Abstract
Carnivory has evolved independently several times in eutherian (including placental) and metatherian (including marsupial) mammals. We used geometric morphometrics to assess convergences associated with the evolution of carnivory across a broad suite of mammals, including the eutherian clades Carnivora and Creodonta and the metatherian clades Thylacoleonidae, Dasyuromorphia, Didelphidae and Borhyaenoidea. We further quantified cranial disparity across eutherians and metatherians to test the hypothesis that the marsupial mode of reproduction has constrained their morphological evolution. This study, to our knowledge the first to extensively sample pre-Pleistocene taxa, analysed 30 three-dimensional landmarks, focused mainly on the facial region, which were digitized on 130 specimens, including 36 fossil taxa. Data were analysed with principal components (PC) analysis, and three measures of disparity were compared between eutherians and metatherians. PC1 showed a shift from short to long faces and seemed to represent diet and ecology. PC2 was dominated by the unique features of sabre-toothed forms: dramatic expansion of the maxilla at the expense of the frontal bones. PC3, in combination with PC1, distinguished metatherians and eutherians. Metatherians, despite common comparisons with felids, were more similar to caniforms, which was unexpected for taxa such as the sabre-toothed marsupial Thylacosmilus. Contrary to previous studies, metatherian carnivores consistently exhibited disparity which exceeded that of the much more speciose eutherian carnivore radiations, refuting the hypothesis that developmental constraints have limited the morphological evolution of the marsupial cranium.
Keywords: marsupials, placentals, diversity, developmental constraints, cranium, morphometrics
1. Introduction
The repeated evolution of hypercarnivory in mammals provides an ideal system for the study of ecomorphological convergence across temporally, biogeographically and reproductively diverse clades. Hypercarnivory has evolved at least twice in eutherian mammals (the clade including placental mammals and their stem relatives), in the extinct order Creodonta (although this may be a polyphyletic clade) and the familiar and extant clade Carnivora [1]. Within Metatheria (the clade including marsupials and their stem relatives), hypercarnivory has evolved in at least three clades, the Australian diprotodontian clade Thylacoleonidae, the Australian Dasyuromorphia and the South American Borhyaenoidea, including thylacosmilids [2]. Qualitative comparisons within and across these clades are popular, with many of the metatherian carnivores given familiar names that refer to placental carnivorans, such as ‘marsupial lion’ (Thylacoleo carnifex) or Tasmanian wolf or tiger (Thylacinus cynocephalus).
In addition to ecomorphological analyses, comparisons among eutherians and metatherians can be used to test long-standing hypotheses of developmental constraints on marsupial evolution. Marsupials are born after a short period of gestation, in some cases as little as a few weeks after conception, and the neonate is equipped with only well-developed oral apparatus, to suckle, and forelimbs, to perform the crawl to the pouch [3–15]. This early ossification has been demonstrated to have constrained the morphological evolution of the forelimb across marsupials [10], but has not been explicitly tested in the cranium. Specifically, it can be hypothesized that marsupial cranial morphology is limited in its evolutionary ‘potential’, relative to that of placental mammals, by the well-established early development of cranial structures (e.g. dentary, premaxilla and maxilla bones) that are necessary to support suckling in the highly altricial marsupial neonate [3].
There have been a few studies directly comparing cranial morphology of extant placental and marsupial carnivores using quantitative approaches. Werdelin [16] conducted a morphometric analysis of six dasyuromorphians and 34 placental carnivorans, using 11 linear measurements of the cranium and mandible. He identified similarities between T. cynocephalus and the red fox, Vulpes vulpes, as well as between the Tasmanian devil (Sarcophilus harrisii) and Hyaenidae. He also noted that carnivorous dasyuromorphians display comparable variability to a single family of Carnivora. In a follow-up paper concentrating on the masticatory apparatus, Werdelin [17] suggested that the lower variability, particularly in the post-canine dentition and jaw morphology, observed in carnivorous marsupials relative to placental carnivorans, may be owing to the dental developmental pattern of marsupials. Specifically, in dasyuromorphians, all molars erupt in the position of the main vertical slicing teeth, the carnassials, and then are pushed forward by the next erupting molar, which usurps the previous molar's position as the main carnassial. For this reason, all of the molars in a dasyuromorphian are specialized carnassial teeth. By contrast, only the last (fourth) upper premolar and first lower molar of a placental carnivoran are specialized carnassials, with post-carnassial teeth either reduced in hypercarnivores, such as felids, or expanded for grinding, as in bears and other hypocarnivorous or herbivorous carnivorans. Thus, it has been argued that placental carnivorans can maintain greater dental and ecological flexibility than their marsupial counterparts.
Correspondingly, temporo-biogeographic analysis has suggested that it is the radiation of large mammalian omnivores, rather than hypercarnivorous forms, that has been most constrained in both Australian and South American faunas, since at least late Oligocene times [2]. However, there is certainly capacity for some molar specialization among dasyuromorphians and borhyaenoids, as shown by correlation between the length and alignment of vertical shearing blades and relative areas of talonid basins [18,19]. We also note that among Australian marsupials this dental constraint appears to have been circumvented by thylacoleonids [20] and probably propleopine kangaroos [18].
Among placental carnivores, creodonts modified more of their post-canine dentition to function as carnassials, again losing their flexibility [21]. Recent studies [22,23] have also assessed the importance of dental flexibility within Carnivora, demonstrating that hypercarnivory and the associated reduction of post-carnassial dentition have resulted in lower morphological and taxonomic diversity in some carnivoran clades, e.g. felids. As all of these studies have focused on dental and mandibular traits, the specific question of constraints on cranial morphology has not yet been addressed.
A recent study of cranial morphology [24] involved three-dimensional geometric morphometric analysis of 43 species of carnivorans (30 species) and marsupials (13 species), including the Thylacoleonidae, Dasyuridae and Peramelidae. The results of that study demonstrated that there were clade-specific constraints, but that both clades showed similar patterns of morphological variation associated with diet. Specifically, the authors of that study found that skull shape, feeding behaviour and bite force were significantly correlated, although more so in the sampled marsupials than in the carnivorans. However, that study neither included pre-Pleistocene carnivorans nor any New World marsupials except for the extant Didelphis virginiana. As noted above, and detailed below, carnivory has evolved in many other clades of mammals, including placental Creodonta and metatherian Borhyaenoidea. Here we expand on these previous, mainly neontological studies with geometric morphometric data for 36 fossil taxa, providing a broad sample of extinct members of the well-studied Carnivora and Australidelphia, as well as Creodonta, Borhyaeonoidea and Didelphoidea, to test if the patterns in cranial shape observed by Wroe & Milne [24] apply across carnivorous mammals. We specifically test for ecological convergences among extinct forms without ecological counterparts in modern ecosystems, as well as using the broader sample of fossil and extant taxa to rigorously test the hypothesis that metatherian carnivores are constrained in cranial morphology, relative to eutherian carnivores, by the early development of the facial region necessitated by their mode of reproduction.
