Abstract
Immune-to-brain communication is essential for an individual to aptly respond to challenging internal and external environments. However, the specificity by which the central nervous system detects or ‘senses’ peripheral immune challenges is still poorly understood. In contrast to post-mortem c-Fos mapping, we recorded neural activity in vivo in two specific cortico-limbic regions relevant for processing visceral inputs and associating it with other sensory signalling, the amygdala (Am) and the insular cortex (IC). Adult rats were implanted with deep-brain monopolar electrodes and electrical activity was monitored unilaterally before and after administration of two different immunogens, the T-cell-independent antigen lipopolysaccharide (LPS) or the T-cell-dependent antigen staphylococcal enterotoxin B (SEB). In addition, the neural activity of the same individuals was analysed after single as well as repeated antigen administration, the latter inducing attenuation of the immune response. Body temperature and circulating cytokine levels confirmed the biological activity of the antigens and the success of immunization and desensitization protocols. More importantly, the present data demonstrate that neural activity of the Am and IC is not only specific for the type of immune challenge (LPS versus SEB) but seems to be also sensitive to the different immune state (naive versus desensitization). This indicates that the forebrain expresses specific patterns of electrical activity related to the type of peripheral immune activation as well as to the intensity of the stimulation, substantiating associative learning paradigms employing antigens as unconditioned stimuli. Overall, our data support the view of an intensive immune-to-brain communication, which may have evolved to achieve the complex energetic balance necessary for mounting effective immunity and improved individual adaptability by cognitive functions.
Keywords: telemetry, cytokines, insular cortex, amygdala, lymphocytes, tolerance
1. Introduction
The cornerstone of the immune-to-brain interactions was the observation in rats that days after the initial inoculation of sheep erythrocytes, when the primary immune response peaks, increased firing rates were recorded in the hypothalamic ventromedial nucleus [1]. This discovery initiated the conceptualization of lymphocytes sharing a common chemical language with neurons for intra- and inter-system communication [2]. Surprisingly, however, after some attempts [3–8], only minor progress has been achieved in understanding the neural processing and encoding of lymphocyte signalling. Another provoking finding was the possibility to induce a behaviourally conditioned immune response employing antigens or immunomodulatory drugs as unconditioned stimuli [9]. In overall, these data raised the hypothesis that the brain has the capacity to recognize, encode and store signalling from the immune system.
Clinical and experimental evidence support the physiological relevance of immune-to-brain communication [10–13], and several underlying pathways have been well identified [14,15]. Vagal afferences were initially implicated as the route by which immune signals access the brain [16,17]; however, opposing data challenge the vagus monopoly, brain-resident macrophages and endothelial cells being essential players on the lymphocyte-to-neuron transduction processes [18–20]. Cytokines are key intercellular messengers orchestrating immune responses, but also have direct and specific neuronal effects [21,22]. They activate discrete networks within the hindbrain-, hypothalamic- and cortico-limbic structures [23,24], consequently affecting behaviour such as lethargy, depression, anorexia, sleepiness and hyperalgesia, collectively termed as ‘sickness behaviour’ [14].
Lipopolysaccharide (LPS) and staphylococcal enterotoxin B (SEB) are bacterial products, both eliciting fast, robust but distinct immune responses. LPS mainly triggers CD14+ myeloid cells, without the initial involvement of T lymphocytes (i.e. T-cell-independent antigen), leading to a particular profile of pro-inflammatory cytokine release (e.g. IL-1β, IL-6 and TNFα) [25]. In contrast, the superantigen SEB strongly activates T lymphocyte clones via the T-cell receptor (V-β8), concomitantly resulting in increases of IL-2 and other cytokines in the blood [26,27]. The magnitude of the immune response to both antigens largely depends on the individual immune history; in contrast to the robust immune response during the first antigen encounter with pronounced cytokine release, repeated inoculations of LPS or SEB lead to desensitization, significantly dampening peripheral immune responses such as cytokine production [28,29]. Peripheral insults activate lymphocytes, leading to local and self-regulated processes, but also are strongly modulated by autonomic regulatory loops [30–32]. Importantly, the levels of circulating cytokines parallel the capacity of LPS to be used as effective unconditioned stimulus in associative learning paradigms [33,34], indicating the relevance of pro-inflammatory cytokines in the immune-to-brain signalling process.
Post-mortem c-Fos mapping, with poor time resolution, has been the choice for documenting a neural network responsive to peripheral immune challenges. So far, this network includes: the nucleus of the solitary tract, area postrema, parabrachial nucleus in the hindbrain, central nucleus of the amygdala (Am) and hypothalamic paraventricular nucleus in the forebrain [23,35–40]. However, longitudinal studies on neuronal responses after one or repeated antigen inoculation are completely lacking. Therefore, in order to analyse how and to what extent two specific immunogens commonly employed as unconditioned stimuli (LPS versus SEB) affect relevant cortico-limbic structures activity, we recorded electrical activity of the Am and in the insular cortex (IC) during the first 200 min after the first or repeated antigen administrations, respectively. We employed a wireless recording method, allowing unrestricted animal behaviour, minimizing stress artefacts and providing in our hands reliable electrophysiological data [41]. The study focused on the Am and IC since these structures have previously been shown to be involved in immune-visceral inputs, independently of the afferent route involved [36,42–44], and because lesion experiments documented the relevance of these two structures during processing immune signals employed as unconditioned stimuli [45–47]. Furthermore, in independent experiments, changes in body temperature and circulating cytokine profiles were monitored to further evaluate the immune reactivity under the various experimental conditions.
