Abstract
Cell shape plays a role in cell growth, differentiation, and death. Herein, we used the hepatocyte, a normal, highly differentiated cell characterized by a long G1 phase, to understand the mechanisms that link cell shape to growth. First, evidence was provided that the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) cascade is a key transduction pathway controlling the hepatocyte morphology. MEK2/ERK2 activation in early G1 phase did not lead to cell proliferation but induced cell shape spreading and demonstration was provided that this MAPK-dependent spreading was required for reaching G1/S transition and DNA replication. Moreover, epidermal growth factor (EGF) was found to control this morphogenic signal in addition to its mitogenic effect. Thus, blockade of cell spreading by cytochalasin D or PD98059 treatment resulted in inhibition of EGF-dependent DNA replication. Our data led us to assess the first third of G1, is exclusively devoted to the growth factor-dependent morphogenic events, whereas the mitogenic signal occured at only approximately mid-G1 phase. Moreover, these two growth factor-related sequential signaling events involved successively activation of MEK2-ERK2 and then MEK1/2-ERK1/2 isoforms. In addition, we demonstrated that inhibition of extracellular matrix receptor, such as integrin β1 subunit, leads to cell arrest in G1, whereas EGF was found to up-regulated integrin β1 and fibronectin in a MEK-ERK–dependent manner. This process in relation to cytoskeletal reorganization could induce hepatocyte spreading, making them permissive for DNA replication. Our results provide new insight into the mechanisms by which a growth factor can temporally control dual morphogenic and mitogenic signals during the G1 phase.
INTRODUCTION
Morphological cell events that occur in liver regeneration as well as in angiogenesis, inflammation conditions, embryonic development, wound repair and tumor metastasis, play a critical role in cell physiology. Adhesion and interaction with extracellular matrix (ECM) proteins are often required for cell progression through the G1 phase, and it is well established that growth in most normal cells requires cell adhesion and stimulation by growth factors to progress in G1 (Assoian, 1997; Giancotti, 1997; Bottazzi et al., 1999). Indeed, cell attachment activates many intracellular signaling pathways, including tyrosine phosphorylation cascades as mitogen-activated protein kinase (MAPK) activation, calcium influx, pH variations, and inositol lipid turnover. The mechanism by which adhesion and cell shape modification induced by soluble factors could be determinant for cell cycle progression needed to be elucidated.
Proliferation of many cells has been shown to be dependent on adhesion and spreading, requiring specific intracellular signaling events. It has been proved that ECM can modulate cell sensitivity to soluble mitogens and regulate cell proliferation in vitro (Renshaw et al., 1997; Moro et al., 1998). The epidermal growth factor (EGF) receptor (EGFr) is a member of the Erb B family of ligand-activated tyrosine kinase receptors, which plays a central role in the proliferation and differentiation of many cells (Zhang et al., 1996; Walker et al., 1998). In addition, growth factor receptors such as EGFr and hepatocyte growth factor receptor elicit increased cell movement upon ligand binding in a wide variety of cells (Chen et al., 1993; Matthay et al., 1993; Block et al.; 1996). One of the mechanisms could involve MEK/extracellular signal-regulated kinase (ERK) activation, and it has been suggested that this pathway could control migration and cell morphology. Both growth factor receptors and integrins promote signaling events that lead to MAPK activity and the induction of cell migration (Klemke et al., 1997). Also, constitutively active MAPK induces rapid morphological changes of fibroblastic cells, which are accompanied by disruption of stress fibers and disappearance of focal adhesion (Gotoh et al., 1999). MAPK activation has been associated with cell spreading rather than cell attachment, implicating this pathway in shape-dependent cell cycle progression (Zhu and Assoian, 1995). A wide variety of extracellular stimuli can induce activation of the MEK/ERK cascade, which transduces proliferation or differentiation signals from the plasma membrane into the nucleus. The activation of Ras/Raf results in activation the protein kinases MEKs, which activate the MAPK ERK1 and ERK2. These kinases phosphorylate a number of substrates that participate in cell cycle regulation, leading to the induction of several genes such as the ones induced during hepatocyte growth progression, i.e., c-fos and cyclin D1 (Albanese et al., 1995; Lavoie et al., 1996; Weber et al., 1997; Fiddes et al., 1998).
The EGF-signaling pathways mediating cell spreading and mitosis seem to be distinct, but no detailed analysis of this bifunctional effect according to cell cycle position has ever been made. The precise location of the cell in the G1 phase could be of prime importance in the growth factor-induced morphological and/or mitotic effect. Primary culture of hepatocytes appears to be a very powerful model to address this question. Indeed, in a normal liver, hepatocytes can remain quiescent for very long periods. However, after tissue disruption of cell-cell contact during cell isolation, the G0/G1 transition takes place (Etienne et al., 1988; Loyer et al., 1996). This mimics the entry into G1 of proliferating hepatocytes, in vivo, in the regenerating liver after partial hepatectomy (PHT), corresponding to the priming step of the regenerating process (Fausto et al., 1995; Michalopoulos and DeFrances, 1997). Entry into and progression through the G1 phase, in vivo and in vitro, are immediately accompanied by several morphological changes associated with anchorage-dependent signals that require growth factors, ECM proteins in relation to integrins and cytoskeletal components, all allowing hepatocyte survival and growth.
We previously reported two peaks of MEK/ERK activations in regenerating liver. One, located in mid-late G1 corresponded to growth factor induction of the mitogen signal necessary for hepatocyte progression in late G1 and S phases in regenerating liver and in growth-stimulated hepatocytes in primary culture (Talarmin et al., 1999). The other occurred early after PHT in vivo and also during hepatocyte remodeling early after seeding in culture. Until now, most studies have been focused on signaling pathway activations involved in hepatocyte proliferation but have not accounted for the importance of cell remodeling occurring in the early G1 phase of hepatocytes. In this study, we focused attention on this first MEK/ERK activation in the early G1 phase and we demonstrated its role in the morphological changes that occur during adhesion and spreading associated with the first step of G1 phase progression. The hypothesis that growth factors, such as EGF, might control early progression in the cell cycle by influencing these morphological events was analyzed. It was questioned how the MAPK pathway involved in this morphogenic signal could coordinately regulate mitogen signal and entry in S phase. Finally, we demonstrated for the first time that an ECM protein, fibronectin, and the β1 integrin subunit were under a MEK/ERK regulation that leads to hepatocyte spreading.
MATERIALS AND METHODS
Animals
Female Sprague-Dawley rats (weighing ∼200 g) were purchased from Charles River France (Saint Aubon Les Elbeuf, France). Animals were given food and water ad lib, and experiments were carried out in accordance with French laws and regulations. Surgical removal of 70% of the liver induces a synchronized growth response involving almost only hepatocytes during the first wave of replication. Remaining liver was collected after PHT according to previously described procedures (Loyer et al., 1994). Laparotomies were performed as controls (sham operations). At different times after PHT, animals were killed; the livers were harvested, immediately frozen in liquid nitrogen, and stored at −80°C until analysis.
Cell Cultures
Hepatocytes were isolated from Sprague-Dawley male (150–200 g) rat livers by the two-step perfusion procedure using 0.025% collagenase (Boehringer-Ingelheim, Gagny, France) buffered with 0.1 M HEPES (pH 7.4) as previously described (Guguen et al., 1975). They were plated at a density of 105 cells/cm2 in 35-mm-diameter dishes in 2 ml of minimal essential medium/medium 199 (3:1, vol/vol) containing penicillin (100 IU/ml), streptomycin (100 μg/ml), insulin (5 μg/ml), and bovine serum albumin (1 mg/ml). The medium was supplemented or not with 10% fetal calf serum (FCS) for 4 h as indicated in the figure legends. Four hours after the cells were plated, the medium was replaced with basal medium without FCS and renewed every day. EGF stimulations were performed at 50 ng/ml. At the indicated times, PD98059, cytochalasin D, or tyrphostin AG1478 solved in dimethyl sulfoxide (DMSO) were added at the defined concentrations. All control cultures containing DMSO at final concentrations of 0.2 or 0.37%, were changed at the same time as that of treated cells. For blocking function assay, we used hamster immunoglobulin (Ig) M against rat integrin β1 subunit (22630D) and isotype control (11130D) from BD-PharMingen, (San Diego, CA) at 25 μg/ml. The spreading was quantified by using Quantity One software developed by Bio-Rad (Hercules, CA) and was a mean of >150 cells from three independent experiments for each point.
Chemicals
[α-32P]dCTP (3000 Ci/mmol) and [methyl-3H]thymidine (5 Ci/mmol) were from Amersham (Buckinghamshire, England); insulin I-5500 was from Sigma (Saint Quentin Fallavier, France); recombinant human EGF was from Promega (Madison, WI); PD98059, SB203580, and tyrphostin AG1478 were from Calbiochem (La Jolla, CA); cytochalasin D was from Alexis (San Diego, CA).
