Abstract
Control of bovine tuberculosis (TB) in cattle has proven particularly challenging where reservoirs of infection exist in wildlife populations. In Britain and Ireland, control is hampered by a reservoir of infection in Eurasian badgers (Meles meles). Badger culling has positive and negative effects on bovine TB in cattle and is difficult, costly and controversial. Here we show that Bacillus Calmette-Guérin (BCG) vaccination of captive badgers reduced the progression, severity and excretion of Mycobacterium bovis infection after experimental challenge. In a clinical field study, BCG vaccination of free-living badgers reduced the incidence of positive serological test results by 73.8 per cent. In common with other species, BCG did not appear to prevent infection of badgers subjected to experimental challenge, but did significantly reduce the overall disease burden. BCG vaccination of badgers could comprise an important component of a comprehensive programme of measures to control bovine TB in cattle.
Keywords: bovine tuberculosis, vaccination, badger, wildlife, Bacillus Calmette-Guérin
1. Introduction
Tuberculosis (bovine TB), caused by infection with Mycobacterium bovis, is a serious zoonotic infection that affects cattle and other wild and domesticated animals. Over the past two decades, the incidence of bovine TB in cattle has increased substantially in parts of Great Britain (GB) creating a significant economic burden on government and the cattle industry [1]. Tuberculin testing and the slaughter of infected cattle has been sufficient to control or even eradicate the disease in parts of the world, but this has proven more challenging where infection persists in a wildlife reservoir.
In 1971, M. bovis infection was first detected in Eurasian badgers (Meles meles) in GB [2]. Since then, epidemiological studies have demonstrated that M. bovis infection is present in badgers across large parts of Britain and Ireland [3,4], that infected badgers may excrete M. bovis [5] and are a source of infection for cattle [6,7]. However, badger culling can have complex epidemiological outcomes, including both positive and negative impacts on the incidence of bovine TB in cattle [8,9]. Furthermore, badger culling as an approach to disease control can be costly, practically difficult and indiscriminate, and remains controversial. Vaccinating badgers may be an alternative or complementary strategy that overcomes some of these challenges [10].
Bacillus Calmette-Guérin (BCG) is an attenuated strain of M. bovis, which is widely used around the world as a vaccine against human TB caused by Mycobacterium tuberculosis. BCG protects against severe childhood forms of TB but has only limited efficacy against adult pulmonary disease [11,12]. Thus, BCG has long been considered to restrict the extent of disease rather than prevent infection [12]. However, this dogma has recently been challenged by observations in humans [13], wild brushtail possums (Trichosurus vulpecula) [14,15], and experimentally and naturally infected cattle [16,17], where vaccination appeared to prevent infection in a proportion of subjects. As the aim of a badger vaccine would be to at least limit transmission from infected badger populations to cattle, we reasoned that a vaccine which prevented disease progression to a point before animals became infectious could still achieve this goal [18]. Previous studies have shown that BCG confers some protection to badgers against experimental challenge with M. bovis when delivered via the subcutaneous or combined intranasal/conjunctival routes [19,20]. Our recent studies have shown that intramuscular (IM) administration of BCG to badgers is both safe and of equivalent immunogenicity to subcutaneous delivery [21]. It also has the practical advantage of being readily administered to trapped badgers without recourse to anaesthesia. Here we present the results of IM vaccination of badgers with BCG from two experimental infection studies and a 4-year clinical field study.
2. Material and methods
(a). General
(i). Anaesthesia
In all cases, badgers were anaesthetized through intra-muscular injection of a combination of ketamine hydrochloride (100 mg ml−1, Vetalar V, Pharmacia & Upjohn, Crawley, UK), medetomidine hydrochloride (1 mg ml−1, Domitor, Pfizer, Sandwich, UK) and butorphanol tartrate (10 mg ml−1, Torbugesic, Fort Dodge Animal Health Ltd, Southampton, UK) at a ratio of 2 : 1 : 2 by volume, respectively [22]. This was supplemented with inhalant isoflurane when necessary.
(ii). Bacillus Calmette-Guérin vaccination
BCG Danish strain 1331 vaccine (Statens Serum Institut (SSI), Copenhagen, Denmark) was supplied at 2–8 × 106 colony-forming units (CFU) per vial. The vaccine was prepared by adding 1 ml of Sauton diluent to each vial. In all studies, the vaccine was injected in the lumbar muscle, following shaving and cleaning of the overlying skin. All vaccinated animals received 1 ml of vaccine that had been reconstituted for less than 4 h. At the end of each vaccination session, residual vaccine was cultured on modified Middlebrook 7H11 agar plates to determine the viable count and titre of the vaccine.
(iii). Immunological assays
Immune responses of badgers in all studies were monitored by gamma interferon (IFNγ) ELISA [23] and by measuring antibodies in serum to any combination of four mycobacterial antigens (MPB83, CFP-10, 38 kDa, Mtb8.4) using the Brock TB Stat-Pak test (Chembio Diagnostic Systems Inc., New York, USA) [24,25]. Blood samples were taken into heparin and SST BD Vacutainer Blood Collection Tubes (BD, Plymouth, UK) and processed on the same day. Antigens used to stimulate blood cultures were PPD-B and PPD-A. After antigen stimulation, supernatants from the blood cultures were used to test for badger IFNγ by ELISA [23]. Quantitative data from the IFNγ ELISA (optical density units) were converted into binary data (positive/negative result) on the basis of a test cut-off determined during the development of the test [23].
