Abstract
Chemical transmitters released from astrocytes, termed gliotransmitters, modulate synaptic transmission and neuronal function. Using astrocyte-specific inducible transgenic mice (dnSNARE mice), we have demonstrated that inhibiting gliotransmission leads to reduced activation of adenosine A1 receptors (A1R) and impaired sleep homeostasis (Halassa et al., 2009;Pascual et al., 2005). Additionally, synaptic N-methyl-D-aspartate receptor (NMDAR) currents are reduced in these astrocyte-specific transgenic animals (Fellin et al., 2009). Because of the importance of adenosine and NMDA receptors to sleep processes we asked whether there is a causal linkage between changes in A1R activation and synaptic NMDA receptors. We show that astrocytic dnSNARE expression leads to reduced tyrosine phosphorylation of Src kinase and NR2 subunits concomitant with the decreased surface expression of the NR2 subunits. To test the role of A1R signaling in mediating these actions, we show that incubation of wildtype (WT) slices with an A1R antagonist reduces tyrosine phosphorylation of Src kinase and NR2B, decreases the surface expression of the NR2B subunits and leads to smaller NMDA component of miniature EPSCs. In dnSNARE mice we could rescue WT phenotype by incubation in an A1R agonist: activation of A1 receptor led to increased tyrosine phosphorylation of Src kinase and NR2B subunits as well as increased the surface expression of the NR2B subunit and increased NMDA component of the synaptic mEPSC. These results provide the first demonstration that astrocytes can affect neuronal excitability on a long time scale by regulating the surface expression of NMDA receptors through the activation of specific intracellular signaling pathways.
Keywords: Astrocyte, NR2, trafficking
Introduction
NMDA receptors are regulated by a diversity of signals and are of importance to numerous biological functions including the regulation of learning and memory, long term potentiation and depression of synaptic transmission, as well as for contributing to excitotoxicity (Dirnagl et al., 1999;Lau & Zukin, 2007;Morris et al., 1986;Salter & Kalia, 2004;Steriade et al., 1993). Astrocytes modulate NMDA receptor on a short timescale by releasing glutamate and by providing the NMDA receptor co-agonist D-serine. Astrocyte-derived D-serine continuously modulates the functional NMDA receptor current and has shown to be important for the induction of synaptic plasticity (Henneberger et al., 2010;Mothet et al., 2000;Panatier et al., 2006;Schell et al., 1995;Schell et al., 1997;Stevens et al., 2003;Wolosker et al., 1999;Yang et al., 2003).
In a recent study we identified the presence of a second pathway of glial control of NMDA receptor current (Fellin et al., 2009). The molecular genetic expression of the SNARE domain of VAMP2 selectively and conditionally in astrocytes leads to an impairment in the formation of the SNARE complex that is required for regulated exocytosis (Pascual et al., 2005). dnSNARE mice exhibit reduced release of ATP/adenosine from astrocytes and a reduction in the surface expression of the NR2A and NR2B subunits of the NMDA receptor (Fellin et al., 2009). How SNARE expression in astrocytes regulates the surface expression of NMDA receptor subunits is unknown. Astrocytic dnSNARE expression leads to an impairment in release of at least two gliotransmitters: ATP/adenosine and D-serine. Given that elevated D-serine has been shown capable of causing an internalization of NMDA receptors we determined whether astrocytic purinergic signaling, by activating A1 receptors promotes increased surface expression of NMDA receptors.
Through a combination of biochemical, electrophysiology and pharmacological studies we demonstrate that the astrocytic activation of neuronal A1R leads to an activation of the Src family tyrosine kinases (SFKs) that then phosphorylates the NR2A and NR2B subunits of the NMDA receptor. Tyrosine phosphorylation in turn regulates the rate of endocytosis of the NMDA receptor: when A1R activity and consequently SFK activation is reduced in dnSNARE mice, a consequent reduction in tyrosine phosphorylation of the NMDA receptor subunits enhances interactions with the AP2 adapter protein that is required for endocytosis of these subunits, and thus leads to a reduced surface expression of the NMDA receptor subunit. Taken together these results provide the first evidence that activation of A1 receptors by astrocytes controls the surface expression of NMDA receptors through a Src-dependent pathway.
Materials and Methods
All procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Tufts University and Institutional Animal Care and Use Committee. dnSNARE transgenic mice have been backcrossed onto a C57BL6/J genotype for more than 10 generations. Consequently, dnSNARE littermates as well as C57BL6/J mice were used as controls.