2. Material and methods
(a). Animals
Male Dark Agouti rats (250–300 g, Harlan, Netherlands) were individually housed under a normal 12 L : 12 D schedule (lights on at 7.00 h) with food and water available ad libitum.
(b). Antigens
Two different antigens were selected: LPS from Escherichia coli (serotype O55 : B5, Sigma, Germany) and Staphylococcus enterotoxin B (SEB, Sigma). Both antigens were diluted in sterile saline and applied intraperitoneally (i.p.).
(c). Study design
Treatment regimen and study design are summarized in table 1. Three independent experiments under identical conditions were performed for acquiring data on (A) neural recordings, (B) cytokines, and (C) body temperature. Immune desensitization was induced as previously described for LPS [48] and SEB [29]. Briefly, rats received i.p. injections of either ascending doses of LPS (0.1, 0.2, 0.5, 0.5 and 0.5 mg kg−1 b.w.) or constant doses of SEB (1.0 mg kg−1 b.w.) over a period of 5 consecutive days, whereas controls were injected with vehicle (1 ml sterile saline). Antigen doses and application intervals were selected based on previous experience in our laboratory [33,34]. After the first and fifth application, neuronal activity (Exp A), cytokine levels (Exp B) and body temperature (Exp C) were analysed.
Table 1.
Study design and variables analysed.
| naive | desensitization | ||||
|---|---|---|---|---|---|
| experimental day | 1 | 2 | 3 | 4 | 5 |
| Veh (saline 1 ml) | X | X | X | X | X |
| LPS (mg kg−1) | 0.1 | 0.2 | 0.5 | 0.5 | 0.5 |
| SEB (mg kg−1) | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| neural recordings | X | X | |||
| temperature | X | X | X | X | X |
| cytokines | X | X |
(d). Neural recordings
Two independent experiments were performed, one for LPS (n = 8) + vehicle (n = 6) and other for SEB (n = 10) + vehicle (n = 7). Deep-brain electrode implantation and neural recordings were performed as described previously [41,49]. Briefly, under deep anaesthesia with xylazine/ketamine i.p. (5 mg kg−1/90 mg kg−1), monopolar stainless steel electrodes were unilaterally implanted into the Am and the ipsilateral IC at coordinates relative to the Bregma (Am: posterior −2.5 mm, lateral 4.3 mm, ventral 8.0 mm; IC: anterior +1.6 mm, lateral 4.8 mm, ventral 6.5 mm). Coordinates were selected based on previous lesion experiments [45], corresponding to the anterior IC and the caudal Am, responsible for visceral processing [42,44,50]. Lateralization was controlled by equally distributing left and right hemispheres across the groups. A stainless steel screw used as an indifferent electrode was positioned on the skull at the surface of the cerebellum. Electrodes were soldered to a socket (TSE-Systems, Bad Homburg, Germany) and fixed with dental cement on the skull. After surgery, re-hydration, analgesics and prophylactic antibiotics were applied. Before any experimental treatment, animals were allowed to recover for a period of at least 14 days, time enough to metabolize peri-operative drugs and to reduce blood–brain barrier leakage. To monitor the neural activity of freely moving rats, a calibrated transmitter (TSE Systems) was fixed on the electrode socket by means of a plug connection. The neural signals were telemetrically received via pulse position modulation and transmitted to a decoder. Data acquisition was coupled online to a computer that transformed the data into real time by means of fast Fourier transformation analysis and displayed them as absolute power (µV) as well as continuous spectra of power density. The mean absolute power was calculated for the total power, summarizing the power of the whole frequency range (0.6–30 Hz). Sampling rate of the digitized neural signals was 128 s−1. By coupling the data acquisition to a SIGMA PLpro EEG device with simultaneous video recording of the behaviour of the rat (Neurowerk, Gelenau, Germany), raw recordings were visualized on the computer screen and inspected to check for the presence of any anomalous patterns. The baseline was composed of three records (5 min every 15 min, 1 h before treatment). After antigen injection, recordings started again for 5 min every 15 min (first time point 20 min post-immune challenge). Data were analysed as power index (total power at a given time point/baseline total power) to account for the changes over time. To compute the magnitude of change, an integrated power index was calculated as the area under the curve (Sigma Plot) during the whole recording period (20–200 min).