(iv). Culture of M. bovis
Clinical samples (tracheal aspirate, laryngeal and rectal swabs, urine and faeces) taken from badgers were not decontaminated before culture with the following exceptions. Rectal swabs were soaked overnight in 0.85 per cent sodium chloride saline solution and faeces were suspended in saline. The saline solution or faeces suspension were decontaminated with 5 per cent final volume oxalic acid for 10 min at room temperature. Material for culture was retrieved by centrifugation and the oxalic acid removed by a wash step using saline.
Tissue samples collected at post-mortem examination were taken aseptically, weighed and frozen at −20°C. Subsequently, they were thawed to room temperature and each tissue type cultured separately. Tissues were homogenized in 10 ml 0.85 per cent saline using IKA tubes (IKA Werke GmbH & Co. KG, Staufen, Germany). All samples were cultured on Middlebrook 7H11 medium and incubated for 12 weeks at 37°C before examination for the presence of bacterial growth. Confirmation of the identity of colonies as the M. bovis challenge strain was obtained by spoligotyping [26].
(b). Mycobacterium bovis experimental infection studies
(i). Mycobacterium bovis
The M. bovis strain used for challenge in the experimental infection model was originally isolated from an infected wild badger in the UK in 1997 (isolate 74/0449/97). This was stored as a first passage stock culture until expanded and stored as frozen aliquots (−80°C) for experimental infection studies. The clonality of the culture was confirmed by demonstrating the spoligotype—SB0140 (VLA type 9) and VNTR type (8-5-5-5-3-3.1) of 10 per cent of colonies grown from a culture of approximately 105 CFU ml−1. The stock vials used for the challenge had not been passaged further and contained approximately 107 CFU ml−1 viable M. bovis.
(ii). Animals and sampling
The first experiment vaccine efficacy study 1 (VES1) involved five badgers and the second experiment vaccine efficacy study 2 (VES2) involved 10 badgers (table 1). The animals were trapped from the wild in a county of England with no reported cases of TB in badgers and very few cases of bovine TB. After capture, badgers were confirmed TB-free on the basis of three consecutive negative results, one month apart, for both IFNγ and culture of clinical samples. The badgers were housed in groups of up to four animals, each of which contained individuals from the same social group of origin wherever possible. Badgers were identified by a unique tattoo on the belly and a subcutaneous microchip with a unique number (AVID PLC, Lewes, UK).
Table 1.
The number of badgers in each treatment group for the two laboratory vaccine efficacy studies (VES), with vaccine and challenge doses and the median lesion score. (CFU, colony-forming units.)
| experiment | treatment group | dose of BCG (CFU ml−1) | dose of M. bovis (CFU ml−1) | number of badgers | median lesion score |
|---|---|---|---|---|---|
| VES1 | BCG | 5.4 × 106 | 4.8 × 103 | 3 | 4 |
| non-vaccinated | n.a. | 4.8 × 103 | 1 | 12.5 | |
| 3.7 ×103 | 1 | ||||
| VES2 | BCG | 3.2 × 106 | 2.6 × 103 | 4 | 4 |
| 2.8 × 103 | 2 | ||||
| non-vaccinated | n.a. | 2.8 × 103 | 4 | 9 |
Each group of badgers was housed in an open-air pen of approximately 50 m2, containing concrete tunnels and wooden pallets for environmental enrichment. The badgers received a diet of dog food, peanuts and occasionally eggs, and had constant access to fresh water. The badgers were moved to an Advisory Committee on Dangerous Pathogens Containment Level 3 facility approximately five to six weeks before challenge.
Once every two to three weeks, the badgers were anaesthetized and examined. Blood was collected by jugular venipuncture and subjected to IFNγ and Stat-Pak tests. Tracheal mucus was collected by aspiration with a flexible urinary catheter (Arnolds Veterinary Products, Shrewsbury, UK) and dispensed into Middlebrook 7H9 broth. Laryngeal and rectal swabs were collected and placed into 7H9 broth and phosphate-buffered saline (PBS), respectively. Urine was collected into sterile 15 ml plastic tubes by manual compression of the bladder.
(iii). Experimental infection with M. bovis
Seventeen weeks post-vaccination, all badgers were infected with M. bovis. One vial of stock M. bovis was thawed and serially diluted in sterile water + 0.05% (v/v) Tween 80 to contain approximately 5 × 103 CFU ml−1. At each dilution, the suspension was vortexed to diminish the risk of bacterial clumping. The final dilution (challenge inoculum) was made in sterile PBS + 0.05% Tween 80. The titre of the challenge inoculum was determined by plating on Middlebrook 7H11 agar a sample from a syringe kept in the same conditions as those used for challenge.
For both experiments, challenge occurred over two separate days, and the viable count of the inoculum was determined on each day. The challenge inoculum was delivered by endobronchial instillation to anaesthetized badgers using a 70 cm fibroscope (Olympus UFR-P2, 3.6 × 1.2 mm canal), targeting the bronchus of the right middle lobe. Mycobacterium bovis suspension in a 1 ml volume was inoculated via a sterile plastic catheter (1 mm × 1 m) and the catheter was flushed with 1 ml PBS. Between animals the fibroscope was disinfected with ortho-phthalaldehyde (Cidex-OPA) and 70 per cent ethanol and then rinsed with sterile water.