Slice preparation
300µm slices containing somatosensory cortex of were prepared from 6 to 15 week-old mice. Mice were deeply anesthetized with isoflurane and decapitated. The brain was rapidly removed from the skull and chilled with cold (4°C) artificial cerebrospinal fluid (ACSF) of the following composition in mM: 120 NaCl, 3.2 KCl, 1 KH2PO4, 26 NaHCO3, 2 MgCl2, 1 CaCl2, 10 glucose, 2 Na-pyruvate, and 0.6 mM ascorbic acid at pH 7.4 (with O2 95%, CO2 5%). The brain was then glued on a plate and cut with a vibratome (VT1000S or VT1200S; Leica, Mannheim, Germany). Before recording, slices were incubated at 32°C for a recovery period of 1 h.
Patch-Clamp Recordings
All recordings were performed at room temperature. Pyramidal Neurons in layer 2/3 of somatosensory cortex were visualized with a 40x objective. Signals were made with an Axopatch 1D amplifier (Axon Instruments) and filter at 2k Hz. Data digitized at 10K Hz with Digidata 1322 (molecular devices), and stored in the computer with Clampex 9.2 (Molecular Devices). Pipette resistance was 3–5 MΩ.
To measure the evoked AMPA/NMDA ratio, pyramidal neurons were held at +40 mV in presence of 20µM bicuculline. Electrodes contained in mM: 120 cesium gluconate, 20 HEPES, 0.4 EGTA, 2.8 NaCl, 5 TEA-Cl, 2 MgCl2, 2.5 MgATP, 0.3GTP (pH 7.2 with CsOH). To minimize polysynaptic activity, slices were superfused continuously in the recording chamber with ACSF containing 4mM Mg2+ and Ca2+ (Moore et al., 2003). After recording a stable baseline of dual EPSCs (AMPA and NMDA), the NMDA receptor antagonist D-AP5 (50 µM) was applied to the bathing solution for 7–10 min to isolate AMPA receptor mediated EPSCs. An average of 10 EPSCs was collected to assess each type of EPSC. The NMDA component was obtained by subtracting the isolated AMPA component from the dual component EPSC. The AMPA/NMDA ratio was obtained by dividing peak of AMPA EPSC by the peak of NMDA.
To measure the AMPA and NMDA components of mEPSCs, cells were recorded at holding potential −70mV in the presence of 1µM Tetrodotoxin (TTX). Intracellular solution contained the following (in mM): 125 K-gluconate, 15 KCl, 0.2 EGTA, 4 Na2ATP, 0.5 NaGTP, and 10 HEPES to pH 7.2 with KOH. To monitor AMPAR mediated mEPSC recordings were made in ACSF containing 1mM Mg2+. mEPSCs having both an AMPAR- and an NMDAR mediated component were recorded in ACSF in the absence of Mg2+.
Surface biotinylation
Slices from the somatosensory cortical region were incubated in ACSF at 32 °C for at least 1 h recovery before experimentation. Various drugs were applied at 32°C. After treatment, slices were then placed on ice and incubated for 30 min with 1 mg/mL NHS-SS-biotin (Pierce). Excess biotin was removed by washing three times in cold ACSF and lysed as described previously (Fellin et al., 2009;Terunuma et al., 2004) Insoluble material was removed by centrifugation, and lysates were incubated with Streptavidin beads (Pierce) for 12 h at 4 °C. Bound material was subjected to SDS-PAGE and then immunoblotted with NMDAR (NR2B, BD Transduction Laboratories; NR2A, Invitrogen; phospho-NR2B Y1472, Sigma), AMPAR (GluR1, Chemicon; phospho-Glu R1 S845, Chemicon), A1 adenosine receptor (Sigma), β-Actin (Sigma), β-Tubulin (Sigma), Src kinase (Src, Santa Cruz; phospho-Src Y416, Cell Signaling) antibodies and visualized by ECL (Pierce). Blots were then quantified using the CCD based FujiFilm LAS 3000 system.
Immunoprecipitation
Somatosensory cortical slices were lysed in 20 mM Tris_HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM NaF, 2 mM Na3VO4, 10 mM Na4P2O7, 10 µg/mL leupeptin, 1 µg/mL aprotinin, 10 µg/mL antipain and 250 µg/mL 4-(2-Aminoethl) benzenesulfonyl fluoride hydrochloride. Soluble material was then immunoprecipitated with anti-NR2B and anti-NR2A antibodies or control IgG coupled to protein A Sepharose (Terunuma et al., 2004). Bound material was then subjected to immunoblotting with NR2B (BD Transduction Laboratories), NR2A (Invitrogen), β-adaptin (BD Transduction Laboratories) or phospho-tyrosine (p-Y) (Santa Cruz) antibodies.
