Abstract
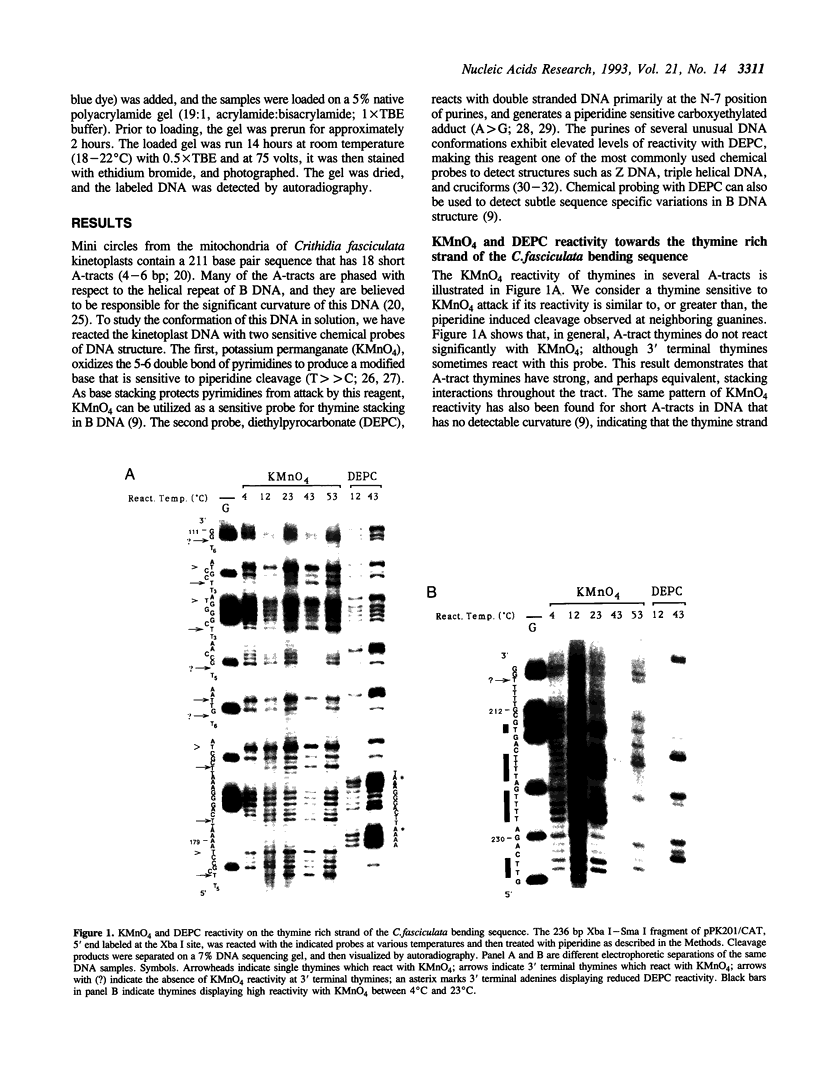
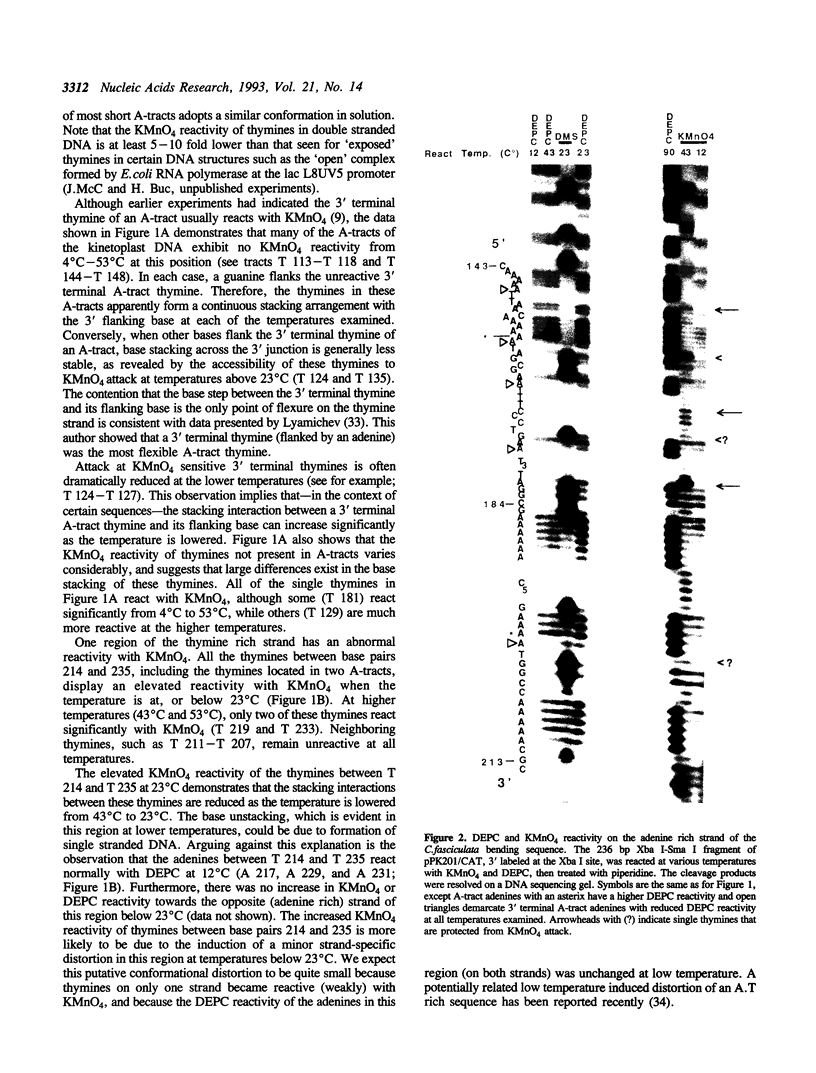
The chemical probes potassium permanganate (KMnO4) and diethylpyrocarbonate (DEPC) have been used to study the conformation of bent kinetoplast DNA from Crithidia fasciculata at different temperatures. Chemical reactivity data shows that the numerous short A-tracts of this bent DNA adopt a similar structure at 43 degrees C. This conformation appears to be very similar to the conformation of A-tracts in DNA exhibiting normal gel mobility. The A-tract structure detected by chemical probing is characterized by a high degree of base stacking on the thymine strand, and by an abrupt conformational change at the 3' end of the adenine strand. In general, no major alteration of this A-tract specific structure was detected between 4-53 degrees C. However, probing with KMnO4 revealed two unusual features of the C. fasciculata sequence that may contribute to the highly aberrant gel mobility of this DNA: 1) the B DNA/A-tract junction 5' dC/A3-6 3'. 5' dT3-6/G 3' is disproportionately represented and is conformationally distinct from other 5' end junctions, and 2) low temperature favors a novel strand-specific conformational distortion over a 20 base pair region of the bent kinetoplast DNA. Presence of the minor groove binding drug distamycin had little detectable effect on the A-tract conformation. However, distamycin did inhibit formation of the novel KMnO4 sensitive low temperature structure and partially eliminated the anomalous gel mobility of the kinetoplast DNA. Finally, we describe a simple and reproducible procedure for the production of an adenine-specific chemical DNA sequence ladder.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abagyan R. A., Mironov V. N., Chernov B. K., Chuprina V. P., Ulyanov A. V. Electrophoretic behavior of d(GGAAAAAAGG)n, d(CCAAAAAACC)n, and (CCAAAAAAGG)n and implications for a DNA bending model. Nucleic Acids Res. 1990 Feb 25;18(4):989–992. doi: 10.1093/nar/18.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeev D. G., Lipanov A. A., Skuratovskii IYa Poly(dA).poly(dT) is a B-type double helix with a distinctively narrow minor groove. 1987 Feb 26-Mar 4Nature. 325(6107):821–823. doi: 10.1038/325821a0. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Bode J., Kohwi Y., Dickinson L., Joh T., Klehr D., Mielke C., Kohwi-Shigematsu T. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science. 1992 Jan 10;255(5041):195–197. doi: 10.1126/science.1553545. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhoff A. M., Tullius T. D. The unusual conformation adopted by the adenine tracts in kinetoplast DNA. Cell. 1987 Mar 27;48(6):935–943. doi: 10.1016/0092-8674(87)90702-1. [DOI] [PubMed] [Google Scholar]