Immunoblotting and Immunocytochemical Analysis
For harvesting, cells were rinsed with phosphate-buffered saline and lysed in homogenization buffer (60 mM β-glycerophosphate, 15 mM p-nitrophenylphosphate, 25 mM 3-(N-morpholino)propanesulfonic acid, pH 7.2, 15 mM EGTA, 15 mM MgCl2, 2 mM dithiothreitol, 1 mM vanadate, 1 mM NaF, 1 mM phenylphosphate, 100 μM benzamidin, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml soybean trypsin inhibitor). The amount of total protein was determined using the Bio-Rad protein assay (Life Science Bio-Rad, Ivry, France). After SDS-PAGE, proteins were transferred onto nitrocellulose membranes by using a Trans-Blot TM cell apparatus (Bio-Rad) for 4 h at 400 mA in buffer (25 mM Tris, 192 mM glycine, 20% methanol. The amounts loaded were checked with Ponceau dye. Subsequently, filters were rinsed in Tris-buffered saline (TBS; pH 7.4), blocked with 3% nonfat dry milk in TBS-2% glycine at room temperature, and incubated overnight at 4°C with primary antibodies diluted in the same buffer. Anti-phospho-MEK1/2, anti-phospho-ERK1/2, and anti-phospho-p38 MAPKs were rabbit polyclonal antisera directed to a synthetic phosphoserine-217/221 peptide corresponding to residues 214–226 of human MEK1, a synthetic phosphotyrosine peptide corresponding to residues 196–209 of human p44 MAPK, and a synthetic phospho-Thr180/Tyr182 peptide corresponding to human p38 MAPK sequence, respectively (New England Biolabs, Beverly, MA). Polyclonal antibodies against MEK1 (sc-219), MEK2 (sc-524), ERK1 (sc-94), ERK2 (sc-154), and p38 MAPK (sc-535) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibody against cyclin D1 was obtained from Neomarkers (Union City, CA). Anti-phosphotyrosine–PY20 mouse IgG2b antibody and anti-integrin β1 mouse IgG1 were purchased from Transduction Laboratories (Lexington, KY). After three washes in TBS, membranes were incubated in 3% nonfat dry milk in TBS-2% glycine for 1 h and with horseradish peroxidase-conjugated secondary antibody for 2 h at room temperature. After five washes in TBS, proteins were detected according to the SuperSignal Ultra Chemiluminescent Substrate procedure (Pierce, Rockford, IL). All experiments described have been done at least three times.
[3H]Thymidine Incorporation
The rate of DNA synthesis was measured in primary cultures by adding 2 μCi of [methyl-3H]thymidine (5 Ci/mmol) for given periods of time before cell harvesting, as indicated. Cells were washed twice in phosphate-buffered saline, scraped off the Petri dish, and aliquoted for protein content determination and [3H]thymidine counting after precipitation and washing in trichloroacetic acid.
Northern Blotting
Cells were lysed at different times of culture, RNA was extracted with the RNeasy kit (QIAGEN, Valencia, CA), and Northern blotting was performed as described previously (Loyer et al., 1996). The murine cyclin D1 cDNA probe was provided by Drs. M. Roussel and C. Sherr (Memphis, TN). The integrin β1 subunit cDNA probe was provided by Dr. B. Clément (Rennes, France)
Reverse Transcriptase and Polymerase Chain Reactions
Total RNA (1 μg) from cultured hepatocytes was used for first-strand cDNA synthesis with murine leukemia virus reverse transcriptase (Promega). The amplification reactions were performed on successive cDNA dilutions to determine the linear range of amplification. Briefly, the denaturation step was for 1 min at 94°C, annealing was for 1 min at 55°C, and elongation was for 1 min at 72°C (30 cycles). Primers for fibronectin were 5′-CCCACAGGGGCAAGTT-TCCAGGTACAGGGT-3′ (sense) and 5′-GATCTCTGGTCCATGAAGATTGGGGTGTGG-3′ (antisense) and for β-actin were 5′-GG-CCATCTCTTGCTG-3′ (sense) and5′-GCCCAGAGCAAGAGAG-3′ (antisense). Amplified cDNA was resolved in a 1% agarose gel stained with ethidium bromide.
RESULTS
Early EGF-dependent MAPK Activation Regulates Hepatocyte Cell Shape
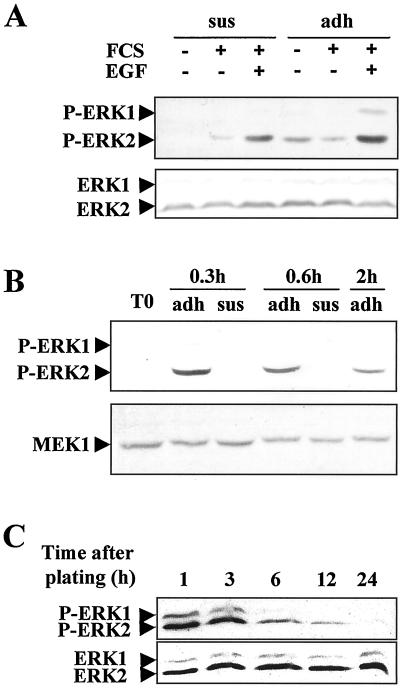
A relationship between the MEK/ERK cascade and the important cell morphology events induced by growth factor stimulation that are known to occur in early G1 progression of hepatocytes was envisioned but has not yet been proved. To test the possible dependence of cell shape evolution from MEK/ERK cascade activation, we used the hepatocyte in vitro system. The kinetics of MAPK pathway activation established by accumulation of the MEK/ERK-phosphorylated forms were analyzed in isolated cells either maintained in suspension or seeded on plastic in a medium with or without FCS and EGF (Figure 1A). In suspended hepatocytes, both EGF and FCS were able to activate the MEK/ERK cascade, indicating that the growth factor signaling did not require cell adhesion and that MAPK activation could precede hepatocyte adhesion. However, an additive effect of the adhesion process onto EGF- and FCS-related MAPK cascade induction was clearly noticed. No variation in the level of total ERK1/2 proteins was detected using a mixture of anti-ERK1 and anti-ERK2 antibodies that recognized all forms (phosphorylated or not) of the two proteins.
Figure 1.
Kinetics of ERK1/2 phosphorylation during early G1 in rat hepatocyte primary cultures analyzed by Western blotting using antibody directed against the phosphorylated forms. (A) Kinetics of ERK1/2 phosphorylation analyzed at the indicated times in suspended (sus) and adherent (adh) hepatocytes. Western blotting analysis was performed 3 h after hepatocyte isolation in suspended (sus) or adherent (adh) cells stimulated (+) or not (−) with FCS or EGF for 15 min. For detection of total ERK 1 and 2, a mixture of equal ratios of anti-ERK1 and anti-ERK2 antibodies was used. (B) Western blotting of ERK1/2 phosphorylation analyzed at the indicated times in hepatocytes seeded on a rigid film of type 1 collagen. T0, freshly isolated hepatocytes. The blot was stripped and reprobed with an anti-MEK1 antibody. (C) Kinetics of ERK1/2 phosphorylation in hepatocyte primary cultures continuously stimulated by FCS from seeding to 24 h. For the kinetics of total ERK1 and ERK2 proteins, a mixture of equal ratios of anti-ERK1 and anti-ERK2 antibodies was used. Experiments were performed at least three times.
Then, to confirm the adhesion-dependent MAPK activation, we analyzed the kinetics of MAPK pathway activation in suspended or adherent cells seeded onto a type 1 collagen film in the absence of FCS and growth factor stimulation (Figure 1B). When seeded on type 1 collagen film, hepatocytes adhered within 20 min and spread up to 2 h such that 3 h later they showed typical epithelioid morphology that did not change thereafter (Rescan, and Baffet, unpublished results). We showed that changes in cell shape during adhesion were associated with MAPK activation and that ERK2 was very rapidly, highly, and transiently phosphorylated, reaching a maximum 20 min after seeding and decreasing thereafter to the basal level. In contrast, no phosphorylation was detected either in freshly isolated cells or in suspended hepatocytes, whereas total MEK1 protein, as a quantitative control, remained unchanged.
We, therefore, further examined the kinetics of activation of the two ERK1/2 forms in presence of FCS. The MEK/ERK cascade was transiently activated, and again ERK2 appeared phosphorylated 1 and 3 h after seeding (Figure 1C). The phosphorylation peak decreased thereafter, reaching a low level 6 h later, and the signal disappeared completely between 12 and 24 h. Quantification analysis of the total ERK proteins performed in parallel showed a constant level of ERK2 protein during this time course.