(e). Cytokine determination
To analyse plasma cytokine concentrations, blood was collected sublingually under light inhalation anaesthesia (Isoflorane) as described previously [33], 2 h after antigen injections, LPS (n = 6) and SEB (n = 6). Time point was selected based on previous experience with both antigens [34,48]. Plasma was collected after centrifugation (5 min, 10 000 rpm, 4°C) and stored at −80°C until cytokine determination. To obtain splenocytes, animals (n = 10) were sacrificed 2 h after the last injection and spleens were collected. Single-cell suspensions of the spleen were obtained as described previously [51]. Briefly, after mechanically disrupting the tissue and erythrolysis, splenocytes were washed, filtered and adjusted to a concentration of 2.5 × 106 cells ml−1 and stimulated in 96-well flat-bottom microtitre plates with 1 µg ml−1 of anti-rat CD3 monoclonal antibody (clone: G4.18; BD Pharmingen) or SEB (10 µg ml−1) for 72 h in a humidified incubator (37°C, 5% CO2).
Employing bead-based multiplex assays, cytokine levels in plasma or culture supernatants were quantified as described previously [51]. Briefly, sample dilutions were incubated with fluorescence-labelled beads that are coupled to monoclonal antibodies against rat IL-1β, IL-2, IL-6, TNFα and IFN-γ. Upon incubation with cytokine-specific detection antibodies, samples were incubated with streptavidin-PE (Becton Dickinson). Per sample, 200 beads were analysed on a dual-laser flow cytometer. Absolute cytokine levels were calculated based on the mean fluorescence intensity of cytokine standards. The detection limits of the assays were 0.1 pg ml−1 for IL-1β, IL-2 and IL-6, 0.5 pg ml−1 for IFN-γ and 1.4 pg ml−1 for TNFα, respectively. To account for inter-individual variability, cytokine levels after the fifth injection were standardized as the intra-individual percentage of the first day.
(f). Body temperature
Body temperature was continuously recorded via implanted data-loggers (mini-Subcue Dataloggers, Calgary, Canada), as described previously [52]. Briefly, data-loggers were surgically i.p. implanted (n = 17) two weeks before starting the experiment and were set to record every 15 min. At the end of the experiment, the data-loggers were explanted, temperature data downloaded and corrected. Temperature data were integrated as the area under the curve (SigmaPlot) between 160 and 630 min post-challenge, expressed as fever index [53] for day 1 and day 5.
(g). Statistical analysis
Data were analysed using SPSS (v. 17.0) for parametric assumptions. When necessary, robust statistic transformations were employed as indicated. Relevant and appropriate tests were employed for each dataset with a common level of significance set at p < 0.05: two-way mixed ANOVA for body temperature; one sample t-test for plasma cytokines; one-way ANOVA for supernatant cytokines; mixed ANOVA and t-tests for neural recordings.
3. Results
(a). Body temperature
LPS and SEB pyrogenicity are well documented and established in our laboratory [52]. Thus, in a first experiment, we analysed body temperature before and after peripheral administration of LPS (n = 5), SEB (n = 6) or Veh (n = 6) after one (naive condition) as well as after repeated antigen challenge (desensitization). Fever was clearly elicited in animals receiving the first LPS or SEB injection (electronic supplementary material, figure S1). Two-way mixed ANOVA, with treatment (Veh, LPS and SEB) and day (first and fifth) as factors yield significant p < 0.05 day effect F1,14 = 21.638 and day × treatment interaction F2,14 = 4.122. These data document the pyrogenic capacities of LPS and SEB under naive conditions and clearly show that after repeated administration, the pyrogenicity of the same antigen is significantly attenuated.
(b). Plasma cytokines
We measured the peripheral cytokine response after single and repeated injections of LPS (n = 6) and SEB (n = 6), respectively. Repeated injections of either LPS or SEB induced a significant attenuation of the cytokine response. For LPS, the pro-inflammatory cytokines TNF-α, IL-6 and IL-1β were significantly decreased after desensitization compared with the levels elicited under the naive condition, confirming our previous reports [33,48]. Simple t-tests indicate that all three cytokines were significantly blunted after the fifth injection of LPS: TNFα, IL-6 and IL-1β (electronic supplementary material, figure S2a, left). Similarly, we could document an in vivo SEB desensitization by means of the blunted production of IL-2 and IL-1β (electronic supplementary material, figure S2a, right). Superantigens, like SEB, induce a strong proliferative response followed by clonal deletion of a substantial portion of defined Vβ T cells [27], and it has been documented that the remaining cells display an in vitro anergy status [26]. Thus, we tested this status of non-responding to the antigen in an in vivo approach. Isolated splenocytes from animals repeatedly injected with either vehicle (n = 5) or SEB (n = 5) (5 × 1 mg kg−1) were challenged in vitro with either anti-CD3 or again with SEB (10 µg ml−1). The robust IL-2 response induced by vehicle in vivo/SEB in vitro challenge was significantly blunted in the SEB in vivo/SEB in vitro condition, as indicated by the Games–Howell post hoc test after one-way ANOVA, F3,7.57,Welch = 13.70 (electronic supplementary material, figure S2b). This effect was specific for SEB reactive clones, since anti-CD3 in vitro challenge was able to elicit a comparable IL-2 production regardless of the in vivo challenge. IFN-γ levels were also significantly affected by SEB in vivo challenge when tested in vitro with either anti-CD3 or SEB, as indicated by the Bonferroni post hoc test after one-way ANOVA, F3,16 = 245.29 (electronic supplementary material, figure S2b). These data confirmed a desensitization of the immune response after repeated exposures to LPS and SEB.