(a). Carnivorous metatherian clades
Dasyuromorphia, as the only extant marsupial clade to include hypercarnivorous species, has been best studied in diet and ecomorphology. Recent representatives are divided into three families [25,26]: Dasyuridae (15 genera), Thylacinidae (one genus), and Myrmecobiidae (one genus). The majority of dasyurids are small, marsupial ‘mice’ that are primarily insectivorous or prey on small vertebrates. There are six species of Dasyurus or ‘native cat’ whose diets comprise variable proportions of small- to medium-sized vertebrate and invertebrate prey, while a single extant genus, Sarcophilus, is known to prey or scavenge on large mammals. The recently extinct Tasmanian tiger, T. cynocephalus, was a larger predator, weighing around 20–30 kg, and the last of the thylacinid radiation ranging back to the Oligocene and comprising at least eight genera [27]. The last known thylacine died in captivity in 1936. The monotypic extant numbat, Myrmecobius faciatus, is a highly specialized insectivore with a greatly reduced dentition. Two species of peremelemorphians, a closely related clade of small to medium-sized insectivorous and omnivorous Australian marsupials, have also been included for comparison.
Another Australian clade, Diprotodontia, includes herbivorous kangaroos, koalas and wombats, but has also given rise to thylacoleonids (including the marsupial lion) and propleopines (giant rat-kangaroos). The three known genera of thylacoleonids, Priscileo, Wakaleo and Thylacoleo, ranged from the late Oligocene to Pleistocene and are probable members of the vombatiform clade [28]. The specializations associated with carnivory in thylacoleonids are remarkable. Instead of the large canines observed in most carnivorous mammals, thylacoleonids modified the enlarged incisors that they share with all diprotodontians into pointed, canine-like teeth. In more derived species, the cheek dentition consisted almost entirely of a single, massive slicing third premolar [20]. Relative to body size, these are the largest carnassials observed in any mammal. Along with this morphology, the most recent species, Thylacoleo carnifex, may have had the greatest bite force, adjusted for size, known among living and extinct mammals and may represent a highly specialized predator of large prey [29–32]. The propleopine kangaroo radiation included three genera ranging in body mass from around 6–47 kg [2,33,34]. Although less marked than in thylacoleonids, the P3 of this subfamily is also a high-crowned blade and varying degrees of carnivory have been inferred from differing relationships between vertical and horizontal shear [18].
The last two clades of metatherian carnivores are the extinct Borhyaeonoidea, which probably lies outside of crown Marsupialia, and the extant Didelphoidea. Borhyaenoids comprised a diverse radiation ranging from the early Palaeocene to the late Pliocene of South America [35,36]. During this lengthy period of isolation, borhyaenoids evolved forms that resemble mustelids, bears, and even sabre-toothed felids in their morphology [37,38]. The largest taxon, Proborhyaena gigantea, had a body mass of up to 600 kg [39], and perhaps the most famous, Thylacosmilus atrox, was arguably the most specialized sabre-toothed mammal to have existed [40]. This animal may have had the unique feature of ever-growing canines with roots that extended above the orbit [35]. Extant didelphoid marsupials, the best known being the species of opossum (Didelphis), are largely insectivorous or omnivorous, but at least one genus, Sparassocynus, is thought to have included a number of more carnivorous species [41].
(b). Carnivorous eutherian clades
There are two major clades of carnivorous eutherians generally recognized: the extant Carnivora and the extinct Creodonta. Creodonts ranged from late Palaeocene to the late Miocene and were the dominant mammalian predators for much of the Cenozoic. Whether creodonts are monophyletic is still unresolved, but they are generally separated into two families, the broad-skulled Oxyaenidae and the more elongate-skulled Hyaenodotidae. Hyaenodontids are particularly noteworthy for including some of the largest known terrestrial mammalian carnivores, such as Megistotherium osteothlastes, for which some body size estimates exceed 800 kg [42]. Because both creodonts and carnivorans bear carnassial teeth, it has been suggested that they share a common ancestor. However, the molars of creodonts form the carnassials, while the carnassials of carnivorans are a premolar–molar combination. It has long been suspected that competition with carnivorans led to the eventual extinction of creodonts, which has been supported by some recent morphometric analyses [43].
The best-known mammalian carnivores are of course members of the extant clade Carnivora. Carnivora is one of the most speciose clades of mammals, with over 260 living species, and is generally divided into two major branches: Feliformia (including cats, linsangs, civets, mongooses, fossas, falanoucs and hyaenas) and Caniformia (encompassing dogs, bears, seals, sea lions, walruses, the red panda, raccoons, skunks, weasels, badgers, otters and wolverines) [1,44,45]. Carnivorans also have an excellent fossil record, with at least 355 extinct genera recognized and stem taxa dating back to the early Palaeocene [46–48]. In addition to representatives of the extant clades, several of the best-preserved fossils represent specialized carnivoran clades that are entirely extinct, including Nimravidae, the ‘false’ sabre-toothed cats, which are probably not closely related to true felids, and Amphicyonidae, the bear-dogs, which are caniforms of uncertain affinities.
Despite their name, carnivorans are an ecologically diverse group, with specialized foliovores, including the giant panda, as well as insectivores, like the aardwolf. While the large-bodied cats, dogs, bears and hyaenas are probably best known, the greater taxonomic and ecological diversity of carnivorans rests in small- to medium-sized members of the caniform Musteloidea (weasels, racoons, red pandas, skunks, badgers and otters), as well as the feliform Viverridae (civets), Herpestidae (mongooses) and Eupleridae (Malagasy carnivores). Carnivora also include a diverse clade of aquatic mammals, the pinnipeds. The number of ecological convergences within Carnivora has provided a rich source for studies of morphological convergence [21,49]. Here, because we are interested in convergences associated with a carnivorous diet across mammals, we concentrate on the terrestrial carnivorous forms within Carnivora, but we also include some closely related more omnivorous or insectivorous forms for comparison.
Metatherians and eutherians diverged by 125 Ma, when their first fossil representatives appear in the record [50,51]. Sparassodonta probably diverged from other metatherians in the Late Cretaceous to earliest Palaeocene, with Didelphoidea diverging from other marsupials within a similar time frame [52]. Diprotodontia, including Thylacoleonidae, Peremelemorphia, and Dasyuromorphia are estimated to have diverged by the middle Eocene [53]. Creodonta and Carnivora must have diverged by the earliest Palaeocene, and, while stem carnivorans appear in the earliest Palaeocene [48], most of the living families, as well as Nimravidae and Amphicyonidae, first appear in the late Eocene, with crown divergence estimates around 42 Ma [54].
