google Scholar]
- Wood JW, Holman DJ, O’Connor KA, Ferrell RJ. Mortality models for paleodemography. In: Hoppa RD, Vaupel JW, editors. Paleodemography: Age distributions from skeletal samples. Cambridge: Cambridge University Press; 2002. pp. 129–168. [Google Scholar]
- Wood JW, Milner GR, Harpending HC, Weiss KM. The Osteological Paradox: Problems of Inferring Prehistoric Health from Skeletal Samples. Current Anthropology. 1992;33:343–370. [Google Scholar]
- Wrigley EA, Schofield RS. The population history of England, 1541–1871 : a reconstruction. London: Edward Arnold; 1981. [Google Scholar]
- Zhou H, He Y, Ohtsuka R. Sex differences in the malnourished status of Chinese children due to schistosomiasis infections and inadequate dietary intake. Environ Sci. 2005;12(3):145–153. [PubMed] [Google Scholar]