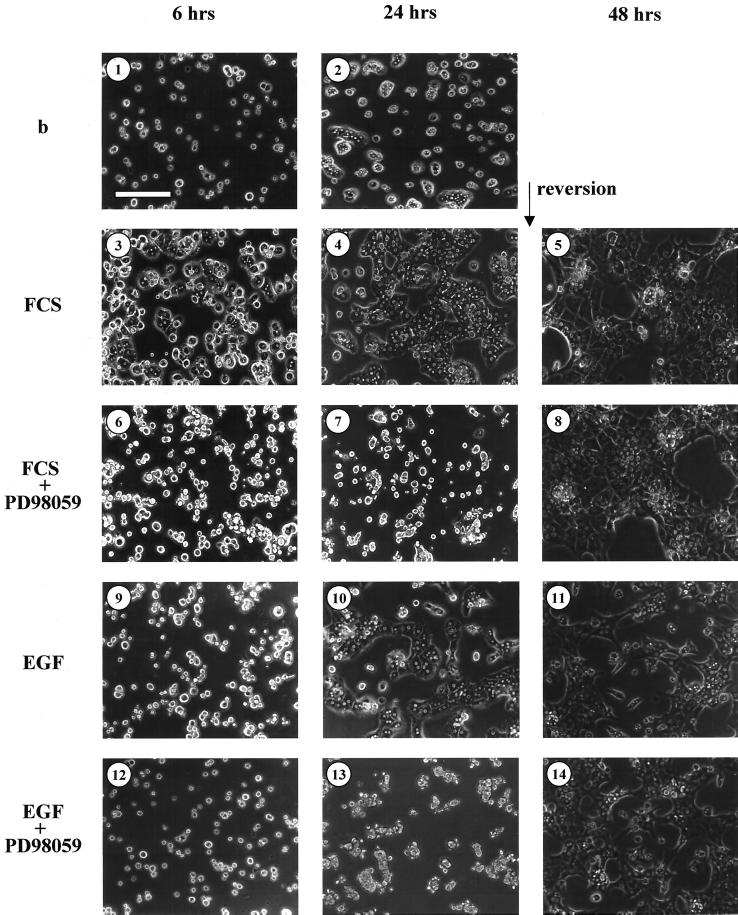
FCS has been reported to support cell spreading, and EGF, as do other growth factors, has a morphogenic effect in many cell types including hepatocytes. To investigate whether the observed MAPK MEK/ERK pathway activation could control the changes of hepatocyte shape, we analyzed the influence of MEK inhibition on hepatocyte adhesion and extension. Previous observations agreed that PD98059 binds to MEK1 and to a lesser extent to MEK2, thus preventing their activation by upstream activators (Alessi et al., 1995; Pang et al., 1995). PD98059 was added or not to freshly isolated hepatocytes immediately after isolation, and cells were allowed to adhere and spread on plastic in the presence of FCS or EGF. In the absence of serum and growth factor stimulation, the basal condition, the cells adhered to the plastic support but underwent spreading with a very low efficiency (Figure 2, 1 and 2). As expected, control cells, in the presence of FCS, adhered and spread within 6 h (Figure 2, 3 and 4). Interestingly, the PD98059 treatment completely inhibited hepatocyte spreading but not adhesion to the support (Figure 2, 6 and 7). A reversion experiment allowed us to demonstrate that blockade of cell spreading by MEK inhibition was not toxic because those hepatocytes could spread within 6 h when PD98059 was withdrawn, the cells undertaking a morphologic appearance close to the control cultures 24 h later (Figure 2, 5–8).
Figure 2.
MAPK pathway inhibition by PD98059 during hepatocyte seeding after FCS and EGF stimulation. Hepatocytes were preincubated for 30 min with solvent (0.2% DMSO) (1, 2, 3, 4, 9, and 10) or inhibitor (75 μM PD98059) (6, 7, 12, and 13) before seeding and then cultured in absence (b; 1 and 2) or in presence of 10% FCS (3, 4, 6, and 7) or 50 ng/ml EGF (9, 10, 12, and 13) and analyzed for extension after 6 or 24 h. Bar, 150 μm. Solvent control (5 and 11) or PD98059 (8 and 14) were then removed after 24 h of exposure, and hepatocytes were observed 24 h later, i.e ,48 h after seeding. These data are representative of three different experiments.
EGF alone was also able to induce important changes in cell shape. Cell spreading started during the first 6 h but was finished only 24 h after stimulation (Figure 2, 9 and 10). We did not notice any differences in the rate of adhesion in the presence or absence of EGF. In the presence of MEK inhibitor, the spreading induced by the growth factor was completely inhibited (Figure 2, 12 and 13). In reversion experiments, when PD98059 was removed 24 h after seeding, hepatocytes rapidly spread on the support and presented a morphology close to control cultures 24 h later (Figure 2, 11 and 14).
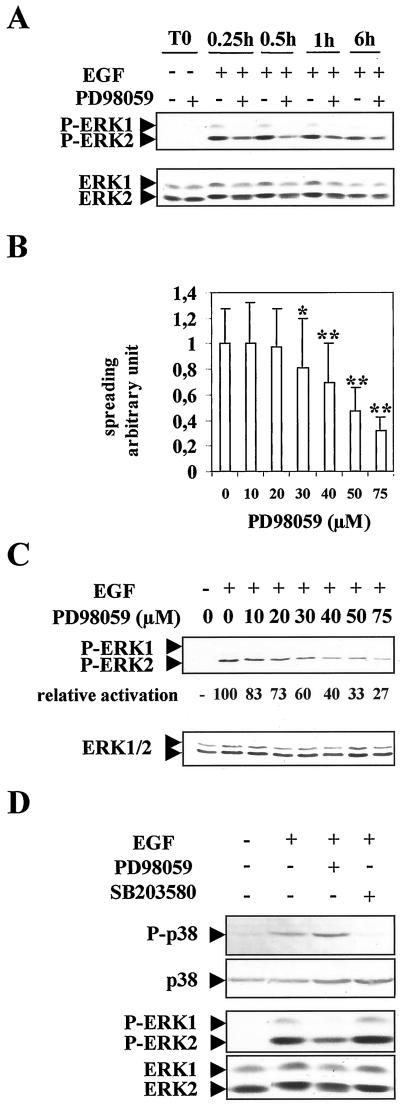
We then, investigated more thoroughly the MAPK pathway mediating the EGF morphogenic effect. First, Western blotting experiments indicated that, again, ERK2 appeared preferentially phosphorylated by the EGF treatment, whereas ERK1 was very poorly activated (Figure 3 A). ERK2 phosphorylation was sustained until at least 6 h. In addition, PD98059 was able to greatly inhibit ERK2 phosphorylation induced by the EGF stimulation. No variation of total ERK1/2 protein amount could be noticed during this time course.
Figure 3.
Dose-dependent inhibition of ERK phosphorylation and cell spreading by PD98059 in EGF-stimulated hepatocytes and specificity of this inhibition. (A) Time course of ERK1/2 phosphorylation after EGF stimulation in the presence or absence of MEK inhibitor 75 μM PD98059. Freshly isolated hepatocytes were allowed to adhere in presence (+) or absence (−) of PD98059 (T0). Then, the cells were stimulated by EGF and analyzed at the indicated times after stimulation. The blot was reprobed with a mixture of anti-ERK1 and anti-ERK2 antibodies to determine for each con dition the amounts of total expressed ERK1/2 proteins. (B) Dose-dependent inhibition of spreading by PD98059 in EGF-stimulated hepatocytes analyzed 24 h after seeding. Error bars indicate SD; *, P < 0.005; **, P < 0.001 (versus control) by Student's t test. (C) Dose-dependent inhibition of ERK1/2 phosphorylation by PD98059 in EGF-stimulated hepatocytes. The membrane was probed first with anti-phospho-ERK and then stripped and reprobed with a mixture of equal ratios of anti-ERK1 and anti-ERK2 antibodies. Quantification study of the dose-dependent inhibition of phospho-ERK2 was shown compared with activation by EGF in absence of inhibitor (level 100). (D) P38 and ERK phosphorylations were analyzed by using anti-phospho-p38 and anti-phospho-ERK antibodies in EGF-stimulated hepatocytes as above. Cells were preincubated for 30 min in the absence (−) or presence (+) of 75 μM PD98059 or 10 μM SB203580. Membranes were reprobed with an anti P38 or an equal ratio of ERK1 and ERK2 antibodies. Experiments described were performed at least three times.
Then, we analyzed the dose-dependent response of PD98059 added at cell seeding. Spreading was quantified as a factor of surface area covered with individual cells over control cells and was measured as described in MATERIALS AND METHODS (Figure 3B). In the presence of EGF, a PD98059 dose-dependent response was obtained. The MEK inhibitor started to inhibit hepatocyte spreading at 30 μM (p < 0.005), and increasing concentrations had drastic effects on cell morphology, leading to 50 and 70% inhibition of spreading at 50 and 75 μM, respectively (p < 0.001). Furthermore, ERK2 phosphorylation was inhibited in a parallel dose-dependent manner, whereas no significant changes in the expression level of both ERK1 and 2 total proteins could be detected (Figure 3C).