2. Material and methods
(a). Specimens
Specimens representing 62 species (table 1) in 10 extinct and extant carnivoran clades (Felidae, Viverridae, Herpestidae, Hyaenidae, Nimravidae, Amphicyonidae, Canidae, Ursidae, Procyonidae and Mustelidae), two hyaenodontid creodonts and 16 species in five metatherian clades (Thylacoleonidae, Dasyuromorphia (including Dasyuridae, Thylacinidae and Myrmecobiidae), Peramelidae, Didelphidae, Borhyaenoidea) were studied. A total of 130 specimens (electronic supplementary material, table S1) were digitized, using an Immersion Microscribe three-dimensional digitizer (Immersion Corp., San Jose, CA, USA). For species in which multiple specimens were sampled, a mean shape was calculated and used in subsequent analyses. Because gender information is limited for many rare marsupial taxa, and impossible to obtain for most fossil taxa, we did not separate male and female specimens in analyses, and only adult specimens were used.
Table 1.
List of taxa used in analyses.
| Eutheria |
|---|
| Creodonta |
| Hyaenodon leptocephalusa |
| Pterodon dasyuroidesa |
| Carnivora |
| Felidae |
| Felis caracal |
| Acinonyx jubatus |
| Panthera tigris |
| Panthera pardus |
| Panthera onca |
| Panthera leo |
| Neofelis nebulosa |
| Smilodon fatalisa |
| Smilodon populatora |
| Homotherium sp.a |
| Panthera atroxa |
| Felis issiodorensisa |
| Dinofelis piveteauia |
| Dinofelis barlowia |
| Viverridae |
| Paradoxurus hermaphroditus |
| Arctictis binturong |
| Viverricula indica |
| Hyaenidae |
| Hyaena brunnea |
| Crocuta crocuta |
| Proteles cristatus |
| Pachycrocuta bellaxa |
| Hyaena makapania |
| Herpestidae |
| Mungos mungo |
| Suricata suricatta |
| Galerella sp. |
| Herpestes ichneumon |
| Mustelidae |
| Gulo gulo |
| Aonyx capensis |
| Mellivora capensis |
| Meles meles |
| Procyonidae |
| Procyon lotor |
| Nasua sp. |
| Amphinasua brevirostrisa |
| Ursidae |
| Ursus arctos |
| Ursus americanus |
| Ursus maritimus |
| Ursus thibetanus |
| Ursus spelaeusa |
| Arctotherium sp.a |
| Arctodus simusa |
| Hemicyon ursinusa |
| Canidae |
| Otocyon megalotis |
| Lycaon pictus |
| Canis mesomelas |
| Canis lupus domesticus |
| Canis adustus |
| Canis latrans |
| Vulpes chama |
| Canis dirusa |
| Dusicyon avusa |
| Theriodictis platensisa |
| Protocyon scagliaruma |
| Mesocyon coryphaeusa |
| Mesocyon josephia |
| Microtomarctus confertaa |
| Enhydrocyon sp.a |
| Amphicyonidae |
| Daphoenus vetusa |
| Nimravidae |
| Nimravus debilisa |
| Dinictis felinaa |
| Hoplophoneus sp.a |
| Metatheria |
| Borhyaenoidea |
| Borhyaenidae |
| Arctodictis sp.a |
| Thylacosmilidae |
| Thylacosmilus atroxa |
| Didelphimorphia |
| Didelphidae |
| Didelphis virginiana |
| Sparassocynus sp.a |
| Dasyuromorphia |
| Thylacinidae |
| Thylacinus cynocephalusa |
| Dasyuridae |
| Sarcophilus harrisii |
| Dasyurus viverrinus |
| Dasyurus maculatus |
| Dasyurus geoffroyii |
| Barinya wangalaa |
| Nimbacinus dicksonia |
| Myrmecobiidae |
| Myrmecobius fasciatus |
| Peramelemorphia |
| Peramelidae |
| Isoodon obesulus |
| Macrotis lagotis |
| Diprotodontia |
| Thylacoleonidae |
| Thylacoleo carnifexa |
| Wakaleo vanderleureia |
aIndicates extinct taxa.
(b). Landmarks
Thirty three-dimensional landmarks were digitized, and every attempt was made to identify landmarks with clear homology, such as sutures and alveoli, across varied morphologies. Because the focus of this study was to assess convergences related to diet and feeding ecology, landmarks are focused on the facial, dental and zygomatic regions, as well as muscle attachment sites, such as the sagittal crest (figure 1; electronic supplementary material, table S2).
Figure 1.
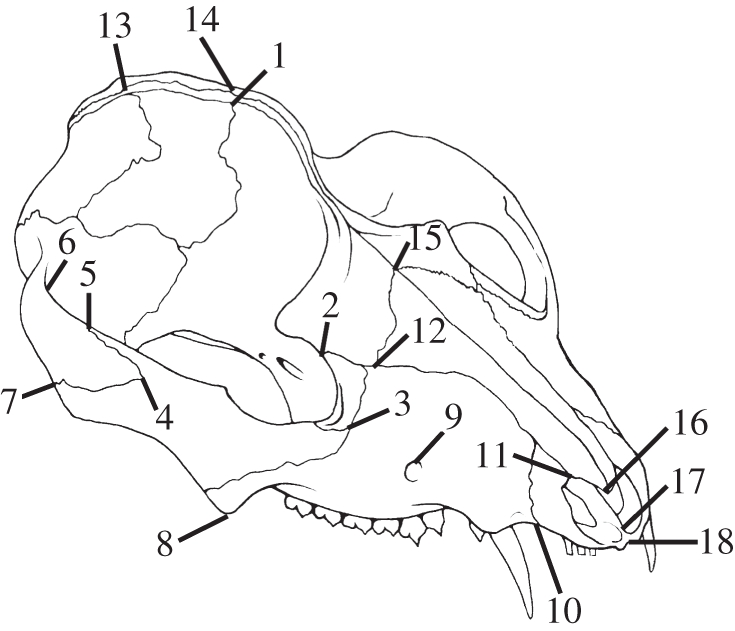
Landmarks captured on each specimen, shown here on Didelphis virginiana (Adapted from [24]). Landmarks are detailed in the electronic supplementary material, table S1.