(iv). Post-mortem examination
Twelve weeks after challenge (29 weeks after vaccination), the badgers were killed humanely with an intravenous overdose of sodium pentobarbitone and immediately subjected to post-mortem examination by pathologists blinded to treatment allocation. A pre-determined set of tissues was collected and examined for gross/visible lesions. Gross lesions were detected by finely slicing lymph nodes (LNs) and organs. Each LN was divided for histology and culture, and for the larger organs such as spleen, approximately 3 cm3 of tissue was submitted for culture and the remainder for histology. Histologically, a TB lesion consisted of one or more granulomas containing acid-fast bacteria (AFB) in Ziehl–Neelsen (ZN)-stained sections. A visible lesion score was derived using a standardized ordinal scoring system of 1–4 (few foci or slight swelling to extensive caseation or areas of coalesced foci) [27,28]. Only visible lesions subsequently confirmed as tuberculous by either isolation of M. bovis from the tissue by culture or the appearance of AFB in ZN-stained histological sections counted towards the final score. The score was derived from the sum of the highest scoring lung lobe plus the scores from all other tissues. The parameters used to assess the severity of gross disease present post-mortem have been used to assess the efficacy of BCG vaccine in white-tailed deer [29] and cattle [17,30], as well as badgers [19].
(v). Data analysis
Each variable under test was evaluated for between study differences using a general linear modelling approach. As no significant differences were obtained between the two studies, the results of experiments VES1 and VES2 were combined to increase the statistical power and give the best estimate of treatment effects. Lesion scores were measured on an ordinal scale and investigated for evidence of a difference between treatment groups (vaccinate or control) and experiments (VES1 or VES2) using the Mann–Whitney non-parametric rank test. Further analysis was conducted to compare treatment groups with respect to the time until M. bovis was isolated from any clinical sample post-challenge. The mean time to a positive culture result was estimated with a 95% confidence interval (CI) using Kaplan–Meier estimates. A non-parametric distribution analysis approach was undertaken using the log-rank test to identify significant differences between times to positive culture. All analyses employed Minitab v. 15.1 (2007) (Minitab Ltd, Coventry, UK) and NCSS v. 7.1.5 (2008) (NCSS, Kaysville, UT, USA). Differences in the proportion of positive and negative culture results between clinical samples were tested using χ2 and between treatment groups in the captive animal studies using Fisher's exact tests.
(c). Field study
(i). Social group identification and treatment allocation
Fieldwork took place from 2006 to 2009 inclusive, over an area of approximately 55 km2 in Gloucestershire, southwest England where a relatively high density of badgers was anticipated. The number of badger social groups and the spatial configuration of their territories were identified from surveys for active setts (communal burrow systems) and bait marking [31].
Active badger setts in the study area were subjected to two consecutive nights of trapping, and the entire area was trapped at least twice each year in all but one year. In 2007, a foot and mouth disease outbreak resulted in restrictions to fieldwork and hence the study area was trapped only once. Allocation of treatments to social groups was carried out after the first trapping session in 2006. Groups were randomly assigned to vaccinate or control treatments at a ratio of 60 : 40, while ensuring, as far as possible, an approximate balance of group sizes and bovine TB prevalence between treatments (see the electronic supplementary material for details of this design process). If a social group was allocated to vaccination, every badger caught from that social group was vaccinated irrespective of prior knowledge of its infection status. Badger social groups can split or merge over time, which would present a problem for treatment allocation where two groups of differing treatment merged. Hence, we determined that any animals in control social groups that merged with vaccinated groups be regarded as new to the study, and that they should be vaccinated and their history prior to vaccination ignored.
(ii). Sampling and vaccination
Badgers were captured in cage traps deployed in the immediate vicinity of sett entrance holes, then transferred into holding cages labelled with the sett name to ensure that each badger was returned to its point of capture at the end of procedures. On arrival at the sampling facility, badgers were anaesthetized and each new individual was marked with a programmed microchip (inserted subcutaneously between the shoulders) and a tattoo on the abdomen with a unique three-digit identification. Clinical samples were taken from all badgers: blood (into Vacutainer tubes of heparin and SST); tracheal mucus (by catheter); urine (by manual expression or catheter); faeces (by Microlax enema); and swabs of any wounds or other samples (e.g. abscesses, discharges). BCG vaccine was administered to badgers in the vaccination treatment group on recapture at a rate of one dose per calendar year. Three diagnostic tests for bovine TB were applied to badgers at each capture event: two immunological blood tests (IFNγ and Stat-Pak) and culture for M. bovis of clinical samples.