To demonstrate the specificity of this inhibition, regarding the MAPK pathway targeted, we analyzed P38 MAPK phosphorylation belonging to another signaling pathway. P38 phosphorylation was induced by EGF treatment, but addition of MEK inhibitor at 75 μM did not influence the phosphorylation activity of this pathway (Figure 3D). Conversely, inhibition of this P38 pathway by the specific inhibitor SB203580 abolished P38 phosphorylation but had no effect on ERK phosphorylation (Figure 3D) and cell spreading (Rescan, and Baffet, unpublished results). In parallel, evidence was provided that the different treatments did not significantly affect the levels of total ERK1/2 and P38 proteins expression.
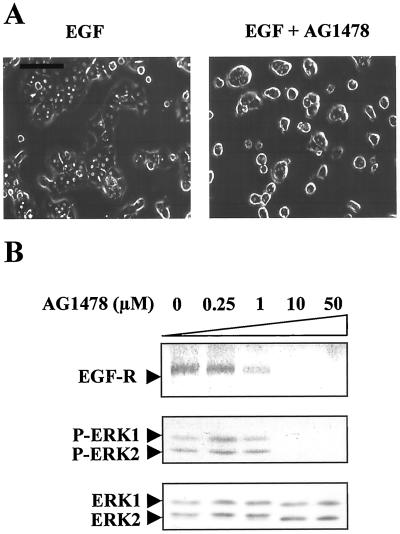
We also verified that EGFr phosphorylation via ERK2 activation had a key role in EGF-induced morphogenesis. For this purpose, we examined the cell shape modifications after tyrphostine AG 1478 treatment. AG 1478 is a highly potent and specific inhibitor of EGFr. Tyrphostine AG 1478 inhibited EGF-induced spreading (Figure 4A). Furthermore, tyrphostine AG 1478 blocked both tyrosine autophosphorylation of the receptor and EGF-dependent ERK activation in a dose-dependent manner in 48-h-old cultures, whereas the expression level of ERK1/2 proteins remained unchanged by the treatment (Figure 4B).
Figure 4.
Influence of receptor autophosphorylation inhibition by tyrphostin AG1478 treatment. EGF induced MAPK activation in suspended and adhered hepatocytes. (A) Freshly isolated hepatocytes were stimulated by EGF in the absence (EGF) or presence of tyrphostin AG1478 (EGF + AG1478) and allowed to adhere and spread for 12 h. Bar, 80 μm. (B) Dose-dependent inhibition of EGFr and ERK1/2 phosphorylation after EGF stimulation (15 min) in the presence of increasing concentrations of tyrphostin AG1478 (0.25, 1, 10, and 50 μM) in 48-h-old cultured hepatocytes. For EGFr analysis, total lysates were analyzed with anti-phospho-tyrosine PY20 antibody. Zero corresponds to the control of solvent inhibitor (0.1% DMSO) added at the highest concentration used in the 50 μM AG 1478 treatment. The blot was reprobed with a mixture of ERK1 and ERK2 antibodies. These data are representative of three different experiments.
Hepatocyte Shape Controls S Phase Entry but Not Progression to Mid-G1
To see whether these MAPK-mediated morphology changes might influence the hepatocyte progression through G1 and S phases, we examined two in vitro situations in which hepatocyte spreading could be modulated: cells were exposed either to cytochalasin D treatment or to MEK inhibitor and then were stimulated to proliferation by addition of EGF.
Actin cytoskeleton integrity has proved to be a major requirement in many integrin-mediated signaling events and a powerful cell shape regulator. Addition of cytochalasin D, a drug that disrupts the integrity of the microfilament lattice, resulted in inhibition of cell spreading in many cell types. In the presence of cytochalasin D added at cell seeding, hepatocyte spreading, but not the adhesion process, was inhibited (Figure 5A). In parallel, cytochalasin D blocked the DNA replication of EGF-stimulated hepatocytes (Figure 5B): [H3]thymidine incorporation remained close to basal level and a peak of labeling was observed as expected between 48 and 60 h in normally spreading hepatocytes. This inhibition was completely reversible because cytochalasin D removal after 48 h of treatment resulted in hepatocyte spreading, and these cells started to replicate DNA concomitantly with control cultures when stimulated by EGF at the same time, i.e., at 48 h (Figure 5C). This experiment showed that actin filaments were actively involved during hepatocyte spreading and that cell shape was an important regulator of hepatocyte replication.
Figure 5.
Inhibition of cell spreading and DNA replication by cytochalasin D treatment in EGF-stimulated hepatocytes. (A) Inhibition of spreading by cytochalasin D. Hepatocytes were stimulated at seeding by EGF in the presence or absence (control) of cytochalasin D at 1 μM and observed 24 h later. (B) Time course of [methyl-3H]thymidine incorporation into DNA in EGF-stimulated cells maintained (open bar) or not (black bar; solvent control) in the presence of cytochalasin D (1 μM) throughout the culture. (C) Reversion experiment in which cytochalasin D was removed after 48 h of treatment and DNA replication analyzed by thymidine incorporation at the indicated times after seeding. Labeled thymidine was added 4 h before cell harvesting. Values expressed in counts per minute per microgram of protein (prot) are means of duplicates from three different experiments.
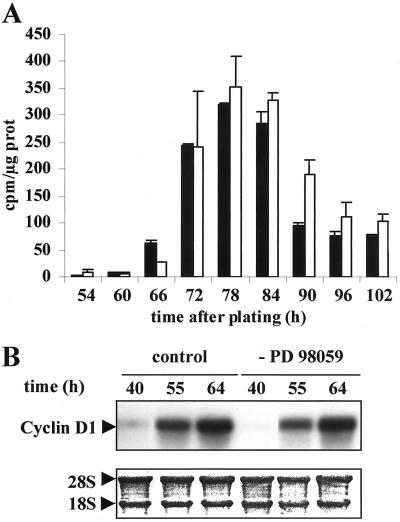
Similar results were obtained with MEK inhibitor by inhibiting cell shape extension. We carried out [H3]thymidine experiments in which cells were exposed to PD98059 up to 42 h of culture and then stimulated by EGF. As expected, continuous exposure to PD98059 completely abolished DNA replication (Talarmin et al., 1999). Untreated control cells stimulated by EGF at 42 h started to replicate DNA at 66–74 h (Figure 6A). Hepatocytes in which spreading was inhibited by PD98059 treatment during 42 h started to replicate DNA by the same time as the control culture, i.e., between 66 and 74 h, indicating that unspread cells progressed to mid-G1 independently of the MEK/ERK pathway.
Figure 6.
DNA replication and cyclin D1 mRNA synthesis after PD98059 treatment. (A) Reversion experiment. Cultures were exposed for 42 h to the solvent control (black bar) or PD98059 (open bar). Then, the drug was removed and hepatocytes were stimulated by EGF. [methyl-3H]Thymidine incorporation into DNA was analyzed at the indicated times after growth factor stimulation. (B) Cyclin D1 mRNA was analyzed by Northern blotting in solvent control and PD98059-treated cells at the indicated times after drug removal. 18S and 28S rRNAs dyed by methylene blue coloration were used as the control. Experiments described were performed at least three times.
In hepatocytes, as in many other cells, the up-regulation of cyclin D1 in mid-late G1 is indicative of G1/S transition and mitogenic response (Koch et al., 1994; Albrecht et al., 1995; Loyer et al., 1996; Albrecht and Hansen, 1999; Talarmin et al., 1999). Cyclin D1 mRNA induction appeared simultaneously in untreated and PD98059-treated hepatocytes stimulated at the same time by EGF, emphasizing again that round hepatocytes had progressed through early G1 in the presence of MEK inhibitor independently of MAPK activation (Figure 6B).