Analysis
Analysis of mEPSC was performed using Minianalysis 6.0 software (Synaptosoft). Events were detected automatically using a threshold of 5 pA for mEPSC mediated by AMPA receptors and 15 pA for mixed AMPA and NMDARs. The average mEPSC waveform of each cell was aligned at their half-maximal rise time. The peak of an average mEPSC was taken as the AMPA peak. NMDA current amplitude was measured 20 ms after the AMPA peak (Myme et al., 2003). Data were analyzed using Clampfit 9.2(molecular devices) and Origin 7.5(OriginLab). Results are expressed as mean ± SEM. Statistical comparisons were performed using two-tailed t-test when two groups were compared, and one way ANOVA followed by Bonferroni-adjusted post-hoc when multiple comparisons were made. Significance was defined as P<0.05.
Results
Astrocytic dnSNARE expression leads to reduced NMDA receptor function
As a first step in determining the role of astrocyte-derived adenosine in the regulation of synaptic NMDA receptors we performed whole cell recordings from cortical pyramidal neurons and measured the properties of evoked and miniature synaptic AMPA and NMDA receptor currents. We recorded the evoked synaptic AMPA/NMDA current ratio from pyramidal neurons in LayerII/III of the slices from the somatosensory cortex in response to stimulation of layer IV. To measure the ratio of evoked AMPA/NMDA currents pyramidal neurons were held at +40 mV in presence of 20µM bicuculline. After recording a stable baseline of dual component EPSCs (AMPA and NMDA), the NMDA receptor antagonist D-AP5 (50µM) was applied to the bathing solution for 7–10 min to isolate AMPA receptor mediated EPSCs. The evoked AMPA/NMDA current ratio was approximately doubled in brain slices obtained from dnSNARE mice compared to WT littermates (WT: 0.20±0.02, n=8; dnSNARE: 0.42±0.04, n=8; P<0.01, t-test) (Figure. 1A).
Figure 1. Astrocytic dnSNARE expression reduces NMDAR function.
(A) AMPA and NMDA mediated EPSCs from WT and dnSNARE mice (8 cells from 3 animals). (B) Representative traces of mEPSCs recorded from WT and dnSNARE mice in presence of 1 mM Mg2+ together with histograms of mEPSC properties (12 cells from 3 WT mice, 10 cells from 4 dnSNARE mice). (C) The NMDA receptor antagonist D-AP5 (50 µM) does not affect the average mEPSC in 1mM Mg2+ ACSF. However, in 0 mM Mg2+ ACSF, D-AP5 reduces the delayed NMDAR component. (D) Average mEPSC in absence of Mg2+ recorded from control and dnSNARE mice. Average AMPA (peak amplitude) and average NMDA component (20 ms after peak) of dual components mEPSCs (Control: n = 8 cells, dnSNARE n = 8). Open bars refer to WTs and black bars to dnSNARE. (E) Western blots showing total and surface expression of NMDA and AMPA receptor subunits in WT and dnSNARE mice. Histograms show normalized surface expression of each subunit (n = 5). Error bars, mean±SEM; * P<0.05, **P<0.01.
We recorded mEPSCs in 1 mM Mg2+ containing ACSF at a holding potential of −70 mV (Figure 1B, C). Consistent with the presence of a Mg2+ block of the NMDA receptor the addition of the NMDAR antagonist, D-2-amino-5-phosphonopentanoate (D-AP5), did not change the kinetics of mEPSC (Figure 1C) while DNQX attenuated the mEPSC so that they are no longer detectable indicating that the mEPSC is mainly mediated by AMPARs under these experimental conditions. The amplitude of the peak mEPSC current was unchanged by dnSNARE expression (WT: 9.82±0.43 pA, n=12; dnSNARE: 10.23±0.90 pA, n=10; P=0.67), although the frequency of mEPSCs was increased by astrocytic dnSNARE expression (WT: 2.50±0.34 Hz, dnSNARE: 4.15±0.74 Hz) (Figure 1B) consistent with the removal of adenosine A1 receptor-mediated presynaptic inhibition. In support of this idea, the mEPSC frequency was significantly greater in WT slices incubated in the A1R antagonist CPT (4.56 ± 0.29 Hz, n=9) compared to vehicle (2.50+0.34 Hz, n = 12; p<0.003) whereas incubation in CPT did not lead to a significant increase in mEPSC frequency in dnSNARE slices (CPT, 4.45±0.69 Hz n=6; Vehicle 4.15 ± 0.74 Hz, n=10).
To enhance the NMDA component of the mEPSC we performed experiments in ACSF lacking Mg2+: to identify the NMDA component of the mixed mEPSC we added D-AP5 and show that the magnitude of the mEPSC 20ms following the peak is a reliable indicator of the NMDAR current contribution (Figure 1C). A comparison of average mEPSCs in dnSNARE and WT slices show that astrocytic dnSNARE expression leads to a selective reduction in the synaptic NMDAR current (Control: 6.06±1.08 pA, n=8; dnSNARE: 3.07±0.48 pA, n=8; P<0.05) with no change in the AMPA component (Control: 17.86±0.69 pA; dnSNARE: 18.51±1.96 pA, P=0.76, t-test) (Figure 1D).