(iii). Data analysis
The purpose of the analyses was to identify any effect of vaccination on the likelihood that an individual would change its diagnostic test status from negative to positive. Such a change in status is termed an ‘incident case’. Hence, we restricted our analyses to include only animals that were negative to all diagnostic tests on their initial capture and that were trapped more than once during the study. The unit of statistical analysis was the badger social group as specified by the cluster-randomized design of the study, and the response variable was the proportion of incident cases within each social group. The binomial data were analysed using a generalized linear model with a logit (log odds ratio) link function and binomial errors, fitted by maximum quasi-likelihood with provision for over-dispersion (i.e. greater than expected binomial variability). Models were fitted using GenStat 12.1 (VSN International, Hemel Hempstead, UK). The model was fitted separately for each diagnostic test and for Stat-Pak and culture combined, to estimate the proportions in each social group (with 95% CI) of test-positive incident cases in vaccinate and control treatments. An approximate F-probability test was used to indicate evidence of any statistically significant difference between treatments.
3. Results
(a). Experimental infection studies
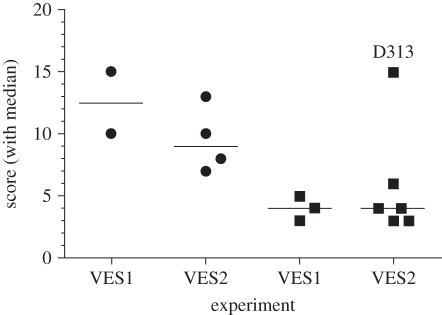
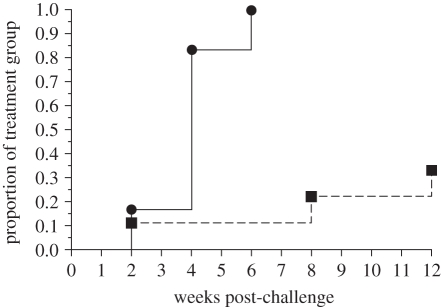
Following experimental challenge with M. bovis, vaccinated badgers had significantly lower lesion scores detected at post-mortem examination than unvaccinated animals (p = 0.013, Mann–Whitney test; table 1 and figure 1). There was no evidence of differences between experiments (p = 1.00, Mann–Whitney test). Although BCG did not prevent infection in vaccinated badgers, it reduced the extent of disease in all but one animal (D313). Mycobacterium bovis was detected by culture of clinical samples in a significantly smaller proportion of vaccinated than unvaccinated badgers (p = 0.028, Fisher's exact test; figure 2). All six non-vaccinated badgers yielded at least one positive culture sample; M. bovis was detected intermittently in the larynx/trachea from four weeks post-challenge in the first experiment and from two weeks post-challenge in the second (see the electronic supplementary material, table S1). By contrast, three of the nine vaccinated animals yielded positive samples at 2, 8 and 12 weeks post-challenge (see the electronic supplementary material, table S1). Mycobacterium bovis was not detected in the urine of any badger, and was only isolated from one faecal sample taken post-mortem from the most heavily infected badger (D313; see the electronic supplementary material, table S1). The average time taken for vaccinated badgers to yield a positive culture sample was significantly longer than in non-vaccinated badgers (p = 0.002, log-rank test).
Figure 1.
BCG vaccination with 2–8 × 106 CFU BCG Danish strain 1331 reduced the severity and progression of experimental bovine TB in badgers. Badgers were vaccinated and then challenged 17 weeks later with endobronchial M. bovis. Disease severity was assessed post-mortem 12 weeks after challenge. Only lesions subsequently confirmed to be caused by M. bovis by bacterial culture or histology were used to calculate the lesion score. The data for individual animals from two separate experiments (VES1 and VES2) are shown together with the group median. Filled circles, non-vaccinated; filled squares, BCG. The score for one animal (D313) is indicated where vaccination failed to protect.
Figure 2.
BCG vaccination reduced the proportion of badgers from which M. bovis was isolated from clinical samples following experimental bovine TB in badgers. Badgers were vaccinated with 2–8 × 106 CFU BCG Danish strain 1331 and then challenged 17 weeks later with endobronchial M. bovis. Samples of tracheal mucus, urine and faeces were collected fortnightly after challenge for 12 weeks. Data from two experiments were combined and the proportion of animals within each treatment group (BCG (filled squares) or non-vaccinated (filled circles)) yielding M. bovis growth from any clinical sample are shown.
Six of the eight vaccinated badgers were positive to the IFNγ test after vaccination. Positive results were recorded from 2 to 17 weeks after vaccination (see the electronic supplementary material, table S2). One of six non-vaccinated badgers recorded a single positive IFNγ test result two weeks prior to challenge with M. bovis. After challenge, all badgers except two (one control, one vaccinated) were positive to the test, and most were intermittently positive (see the electronic supplementary material, table S2). There was no obvious association between the frequency of test positivity after challenge and the extent of pathology post-mortem. A single (vaccinated) badger was positive by Stat-Pak at all time points. After challenge, four of the six non-vaccinated badgers and eight of the nine vaccinated badgers became Stat-Pak-positive (see the electronic supplementary material, table S3).
(b). Field study
During the lifetime of the study, 844 badgers were caught and sampled. For the purpose of the analyses presented here, we identified 262 individuals which had been captured more than once and were test negative on initial capture. These comprised 179 vaccinated badgers (from 38 social groups) and 83 controls (from 26 social groups) that remained unvaccinated.