- Coll M., Frederick C. A., Wang A. H., Rich A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8385–8389. doi: 10.1073/pnas.84.23.8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann S., Mazzarelli J. M., McLaughlin L. W., von Kitzing E., Travers A. A. DNA curvature does not require bifurcated hydrogen bonds or pyrimidine methyl groups. J Mol Biol. 1992 Jun 5;225(3):729–738. doi: 10.1016/0022-2836(92)90397-3. [DOI] [PubMed] [Google Scholar]
- Furlong J. C., Lilley D. M. Highly selective chemical modification of cruciform loops by diethyl pyrocarbonate. Nucleic Acids Res. 1986 May 27;14(10):3995–4007. doi: 10.1093/nar/14.10.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai X. X., Lipson K. E., Prystowsky M. B. Unusual DNA binding characteristics of an in vitro translation product of the CCAAT binding protein mYB-1. Nucleic Acids Res. 1992 Feb 11;20(3):601–606. doi: 10.1093/nar/20.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser S. M., Laemmli U. K. Cohabitation of scaffold binding regions with upstream/enhancer elements of three developmentally regulated genes of D. melanogaster. Cell. 1986 Aug 15;46(4):521–530. doi: 10.1016/0092-8674(86)90877-9. [DOI] [PubMed] [Google Scholar]
- Griffith J., Bleyman M., Rauch C. A., Kitchin P. A., Englund P. T. Visualization of the bent helix in kinetoplast DNA by electron microscopy. Cell. 1986 Aug 29;46(5):717–724. doi: 10.1016/0092-8674(86)90347-8. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Evidence for the existence of stable curvature of DNA in solution. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4632–4636. doi: 10.1073/pnas.81.15.4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P. J. Pyrimidine 5-methyl groups influence the magnitude of DNA curvature. Biochemistry. 1990 Feb 27;29(8):1980–1983. doi: 10.1021/bi00460a003. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence dependence of the curvature of DNA: a test of the phasing hypothesis. Biochemistry. 1985 Dec 3;24(25):7033–7037. doi: 10.1021/bi00346a001. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence-directed curvature of DNA. Annu Rev Biochem. 1990;59:755–781. doi: 10.1146/annurev.bi.59.070190.003543. [DOI] [PubMed] [Google Scholar]
- Hanvey J. C., Shimizu M., Wells R. D. Intramolecular DNA triplexes in supercoiled plasmids. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6292–6296. doi: 10.1073/pnas.85.17.6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Corbin V., Gilbert W. Nucleotide sequence of the 3' half of AKV. Nucleic Acids Res. 1982 Nov 11;10(21):6931–6944. doi: 10.1093/nar/10.21.