To further evaluate the specificity of this action we used biotinylation assays to measure the surface NMDA and AMPA subunits. Surface biotinylation experiments show that astrocytic dnSNARE expression selectively reduced normalized surface expression of NR2A and NR2B subunits of NMDARs, but not GluR1 subunits of AMPA(NR2A: 51.4±18.1%, P<0.05; NR2B: 62.1 ±9.1%, P<0.01; GluR1: 92.9±18.9%, P>0.05; n=5, t-test) (Figure 1E) in agreement with previous results (Fellin et al., 2009).
Astrocytic dnSNARE expression leads to reduced activation of the Src family kinase pathway and tyrosine phosphorylation of NR2 subunits
SFKs act as a molecular hub for control of NMDARs (Salter & Kalia, 2004). NR2A and NR2B but not NR1 subunits are tyrosine-phosphorylated by SFKs (Lau & Huganir, 1995;Moon et al., 1994). We therefore examined whether dnSNARE sensitive gliotransmitters regulated NR2 subunits via Src kinase activity. Src kinase is activated by autophosphorylation of tyrosine 416 (Salter & Kalia, 2004). Compared with control animals, dnSNARE showed a nearly 50% reduction in phosphorylation level at tyrosine 416 of Src kinase (62.±9.1%, n=4, *p<0.05, t-test) (Figure 2A). Src kinase promotes surface expression of NR2 subunits by phosphorylation of NR2 subunits and enhances NMDAR activity (Prybylowski et al., 2005;Thornton et al., 2003). There are three Src-mediated phosphorylation sites in NR2A and NR2B subunits (Nakazawa et al., 2001;Prybylowski et al., 2005;Yang & Leonard, 2001) with tyrosine 1472 being the main phosphorylation site in NR2B subnunits (Nakazawa et al., 2001). Astrocytic dnSNARE expression led to reduced tyrosine phosphorylation of NR2B (Y1472) and to reduced tyrosine phosphorylation of NR2A (pNR2B (Y1472): 72.5±9.9%, p<0.05, n=4, t-test, pNR2A (pY): 58.9±15.7%, *p<0.05, n=3, t-test) (Figure 2B and C), while phosphorylation of GluR1 at serine 845 (Ser-845), which regulates trafficking and surface expression of GluR1 (Ehlers, 2000;Oh et al., 2006), was unchanged (95.7±2.9%, n=4, P>0.05, t-test) (Figure 2D). Additionally, probing cell surface NR2B subunit confirmed a decrease in tyrosine phosphorylation of NR2B in slices obtained from dnSNARE mice (71.6±8.0%, *p<0.05, n=3, t-test). Because of the availability of a residue specific antibody for NR2B, but not NR2A, that recognizes Y1472 and is sensitive to phosphorylation of this residue, subsequent biochemical experiments were limited to this subunit.
Figure 2. Astrocytic expression of dnSNARE leads to decreased tyrosine phosphorylation of Src kinase and NMDAR subunits.
(A) Somatosensory cortical slices were lysed and soluble material was immunoblotted with anti-Src and phospho-Src Y416 (p-Src)antibodies. The normalized amount of p-Src in nSNARE was significantly smaller than WT (n= 4). (B) NR2A was immunoprecipitated (IP) then probed with anti-p-Y antibody. In dnSNARE there was significantly reduced Y-phosphorylation of NR2A compared to WT (n=3). (C) Soluble material was immunoblotted with anti-NR2B and anti-NR2B phospho-NR2B Y1472 (p-NR2B) antibodies. dnSNARE led to reduced phosphorylation of Y1472 of the NR2B subunit (n=4). (D) Soluble material was immunoblotted with anti-GluR1 and phospho-GluR1 S845 (p-GluR1).p-GluR1. The normalized amount of p-GluR1 in dnSNARE was not difference from WT (n= 4). (E) Immunoblots of IP obtained with anti-NR2B antibody. Blots probed with anti-NR2B and anti-β adaptin antibodies. The amount of AP-2 in dnSNARE was normalized to WT. The amount of AP-2 in dnSNARE was significantly larger than in WT (n=4). Error bars, mean±SEM; * P<0.05.
The intracellular tails of NR2 subunits contain internalization motifs that interact with the clathrin adaptor protein (AP-2) that is important for clathrin-dependent receptor endocytosis (Roche et al., 2001). In the case of the NR2B subunit, phosphorylation of Y1472 at the YEKL sequence that is located on its distal c-terminal, prevents the AP-2 adaptor from binding to the YEKL sequence thereby preventing internalization (Prybylowski et al., 2005). Immunoprecipitation assays demonstrate that astrocytic expression of dnSNARE significantly increased the association between AP-2 and the NR2B subunit (297.0±71.9%, n=4, P<0.05) (Figure 2E). These results suggest that in the dnSNARE mice reduced SFK activity leads to decreased tyrosine-phosphorylation of NR2 subunits and as a consequence enhanced interaction with AP2 leading to enhanced endocytosis and to decreased cell surface expression of the NR2 subunits.