(c). Data analysis
Landmarks were subjected to Generalized Procrustes analysis, to remove the effects of rotation, translation and size [55]. Principal components (PC) analysis was then used to identify the major components of variation across taxa [56] and to assess ecomorphological convergences, based on position in morphospace, across the clades of interest. Analyses were conducted in Morphologika 2.5 [57]. Although unequal sampling of clades has the potential to skew analyses, we did not perform weighting or correction, primarily because the phylogenetic positions of many of the fossil taxa are currently ambiguous, complicating any attempt at phylogenetic correction. Because all of the clades overlap considerably in morphospace, we consider it unlikely that the larger sample of Carnivora is substantially skewing the results.
To test if metatherian carnivores are constrained in cranial morphology relative to their more speciose eutherian counterparts, we compared two measures of morphological disparity, variance and mean pairwise dissimilarity (MPD) [58,59], between both clades. Sample variance of the two groups (metatherians and eutherians) was calculated from Procrustes distances of specimens relative to the mean shape of each group and compared using Integrated Morphometrics Package Simple3D [60]. A delta variance permutation test, which randomly swaps residuals (900 repetitions, in this case) from the means of each group to assess whether the observed difference in variance between two groups differs from a random expectation, was used to assess significance. MPD, measured among all pairs of specimens, is more robust to sample size [59] and was calculated in two ways: (i) from partial Procrustes distances, which takes into account all of the variation in the dataset; and (ii) from Euclidean distances between species across the first five PCs, those representing approximately 5 per cent or more of the total variance in the dataset. Significances of both measures were assessed with a permutation test (1000 repetitions) used to assess significance, in Mathematica 7.0 (Wolfram Inc., Urbana, IL, USA).
3. Results
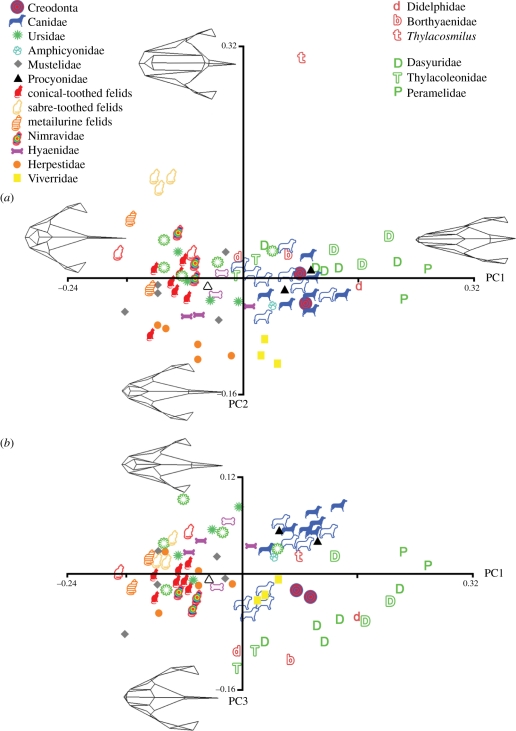
The first PC explained 35.5 per cent of the variance, and represented a shift from a short, wide and high cat-like skull on the negative end to a long, narrow and flatter skull on the postitive end (figure 2a). The negative end was unsurprisingly dominated by felids, both saber-toothed and conical-toothed, as well as some extant mustelids, such as Gulo gulo, the wolverine, and extinct bears, such as Arctodus simus, the giant short-faced bear. The positive end of PC1 was occupied exclusively by metatherians, primarily insectivorous peramelids and dasyurids. Metatherians and eutherians overlapped extensively in the central region of PC1. Among eutherians, most extant and extinct canids, creodonts, amphicyonids, procyonids and viverrids fell on the positive side of PC1, in the same region as most dasyurids, thylacoleonids and borhyaenids. The slightly negative region of PC1 was occupied mainly by extant and extinct hyaenids, herpestids, extant and extinct bears, nimravids, and some procyonids and mustelids, as well as extinct borhyaenids and thylacoleonids.
Figure 2.
PC analyses. (a) PCs1 and 2. Metatherians are represented by letters, and eutherians by symbols, as detailed. Open symbols represent extinct taxa, and closed symbols denote extant taxa. Wireframe models of cranial shapes at the end of each axis are shown in dorsal view. PC1 represents a shift from short-faced, generally hypercarnivorous forms on the negative end to long-faced insectivorous forms on the positive end. Metatherians are concentrated on the positive end of PC1, and felids define the negative end. The positive extreme of PC2 is defined by the isolated sabre-toothed metatherian, Thylacosmilus atrox, with sabre-toothed felids (open yellow cats) falling in an intermediate region between Thylacosmilus and all other sampled taxa. Interestingly, the third group of sabre-toothed carnivores, nimravids (multicoloured cats) fall with the rest of carnivores, rather than with other sabre-toothed forms on PC2. Overlap is strongest between caniforms and metatherians. (b) PCs1 and 3, with wireframe models shown for the extremes of PC3. There is clearer separation between metatherians and eutherians in this graph. Creodonts (multicoloured circles) fall between most metatherians and eutherians, though caniforms again overlap with some metatherians.
PC2 (13% of the total variance) was dominated by the extreme morphology of the sabre-toothed metatherian, Thylacosmilus, which defined the positive end of this axis (figure 2a). Sabre-toothed felids, not including metailurines, were also strongly positive on PC2, intermediate between Thylacosmilus and the other taxa. Metailurines were removed from their sabre-toothed relatives because their canines, while flattened, are not greatly elongated, probably reflecting ecological differences from the ‘fully’ sabre-toothed felids. Interestingly, Dinofelis piveteaui fell between the fully sabre-toothed felids and the conical-toothed cats, while the other metailurine, D. barlowi, sat among the conical-toothed felids, consistent with previous analyses of variation across Dinofelis species [61]. The third group of sabre-toothed forms, the nimravids, fell with most of the other taxa near the centre of PC2. The negative end of PC2 was occupied by viverrids and herpestids. The variance described by PC2 relates to relative cranial height, particularly in the anterior region, which is greatly enlarged in sabre-toothed forms. In particular, PC2 clearly showed the enlargement of the maxilla and nasal bones at the expense of the frontal bones in sabre-toothed forms.
PC3 represented 8.2 per cent of the variance and showed a shift from a broader, more robust skull with a shorter face on the negative end to a pointier, narrower skull on the positive end (figure 2b). Metatherians generally fell on the negative end of PC3, while the positive end was dominated by caniforms, in contrast to the similarity of their positions on PC2. There was much overlap among species on PC3, but in combination with PC1 (figure 2b) there was substantial separation of carnivoran and metatherian species, with the creodonts occupying an intermediate position.
PC4 (5.9% of the variance) mainly described shifts in the relative sizes of the premaxilla and maxilla and the robustness of the zygomatic arch, without any strong phylogenetic signal apparent in the distribution of taxa. PC5 (4.7% of the variance) reflected the flexion of the cranium, and also showed little apparent phylogenetic signal, with metatherians falling near zero among a wide scatter of eutherian carnivores.