(c). Neural activity
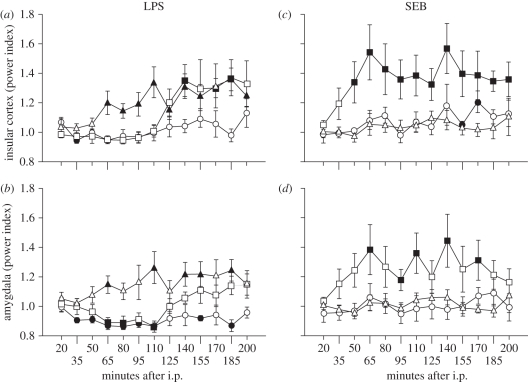
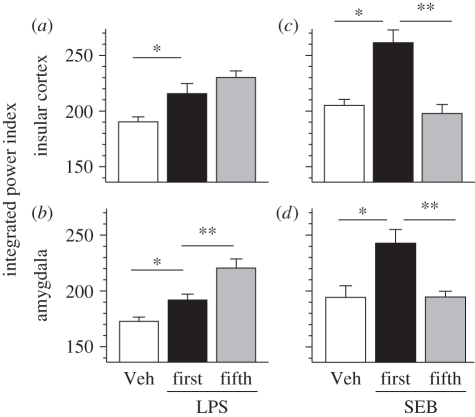
We analysed the neural activity in the IC and Am via radio-telemetry in the following groups of animals: vehicle n = 13, LPS n = 8, SEB n = 10. Recordings of the baseline are summarized in table 2, corresponding to 31 (n = 13 vehicle/n = 8 LPS/n = 10 SEB) recordings to the first day and 18 to the fifth day (n = 8 LPS/n = 10 SEB). There was no significant difference between left and right hemispheres for IC, t47 = 1.605, as well as for Am, t47 = 1.567. Therefore, data of both hemispheres were pooled for each brain structure for further analysis. Under naive condition (first injection), LPS significantly affected neural activity in the IC and Am (figure 1a,b). The IC positively responded to a 0.1 mg kg−1 LPS challenge with a latency of 140 min, peaking at 185 min (table 3). When integrated, the magnitude of change was significantly different from vehicle, t13 = −2.403, p < 0.05 (figure 2a). The Am also responded to LPS around 110 min after inoculation (figure 1b). When integrated, the magnitude of change was significantly higher than the integrated power index of vehicle (figure 2b). Regarding SEB, the IC reacts faster, with a latency of 50 min (figure 1c, table 3). The magnitude of change was the largest of all of the conditions tested, being significantly different from the vehicle response t13 = −3.777, p < 0.05. For this antigen, the Am mostly parallels the activity of the IC, with a latency of 65 min and sustained increments (figure 1d, table 3), and a significant magnitude of response (figure 2d) t9 = 3.889, p < 0.05.
Table 2.
Baseline neural activity (total power) in the left and right hemispheres before any i.p. injection. Units: micro-volts, mean ± s.e.m. Data include a total of 49 recordings, including data collected on the first (n = 31) and fifth day (n = 18).
| insular cortex |
amygdala |
||
|---|---|---|---|
| left (n = 24) | right (n = 25) | left (n = 24) | right (n = 25) |
| 5314 ± 244 | 4811 ± 199 | 6469 ± 288 | 5800 ± 313 |
Figure 1.
Cortico-limbic dynamics during early phase of the immune response. Insular cortex and amygdala activity were followed with a wireless method; monopolar deep-brain electrodes coupled to telemetry system. The baseline was composed of three recordings (5 min every 15 min, 1 h before treatment). After treatment (first day: Veh n = 13, LPS n = 8, SEB n = 10; fifth day: LPS n = 8, SEB n = 10), recordings started again for 5 min every 15 min with the first time point 20 min post-challenge. Data expressed as power index (time point total power/baseline total power) over time. Challenge under naive = first injection (squares), or after desensitization = fifth injection (triangles). As negative control, vehicle (circles) was administered. Closed symbols depict p < 0.05 versus baseline, one-sample t-test.
Table 3.