Distinct MEK1/2 and ERK1/2 Phosphorylation Patterns Related to EGF Morphogenic and Mitogenic Functions
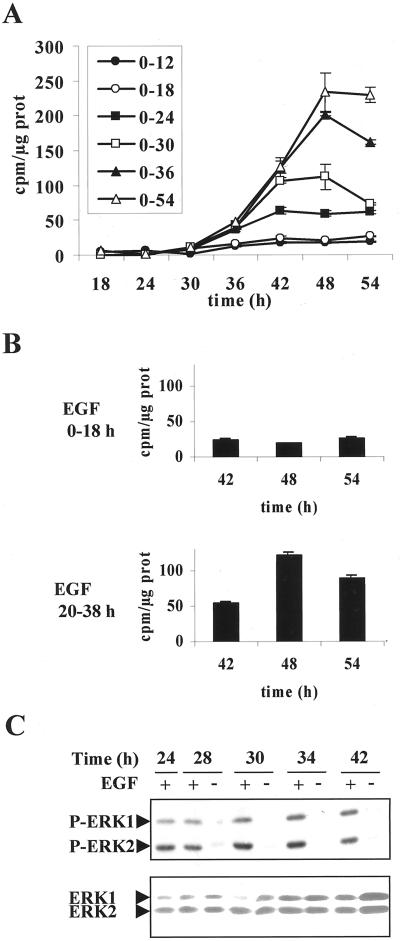
To progress in understanding the dual controls mediated by EGF onto mitogenic and morphogenic functions of the cells, we devised experiments to determine both the sequence of signaling events and their degree of interplay. To cut down first the time window during G1 phase in which EGF exerted its morphogenic effect, we examined DNA replication in a series of cultures stimulated by EGF for increasing periods from 12 to 54 h. The 12- and 18-h periods of stimulation were sufficient to induce hepatocyte spreading, as shown in Figure 2, but not for detecting DNA replication (Figure 7A). In contrast, periods of stimulation longer than 18 h resulted in the induction of DNA replication. These results evidenced that EGF induced a morphogenic signal in early G1, which was completely distinct and preceded the mitogenic effect occurring in the mid-G1 phase. We verified that an 18-h period of EGF stimulation was sufficient to induce DNA replication when this stimulation was located after 20 h of culture (Figure 7B), confirming that the mitogenic effect of EGF could take place only when the cells have reached mid-G1 phase.
Figure 7.
Sequential induction of EGF-dependent morphogenic and mitogenic effect. (A) Time course of [methyl-3H]thymidine incorporation into DNA after EGF stimulation over different time periods along the G1 phase (0–12, 0–18, 0–24, 0–30, 0–36, and 0–54 h). (B) [methyl-3H]Thymidine incorporation into DNA analyzed at the indicated times in hepatocytes stimulated by EGF for 18 h at two different times (EGF 0–18 and 20–38 h) during G1 phase progression. (C) Kinetics of EGF-induced ERK1/2 phosphorylation in hepatocytes stimulated by EGF throughout the 42 h of culture (+) or only during the first 24 h (−) and analyzed by Western blot at the indicated times. After stripping, a mixture of equal ratios of anti-ERK1 and anti-ERK2 antibodies was used to detect total ERK1/2 proteins. These data are representative of three different experiments.
Second, we precisely analyzed the MAPK cascade activation all along the G1 phase progression. ERK1/2 phosphorylation status was determined in hepatocytes continuously stimulated by EGF from seeding to 42 h (Figure 7C). The MAPK phosphorylation was sustained as long as the growth factor was present. Interestingly, we observed that ERK1 phosphorylation increased during the time course analyzed. When EGF was withdrawn at 24 h, phosphorylation of the two forms, ERK1 and ERK2, was rapidly abolished, showing that the kinase activation was dependent on the presence of the growth factor. Nevertheless, a fraction of cells replicated DNA, indicating that cells that have received the activation signaling did not further need the presence of growth factor and maintenance of the kinase activation for progressing to S phase. However, a continuous EGF stimulation up to 42 h activated ERK1/2 persistently and increasingly, and DNA replication increased to a maximum in parallel. The expression level of the total ERK1/2 proteins examined in parallel evidenced an accumulation of ERK1 at 24–30 h, whereas no significant change of ERK2 was noticed.
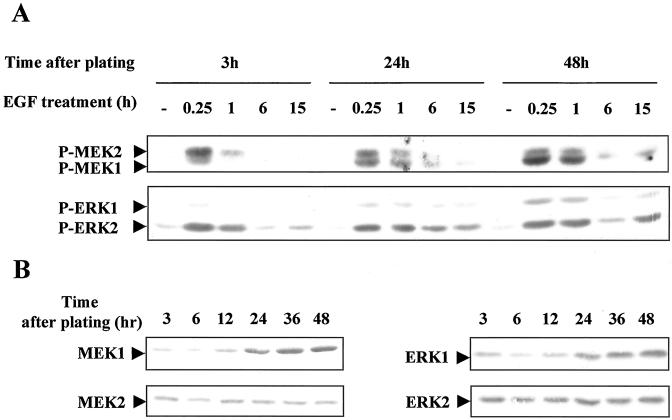
These results led us to postulate that EGF-dependent mitogenic signal recruited a pattern of activated MAPKs distinct from that involved in the morphogenic effects. We analyzed in detail the different forms of MEKs and ERKs activated during G1 phase progression in hepatocytes stimulated by EGF either immediately or 24 and 48 h later: the first stimulation located in early G1 targeted only the EGF morphogenic signal, whereas the second and the third stimulations, occurring in cells that have progressed up to mid- and mid-late G1, allowed DNA replication in a fraction of hepatocytes or nearly all the population, respectively. Phosphorylation patterns of MEKs and ERKs were analyzed 0.25, 1, 6, and 15 h after the growth factor stimulation performed at 3, 24, and 48 h after plating (Figure 8A). A gradual recruitment of MEK1- and ERK1-phosphorylated forms appeared, whereas the MEK2- and ERK2-phosphorylated forms were not significantly modified. Interestingly, quantification analysis of total amounts of MEK1/2 and ERK1/2 proteins evidenced a parallel increase of MEK1 and ERK1 expression (2.3 and 1.9 ± 0.2-fold increase, respectively) and no significant change of MEK2 and ERK2 expression levels (Figure 8B). This increase of MEK1 and ERK1 protein expression levels appeared to strictly parallel that of their phosphorylation patterns (2.4 and 1.8 ± 0.1-fold increase, respectively), indicating that a regulation at/or upstream of the protein synthesis was likely associated with the sequential effect of the growth factor. Moreover, these results mainly provided evidence that the two morphogenic and mitogenic functions of EGF involved distinct MAPK phosphorylation patterns in relation to protein expression.
Figure 8.
Phosphorylation of different forms of MEK1/2 and ERK1/2 according to EGF stimulation in the G1 phase. (A) Hepatocytes were stimulated by EGF at different times in G1 phase progression (3, 24, and 48 h after plating). Phosphorylation of MEK1/2 and ERK1/2 was analyzed 0.25, 1, 6, and 15 h after the growth factor stimulation. −, control cells before stimulation at the indicated times. (B) Detection of total MEK1, MEK2, ERK1, and ERK2 proteins in hepatocytes during G1 progression analysis. Western blottings were performed using antibodies directed against each isoform. These data are representative of three different experiments.
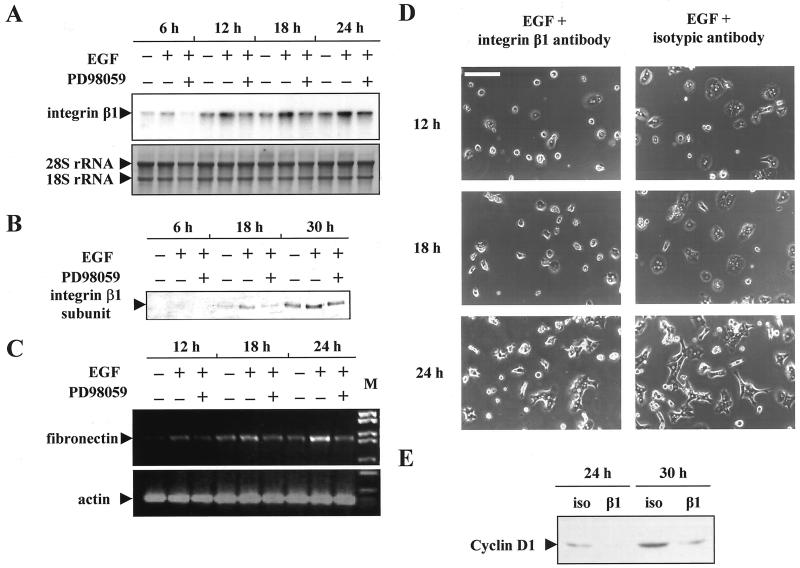
EGF Up-regulated Integrin β1 Subunit and Fibronectin by a Mechanism That Is MEK/ERK Dependent
Early changes in gene expression during liver regeneration have been associated with ECM and cell shape remodeling, and some growth factors are thought to have a profound effect on the synthesis of ECM and their receptor (Mars et al., 1995; Watanabe et al., 1997). The implication of the MEK/ERK pathway in this regulation was therefore investigated. We looked at the level of integrin β1 in regenerating liver and in freshly isolated hepatocytes after EGF stimulation and analyzed the regulatory mechanism that leads to hepatocyte spreading.