Vaccination was associated with significant reductions in the incidence of positive Stat-Pak results (73.8%; p < 0.001) and combined Stat-Pak and culture positives (61.4%; p < 0.01; table 2). The incidence of IFNγ test-positivity was not significantly reduced by vaccination (19.7%; p = 0.41; table 2). Mycobacterium bovis was isolated by culture from 189 of 4854 clinical samples, most frequently from abscesses or wound sites although these types of sample represented only 3.4 per cent of all clinical samples taken. Of the remainder, M. bovis was more frequently detected in tracheal mucus (4.1%) than in urine (2.6%) or faeces (2.2%) (see the electronic supplementary material, table S4). These differences were significant (p < 0.01, χ2-test) and are consistent with the predominantly respiratory nature of the infection [5]. The incidence of culture positivity in vaccinated groups was not significantly lower than in control groups (27.1%; p = 0.50; table 2).
Table 2.
Overall incidence of positive results to diagnostic tests for TB in BCG-vaccinated and control (non-vaccinated) badgers in the clinical field study. (Approximate F probability values <0.05 identify statistically significant differences between treatments from generalized linear models applied to data aggregated by social group.)
| test | treatment | % of incident cases (95% CI) | test-positive badgers | total number of badgers | approximate F probability |
|---|---|---|---|---|---|
| Stat-Pak | BCG | 4.5 (2.4–8.2) | 8 | 179 | 0.001 |
| control | 17.1 (10.8–25.9) | 14 | 82 | ||
| culture | BCG | 6.1 (3.4–10.8) | 11 | 179 | 0.50 |
| control | 8.4 (4.0–16.7) | 7 | 83 | ||
| Stat-Pak or culture | BCG | 8.4 (4.9–14.0) | 15 | 179 | 0.009 |
| control | 21.7 (13.5–32.9) | 18 | 83 | ||
| IFNγ | BCG | 28.1 (20.5–37.2) | 50 | 178 | 0.38 |
| control | 35.0 (23.1–49.2) | 28 | 80 |
4. Discussion
Vaccination of wildlife has been studied for at least 50 years since the earliest attempts at oral vaccination against classical swine fever (CSF) (reviewed by Kaden et al. [32]). Although routine vaccination against CSF is prohibited in domestic pigs within the European Union, emergency vaccination is permitted. Vaccination has become more widely accepted as a potential option for the management of disease in wildlife populations following its successful employment in the control of sylvatic rabies [33]. Although currently very few vaccines for wildlife species have been licensed, work is on-going in a number of areas. Recent examples include experimental vaccination of wild white-footed mice (Peromyscus leucopus) [34], a reservoir host species of the human Lyme disease agent (Borrelia burgdorferi), American robins (Turdus migratorius) [35], an amplification host for West Nile Virus, and bison (Bison bison) and elk (Cervus elaphus) against brucellosis [36]. A more extensive review of the subject can be found in Delahay et al. [37].
In the UK and Ireland, it is recognized that vaccination of badgers may play an important role in managing the risks of TB infection in cattle. The results presented here demonstrate that the IM administration of BCG to badgers can reduce the severity and progression of experimentally induced TB and the frequency of excretion of M. bovis. Furthermore, our field study provides, to our knowledge, the first evidence for a beneficial effect of BCG on M. bovis infection in free-living badgers.
As a decision was taken by the sponsors of the work not to kill the badgers at the end of the study, we used three different diagnostic tests to assess the TB status of badgers at the time of capture: IFNγ and Stat-Pak blood tests and isolation by culture of M. bovis from clinical samples. The IFNγ test measures the production of IFNγ following stimulation of whole heparinized blood with bovine and avian tuberculin, with estimated specificity of 93.6 per cent and sensitivity of 80.9 per cent [23]. The Stat-Pak is a lateral flow assay for the presence of antibodies to M. bovis antigens in serum [25] with estimated specificity of 93.1 per cent and sensitivity of 34.4 per cent in infected badgers with no visible TB lesions, rising to 78.1 per cent in cases of more advanced disease, including where M. bovis is being excreted [24]. Although less sensitive than the IFNγ test, in a wide variety of species including badgers, the appearance of serum antibodies to mycobacterial antigens during natural and experimental infection, correlates with advanced disease (reviewed in [38]) and has previously been used as a surrogate for BCG-mediated protection against experimental M. bovis infection in badgers [20]. Furthermore, the Stat-Pak test is able to discriminate between M. bovis-infected and BCG-vaccinated individuals, as it relies on antibody recognition of antigens that are either poorly (MPB83) or not (CFP-10) expressed by BCG Danish; or are poorly immunogenic (38 kDa antigen and Mtb8.4) in vaccinated badgers [25].
While the isolation of M. bovis from a clinical sample is definitive for bovine TB infection, a precise estimate of the sensitivity of the culture of clinical samples is unknown. On the basis of the reported sensitivities for the two immunological tests and the total number of positive test results we estimated it to be in the range of 20–24% for the field study, which is consistent with a previous estimate [39].
The highly significant reduction in the incidence of positive Stat-Pak results observed as a result of vaccinating wild badgers with IM BCG is consistent with a protective effect of vaccination, as antibody production is positively correlated with the extent and severity of TB infection in both humans and badgers [24,40]. Vaccination had no influence on Stat-Pak positivity post-challenge in the experimental studies, despite this being observed previously when BCG was evaluated experimentally in badgers [20]. The number of viable M. bovis organisms used in the challenge inoculum is likely to be substantially higher than that encountered during natural exposure, and while this is a stringent test of vaccine-induced protection, the large antigenic dose it represents probably accounts for why 12 out of 15 (80%) of the captive badgers became seropositive following challenge.