Because of the highly anomalous morphology of Thylacosmilus, we also conducted analyses without this taxon. Results were very similar, with the first five PCs comprising 37.4, 10.2, 8.7, 6.3 and 5.0 per cent of the total variance, respectively. The distribution of taxa along these axes was relatively unchanged, and the main difference was a slightly greater separation of the sabre-toothed felids from the other cat-like forms (true felids and nimravids) on PC2.
Comparisons of the three measures of disparity between these samples of metatherian and eutherian carnivores showed that, when Thylacosmilus was included, variance was significantly greater in metatherians (0.099) than in eutherians (0.022, p ≪ 0.01). However, when Thylacosmilus was excluded, the variances of the metatherian and eutherian samples were not significantly different (0.027 and 0.022, respectively; p = 0.12). Results for MPD, calculated across the first five PCs were similar, with metatherians showing greater disparity when Thylacosmilus was included (p < 0.001). However, when Thylacosmilus was removed, MPD was near equal for the two groups (p = 0.26). When MPD was calculated from partial Procrustes distances, disparity was significantly greater in metatherians than in eutherians, whether T. atrox was included (metatherian MPD = 0.242, placental MPD = 0.203, p < 0.001) or not (metatherian MPD = 0.221, p < 0.001).
4. Discussion
A previous study demonstrated consistent shifts in cranial shape associated with diet in extant marsupials and placentals [24]. While diet cannot be explicitly tested with all the taxa studied here, because of the inevitable lack of reliable dietary reconstructions for many of the extinct taxa, some general patterns were clear. PC1 showed a shift from hypercarnivorous forms to more omnivorous and insectivorous forms. This pattern was reflected both across the full range of taxa, as well as within each clade. For example, several of the extinct bears, such as Arctodus, occupied the short-faced, generally hypercarnivorous region of morphospace, consistent with previous reconstructions of some of these taxa as carnivores ([62], but see [63]). Interestingly, while most ursids overlapped with felids on PC1, the early ‘dogbear’, ursid Hemicyon, fell near canids on PC1.
Both canids and dasyurids occupied a large range on PC1, probably reflecting the broader ecological diversity in these clades than in, for example, felids. Extinct dasyurids all fell closer to the insectivore side of morphospace, while most of the extant forms overlapped strongly with extant canids. By contrast, extinct canids, which occupied a noticeably larger range of morphospace than the extant forms, showed more forms, such as Enhydrocyon and Theriodictis falling near the hypercarnivorous end of morphospace. This result is particularly interesting, as analyses based on dental characters and body size of extant taxa have suggested that living canids have low disparity relative to other carnivoran clades [64]. Among metatherians, thylacoleonids, borhyaenids, the extinct didelphoid Sparassocynus, and the recently extinct marsupial wolf, T. cynocephalus, fell closer to the hypercarnivorous end of morphospace than the other taxa (other dasyuromorphians, didelphoids, and peramelids), supporting reconstructions of these taxa as the more carnivorous within the clade. All of the main clades of metatherian carnivores, dasyuromorphians, boryhaenoids and thylacoleonids, as well as Sparassocynus, fell into the same region of morphospace (PCs1 and 2) as most caniforms, as did Thylacosmilus on PC1. Thus, ‘dog space’ has been converged upon independently by at least four lineages of metatherian carnivores. By contrast, it appears that ‘cat space’ has been left relatively unexplored by extant or extinct metatherian carnivores, despite the frequent application of felid common names to metatherian carnivores, such as the marsupial lion and native cat. One striking example of morphological convergence was the strong overlap in cranial shape of Thylacoleo and Enhydrocyon, a ‘cat-like’ hypercarnivorous hesperocyonine canid [49], despite their markedly different dentitions. However, neither the cat-like canid nor the marsupial lion overlapped with any felids in morphospace, and it appears that, in the drastic shortening of the rostrum, as well as the related reduction of the post-canine dentition, cats, as well as the odd mustelid, are unique among carnivorous mammals.
While sabre-toothed felids clustered separately from the rest of the placental taxa, members of the other placental sabre-toothed clade, the Nimravidae, overlapped with most of the other taxa in the centre of PC2. It is interesting that the cranial morphology of sabre-toothed nimravids and felids is not as strongly convergent as their dentition would suggest. This pattern is consistent with studies of cranial modularity [65], which have demonstrated that nimravids show a typical placental mammal pattern, while sabre-toothed felids show a reduced integration of the anterior facial region that is unusual for mammals.
The distribution of taxa on PCs1 and 3 (figure 2b) was very similar to that of PCs1 and 2 in the study of Wroe & Milne (fig. 2 in [24]). Thus, while PC1 strongly reflected diet, PC2 here represented variation in fossil taxa that is not represented in extant forms. That PC2 was driven primarily by the inclusion of sabre-toothed felids, nimravids and metatherians highlights the importance of incorporating fossil taxa when testing hypotheses of morphological evolution and diversity. The intriguing placement of creodonts intermediately between metatherians and carnivorans in figure 2b further suggests that the focus on Carnivora has led to underestimation of eutherian carnivore diversity.
In functional terms, a relatively short cranium, which contributed to the variation on PC1, confers a reduced distance between the temporomandibular joint and bite points in the dentition, and hence a shorter outlever and higher bite forces. Similarly, a broader cranium, one of the aspects of shape captured by PC3, typically correlates with larger jaw-adducting musculatures, an ability to generate higher bite forces, and a capacity to resist high stresses generated in the killing of large prey or biting into hard material such as bone [29]. Our within-group results are broadly consistent with these interpretations for living placental and metatherian carnivores. Thus, among marsupials, the osteophageous Tasmanian devil (S. harrisii) recorded the lowest values for PC1 and PC3, and insectivorous peramelids recorded the highest. Likewise, among extant placentals, low values for PC1 and PC3 were found among hyaenids and conical-toothed felids, and high values were generated for canids that take relatively small prey.
Phylogeny is clearly a major factor in cranial morphology, and some phylogenetic groupings were evident in this dataset. Nearly all of the family-level clades grouped together strongly in morphospace, from felids and canids to creodonts, thylacoleonids and nimravids. This phylogenetic clustering is particularly interesting for some of the most ecologically diverse clades, such as ursids, for which herbivorous/omnivorous to hypercarnivorous forms were sampled. The relatively close clustering of the social insectivorous hyaenid Proteles with the carnivorous and bone-cracking hyaenas was also surprising, although it did fall farther towards the positive, insectivorous end of PC1 than its relatives.