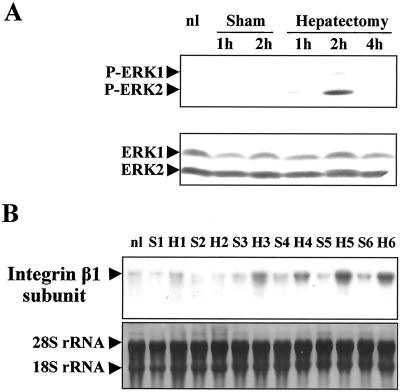
As already shown in a previous report (Talarmin et al., 1999), a biphasic MEK/ERK activation was observed in the G1 phase of regenerating liver. One occurred in the early G1 phase and the other in the mid-late G1 phase. Here we evidenced that in the early G1 phase, ERK2 was the MAPK form predominantly phosphorylated after PHT (Figure 9A) in contrast to livers from sham-operated control and normal animals for which no phosphorylation of the pathway could be detected. Meanwhile, the expression level of the proteins remained unchanged. To see whether the MEK/ERK cascade activation could be correlated with proteins involved in hepatocyte remodeling, we analyzed the integrin β1 subunit expression by Northern blotting between 1 and 6 h after PHT at times surrounding ERK2 phosphorylation (Figure 9B). In sham-operated animals the β1 integrin mRNA level was low whatever the time analyzed. In contrast, we showed that the amount of β1 subunit mRNA increased very rapidly 3 h after PHT and remained high thereafter.
Figure 9.
Kinetics of ERK1/2 phosphorylation during early G1 in regenerating liver and induction of integrin β1 subunit. (A) Kinetics of phosphorylation of ERK1/2 was performed in quiescent liver (nl) and after PHT or sham surgery (nonregenerating livers) at the indicated times. The blot was reprobed with a mixture of ERK1 and ERK2 antibodies. (B) Northern blotting of integrin β1 subunit. Total RNAs were prepared from remnant livers of 70% partially hepatectomized rats (H) and sham operated rats (S) at the indicated times (from 1 to 6 h) or quiescent liver (nl) as described in MATERIALS AND METHODS. Experiments described were performed at least three times.
Because hepatocytes were known to produce ECM proteins, our next question was whether the EGF-dependent MAPK activation that induced spreading in vitro had an effect on the synthesis of ECM and/or receptor regulation. We examined the mRNA transcripts of fibronectin and integrin β1 in hepatocyte primary cultures stimulated by EGF and treated or not by the MEK inhibitor (Figure 10A,C). EGF increased integrin β1 and fibronectin mRNA levels. The two mRNAs accumulation increased within 12 h after EGF stimulation and remained high thereafter. Interestingly, the MEK inhibitor was able to inhibit this induction. In presence of PD98059 and EGF, fibronectin and integrin β1 mRNAs remained at control level (non-growth factor stimulated hepatocytes), showing that up-regulation of these two mRNAs was dependent on the MEK/ERK cascade.
Figure 10.
Up-regulation of integrin β1 and fibronectin expression by EGF. Requirement of MEK/ERK pathway activation. (A) Northern blot analysis of integrin β1 subunit expression in hepatocytes stimulated (+) or not (−) by EGF in presence (+) or absence (−) of MEK inhibitor. When required, EGF and PD98059 were added at cell seeding and hepatocytes were harvested and analyzed at the indicated times after plating. (B) Western blot analysis of integrin β1 subunit expression in hepatocytes stimulated or not by EGF in presence or absence of MEK inhibitor and analyzed at the indicated times after plating. (C) Kinetics of the fibronectin expression in EGF- and/or PD98059-treated hepatocytes was performed at the indicated times. Normalization was done with actin primers. (D) Partial inhibition of hepatocyte spreading by addition of anti-integrin β1 subunit antibody. Hepatocytes were stimulated at seeding with EGF in the presence of antibody against β1 integrin or isotypic control and analyzed at the indicated times after plating. Bar, 120 μm. (E) Western blot of cyclin D1 expression in EGF-stimulated hepatocytes cultured in the presence of β1 integrin antibody (β1) or isotypic antibody control (iso), at the indicated times after seeding. These data are representative of three different experiments.
Furthermore, we extended these results to the corresponding protein level by demonstrating that the regulation mediated by the MEK/ERK pathway could also affect the expression of the β1 integrin subunit (Figure 10B).
Therefore, we tested the possibility that cell spreading induced by EGF was, at least in part, mediated by this target ECM protein. Hepatocytes were allowed to spread and were observed 12, 18, and 24 h after growth factor stimulation in the presence of either anti-rat integrin β1 antibody or isotypic Ig control. In the presence of β1 integrin antibody, hepatocyte spreading induced by EGF was highly reduced (Figure 10D), whereas the cells spreading occurred normally with the isotypic control antibody. The mean surface of integrin β1 antibody-treated hepatocytes was <45% of their control counterparts 12 and 18 hours after seeding. The inhibition was less efficient 24 h after seeding and cannot be maintained thereafter.
Finally, we looked at the effects of integrin β1 antibody on hepatocyte cell cycle progression in response to EGF. Cyclin D1 was used again as indicative of the G1/S transition signal. Detection of cyclin D1 protein was performed by Western blotting in hepatocytes treated with the β1 antibody or the control isotypic antibody. Cyclin D1 protein expression increased between 24 and 30 h in the presence of control isotypic antibody, whereas its expression was highly inhibited in the presence of β1 integrin antibody (Figure 10E). A similar result was also obtained by immunolocalization (Rescan, Coutant, Talarmin, Theret, Glaise, Guguen-Guillouzo, and Baffet, unpublished results) indicating that cells blocked in spreading by β1 antibody appeared unable to efficiently respond to mitogenic signal.
DISCUSSION
Cell shape evolution determines whether an individual cell will grow, differentiate, or die in response to tissue microenvironment and growth factor. The mechanisms involved in these processes of regulation appear to be crucial in the balance between proliferation, differentiation, and apoptosis. The present study was carried out to analyze how the MAPK MEK/ERK pathway regulates morphological shape and cell progression in the G1 and S phases of hepatocytes used as highly normal differentiated cells.
Hepatocytes are anchorage-dependent cells that needs to adhere for survival and progression in the G1 phase. The G0/G1 transition takes place during the liver dissociation by collagenase treatment. The disruption of cell-cell interaction is rapidly accompanied by induction of c-fos, c-jun, and B-jun that reaches a maximum in freshly isolated cells and drastically decreases thereafter (Etienne et al., 1988). This early G1 progression is accompanied by cell adhesion and spreading. In this study, we found that adhesion transiently activated the MAPK pathway, and this activation was related to cell capability to spread. Inhibition of the MAPK pathway totally abolished cell spreading but not the adhesion process, indicating that MEK/ERK activation is a key pathway involved in the control of cell remodeling rather than cell attachment itself. Several groups have analyzed the effect of cell adhesion on early growth factor ERK activation, and conflicting results were reported. In a suspension of fibroblasts NIH 3T3 and endothelial cells that were prevented from spreading, growth factors activated ERKs (Huang et al., 1998, Zhu and Assoian, 1995). On the other hand, Renshaw et al. (1999;1997) found that activation of ERK2 by growth factor was in fact strongly dependent on adhesion to ECM. Here, we show that MAPK activation could occur in a hepatocyte suspension stimulated by EGF, although to a lesser extent than in their adhering counterparts. Adhesion could mainly provide a survival signal involving an anchorage-dependent activation pathway distinct from MAPK activation (Le Gall et al., 1998; Oktay et al., 1999). In agreement, adhesion to the support could activate the PI3K pathway as shown by phosphorylation of survival factor AKT, an activation pathway independent of MEK/ERK in our model (Coutant, and Baffet, unpublished results).
Evidence has been provided that EGF can also support a morphogenic effect in the early G1 phase via MEK/ERK activation preceding its mitogenic effect that occurs in mid-late G1. These results emphasize the role of EGF at two important checkpoints that are cell shape and the restriction point in mid-late G1 phase by a mechanism that involves MAPK activation. In agreement, reports showed that the ability of glioblastoma cells to spread at normal rate was partially rescued by activated MEK1 (Gu et al., 1998). On the other hand, constitutive active MAPK induced rapid morphological changes of fibroblastic cells, which are accompanied by disruption of stress fibers and disappearance of focal adhesion and anchorage-independent growth (Greulich and Erikson, 1998; Gotoh et al., 1999). Furthermore, MEK inhibitor was found to block the ability of colon cell carcinoma to scatter and to suppress growth of colon tumors in vivo, in agreement with reports indicating that MAPK can regulate cell motility (Klemke et al., 1997; Sebolt-Leopold et al., 1999; Fincham et al., 2000).
This bifunctional role of EGF raises the question of the relationship between the two concerned functions. Demonstration was provided that the mitogenic function evidenced by DNA replication is dependent on that controlling the cell shape. function. The use of cytochalasin D revealed that microfilament integrity was absolutely required for hepatocyte spreading and also for progression in the S phase. It is interesting to note that hepatocytes behaved the same way when plated on RGD-coated dishes, acting as an integrin ligand but not allowing hepatocyte extension (Hansen et al., 1994). By a refined experimental procedure, Huang et al. (1998) demonstrated that cell shape and cytoskeletal tension controlled cell cycle progression and S phase entry in human capillary endothelial cells. Very recently, Hansen and Albrecht (1999) showed that hepatocytes seeded on collagen gel remained rounded and quiescent in association with low cyclin D1 expression after growth factor stimulation, and cyclin D1 overexpression allowed shape-independent S phase entry.