A1R antagonist phenocopies dnSNARE expression by reducing activation of the Src family kinase pathway and reducing NMDAR function
Astrocytic dnSNARE expression leads to a reduction in both adenosine and D-serine (Fellin et al., 2009;Halassa et al., 2009). Since elevated D-serine causes an internalization of NMDARs (Nong et al., 2003) and exogenous D-serine does not restore the NMDA current in dnSNARE mice (Fellin et al., 2009) we asked whether dnSNARE sensitive adenosine and A1R activation regulates SFK and NMDA receptor function. Incubating slices of WT animals with the A1R antagonist CPT (32 °C, 200 nM) for 3 hours led to reduced tryosine phosphorylation of Src kinase and NR2B subunits, decreased surface expression of the NR2B subunits without a change in the AMPA GluR1 subunit (p-Src: 73.9±7.0% P<0.05; p-NR2B, 87.0±1.6%; p<0.01, surface expression of NR2B: 53.1±15.3%, surface expression of GluR1: 106.1±33.0%, P>0.05; n=3, t-test)(Figure 3A, B and C).
Figure 3. An A1 receptor antagonist down regulates NMDAR function.
Slices from WT incubated in CPT for 3hours. CPT significantly decreased tyrosine phosphorylation of Src kinase (A) and NR2B (B) (n=3) (C) CPT significantly decreased surface expression of NR2B but not the AMPA receptor subunits GluR1 (n=3). (D) NMDA components of mixed mEPSC significantly reduced after CPT incubation more than 3 hours (Control: 8 cells from 7 mice; CPT: 9 cells from 7 mice). *p<0.05, **P<0.01.
We sought electrophysiological corroboration of these biochemical data by measuring the AMPA and NMDA components of the mEPSC in the presence of the A1R antagonist CPT. Since preliminary studies did not reveal immediate effects of A1R pharmacology on NMDA receptor currents, we used pre-incubation of slices in CPT (3 hrs). Recordings from slices following pre-incubation revealed a selective reduction in the delayed (20 ms) NMDA component of mixed mEPSC with no effect on the peak, AMPA component (NMDA in Control: 6.5±0.6 pA, NMDA in CPT, 4.4±0.6 pA; P<0.05. AMPA in Control: 18.3±0.7 pA; AMPA in CPT: 18.8±0.6 pA, p=0.56. Control: n=8, CPT, n=9; t-test) (Figure 3D).
Activation of A1R in dnSNARE slices causes an SFK-dependent increase in NMDA function
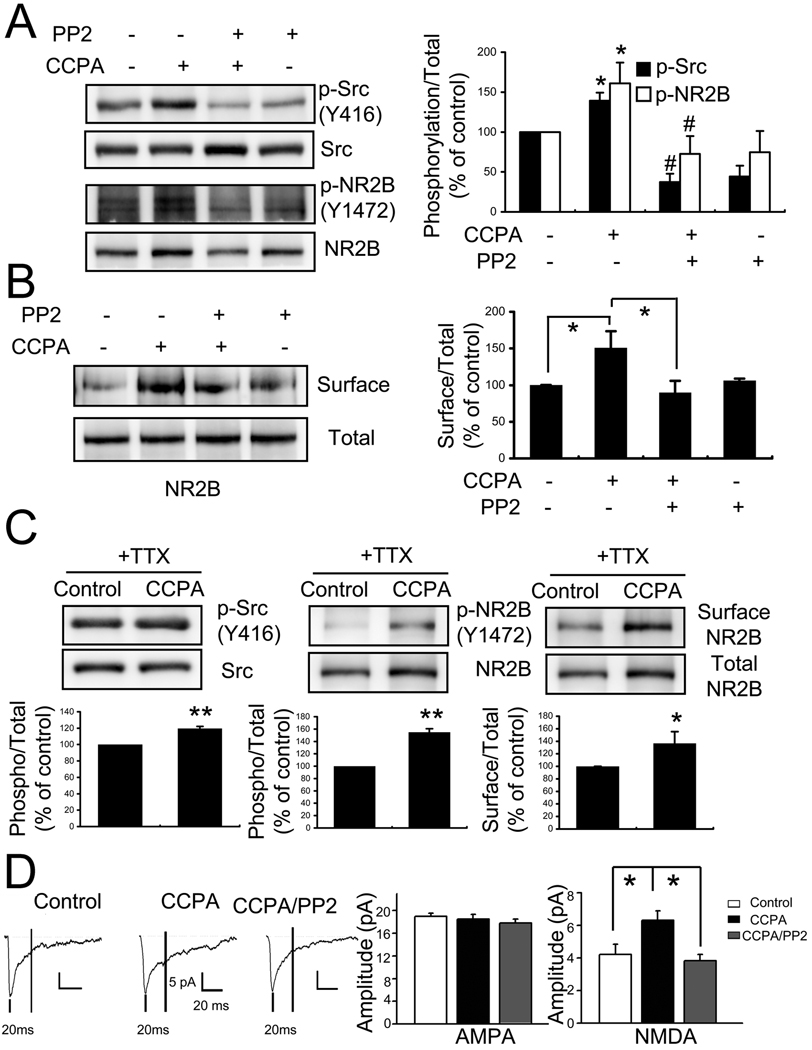