While the eutherian carnivoran sample was nearly four times larger than the metatherian sample, the selection of specimens represents nearly the full morphological and ecological range of carnivorous forms in both clades. A lack of complete cranial specimens for many borhyaenid and all propleopine taxa prohibited more comprehensive coverage of those metatherian clades. Among eutherians, creodonts were represented solely by hyaenodontids, and inclusion of oxyaenids would improve this analysis. The mesonychid ‘condylarths’ represent another group of carnivorous eutherians that could be considered in such an analysis. However, the sampling in this study demonstrates several important conclusions. First, metatherian carnivores converge strongly with caniform carnivorans, as do hyaenodontid creodonts. Second, and consistent with previous analyses of mainly extant taxa [24], within clades, the shape changes associated with hypercarnivorous to insectivorous diets remain consistent when fossil taxa are included, providing a valuable tool for reconstructions of diet in extinct metatherians and eutherians. Finally, as noted above, previous hypotheses based on cranial and postcranial developmental maturity of the marsupial neonate have suggested that marsupials may be constrained relative to placentals by their mode of reproduction [3]. While these hypotheses on marsupial evolutionary ‘potential’ are supported by postcranial data [10], developmental constraints on morphological evolution are not evident in the cranial data presented here. Cranial disparity of metatherian carnivores is significantly greater than that of eutherian carnivores, although admittedly this is driven by a single taxon, Thylacosmilus. However, even when Thylacosmilus is removed from the analysis, disparity of metatherian and eutherian carnivores was either near equal and not significantly different or, when disparity was calculated from pairwise partial Procrustes distances, still significantly greater in metatherians. This result suggests that the marsupial mode of development has not constrained the morphological evolution of the cranium in marsupial carnivores. Specifically, the early ossification of the facial bones and their usage during suckling in the highly altricial marsupial neonate does not appear to have limited the ability of the cranium to evolve morphologies highly specialized for carnivory, including some of most extreme forms encountered in the mammalian record.
Acknowledgements
We thank collections managers and curators at the Australian Museum, Western Australian Museum, South Australian Museum, Capetown Museum, Witwatersrand University, Transvaal Museum and Museo de La Plata for access to specimens. V. Weisbecker, J. Finarelli, P. D. Polly and L. Werdelin provided helpful discussions and reviews of this manuscript. This work was funded by Australian Research Council (DP0666374 and DP0987985) and University of New South Wales Internal Strategic Initiatives grant to S.W.
References
- 1.Goswami A. 2010. Introduction to Carnivora. In Carnivoran evolution: new views on phylogeny, form, and function (eds Goswami A., Friscia A.), pp. 1–24 Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Wroe S., Argot C., Dickman C. 2004. On the rarity of big fierce carnivores and primacy of isolation and area: tracking large mammalian carnivore diversity on two isolated continents. Proc. R. Soc. Lond. B 271, 1203–1211 10.1098/rspb.2004.2694 (doi:10.1098/rspb.2004.2694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lillegraven J. A. 1975. Biological considerations of the marsupial-placental dichotomy. Evolution 29, 707–722 10.2307/2407079 (doi:10.2307/2407079) [DOI] [PubMed] [Google Scholar]
- 4.Smith K. K. 1996. Integration of craniofacial structures during development in mammals. Am. Zool. 36, 70–79 [Google Scholar]
- 5.Smith K. K. 1997. Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution 51, 1663–1678 10.2307/2411218 (doi:10.2307/2411218) [DOI] [PubMed] [Google Scholar]
- 6.Smith K. K. 2001. Early development of the neural plate, neural crest and facial region of marsupials. J. Anat. 199, 121–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith K. K. 2001. Heterochrony revisited: the evolution of developmental sequences. Biol. J. Linn. Soc. 73, 169–186 10.1111/j.1095-8312.2001.tb01355.x (doi:10.1111/j.1095-8312.2001.tb01355.x) [DOI] [Google Scholar]
- 8.Sánchez-Villagra M. R. 2002. Comparative patterns of postcranial ontogeny in therian mammals: an analysis of relative timing of ossification events. J. Exp. Zool. Mol. Dev. Evol. 294B, 264–273 10.1002/jez.10147 (doi:10.1002/jez.10147) [DOI] [PubMed] [Google Scholar]
- 9.Smith K. K. 2002. Sequence heterochrony and the evolution of development. J. Morphol. 252, 82–97 10.1002/jmor.10014 (doi:10.1002/jmor.10014) [DOI] [PubMed] [Google Scholar]
- 10.Sears K. E. 2004. The role of constraints in the morphological evolution of marsupial shoulder girdles: evidence from comparative anatomy, paleontology, and embryology. Evolution 58, 2353–2370 10.1111/j.0014-3820.2004.tb01609.x (doi:10.1111/j.0014-3820.2004.tb01609.x) [DOI] [PubMed] [Google Scholar]
- 11.Smith K. K. 2006. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Dev. Dyn. 235, 1181–1193 10.1002/dvdy.20676 (doi:10.1002/dvdy.20676) [DOI] [PubMed] [Google Scholar]
- 12.Goswami A. 2007. Cranial modularity and sequence heterochrony in mammals. Evol. Dev. 9, 290–298 10.1111/j.1525-142X.2007.00161.x (doi:10.1111/j.1525-142X.2007.00161.x) [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Villagra M. R., Goswami A., Weisbecker V., Mock O., Kuratani S. 2008. Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evol. Dev. 10, 519–530 10.1111/j.1525-142X.2008.00267.x (doi:10.1111/j.1525-142X.2008.00267.x) [DOI] [PubMed] [Google Scholar]
- 14.Weisbecker V., Goswami A., Wroe S., Sánchez-Villagra M. R. 2008. Ossification heterochrony in the therian postcranial skeleton and the marsupial-placental dichotomy. Evolution 59, 2691–2704 [DOI] [PubMed] [Google Scholar]