Latency and peak of insular cortex and amygdala activity after immune challenge. Data are expressed as minutes; latency, first significant change from baseline; peak, maximal deviation from baseline; (+) or (−) signs indicate the direction of change in comparison to baseline; n.s., not significant.
| naive (day 1) |
desensitization (day 5) |
|||||||
|---|---|---|---|---|---|---|---|---|
| insular cortex |
amygdala |
insular cortex |
amygdala |
|||||
| latency | peak | latency | peak | latency | peak | latency | peak | |
| LPS | 140 (+) | 185 (+) | 65 (−) | n.s. | 65 (+) | 140 (+) | 65 (+) | 110 (+) |
| SEB | 50 (+) | 140 (+) | 65 (+) | 140 (+) | n.s. | n.s. | n.s. | n.s. |
Figure 2.
Cortico-limbic magnitude during early immune response (first day: Veh n = 6 + 7, LPS n = 8, SEB n = 10; fifth day: LPS n = 8, SEB n = 10). Data expressed as integrated power index (area under the curve 20–200 min post-injection; trapezoid method). Challenge under naive = first injection (filled bars), or after desensitization = fifth injection (grey bars). As negative control, vehicle (open bars) was administered. *p < 0.05 first versus vehicle (Veh), independent t-test; **p < 0.05 first versus fifth injection, dependent t-test.
Five days later, after the same subjects were submitted to repeated injections of the same antigen (either LPS or SEB), their neural activity was again monitored and analysed for dynamics and magnitude changes. Regarding LPS, the latency in the IC was shorter (65 min) than in the naive condition (figure 1a, table 3). However, the total magnitude was not affected (figure 2a). As on the first day, the Am followed a similar dynamic (table 3 and figure 1b); however, in this structure, the magnitude was significantly higher compared with the first encounter with LPS (figure 2b), t7 = −2.857, p < 0.05. Importantly, repeated injections of SEB evoked a response completely opposite to LPS, resulting in blunted neural activity (dynamics/magnitude), being even now similar to the vehicle condition (table 3 and figures 1 and 2c,d).
4. Discussion
Anatomical and functional evidence accumulated during the last decades document a close interaction of the immune and nervous systems on homeostasis maintenance [10,12]. Our data extend the view of the specificity of immune-to-brain communication during the early minutes of the immune response. Already within the first 200 min after antigen inoculation, the dynamics and magnitude of the electrical activity recorded from the IC and Am were different depending on the antigen administered: T-cell-dependent (SEB) versus T-cell-independent (LPS) antigen. Moreover, in the same individual, we documented that the immune history significantly affects the neural response to the same antigen challenge; i.e. after desensitization, both antigens induced a different neural response, indicating a different perception of the same antigenic challenge. Furthermore, these data provide the electrophysiological substrate for associative learning paradigms employing these antigens as unconditioned stimuli. In summary, our data highlight cortico-limbic dynamics and specificity related to the peripheral immune reactivity within its very early stages.
Hypothalamic nuclei were initially screened and found to be reactive during the effector phase of the humoral immune response (days after inoculation) in anaesthetized [1] and in conscious rats [6,7,54,55]. We took advantage of two stereotypic bacterial antigens that elicit fast and well-characterized immune responses [25,56]. Additionally, these antigens induce corticosterone release [34,57–59], considered to be part of the adaptive suppressive neuroendocrine response necessary to control immune reactivity [30]. Furthermore, both antigens have been also employed as unconditioned stimuli in associative learning protocols, inducing a conditioned taste avoidance as well as other conditioned responses; fever, sympathetic activity, glucocorticoids and cytokines among other parameters [34,48,60,61]. We further focused on the IC and Am as two structures known to be involved in immune-to-brain communication in general [42,44,62–64], and in the behaviourally conditioned immune response in particular [45–47]. We found no lateralization of the baseline neural activity, and a short latency for the two antigens tested (65–140 min for LPS, 50–65 min for SEB). This time frame parallels pro-inflammatory cytokine production and release into circulation for SEB [59,65] and for LPS [48]. Because neither of the antigens employed cross the blood–brain barrier in appreciable concentrations [66], and deep-brain recordings took place long enough to recover the blood–brain barrier integrity, an alternative afferent route would be of relevance with likely intermediate messengers such as cytokines or prostaglandins [17,39,67–70]. In the case of LPS, substantial evidence indicates that circulating cytokines can be monitored by non-neuronal cells of the cerebral vasculature, thereupon releasing prostaglandins to reach neurons, independently of vagal afferences [39,69,71]. In contrast, disruptions of interoceptive signalling by area postrema lesions or vagotomy markedly attenuated SEB- but not LPS-stimulated brain c-Fos expression [24,39] indicating that LPS and SEB appear to use distinct mechanisms to signal the brain. In addition to the different afferent routes employed, our data document a distinct neural processing of these two stereotypic antigens; the dynamics and magnitude of cortical and amygdaloidal activity differ during the particular immune responses elicited, confirming the proposed specificity of the immune signalling processes [23,72]. Furthermore, this is the first longitudinal in vivo evidence documenting how rapidly the brain reacts to peripheral immune challenges, supported on many other post-mortem metabolic mapping reports [24,35,39,40]. In this regard, our recordings on the IC are the first to indicate a clear involvement of this neocortical area in the early processing of peripheral immune inputs, which was not previously allocated by c-Fos expression. However, it should be mentioned that the IC has been widely involved in processing visceral inputs [62,63] as well as in associating immune stimuli with other exteroceptive stimulation within the acquisition phase of Pavlovian conditioning [45–47]. Substantial evidence supports the Am as relaying visceral inputs [64,73] in addition to its well-known function in arousal [74]. Our electrophysiological recordings from the Am are in agreement with data from chinchilla rabbits following lateral Am activity after immunization with different antigens (e.g. sheep erythrocytes, rat erythrocytes and horse globulin) [4,75], confirming a prominent role of this limbic structure within the processing of signals released during the early phase of an immune response [36]. Electrophysiological recordings, while offering in vivo neural activity assessments and longitudinal experimental designs, limit the number of regions to be analysed in comparison to post-mortem metabolic mapping. In this regard, it is well known that severe ablations of the IC and the Am might interfere with peripheral immune reactivity [76,77]. However, our controls have been treated in the same way; thus, comparisons are valid but might differ from completely intact rats. Another potential confounder is the stress response induced by the electrophysiological recording apparatus. In this regard, we took advantage of wireless technology allowed by radio-telemetry to acquire electrical neural activity in completely free-behaving rats [49,78,79], which might reduce stress artefacts.