Although the incidence of culture positivity in vaccinated groups of wild badgers was lower, the lack of statistical significance was not altogether surprising given the estimated low sensitivity of the method and the reported infrequency with which infected badgers appear to excrete M. bovis [39]. Similarly, the incidence of IFNγ-positivity in vaccinated groups was lower but not significantly so, either as a consequence of the relatively low power of the field study to detect a significant difference or because of false-positive test results associated with the vaccine itself. The latter appears to be the case since in our experimental studies, six out of eight captive vaccinated badgers yielded positive IFNγ results between 2 and 17 weeks after vaccination. As a result, the effect of vaccination on TB transmission in the field study will have been underestimated in our analysis.
5. Conclusion
Our results demonstrate that IM BCG vaccination reduced the severity and progression of experimental bovine TB infection in captive badgers and the frequency of M. bovis isolation from clinical samples. The endobronchial M. bovis infection model is particularly suitable for the experimental evaluation of vaccine efficacy as the respiratory route is considered the primary route of M. bovis infection in wild badgers [5].
IM BCG in free-living, wild badgers significantly reduced their likelihood of yielding a positive result to a serological test associated with progressive/severe disease [24]. Assays that distinguish IFNγ responses to infection from those induced by vaccination will help in measuring the impact of BCG on transmission in badger populations, but from a disease-management perspective, the key question will be to determine whether vaccination has an impact on the spread of M. bovis to cattle. What is clear is that while vaccination of badgers is unlikely to be the sole solution to this disease problem, the advent of the first licensed BCG vaccine for use in wildlife could provide a new and important component of a comprehensive programme of bovine TB control for cattle in the UK and Ireland.
Acknowledgements
Licenses from The Home Office, Natural England, and the Veterinary Medicines Directorate covered various aspects of this work. All animal work was cleared following review by institutional ethical review panels.
We are indebted to the numerous staff who contributed to this work from the VLA (Pathology Department, TB Diagnostic Section, Animal Services Unit, Centre for Epidemiology and Risk Analysis and Quality Management Group) and from Fera (in particular, the commitment and hard work of the field team). We are grateful to Douglas Young and members of the Department for Environment, Food and Rural Affairs (Defra) Vaccine Programme Advisory Group for discussion and advice, and to the landowners and tenants in the field study area for allowing us access. We thank members of the Food and Farming Group at Defra for their support and for funding this work. © British Crown Copyright 2010
References
- 1.Sheppard A., Turner M. 2005. An economic impact assessment of bovine tuberculosis in south west England. Exeter, UK: Centre for Rural Research, University of Exeter [Google Scholar]
- 2.Muirhead R. H., Burns K. J. 1974. Tuberculosis in wild badgers in Gloucestershire: epidemiology. Vet. Rec. 95, 552–555 10.1136/vr.95.24.552 (doi:10.1136/vr.95.24.552) [DOI] [PubMed] [Google Scholar]
- 3.Cheeseman C. L., Wilesmith J. W., Stuart F. A. 1989. Tuberculosis: the disease and its epidemiology in the badger, a review. Epidemiol. Infect. 103, 113–125 10.1017/S0950268800030417 (doi:10.1017/S0950268800030417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolan L. A. 1993. Badgers and bovine tuberculosis in Ireland: a review. In The badger (ed. Hayden T. J.), pp. 108–116 Dublin, Ireland: Royal Irish Academy [Google Scholar]
- 5.Clifton-Hadley R. S., Wilesmith J. W., Stuart F. A. 1993. Mycobacterium bovis in the European badger (Meles meles): epidemiological findings in tuberculous badgers from a naturally infected population. Epidemiol. Infect. 111, 9–19 10.1017/S0950268800056624 (doi:10.1017/S0950268800056624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White P. C., Brown J. A., Harris S. 1993. Badgers (Meles meles), cattle and bovine tuberculosis (Mycobacterium bovis): a hypothesis to explain the influence of habitat on the risk of disease transmission in southwest England. Proc. R. Soc. Lond. B 253, 277–284 10.1098/rspb.1993.0114 (doi:10.1098/rspb.1993.0114) [DOI] [PubMed] [Google Scholar]
- 7.Wilesmith J. W. 1991. Epidemiological methods for investigating wild animal reservoirs of animal disease. Rev. Sci. Tech. 10, 205–214 [DOI] [PubMed] [Google Scholar]