- 15.Goswami A., Weisbecker V., Sánchez-Villagra M. R. 2009. Developmental modularity and the marsupial-placental dichotomy. J. Exp. Zool. 312B, 186–195 10.1002/jez.b.21283 (doi:10.1002/jez.b.21283) [DOI] [PubMed] [Google Scholar]
- 16.Werdelin L. 1986. Comparison of skull shape in marsupial and placental carnivores. Aust. J. Zool. 34, 109–117 10.1071/ZO9860109 (doi:10.1071/ZO9860109) [DOI] [Google Scholar]
- 17.Werdelin L. 1987. Jaw geometry and molar morphology in marsupial carnivores: analysis of a constraint and its macroevolutionary consequences. Palaeobiology 13, 342–350 [Google Scholar]
- 18.Wroe S., Brammall J., Cooke B. 1998. The skull of Ekaltadeta ima (Marsupialia, Hypsiprymnodontidae) an analysis of some marsupial cranial features and a reinvestigation of propleopine phylogeny, with notes on the inference of carnivory in mammals. J. Paleontol. 72, 735–751 [Google Scholar]
- 19.Jones M. E. 2003. Convergence in ecomorphology and guild structure among marsupial and placental carnivores. In Predators with pouches: the biology of carnivorous marsupials (eds Jones M. E., Dickman C., Archer M.), pp. 285–296 Collingwood, Australia: CSIRO Publishing [Google Scholar]
- 20.Werdelin L. 1988. Circumventing a constraint: the case of Thylacoleo (Marsupialia, Thylacoleonidae). Aust. J. Zool. 36, 565–571 10.1071/ZO9880565 (doi:10.1071/ZO9880565) [DOI] [Google Scholar]
- 21.Van Valkenburgh B. 1999. Major patterns in the history of carnivorous mammals. Annu. Rev. Earth Planet. Sci. 27, 463–493 10.1146/annurev.earth.27.1.463 (doi:10.1146/annurev.earth.27.1.463) [DOI] [Google Scholar]
- 22.Holliday J. A., Steppan S. J. 2004. Evolution of hypercarnivory: the effect of specialization on morphological and taxonomic diversity. Palaeobiology 30, 108–128 (doi:10.1666/0094-8373(2004)030<0108:EOHTEO>2.0.CO;2) [DOI] [Google Scholar]
- 23.Holliday J. A. 2010. Evolution in Carnivora: identifying a morphological bias. In Carnivoran evolution: new views on phylogeny, form, and function (eds Goswami A., Friscia A.), pp. 189–224 Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Wroe S., Milne N. 2007. Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution 61, 1251–1260 10.1111/j.1558-5646.2007.00101.x (doi:10.1111/j.1558-5646.2007.00101.x) [DOI] [PubMed] [Google Scholar]
- 25.Krajewski C., Wroe S., Westerman M. 2000. Molecular evidence for the pattern and timing of cladogenesis in dasyurid marsupials. Zool. J. Linn. Soc. 130, 375–404 10.1111/j.1096-3642.2000.tb01635.x (doi:10.1111/j.1096-3642.2000.tb01635.x) [DOI] [Google Scholar]
- 26.Wroe S., Ebach M., Ahyong S., de Muizon C., Muirhead J. 2000. Cladistic analysis of dasyuromorphian (Marsupialia) phylogeny using cranial and dental characters. J. Mammal. 81, 1008–1024 (doi:10.1644/1545-1542(2000)081<1008:CAODMP>2.0.CO;2) [DOI] [Google Scholar]
- 27.Muirhead J., Wroe S. 1998. A new genus and species, Badjcinus turnbulli (Thylacinidae: Marsupialia), from the Late Oligocene of Riversleigh, northern Australia, and an investigation of thylacinid phylogeny. J. Vertebr. Paleontol. 18, 612–626 10.1080/02724634.1998.10011088 (doi:10.1080/02724634.1998.10011088) [DOI] [Google Scholar]
- 28.Wroe S., Myers T., Wells R. T., Gillespie A. 1999. Estimating the weight of the Pleistocene marsupial lion (Thylacoleo carnifex: Thylacoleonidae): implications for the ecomorphology of a marsupial super-predator and hypotheses of impoverishment of Australian marsupial carnivore faunas. Aust. J. Zool. 47, 489–498 10.1071/ZO99006 (doi:10.1071/ZO99006) [DOI] [Google Scholar]
- 29.Wroe S., McHenry C., Thomason J. 2005. Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proc. R. Soc. B 272, 619–625 10.1098/rspb.2004.2986 (doi:10.1098/rspb.2004.2986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wroe S. 2008. Cranial mechanics compared in extinct marsupial and extant African lions using a finite-element approach. J. Zool. 274, 332–339 10.1111/j.1469-7998.2007.00389.x (doi:10.1111/j.1469-7998.2007.00389.x) [DOI] [Google Scholar]
- 31.Wroe S., Lowry M. B., Anton M. 2008. How to build a mammalian super-predator. Zoology 111, 196–203 10.1016/j.zool.2007.07.008 (doi:10.1016/j.zool.2007.07.008) [DOI] [PubMed] [Google Scholar]
- 32.Wroe S. 2010. Cranial mechanics of mammalian carnivores: recent advances using a finite element approach. In Carnivoran evolution: new views on phylogeny, form, and function (eds Goswami A., Friscia A.), pp. 466–485 Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Archer M., Flannery T. F. 1985. Revision of the extinct gigantic rat kangaroos (Potoroidae: Marsupialia), with a description of a new Miocene genus and species and a new Pliestocene species of Propleopus. J. Paleontol. 59, 1331–1349 [Google Scholar]
- 34.Wroe S., Myers T., Seebacher F., Kear B., Gillespie A., Crowther M., Salisbury S. 2003. An alternative method for predicting body-mass: the case of the marsupial lion. Paleobiology 29, 404–412 (doi:10.1666/0094-8373(2003)029<0403:AAMFPB>2.0.CO;2) [DOI] [Google Scholar]
- 35.Marshall L. G. 1978. Evolution of the Borhyaenidae, extinct South American predaceous marsupials. Berkeley, CA: University of California Press [Google Scholar]
- 36.de Muizon C., Cifelli R. L., Paz R. C. 1997. The origin of the dog-like borhyaenoid marsupials of South America. Nature 389, 486–489 10.1038/39029 (doi:10.1038/39029) [DOI] [PubMed] [Google Scholar]
- 37.Argot C. 2004. Evolution of South American mammalian predators (Borhyaenoidea): anatomical and palaeobiological implications. Zool. J. Linn. Soc. 140, 487–521 10.1111/j.1096-3642.2004.00110.x (doi:10.1111/j.1096-3642.2004.00110.x) [DOI] [Google Scholar]
- 38.Argot C. 2004. Functional-adaptive features and palaeobiologic implications of the postcranial skeleton of the late Miocene sabretooth borhyaenoid Thylacosmilus atrox (Metatheria). Alcheringa 28, 229–266 10.1080/03115510408619283 (doi:10.1080/03115510408619283) [DOI] [Google Scholar]