Distinct neuro-behavioural responses have been documented when comparing the same individual under antigen-naive versus antigen-experienced conditions [33,80,81]. We have reported that LPS alienates its capacity as an unconditioned stimulus in endotoxin-tolerant animals, which might be due to the absence of pro-inflammatory cytokines [33]. Against this background, we tested the hypothesis that differences in the immune response would be also reflected in neural activity of the IC and Am. The blunted cytokine levels and attenuated fever response confirm desensitization after repeated injections of either SEB or LPS, supported by previous reports [29,48,53,80]. Importantly, cortico-limbic activity also was modified by the immune desensitization occurring in the periphery. For SEB, both IC and Am neural activity were significantly reduced after repeated inoculations, supporting the blunted cytokine response in the periphery after repeated applications. Our data support desensitization effects previously reported for repeated and frequent inoculations of this superantigen [28,29]; however, it should be mentioned that not all cytokines were blunted. Interestingly, for the staphylococcal enterotoxin A, the interval between immunizations may be an important factor of the magnitude of desensitization or even sensitization effects [82]. The immunological mechanisms underlying such effects remain elusive, but are likely to be dependent on the kinetics of the T-cell proliferative response after single versus repeated and frequent superantigen injections.
However, for LPS, we observed a paradoxical response, since the IC latency was shortened and the magnitude of the neural response was similar to that observed when LPS was administered in naive immune condition. In this regard, Am activity also showed increased electrical activity after repeated LPS injections when circulating cytokines were blunted, additionally demonstrating significantly higher activity than under naive condition. Potential explanations for this phenomenon are: (i) the used LPS regimen implies increasing doses, starting with 0.1 mg kg−1 on day 1 reaching 0.5 mg kg−1 on day 5, which may lead to a potentially confounding dose factor, (ii) other messengers than cytokines may be involved in the afferent communication on day 5, for instance, prostaglandins released by endothelial and/or perivascular cells as a response to circulating LPS [20,39], and (iii) plasticity phenomena leading to neuro-immune sensitization [83] or associative learning [48]. Finally, whether and to what extent the observed changes are ‘immune specific’ can only be provided in another set of experiments, in which the activity of immunocompetent cells and/or peripheral cytokines will be abolished with concurrent neural recordings. Supporting this argument, we have reported that when using a protein antigen as immune challenge, brain c-Fos expression is completely blunted by a general immunosuppressive pre-treatment [38]. Furthermore, amygdaloid c-Fos mRNA is also blunted after repeated LPS administrations [84].
The immune → neuro → immune loop is essential for energy homeostasis [85]. Immunity involves a substantial energy investment [86]; therefore, lymphocytic activity is subject to a trade-off between other energy-demanding processes, such as reproduction, foraging/hunting, social interaction, resulting at the end in the so-called ‘sickness syndrome’ [14]. On the afferent arm, as mobile sentinel, immune cells are well positioned to constantly survey the organism and to detect pathogenic insults (either self or non-self) responding to it, but simultaneously via immunotransmitters (cytokines, prostaglandins, or neurotransmitters) informing the brain by means of neural and humoral afferent pathways [15]. Theoretically, this immune-to-brain interaction may also codify stimulus modalities (intensity, location and duration). Our data comparing two stereotypic immunogens, LPS versus SEB, substantiate the view of an immunoception process [23], and support the concept of an immunological homunculus [72]. We hypothesize that the sensory capacity to detect relevant immune-borne molecules may lead to the evolutionary advantage of gathering appropriate information for the brain to integrate it with exteroceptive stimuli and/or previous experiences, and to aptly respond, for instance, by allocating fuel storages to energy-demanding cells, i.e. lymphocytes under clonal expansion [85], as well as to learn and anticipate environmental threat and prepare the body for counteraction by associative learning processes [87].