- 8.Donnelly C. A., et al. 2006. Positive and negative effects of widespread badger culling on tuberculosis in cattle. Nature 439, 843–846 10.1038/nature04454 (doi:10.1038/nature04454) [DOI] [PubMed] [Google Scholar]
- 9.Griffin J. M., Williams D. H., Kelly G. E., Clegg T. A., O'Boyle I., Collins J. D., More S. J. 2005. The impact of badger removal on the control of tuberculosis in cattle herds in Ireland. Prev. Vet. Med. 67, 237–266 10.1016/j.prevetmed.2004.10.009 (doi:10.1016/j.prevetmed.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 10.Delahay R. J., Wilson G. J., Smith G. C., Cheeseman C. L. 2003. Vaccinating badgers (Meles meles) against Mycobacterium bovis: the ecological considerations. Vet. J. 166, 43–51 10.1016/S1090-0233(03)00071-6 (doi:10.1016/S1090-0233(03)00071-6) [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues L. C., Diwan V. K., Wheeler J. G. 1993. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int. J. Epidemiol. 22, 1154–1158 10.1093/ije/22.6.1154 (doi:10.1093/ije/22.6.1154) [DOI] [PubMed] [Google Scholar]
- 12.Sutherland I., Lindgren I. 1979. The protective effect of BCG vaccination as indicated by autopsy studies. Tubercle 60, 225–231 10.1016/0041-3879(79)90003-5 (doi:10.1016/0041-3879(79)90003-5) [DOI] [PubMed] [Google Scholar]
- 13.Soysal A., et al. 2005. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet 366, 1443–1451 10.1016/S0140-6736(05)67534-4 (doi:10.1016/S0140-6736(05)67534-4) [DOI] [PubMed] [Google Scholar]
- 14.Corner L. A., Pfeiffer D. U., de Lisle G. W., Morris R. S., Buddle B. M. 2002. Natural transmission of Mycobacterium bovis infection in captive brushtail possums (Trichosurus vulpecula). NZ Vet. J. 50, 154–162 [DOI] [PubMed] [Google Scholar]
- 15.Tompkins D. M., Ramsey D. S., Cross M. L., Aldwell F. E., de Lisle G. W., Buddle B. M. 2009. Oral vaccination reduces the incidence of tuberculosis in free-living brushtail possums. Proc. R. Soc. B 276, 2987–2995 10.1098/rspb.2009.0414 (doi:10.1098/rspb.2009.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ameni G., Vordermeier M., Aseffa A., Young D., Hewinson R. 2010. Field evaluation of the efficacy of Bacille Calmette Guerin (BCG) against bovine tuberculosis in neonatal calves in Ethiopia. Clin. Vaccine Immunol. 17, 1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vordermeier H. M., Chambers M. A., Cockle P. J., Whelan A. O., Simmons J., Hewinson R. G. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70, 3026–3032 10.1128/IAI.70.6.3026-3032.2002 (doi:10.1128/IAI.70.6.3026-3032.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Leeuw A. N., Forrester G. J., Spyvee P. D., Brash M. G., Delahay R. J. 2004. Experimental comparison of ketamine with a combination of ketamine, butorphanol and medetomidine for general anaesthesia of the Eurasian badger (Meles meles L.). Vet. J. 167, 186–193 10.1016/S1090-0233(03)00113-8 (doi:10.1016/S1090-0233(03)00113-8) [DOI] [PubMed] [Google Scholar]
- 19.Corner L. A. L., Costello E., Lesellier S., O'Meara D., Gormley E. 2008. Vaccination of European badgers (Meles meles) with BCG by the subcutaneous and mucosal routes induces protective immunity against endobronchial challenge with Mycobacterium bovis. Tuberculosis (Edinb.) 88, 601–609 10.1016/j.tube.2008.03.002 (doi:10.1016/j.tube.2008.03.002) [DOI] [PubMed] [Google Scholar]
- 20.Lesellier S., et al. 2009. Immunological responses and protective immunity in BCG vaccinated badgers following endobronchial infection with Mycobacterium bovis. Vaccine 27, 402–409 10.1016/j.vaccine.2008.10.068 (doi:10.1016/j.vaccine.2008.10.068) [DOI] [PubMed] [Google Scholar]
- 21.Lesellier S., Palmer S., Dalley D. J., Dave D., Johnson L., Hewinson R. G., Chambers M. A. 2006. The safety and immunogenicity of Bacillus Calmette-Guerin (BCG) vaccine in European badgers (Meles meles). Vet. Immunol. Immunopathol. 112, 24–37 10.1016/j.vetimm.2006.03.009 (doi:10.1016/j.vetimm.2006.03.009) [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson D., Smith G. C., Delahay R. J., Cheeseman C. L. 2004. A model of bovine tuberculosis in the badger, Meles meles: an evaluation of different vaccination strategies. J. Appl. Ecol. 41, 492–501 10.1111/j.0021-8901.2004.00898.x (doi:10.1111/j.0021-8901.2004.00898.x) [DOI] [Google Scholar]
- 23.Dalley D., Dave D., Lesellier S., Palmer S., Crawshaw T., Hewinson R. G., Chambers M. 2008. Development and evaluation of a gamma-interferon assay for tuberculosis in badgers (Meles meles). Tuberculosis (Edinb.) 88, 235–243 10.1016/j.tube.2007.11.001 (doi:10.1016/j.tube.2007.11.001) [DOI] [PubMed] [Google Scholar]