After performing a western blot analysis confirming that there are no significant changes in expression of A1R in dnSNARE mice (89.67 ± 7.20% of WT , n=5, p=0.112), in agreement with our previous report concerning the unaltered sensitivity of the A1R pathway to A1R agonist (Halassa et al., 2009), we incubated dnSNARE slices in the A1R agonist CCPA (100nM, 1hr at 32 °C) to ask whether we could rescue the dnSNARE phenotype by pharmacological activation of A1R. CCPA caused an increase in phosphorlyation of both Src kinase and the NR2B subunit of the NMDA receptor, as well as caused an increase in the surface expression of this subunit (p-Src: 139.3±10.1%, n=3, P<0.05, t-test; pNR2B: 160.8±26.1%, n=3, p<0.05, t-test; surface expression of NR2B: 151.1±22.6%, n=4, p<0.05, t-test) (Figure 4A and B)
Figure 4. Adenosine A1 receptor activation enhances NMDAR current via tyrosine phosphorylation of Src Kinase in dnSNARE mice.
(A–C), Slices from dnSNARE mice were incubated in CCPA (100nM) for 1hour or pre-incubated with Src kinase inhibitor PP2 (10 µM) 30 min. (A) Representative western blots using anti-Src, anti-p-Src, anti-p-NR2B antibodies. The levels of tyrosine phosphorylation of Src kinase and NR2B were significantly increased by incubation with CCPA. PP2 prevented these CCPA induced increases in phosphorylation. (n=3, *p<0.05 control compared with CCPA; #p<0.05 CCPA compared with CCPA/PP2). (B) Representative western blots using anti-NR2B antibody to detect the total and surface expression of NR2B. CCPA significantly increased surface expression of NR2B and the increase was inhibited by preincubation of PP2 (n=4, *p<0.05). (C) In the presence of TTX, CCPA significantly increased tyrosine phosphorylation of Src and NR2B, as well as the surface expression of NR2B (n=3, *p<0.05). (D) In slices from dnSNARE, NMDA components of mixed mEPSC significantly increased after CCPA incubation and the increase was blocked by PP2 (control: 13 cells from 7 mice, CCPA: 11 cells from 7 mice, CCPA/PP2: 11 cells from 6 mice).
To determine whether the ability of CCPA to augment phosphorylation and surface expression of NR2B required SFK activity we incubated slices in 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) a small molecule inhibitor of SFK. PP2 prevented the ability of A1R activation to increase phosphorylation of both src and the NR2B subunit and prevented the A1R induced increase in surface expression of the NR2B subunit (Figure 4A and B). Because CCPA can have powerful inhibitory effects in the nervous system we repeated these experiments in the presence of TTX where we obtained similar results (Figure 4 C).