In summary, we demonstrated in an electrophysiological longitudinal in vivo study that two relevant cortico-limbic structures, the IC and the Am, are differentially reacting after administration of two different antigens: LPS versus SEB. In addition, the IC and the Am were also sensitive to the individual immune history in naive versus desensitization state. These data support the view of the immune system as a sensory organ, substantiate associative learning paradigms employing antigens as unconditioned stimuli and provide strong evidence of the necessity of moving from post-mortem metabolic mapping to in vivo longitudinal studies for a better understand the complex physiology occurring during immune-to-brain interactions.
Acknowledgements
The experiments were carried out following the current Swiss and German regulations for animal experimentation and were approved by relevant local animal ethics committees.
The authors thank Anne-Kathrin Krause for the excellent surgical assistance. The study was supported by the German Research Foundation (SCHE 341/12-1; KR 3614/2-1).
References
- 1.Besedovsky H., Sorkin E., Felix D., Haas H. 1977. Hypothalamic changes during the immune response. Eur. J. Immunol. 7, 323–325 10.1002/eji.1830070516 (doi:10.1002/eji.1830070516) [DOI] [PubMed] [Google Scholar]
- 2.Blalock J. E. 1984. The immune system as a sensory organ. J. Immunol. 132, 1067–1070 [PubMed] [Google Scholar]
- 3.Dafny N. 1983. Interferon modifies EEG and EEG-like activity recorded from sensory, motor, and limbic system structures in freely behaving rats. Neurotoxicology 4, 235–240 [PubMed] [Google Scholar]
- 4.Grigor'ev V. A., Klimenko V. M. 1986. Spatial–temporal organization of subcortical brain functions during immune responses. Neurosci. Behav. Physiol. 16, 305–313 10.1007/BF01148174 (doi:10.1007/BF01148174) [DOI] [PubMed] [Google Scholar]
- 5.Korneva E. A. 1987. Electrophysiological analysis of brain reactions to antigen. Ann. N Y Acad. Sci. 496, 318–337 10.1111/j.1749-6632.1987.tb35784.x (doi:10.1111/j.1749-6632.1987.tb35784.x) [DOI] [PubMed] [Google Scholar]
- 6.Saphier D. 1989. Neurophysiological and endocrine consequences of immune activity. Psychoneuroendocrinology 14, 63–87 10.1016/0306-4530(89)90056-5 (doi:10.1016/0306-4530(89)90056-5) [DOI] [PubMed] [Google Scholar]
- 7.Saphier D., Abramsky O., Mor G., Ovadia H. 1987. Multiunit electrical activity in conscious rats during an immune response. Brain Behav. Immun. 1, 40–51 10.1016/0889-1591(87)90005-5 (doi:10.1016/0889-1591(87)90005-5) [DOI] [PubMed] [Google Scholar]
- 8.Saphier D., Kidron D., Ovadia H., Trainin N., Pecht M., Burstein Y., Abramsky O. 1988. Neurophysiological changes in the brain following central administration of immunomodulatory factors. Isr. J. Med. Sci. 24, 261–263 [PubMed] [Google Scholar]
- 9.Ader R. 1974. Behaviorially conditioned immunosuppression. Psychosom. Med. 36, 183–184 [DOI] [PubMed] [Google Scholar]
- 10.Kioussis D., Pachnis V. 2009. Immune and nervous systems: more than just a superficial similarity? Immunity. 31, 705–710 10.1016/j.immuni.2009.09.009 (doi:10.1016/j.immuni.2009.09.009) [DOI] [PubMed] [Google Scholar]
- 11.Meisel C., Schwab J. M., Prass K., Meisel A., Dirnagl U. 2005. Central nervous system injury-induced immune deficiency syndrome. Nat. Rev. Neurosci. 6, 775–786 10.1038/nrn1765 (doi:10.1038/nrn1765) [DOI] [PubMed] [Google Scholar]
- 12.Steinman L. 2004. Elaborate interactions between the immune and nervous systems. Nat. Immunol. 5, 575–581 10.1038/ni1078 (doi:10.1038/ni1078) [DOI] [PubMed] [Google Scholar]
- 13.Tracey K. J. 2009. Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 10.1038/nri2566 (doi:10.1038/nri2566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dantzer R., O'Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 10.1038/nrn2297 (doi:10.1038/nrn2297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quan N., Banks W. A. 2007. Brain–immune communication pathways. Brain Behav. Immun. 21, 727–735 10.1016/j.bbi.2007.05.005 (doi:10.1016/j.bbi.2007.05.005) [DOI] [PubMed] [Google Scholar]