- 39.Sorkin B. 2008. A biomechanical constraint on body mass in terrestrial mammalian predators. Lethaia 41, 333–347 10.1111/j.1502-3931.2007.00091.x (doi:10.1111/j.1502-3931.2007.00091.x) [DOI] [Google Scholar]
- 40.Turnbull W. D., Segall W. 1984. The ear region of the marsupial sabertooth, Thylacosmilus: influence of the sabertooth lifestyle upon it, and convergence with placental sabertooths. J. Morphol. Embryol. 181, 239–270 [DOI] [PubMed] [Google Scholar]
- 41.Reig O. A., Simpson G. G. 1972. Sparassocynus (Marsupialia, Didelphidae), a peculiar mammal from the late Cenozoic of Argentina. J. Zool. 167, 511–539 10.1111/j.1469-7998.1972.tb01742.x (doi:10.1111/j.1469-7998.1972.tb01742.x) [DOI] [Google Scholar]
- 42.Rasmussen D. T., Tilden C. D., Simons E. L. 1989. New specimens of the giant creodont Megistotherium (Hyaenodontidae) from Moghara, Egypt. J. Mammal. 70, 442–447 10.2307/1381539 (doi:10.2307/1381539) [DOI] [Google Scholar]
- 43.Wesley-Hunt G. D. 2005. The morphological diversification of carnivores in North America. Palaeobiology 31, 35–55 (doi:10.1666/0094-8373(2005)031<0035:TMDOCI>2.0.CO;2) [DOI] [Google Scholar]
- 44.Wozencraft W. C. 2005. Order carnivora. In Mammal species of the world: a taxonomic and geographic reference (eds Wilson D. E., Reeder D. M.), pp. 532–628 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 45.Myers P., Espinosa R., Parr C. S., Jones T., Hammond G. S., Dewey T. A. The animal diversity web. 2008 (online). Ann Arbor, MI: University of Michigan. See: http://animaldiversity.ummz.umich.edu/site/accounts/information/Carnivora.html . [Google Scholar]
- 46.McKenna M. C., Bell S. K. 1997. Classification of mammals above the species level. New York, NY: Columbia University Press [Google Scholar]
- 47.Flynn J. J., Wesley-Hunt G. D. 2005. Carnivora. In The rise of placental mammals: origins and relationships of the major extant clades (eds Archibald D., Rose K.), pp. 175–198 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 48.Flynn J. J., Finarelli J. A., Spaulding M. 2010. Phylogeny of the Carnivora and Carnivoramorpha, and the use of the fossil record to enhance understanding of evolutionary transformations. In Carnivoran evolution: new views on phylogeny, form, and function (eds Goswami A., Friscia A.), pp. 25–63 Cambridge, UK: Cambridge University Press [Google Scholar]
- 49.Van Valkenburgh B. 2007. Deja vu: the evolution of feeding morphologies in the Carnivora. Integr. Comp. Biol. 47, 147–163 10.1093/icb/icm016 (doi:10.1093/icb/icm016) [DOI] [PubMed] [Google Scholar]
- 50.Luo Z.-X., Ji Q., Wible J. R., Yuan C.-X. 2001. An early Cretaceous tribosphenic mammal and metatherian evolution. Science 302, 1934–1940 10.1126/science.1090718 (doi:10.1126/science.1090718) [DOI] [PubMed] [Google Scholar]
- 51.Ji Q., Luo Z.-X., Yuan C.-X., Wible J. R., Zhang J.-P., Georgi J. A. 2002. The earliest known eutherian mammal. Nature 416, 816–822 10.1038/416816a (doi:10.1038/416816a) [DOI] [PubMed] [Google Scholar]
- 52.Horowitz I., Martin T., Bloch J., Ladavèze S., Kurz C., Sánchez-Villagra M. R. 2009. Cranial anatomy of the earliest marsupials and the origin of opossums. PLoS ONE 4, e8278. 10.1371/journal.pone.0008278 (doi:10.1371/journal.pone.0008278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsson M. A., Arnason U., Spencer P. B. S., Janke A. 2004. Marsupial relationships and a timeline for marsupial radiation in South Gondwana. Gene 340, 189–196 10.1016/j.gene.2004.07.040 (doi:10.1016/j.gene.2004.07.040) [DOI] [PubMed] [Google Scholar]
- 54.Polly P. D., Wesley-Hunt G. D., Heinrich R. E., Davis G., Houde P. 2006. Earliest known carnivoran auditory bulla and support for a recent origin of crown-group Carnivora (Eutheria, Mammalia). Palaeontology 49, 1019–1027 10.1111/j.1475-4983.2006.00586.x (doi:10.1111/j.1475-4983.2006.00586.x) [DOI] [Google Scholar]
- 55.Rohlf F. J. 1990. Rotational fit (Procrustes) methods. In Proceedings of the Michigan morphometrics workshop (eds Rohlf F. J., Bookstein F. L.), pp. 227–236 Ann Arbor, MI: University of Michigan Museum of Zoology [Google Scholar]
- 56.Zelditch M., Swiderski D. L., Sheets H. D., Fink W. L. 2004. Geometric morphometrics for biologists: a primer. Boston, MA: Elsevier Academic Press [Google Scholar]
- 57.O'Higgins P., Jones N. 2006. Morphologika: tools for statistical shape analysis. York, UK: Hull York Medical School [Google Scholar]
- 58.Foote M. 1993. Discordance and concordance between morphological and taxonomic diversity. Paleobiology 19, 185–204 [Google Scholar]
- 59.Foote M. 1997. The evoution of morphological diversity. Annu. Rev. Ecol. Syst. 28, 129–152 10.1146/annurev.ecolsys.28.1.129 (doi:10.1146/annurev.ecolsys.28.1.129) [DOI] [Google Scholar]
- 60.Sheets H. D. 2010. Integrated Morphometrics Package (IMP) 7. See http://www3.canisius.edu/~\sheets/morphsoft.html
- 61.Werdelin L., Lewis M. E. 2001. A revision of the genus Dinofelis (Mammalia, Felidae). Zool. J. Linn. Soc. 132, 147–258 10.1111/j.1096-3642.2001.tb02465.x (doi:10.1111/j.1096-3642.2001.tb02465.x) [DOI] [Google Scholar]
- 62.Kurtén B. 1967. Pleistocene bears of North America. II. Genus Arctodus, short-faced bears. Acta Zool. Fenn. 117, 1–60 [Google Scholar]
- 63.Figueirido B., Pérez-Claros J. A., Torregrosa V., Martín-Serra A., Palmqvist P. 2010. Demythologizing Arctodus simus, the ‘short-faced’ long-legged and predaceous bear that never was. J. Vertebr. Paleontol. 30, 262–275 10.1080/02724630903416027 (doi:10.1080/02724630903416027) [DOI] [Google Scholar]
- 64.Werdelin L., Wesley-Hunt G. 2010. The biogeography of carnivore ecomorphology. In Carnivoran evolution: new views of phylogeny, form, and function (eds Goswami A., Friscia A.), pp. 225–245 Cambridge, UK: Cambridge University Press [Google Scholar]
- 65.Goswami A. 2006. Cranial modularity shifts during mammalian evolution. Am. Nat. 168, 270–280 10.1086/505758 (doi:10.1086/505758) [DOI] [PubMed] [Google Scholar]