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Diethyl pyrocarbonate: a chemical probe for secondary structure in negatively supercoiled DNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8009–8013. doi: 10.1073/pnas.82.23.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi K., Nakayama A., Hishinuma F. Sequence-directed bends of DNA helix axis at the upstream activation sites of alpha-cell-specific genes in yeast. Nucleic Acids Res. 1988 Jul 25;16(14B):6693–6711. doi: 10.1093/nar/16.14.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintanar A., Klevit R. E., Reid B. R. Two-dimensional NMR investigation of a bent DNA fragment: assignment of the proton resonances and preliminary structure analysis. Nucleic Acids Res. 1987 Jul 24;15(14):5845–5862. doi: 10.1093/nar/15.14.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchin P. A., Klein V. A., Ryan K. A., Gann K. L., Rauch C. A., Kang D. S., Wells R. D., Englund P. T. A highly bent fragment of Crithidia fasciculata kinetoplast DNA. J Biol Chem. 1986 Aug 25;261(24):11302–11309. [PubMed] [Google Scholar]
- Kohwi Y., Kohwi-Shigematsu T. Magnesium ion-dependent triple-helix structure formed by homopurine-homopyrimidine sequences in supercoiled plasmid DNA. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3781–3785. doi: 10.1073/pnas.85.11.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H. S., Crothers D. M. Calibration of DNA curvature and a unified description of sequence-directed bending. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1763–1767. doi: 10.1073/pnas.85.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy G., Polte T., Rooney T., Hogan M. E. A photochemical method to map ethidium bromide binding sites on DNA: application to a bent DNA fragment. Biochemistry. 1990 Jan 30;29(4):981–988. doi: 10.1021/bi00456a021. [DOI] [PubMed] [Google Scholar]
- Käs E., Izaurralde E., Laemmli U. K. Specific inhibition of DNA binding to nuclear scaffolds and histone H1 by distamycin. The role of oligo(dA).oligo(dT) tracts. J Mol Biol. 1989 Dec 5;210(3):587–599. doi: 10.1016/0022-2836(89)90134-4. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., McDonald J. J., Henderson R. E., Reichmann M. E. Reaction of diethyl pyrocarbonate with nucleic acid components. Adenosine. Biochemistry. 1971 Aug 31;10(18):3335–3342. doi: 10.1021/bi00794a003. [DOI] [PubMed] [Google Scholar]
- Leroy J. L., Charretier E., Kochoyan M., Guéron M. Evidence from base-pair kinetics for two types of adenine tract structures in solution: their relation to DNA curvature. Biochemistry. 1988 Dec 13;27(25):8894–8898. doi: 10.1021/bi00425a004. [DOI] [PubMed] [Google Scholar]