Finally, we sought electrophysiological evidence to corroborate these biochemical results as well as to ask whether functional NMDAR currents are rescued by CCPA in dnSNARE mice. Slices from dnSNARE mice were incubated either in CCPA or in vehicle and consequences on the mixed AMPA/NMDA mEPSC were examined. CCPA caused a significant increase in the NMDA but not AMPA component of mixed mEPSC. In agreement with our biochemical observations in which we demonstrate a role for SFK in mediating the increased phosphorylation and surface expression of the NR2B subunit, the ability of CCPA to augment the NMDA current required SFK since incubation with PP2 prevented this action (AMPA in dnSNARE 19.0±0.6 pA, n=13, AMPA in dnSNARE/CCPA: 18.5±0.84 pA, n=11, AMPA in dnSNARE/CCPA/PP2: 17.8±0.7 pA, n=11, P>0.05, one-way ANOVA) (NMDA in dnSNARE 4.2±0.6 pA; NMDA in dnSNARE/CCPA: 6.3±0.6 pA; NMDA in dnSNARE/CCPA/PP2: 3.8±0.4 pA; p<0.05, dnSNARE/CCPA vs dnSNARE or dnSNARE/CCPA/PP2, one way ANOVA followed by Bonferroni-adjusted post-hoc)
Discussion
Taken together the electrophysiological and biochemical studies provide a mechanistic link between the astrocytic regulation of activation of adenosine A1 receptors and the trafficking of NMDA receptor subunits. Using a conditional mouse model in which the SNARE domain of VAMP2 is expressed selectively in astrocytes we demonstrate a causal relationship between the consequent reduction in A1R activity, and the previously noted parallel reduction in NMDA-mediated synaptic transmission. In wildtype mice we show that pharmacological antagonism of A1R phenocopies the dnSNARE mice by demonstrating a CPT dependent reduction in surface expression, and tyrosine phosphorylation of the NR2B subunit of the NMDA receptor as well as reduced NMDA component of the synaptic current. In dnSNARE mice we show the reverse, that an A1R agonist, CCPA, rescues the SNARE phenotype by increasing surface expression and tyrosine phosphorylation of the NR2B subunit and augmenting the NMDA component of synaptic currents. Src family tyrosine kinases are known to phosphorylate NR2 subunits and, as a consequence, regulate trafficking of these receptors. Our results mechanistically link SFK with the astrocytic A1R regulation of the NMDA subunits. We demonstrate that in the dnSNARE mouse, and in wildtype mice in the presence of an A1R antagonists, that SFK are less active, and conversely that an A1R agonist enhances SFK activation. When SFK are inhibited by the small molecule inhibitor PP2, we show that the ability to rescue wildtype phenotype in dnSNARE mice by A1R agonist is prevented.
D-serine is a well known co-agonist of the NMDA receptor that interacts with the NR1 subunit Johnson & Ascher, 1987;Mothet et al., 2000;Yang et al., 2003). Previously we have shown that astrocytic dnSNARE expression leads to reduced D-serine regulation of NMDA receptors: in dnSNARE mice exogenous D-serine led to a greater augmentation of the NMDA receptor mediated current than in WT littermates. However, exogenous D-serine did not fully rescue the dnSNARE phenotype (Fellin et al., 2009). Surface biotinylation studies show that astrocytic dnSNARE expression also leads to reduced surface expression of NR2A and NR2B subunits, with unchanged expression of the NR1 subunit. Since D-serine binds to the NR1 and not to either NR2A or NR2B subunit, because exogenous D-serine does not rescue the dnSNARE phenotype and because D-serine is known to cause internalization of NMDA receptor subunits (Nong et al., 2003) (the opposite of the observed phenotype) we identified the presence of an additional glial-modulated mechanism that regulates NR2 trafficking.
The adenosine-dependent regulation of NMDA receptor trafficking has important implications for the control of sleep/wake cycles. Previously we have demonstrated that sleep homeostasis is modulated by an astrocyte-dependent A1R mediated process. Expression of astrocytic dnSNARE attenuated responses to sleep deprivation including the increase in compensatory sleep time, non rapid eye movement sleep (NREM) bout duration as well as the power of slow wave activity during NREM sleep (Halassa et al., 2009). It is known that there are wakefulness-dependent changes in NMDA receptor expression and in particular sleep deprivation dependent changes in NR2A mediated synaptic transmission are thought to contribute to changes in plasticity associated with sleep loss (Cirelli & Tononi, 2000;Kopp et al., 2006;Longordo et al., 2009;McKenna et al., 2007;Vyazovskiy et al., 2008). The mechanism underlying the regulation of the NMDA receptor during sleep loss is unknown. Given that sleep loss leads to augmented adenosine levels and A1R activation, our data lead us to propose that the astrocytic regulation of A1R activity during sleep wake cycles acts through the SFK to regulate NMDA receptor trafficking.
Given that astrocytes can regulate functional NMDA receptor currents through the release of D-serine one must ask whether there are any added benefits of an adenosine-dependent pathway that regulates NMDA receptors. Our interpretation is that these two pathways allow the NMDA receptor to be regulated over different time courses. The release of D-serine can be dynamic and provides the potential for second to second changes in NMDA receptor mediated currents. In contrast, because A1R activity regulates trafficking of the NMDA receptor, this is a slower process. Indeed prolonged incubation of pharmacological agonists and antagonists were required to observe functional changes in NMDA receptor currents. Therefore we propose that the adenosine-dependent regulation of NMDA receptors is linked to slow processes such as sleep wake cycles in which astrocyte-derived adenosine is known to play important roles, whereas the D-serine regulation is important for dynamic activity-dependent changes in processes such as synaptic plasticity and learning.