We have demonstrated that PD98059 treated nonspread hepatocytes and untreated well spread cells simultaneously exhibit similar DNA replication and cyclin D1 expression in reversion experiments. It indicates that round cells can progress until mid-late G1 but are unable to respond to a mitogenic signal. It also strongly suggests that hepatocytes can progress to mid-G1 phase independently of MEK/ERK activation, whereas a cell shape-dependent activation of this pathway is necessary for regulating S phase entry by making the cells permissive to mitogen induction, which occurs at the restriction point in mid-late G1. In agreement, other studies demonstrated that spreading of human pulmonary CE cells (Huang et al., 1998) and ERK activation in smooth muscle cells (Ravenhall et al., 2000) are not required for cell cycle entry to mid-G1, whereas cells prevented from spreading fail to progress to the late G1 and S phases. Other pathways such as PI3K, STAT3, JNK, and p38 can be potential players for hepatocyte progression toward mid-G1. Although dominant-negative PI3K did not inhibit DNA synthesis in primary culture (Auer et al., 1998), it could play a role in the G1 progression and/or cell survival as an anchorage-dependent signal. In vivo, signaling through TNF-R receptor type 1/STAT3 is required for initiation of liver regeneration (Webber et al., 1998; Yamada et al., 1998). JNK and p38 are both activated in response to external stimuli in numerous cell types as well as hepatocytes in vivo and in vitro (Gines et al., 1996; Mendelson et al., 1996; Westwick et al., 1996; Jarvis et al., 1997; Spector et al., 1997; Chen et al., 1998). These activations might contribute to a complex sequential regulation of the G1 progression.
Thus, the orderly EGF morphogenic and mitogenic events appear to be dependent on a cellular clock by a temporal control of intracellular signaling in which the MEK/ERK pathway could play a central role. This has led us to further analyze the MAPK activation cascade all along the G1 phase according to the EGF stimulation. To our surprise, we showed that the growth factor-related sequential signaling events involved distinct patterns of activated kinases. In early G1 phase, EGF stimulation that induced only morphological effects involved principally MEK2/ERK2 activation. ERK2 is also the main MAPK-phosphorylated target in the early G1 phase of regenerating liver. In contrast, a stimulation occurring 24 h later, i.e., in cells in the mid- and mid-late G1 phase, which may respond to EGF mitogen effect, was found associated with MEK1/ERK1 phosphorylation, whereas ERK2 remained constantly and highly phosphorylated. We have already reported that specific activation of MEK1 was mitogenic for hepatocytes after EGF stimulation in the mid-late G1 phase. PD98059 treatment abolished DNA replication and inhibited MEK1 activation as well as cyclin D1 induction (Talarmin et al., 1999). It can be noticed that total MEK2 and ERK2 proteins were constantly expressed in G1, indicating that the regulation of these isoforms is mainly under a posttranslational control. In contrast, the amounts of MEK1 and ERK1 proteins and their degree of phosphorylation increased in parallel in the mid- to late G1 phase, suggesting a pretranslational control of these kinase isoforms occurring in the mid-G1 phase.
Taken together, our data emphasize the recent report by Fink et al. (1999), which, in combining experimental data and computer modeling, demonstrates that cellular geometry is important in the spatiotemporal control of intracellular signaling. In hepatocytes during G1 progression two distinct stages of controls could be defined: 1) from early to mid-G1 phase, a growth factor-mediated morphological effect affecting hepatocyte spreading via principally ERK2 activation; 2) a window from mid- to mid-late G1 in which MEK1, ERK1, and 2 are phosphorylated, corresponding to the time needed for that near all the cells are targeted by growth factor mitogenic signal. Later on, in the late G1 phase, cells become independent from growth factor stimulation and progress up to the G1/S transition.
Reports have shown that some ECM proteins as well as matrix metalloproteinases and urokinase are under growth factor regulations and implicated in the early G1 phase of regenerating liver in vivo (Mars et al., 1995; Watanabe et al., 1997; Haruyama et al., 2000). In many cells as well, matrix/integrin interactions could account for the diversity of integrin-dependent cell functions (Morino et al., 1995; Zhu and Assoian, 1995; Renshaw et al., 1997). Here, we show that EGF leads to a positive feedback loop on ECM and integrin expression that induces cell spreading. We demonstrate for the first time that an ECM protein and one of its transmembrane receptor subunit are under a MEK/ERK regulation: 1) MAPK activation preceded integrin subunit induction in the early G1 phase of regenerating liver; 2) in vitro, fibronectin and integrin β1 subunit were rapidly induced after growth factor stimulation; 3) these inductions and cell spreading were inhibited by the MEK inhibitor. In addition, antibodies against β1 subunit were able to reduce cell spreading and consequently to inhibit the response to mitogenic signal.
Complexity in the interplay is even higher if we take into account emerging evidences suggesting a role of MAPKs in mediating integrin-induced differentiation that creates an appropriate extracellular environment. The MAPK-dependent differentiation of PC12 cells is accompanied by up-regulation of the α1β1 receptor and in smooth muscle cells ERK1/2 appears to be essential for the transcription of tenascin (Boudreau and Jones, 1999; Jones et al., 1999). Furthermore, cytoskeleton modulators such as MLCK can be phosphorylated by ERK, which consequently influences cell migration on the ECM and pseudopod formation (Klemke et al., 1997; Mansfield et al., 2000). Together with our data, these results converge to a concept in which ECM, integrins, and cytoskeletal components reorganization, regulated by the MEK/ERK pathway, may all have a key role in cell shape, making them permissive for DNA replication or differentiation.
Disturbed communication between cells and the ECM may also play an important role in malignant transformation, and the MEK/ERK pathway is overactivated in a wide variety of tumor cells in vivo. The motility of many cells was correlated with both oncogenic invasiveness and metastatic potential, and overregulated EGFr signaling in tumors was associated with progression to invasion and metastasis. In this context, we are presently looking at the MAPK MEK/ERK activation regarding hepatocyte motility/spreading potential in hepatocarcinogenesis.
ACKNOWLEDGMENTS
We thank Dr. G. L'Allemain for fruitful suggestions and Drs. P. Loyer and J.C. Andrieux for critical reading of the manuscript. This research was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM), by European Economic Community grant BIO4-CT 960052, and by the Association pour la Recherche contre le Cancer. C. R. is a recipient of fellowship from the Ministère de l'Education nationale, de la Recherche et de la Technologie.