- Lyamichev V. Unusual conformation of (dA)n.(dT)n-tracts as revealed by cyclobutane thymine-thymine dimer formation. Nucleic Acids Res. 1991 Aug 25;19(16):4491–4496. doi: 10.1093/nar/19.16.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini J. C., Effron P. N., Goodman T. C., Singleton C. K., Wells R. D., Wartell R. M., Englund P. T. Physical characterization of a kinetoplast DNA fragment with unusual properties. J Biol Chem. 1984 Jul 25;259(14):8974–8979. [PubMed] [Google Scholar]
- Marini J. C., Levene S. D., Crothers D. M., Englund P. T. Bent helical structure in kinetoplast DNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7664–7668. doi: 10.1073/pnas.79.24.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. G., Rich A. Detection of an unusual distortion in A-tract DNA using KMnO4: effect of temperature and distamycin on the altered conformation. Nucleic Acids Res. 1991 Jun 25;19(12):3421–3429. doi: 10.1093/nar/19.12.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. G., Williams L. D., Rich A. Chemical reactivity of potassium permanganate and diethyl pyrocarbonate with B DNA: specific reactivity with short A-tracts. Biochemistry. 1990 Jun 26;29(25):6071–6081. doi: 10.1021/bi00477a027. [DOI] [PubMed] [Google Scholar]
- Milton D. L., Casper M. L., Gesteland R. F. Saturation mutagenesis of a DNA region of bend. Base steps other than ApA influence the bend. J Mol Biol. 1990 May 5;213(1):135–140. doi: 10.1016/S0022-2836(05)80126-3. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Nadeau J. G., Crothers D. M. Structural basis for DNA bending. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2622–2626. doi: 10.1073/pnas.86.8.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Ryder K., Silver S., DeLucia A. L., Fanning E., Tegtmeyer P. An altered DNA conformation in origin region I is a determinant for the binding of SV40 large T antigen. Cell. 1986 Mar 14;44(5):719–725. doi: 10.1016/0092-8674(86)90838-x. [DOI] [PubMed] [Google Scholar]
- Shatzky-Schwartz M., Hiller Y., Reich Z., Ghirlando R., Weinberger S., Minsky A. Attenuation of DNA-protein interactions associated with intrinsic, sequence-dependent DNA curvature. Biochemistry. 1992 Mar 3;31(8):2339–2346. doi: 10.1021/bi00123a019. [DOI] [PubMed] [Google Scholar]
- Snyder M., Buchman A. R., Davis R. W. Bent DNA at a yeast autonomously replicating sequence. Nature. 1986 Nov 6;324(6092):87–89. doi: 10.1038/324087a0. [DOI] [PubMed] [Google Scholar]
- Ulanovsky L., Bodner M., Trifonov E. N., Choder M. Curved DNA: design, synthesis, and circularization. Proc Natl Acad Sci U S A. 1986 Feb;83(4):862–866. doi: 10.1073/pnas.83.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zahn K., Blattner F. R. Sequence-induced DNA curvature at the bacteriophage lambda origin of replication. Nature. 1985 Oct 3;317(6036):451–453. doi: 10.1038/317451a0. [DOI] [PubMed] [Google Scholar]