Sleep is a process that occurs over a period of about ~8 hours in humans leading us to ask whether the time course of changes in NMDA receptor trafficking (hours) is appropriate to mediate changes in the sleep/wake cycles. In thinking about sleep and its underlying mechanisms it is important to remember that sleep is not a one stage process. Rather during different phases of sleep discrete events are taking place. The onset of sleep consists of slow wave NREM sleep during which time slow oscillations and their associated spindles and ripples dominate. Ripples are known to drive the replay of neuronal activity that was elicited during wakefulness and this replay is believed to help consolidate memories by reactivating neurons and strengthening their relevant synapses (Diekelmann & Born, 2010;Diekelmann et al., 2011). Thus the timing of A1R-dependent NMDA receptor trafficking is well suited to support synaptic strengthening during early phases of sleep given that it takes several hours for NMDA receptor density to decline following reductions in adenosine that are known to occur during sleep.
In conclusion, we demonstrate a newly identified astrocyte-mediated pathway of NMDA receptor regulation. The astrocytic regulation of extracellular adenosine and A1R signaling causes a SFK-dependent phosphorylation of the NR2A and NR2B subunits which regulates endocytosis of these receptors. With the activation of the A1R pathway, increased tyrosine phosphorylation of the NMDA NR2A subunits reduces the rate of endocytosis, leading to larger NMDA receptor component of synaptic transmission. Since the astrocytic regulation of A1R is important in the modulation of sleep homeostasis we propose that this glial modulation permits a slow regulation of NMDA receptors to be timed with sleep wake cycles.
ACKNOWLEDGMENTS
We are grateful to the members of the Haydon laboratory for discussions during the course of this study. This work was supported by grants from the NIH 5R01NS037585, 5R01DA025967. QD is 2009 NARSAD Sidney R. Baer, Jr. Foundation Investigator
Footnotes
Any Conflict of Interest: none
Reference List
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–333. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat.Rev.Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Buchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat.Neurosci. 2011 doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in Neurosciences. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, Moss SJ, Haydon PG. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc.Natl.Acad.Sci.U.S.A. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SHR, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463 doi: 10.1038/nature08673. 232-U120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine Potentiates the Nmda Response in Cultured Mouse-Brain Neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kopp C, Longordo F, Nicholson JR, Luthi A. Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J.Neurosci. 2006;26:12456–12465. doi: 10.1523/JNEUROSCI.2702-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature Reviews Neuroscience. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-D-aspartate receptor subunits. J.Biol.Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- Longordo F, Kopp C, Mishina M, Lujan R, Luthi A. NR2A at CA1 Synapses Is Obligatory for the Susceptibility of Hippocampal Plasticity to Sleep Loss. Journal of Neuroscience. 2009;29:9026–9041. doi: 10.1523/JNEUROSCI.1215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1473. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc.Natl.Acad.Sci.U.S.A. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc.Natl.Acad.Sci.U.S.A. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective Impairment of Learning and Blockade of Long-Term Potentiation by An N-Methyl-D-Aspartate Receptor Antagonist, Ap5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myme CIO, Sugino K, Turrigiano GG, Nelson SB. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. Journal of Neurophysiology. 2003;90:771–779. doi: 10.1152/jn.00070.2003. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J.Biol.Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature. 2003;422:302–307. doi: 10.1038/nature01497. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. Journal of Biological Chemistry. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, Standley S, McCallum J, Dune LC, Ehlers MD, Wenthold RJ. Molecular determinants of NMDA receptor internalization. Nat.Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat.Rev.Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Brady RO, Molliver ME, Snyder SH. D-Serine as a neuromodulator: Regional and developmental localizations in rat brain glia resemble NMDA receptors. Journal of Neuroscience. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-Serine, An Endogenous Synaptic Modulator - Localization to Astrocytes and Glutamate-Stimulated Release. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. A Novel Slow (Less-Than-1 Hz) Oscillation of Neocortical Neurons In-Vivo - Depolarizing and Hyperpolarizing Components. Journal of Neuroscience. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terunuma M, Jang IS, Ha SH, Kittler JT, Kanematsu T, Jovanovic JN, Nakayama KI, Akaike N, Ryu SH, Moss SJ, Hirata M. GABAA receptor phospho-dependent modulation is regulated by phospholipase C-related inactive protein type 1, a novel protein phosphatase 1 anchoring protein. J.Neurosci. 2004;24:7074–7084. doi: 10.1523/JNEUROSCI.1323-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-D-aspartate receptor function via inhibition of Src tyrosine kinase activity. J.Biol.Chem. 2003;278:23823–23829. doi: 10.1074/jbc.M302389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat.Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Leonard JP. Identification of mouse NMDA receptor subunit NR2A C-terminal tyrosine sites phosphorylated by coexpression with v-Src. J.Neurochem. 2001;77:580–588. doi: 10.1046/j.1471-4159.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M, Duan S. Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc.Natl.Acad.Sci.U.S.A. 2003;100:15194–15199. doi: 10.1073/pnas.2431073100. [DOI] [PMC free article] [PubMed] [Google Scholar]