- 24.Chambers M. A., Crawshaw T., Waterhouse S., Delahay R., Hewinson R. G., Lyashchenko K. P. 2008. Validation of the BrockTB Stat-Pak assay for detection of tuberculosis in Eurasian badgers (Meles meles) and influence of disease severity on diagnostic accuracy. J. Clin. Microbiol. 46, 1498–1500 10.1128/JCM.02117-07 (doi:10.1128/JCM.02117-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenwald R., et al. 2003. Improved serodetection of Mycobacterium bovis infection in badgers (Meles meles) using multiantigen test formats. Diagn. Microbiol. Infect. Dis. 46, 197–203 10.1016/S0732-8893(03)00046-4 (doi:10.1016/S0732-8893(03)00046-4) [DOI] [PubMed] [Google Scholar]
- 26.Kamerbeek J., et al. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35, 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corner L. A. L., Costello E., Lesellier S., O'Meara D., Sleeman D. P., Gormley E. 2007. Experimental tuberculosis in the European badger (Meles meles) after endobronchial inoculation of Mycobacterium bovis. I. Pathology and bacteriology. Res. Vet. Sci. 83, 53–62 10.1016/j.rvsc.2006.10.016 (doi:10.1016/j.rvsc.2006.10.016) [DOI] [PubMed] [Google Scholar]
- 28.Crawshaw T. R., Griffiths I. B., Clifton-Hadley R. S. 2008. Comparison of a standard and a detailed postmortem protocol for detecting Mycobacterium bovis in badgers. Vet. Rec. 163, 473–477 10.1136/vr.163.16.473 (doi:10.1136/vr.163.16.473) [DOI] [PubMed] [Google Scholar]
- 29.Palmer M. V., Thacker T. C., Waters W. R. 2007. Vaccination of white-tailed deer (Odocoileus virginianus) with Mycobacterium bovis bacillus Calmette Guerin. Vaccine 25, 6589–6597 10.1016/j.vaccine.2007.06.056 (doi:10.1016/j.vaccine.2007.06.056) [DOI] [PubMed] [Google Scholar]
- 30.Waters W. R., et al. 2009. Efficacy and immunogenicity of Mycobacterium bovis DeltaRD1 against aerosol M. bovis infection in neonatal calves. Vaccine 27, 1201–1209 10.1016/j.vaccine.2008.12.018 (doi:10.1016/j.vaccine.2008.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delahay R. J., Brown J., Mallinson P. J., Spyvee P. D., Handoll D., Rogers L. M., Cheeseman C. L. 2000. The use of marked bait in studies of the territorial organisation of the European badger (Meles meles). Mamm. Rev. 30, 73–87 10.1046/j.1365-2907.2000.00058.x (doi:10.1046/j.1365-2907.2000.00058.x) [DOI] [Google Scholar]
- 32.Kaden V., Lange E., Fischer U., Strebelow G. 2000. Oral immunisation of wild boar against classical swine fever: evaluation of the first field study in Germany. Vet. Microbiol. 73, 239–252 10.1016/S0378-1135(00)00148-6 (doi:10.1016/S0378-1135(00)00148-6) [DOI] [PubMed] [Google Scholar]
- 33.Blancou J., et al. 2009. Options for the control of disease: targeting the infectious or parasitic agent. In Management of disease in wild mammals (eds Delahay R. J., Smith G. C.), pp. 97–120 Tokyo, Japan: Springer [Google Scholar]
- 34.Tsao J. I., Wootton J. T., Bunikis J., Luna M. G., Fish D., Barbour A. G. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl Acad. Sci. USA 101, 18 159–18 164 10.1073/pnas.0405763102 (doi:10.1073/pnas.0405763102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilpatrick A. M., Dupuis A. P., Chang G. J., Kramer L. D. 2010. DNA vaccination of American robins (Turdus migratorius) against West Nile virus. Vector Borne Zoonotic Dis. 10, 377–380 10.1089/vbz.2009.0029 (doi:10.1089/vbz.2009.0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis D. S., Elzer P. H. 2002. Brucella vaccines in wildlife. Vet. Microbiol. 90, 533–544 10.1016/S0378-1135(02)00233-X (doi:10.1016/S0378-1135(02)00233-X) [DOI] [PubMed] [Google Scholar]
- 37.Delahay R. J., Smith G. C., Hutchings M. R. 2009. Management of disease in wild mammals. Tokyo, Japan: Springer [Google Scholar]
- 38.Chambers M. A. 2009. Review of the diagnosis and study of tuberculosis in non-bovine wildlife species using immunological methods. Transbound Emerg. Dis. 56, 215–227 10.1111/j.1865-1682.2009.01076.x (doi:10.1111/j.1865-1682.2009.01076.x) [DOI] [PubMed] [Google Scholar]
- 39.Chambers M. A., Pressling W. A., Cheeseman C. L., Clifton-Hadley R. S., Hewinson R. G. 2002. Value of existing serological tests for identifying badgers that shed Mycobacterium bovis. Vet. Microbiol. 86, 183–189 10.1016/S0378-1135(02)00012-3 (doi:10.1016/S0378-1135(02)00012-3) [DOI] [PubMed] [Google Scholar]
- 40.Demkow U., Filewska M., Michalowska-Mitczuk D., Kus J., Jagodzinski J., Zielonka T., Zwolska Z., Wasik M., Rowinska-Zakrzewska E. 2007. Heterogeneity of antibody response to mycobacterial antigens in different clinical manifestations of pulmonary tuberculosis. J. Physiol. Pharmacol. 58(Suppl. 5), 117–127 [PubMed] [Google Scholar]