Abbreviations used:
- DMSO
dimethyl sulfoxide
- ECM
extracellular matrix EGF, epidermal growth factor
- EGFr
EGF receptor
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- Ig
immunoglobulin
- MEK
mitogen-activated protein kinase kinase
- PHT
partial hepatectomy
- PI3K
phosphatidylinositol 3-kinase
- TBS
Tris-buffered saline
REFERENCES
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ. 1999;10:397–404. [PubMed] [Google Scholar]
- Albrecht JH, Hu MY, Cerra FB. Distinct patterns of cyclin D1 regulation in models of liver regeneration and human liver. Biochem Biophys Res Commun. 1995;209:648–655. doi: 10.1006/bbrc.1995.1548. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Assoian RK. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer KL, Contessa J, Brenz-Verca S, Pirola L, Rusconi S, Cooper G, Abo A, Wymann MP, Davis RJ, Birrer M, Dent P. The Ras/Rac1/Cdc42/SEK/JNK/c-Jun cascade is a key pathway by which agonists stimulate DNA synthesis in primary cultures of rat hepatocytes. Mol Biol Cell. 1998;9:561–573. doi: 10.1091/mbc.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by H.G.F/S.F., E.G.F. and T.G.F. alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi ME, Zhu X, Bohmer RM, Assoian RK. Regulation of p21(cip1) expression by growth factors and the extracellular matrix reveals a role for transient ERK activity in G1 phase. J Cell Biol. 1999;146:1255–1264. doi: 10.1083/jcb.146.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau NJ, Jones PL. Extracellular matrix and integrin signaling: the shape of things to come. Biochem J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ishac EJ, Dent P, Kunos G, Gao B. Effects of ethanol on mitogen-activated protein kinase and stress-activated protein kinase cascades in normal and regenerating liver. Biochem J. 1998;334:669–676. doi: 10.1042/bj3340669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JD, Kim JP, Zhang K, Sarret Y, Wynn KC, Kramer RH, Woodley DT. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993;209:216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Etienne PL, Baffet G, Desvergne B, Boisnard-Rissel M, Glaise D, Guguen-Guillouzo C. Transient expression of c-fos and constant expression of c-myc in freshly isolated and cultured normal adult rat hepatocytes. Oncogene Res. 1988;3:255–262. [PubMed] [Google Scholar]
- Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995;9:1527–1536. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- Fiddes RJ, Janes PW, Sivertsen SP, Sutherland RL, Musgrove EA, Daly RJ. Inhibition of the MAP kinase cascade blocks heregulin-induced cell cycle progression in T-47D human breast cancer cells. Oncogene. 1998;16:2803–2813. doi: 10.1038/sj.onc.1201815. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP. kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Slepchenko B, Moraru II, Schaff J, Watras J, Loew LM. Morphological control of inositol-1,4,5-trisphosphate-dependent signals. J Cell Biol. 1999;147:929–936. doi: 10.1083/jcb.147.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG. Integrin signaling: specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- Gines P, Li X, Brown SE, Nakamura T, Guzelian PS, Heasley LE, Schrier RW, Nemenoff RA. Inhibitory actions of cyclic adenosine monophosphate and pertussis toxin define two distinct epidermal growth factor-regulated pathways leading to activation of mitogen-activated protein kinase in rat hepatocytes. Hepatology. 1996;23:1167–1173. doi: 10.1002/hep.510230535. [DOI] [PubMed] [Google Scholar]
- Gotoh I, Fukuda M, Adachi M, Nishida E. Control of the cell morphology and the S phase entry by mitogen-activated protein kinase kinase: a regulatory role of its N-terminal region. J Biol Chem. 1999;274:11874–11880. doi: 10.1074/jbc.274.17.11874. [DOI] [PubMed] [Google Scholar]
- Greulich H, Erikson RL. An analysis of Mek1 signaling in cell proliferation and transformation. J Biol Chem. 1998;273:13280–13288. doi: 10.1074/jbc.273.21.13280. [DOI] [PubMed] [Google Scholar]
- Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375–1383. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guguen C, Guillouzo A, Boisnard M, Le Cam A, Bourel M. Ultrastructural study of monolayer hepatocytes in adult rat cultures in the presence of hydrocortisone hemisuccinate. Biol Gastroenterol. 1975;8:223–231. [PubMed] [Google Scholar]
- Hansen LK, Albrecht JH. Regulation of the hepatocyte cell cycle by type I collagen matrix: role of cyclin D1. J Cell Sci. 1999;112:2971–2981. doi: 10.1242/jcs.112.17.2971. [DOI] [PubMed] [Google Scholar]
- Hansen LK, Mooney DJ, Vacanti JP, Ingber DE. Integrin binding and cell spreading on extracellular matrix act at different points in the cell cycle to promote hepatocyte growth. Mol Biol Cell. 1994;5:967–975. doi: 10.1091/mbc.5.9.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruyama T, Ajioka I, Akaike T, Watanabe Y. Regulation and significance of hepatocyte-derived matrix metalloproteinases in liver remodeling. Biochem Biophys Res Commun. 2000;272:681–686. doi: 10.1006/bbrc.2000.2837. [DOI] [PubMed] [Google Scholar]
- Huang S, Chen CS, Ingber DE. Control of cyclin D1, P27Kip1, and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis WD, Auer KL, Spector M, Kunos G, Grant S, Hylemon P, Mikkelsen R, Dent P. Positive and negative regulation of JNK1 by protein kinase C and p42(MAP kinase) in adult rat hepatocytes. FEBS Lett. 1997;412:9–14. doi: 10.1016/s0014-5793(97)00705-9. [DOI] [PubMed] [Google Scholar]
- Jones PL, Jones FS, Zhou B, Rabinovitch M. Induction of vascular smooth muscle cell tenascin-C gene expression by denatured type I collagen is dependent upon a beta3 integrin-mediated mitogen-activated protein kinase pathway and a 122-base pair promoter element. J Cell Sci. 1999;112:435–445. doi: 10.1242/jcs.112.4.435. [DOI] [PubMed] [Google Scholar]
- Kim TH, Mars WM, Stolz DB, Petersen BE, Michaloupolos GK. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology. 1997;26:896–904. doi: 10.1002/hep.510260415. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KS, Lu XP, Leffert HL. Primary rat hepatocytes express cyclin D1 messenger RNA during their growth cycle and during mitogenic transitions induced by transforming growth factor-alpha. Biochem Biophys Res Commun. 1994;204:91–97. doi: 10.1006/bbrc.1994.2430. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Rivard N, L'Allemain G, Pouyssegur J. A temporal and biochemical link between growth factor-activated MAP kinases, cyclin D1 induction and cell cycle entry. Prog Cell Cycle Res. 1996;2:49–58. doi: 10.1007/978-1-4615-5873-6_5. [DOI] [PubMed] [Google Scholar]
- Le Gall M, Grall D, Chambard JC, Pouyssegur J, Van Obberghen-Schilling E. An anchorage-dependent signal distinct from p42/44 MAP kinase activation is required for cell cycle progression. Oncogene. 1998;17:1271–1277. doi: 10.1038/sj.onc.1202057. [DOI] [PubMed] [Google Scholar]
- Loyer P, Cariou S, Glaise D, Bilodeau M, Baffet G, Guguen-Guillouzo C. Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro: evidence of a mitogen restriction point in mid-late G1. J Biol Chem. 1996;271:11484–11492. doi: 10.1074/jbc.271.19.11484. [DOI] [PubMed] [Google Scholar]
- Loyer P, Glaise D, Cariou S, Baffet G, Meijer L, Guguen-Guillouzo C. Expression and activation of cdks (1 and 2) and cyclins in the cell cycle progression during liver regeneration. J Biol Chem. 1994;269:2491–2500. [PubMed] [Google Scholar]
- Mansfield PJ, Shayman JA, Boxer LA. Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood. 2000;95:2407–2412. [PubMed] [Google Scholar]
- Mars WM, Liu ML, Kitson RP, Goldfarb RH, Gabauer MK, Michalopoulos GK. Immediate early detection of urokinase receptor after partial hepatectomy and its implications for initiation of liver regeneration. Hepatology. 1995;21:1695–1701. [PubMed] [Google Scholar]
- Matthay MA, Thiery JP, Lafont F, Stampfer F, Boyer B. Transient effect of epidermal growth factor on the motility of an immortalized mammary epithelial cell line. J Cell Sci. 1993;106:869–878. doi: 10.1242/jcs.106.3.869. [DOI] [PubMed] [Google Scholar]
- Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci USA. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix-integrin interaction activates the mitogen-activated protein kinase, p44 erk-1 and p42 erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktay M, Wary KK, Dans M, Birge RB, Giancotti FG. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J Cell Biol. 1999;145:1461–1469. doi: 10.1083/jcb.145.7.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Ravenhall C, Guida E, Harris T, Koutsoubos V, Stewart A. The importance of ERK activity in the regulation of cyclin D1 levels and DNA synthesis in human cultured airway smooth muscle. Br J Pharmacol. 2000;131:17–28. doi: 10.1038/sj.bjp.0703454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Price LS, Schwartz MA. Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J Cell Biol. 1999;147:611–618. doi: 10.1083/jcb.147.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- Spector MS, Auer KL, Jarvis WD, Ishac EJ, Gao B, Kunos G, Dent P. Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol. 1997;17:3556–3565. doi: 10.1128/mcb.17.7.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signaling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker F, Kato A, Gonez LJ, Hibbs ML, Pouliot N, Levitzki A, Burgess AW. Activation of the Ras/mitogen-activated protein kinase pathway by kinase-defective epidermal growth factor receptors results in cell survival but not proliferation. Mol Cell Biol. 1998;18:7192–7204. doi: 10.1128/mcb.18.12.7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Osaki H, Akaike T. TNF-alpha bifunctionally induces proliferation in primary hepatocytes: role of cell anchorage and spreading. J Immunol. 1997;159:4840–4847. [PubMed] [Google Scholar]
- Webber EM, Bruix J, Pierce RH, Fausto N. Tumor necrosis factor primes hepatocytes for DNA replication in the rat. Hepatology. 1998;28:1226–1234. doi: 10.1002/hep.510280509. [DOI] [PubMed] [Google Scholar]
- Weber JD, Raben DM, Phillips PJ, Baldassare JJ. Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem J. 1997;326:61–68. doi: 10.1042/bj3260061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westwick JK, Fleckenstein J, Yin M, Yang SQ, Bradham CA, Brenner DA, Diehl AM. Differential regulation of hepatocyte DNA synthesis by cAMP in vitro in vivo. Am J Physiol. 1996;271:G780–790. doi: 10.1152/ajpgi.1996.271.5.G780. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Webber EM, Kirillova I, Peschon JJ, Fausto N. Analysis of liver regeneration in mice lacking type 1 or type 2 tumor necrosis factor receptor: requirement for type 1 but not type 2 receptor. Hepatology. 1998;28:959–970. doi: 10.1002/hep.510280410. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sun J, Liu N, Wen D, Chang D, Thomason A, Yoshinaga SK. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem. 1996;271:3884–3890. [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Biol Cell. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]