- 16.Wan W., Wetmore L., Sorensen C. M., Greenberg A. H., Nance D. M. 1994. Neural and biochemical mediators of endotoxin and stress-induced c-fos expression in the rat brain. Brain Res. Bull. 34, 7–14 10.1016/0361-9230(94)90179-1 (doi:10.1016/0361-9230(94)90179-1) [DOI] [PubMed] [Google Scholar]
- 17.Watkins L. R., Goehler L. E., Relton J. K., Tartaglia N., Silbert L., Martin D., Maier S. F. 1995. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 183, 27–31 10.1016/0304-3940(94)11105-R (doi:10.1016/0304-3940(94)11105-R) [DOI] [PubMed] [Google Scholar]
- 18.Ericsson A., Arias C., Sawchenko P. E. 1997. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J. Neurosci. 17, 7166–7179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romanovsky A. A., Simons C. T., Szekely M., Kulchitsky V. A. 1997. The vagus nerve in the thermoregulatory response to systemic inflammation. Am. J. Physiol. 273, R407–R413 [DOI] [PubMed] [Google Scholar]
- 20.Serrats J. 2010. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65, 94–106 10.1016/j.neuron.2009.11.032 (doi:10.1016/j.neuron.2009.11.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imeri L., Opp M. R. 2009. How (and why) the immune system makes us sleep. Nat Rev Neurosci 10, 199–210 10.1038/nrn2576 (doi:10.1038/nrn2576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAfoose J., Baune B. T. 2009. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 33, 355–366 10.1016/j.neubiorev.2008.10.005 (doi:10.1016/j.neubiorev.2008.10.005) [DOI] [PubMed] [Google Scholar]
- 23.Goehler L. E., Gaykema R. P., Hansen M. K., Anderson K., Maier S. F., Watkins L. R. 2000. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton. Neurosci. 85, 49–59 10.1016/S1566-0702(00)00219-8 (doi:10.1016/S1566-0702(00)00219-8) [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Wang B. R., Zhang X. J., Duan X. L., Guo X., Ju G. 2004. Fos expression in the rat brain after intraperitoneal injection of Staphylococcus enterotoxin B and the effect of vagotomy. Neurochem. Res. 29, 1667–1674 10.1023/B:NERE.0000035801.81825.2a (doi:10.1023/B:NERE.0000035801.81825.2a) [DOI] [PubMed] [Google Scholar]
- 25.Beutler B. 2003. Innate immune responses to microbial poisons: discovery and function of the toll-like receptors. Annu. Rev. Pharmacol. Toxicol. 43, 609–628 10.1146/annurev.pharmtox.43.100901.135729 (doi:10.1146/annurev.pharmtox.43.100901.135729) [DOI] [PubMed] [Google Scholar]
- 26.del Rey A., Kabiersch A., Petzoldt S., Besedovsky H. O. 2002. Involvement of noradrenergic nerves in the activation and clonal deletion of T cells stimulated by superantigen in vivo. J. Neuroimmunol. 127, 44–53 10.1016/S0165-5728(02)00096-6 (doi:10.1016/S0165-5728(02)00096-6) [DOI] [PubMed] [Google Scholar]
- 27.Hong S. C., Waterbury G., Janeway C. A., Jr 1996. Different superantigens interact with distinct sites in the Vbeta domain of a single T cell receptor. J. Exp. Med. 183, 1437–1446 10.1084/jem.183.4.1437 (doi:10.1084/jem.183.4.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas S. K., Lopez-Collazo E. 2009. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 10.1016/j.it.2009.07.009 (doi:10.1016/j.it.2009.07.009) [DOI] [PubMed] [Google Scholar]
- 29.Jie Y., Pan Z., Chen Y., Wei Y., Zhang W., Wu Y., Peng H., Xu L. 2005. Non-specific tolerance induced by staphylococcal enterotoxin B in treating high risk corneal transplantation in rats. Br. J. Ophthalmol. 89, 364–368 10.1136/bjo.2004.048959 (doi:10.1136/bjo.2004.048959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besedovsky H. O., del Rey A. 1996. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr. Rev. 17, 64–102 [DOI] [PubMed] [Google Scholar]
- 31.Elenkov I. J., Chrousos G. P. 2006. Stress system—organization, physiology and immunoregulation. Neuroimmunomodulation 13, 257–267 10.1159/000104853 (doi:10.1159/000104853) [DOI] [PubMed] [Google Scholar]
- 32.Elenkov I. J., Wilder R. L., Chrousos G. P., Vizi E. S. 2000. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 52, 595–638 [PubMed] [Google Scholar]
- 33.Pacheco-López G., Niemi M. B., Engler H., Engler A., Riether C., Doenlen R., Espinosa E., Oberbeck R., Schedlowski M. 2008. Weakened taste-LPS association during endotoxin tolerance. Physiol. Behav. 93, 261–266 10.1016/j.physbeh.2007.08.022 (doi:10.1016/j.physbeh.2007.08.022) [DOI] [PubMed] [Google Scholar]
- 34.Pacheco-López G., Niemi M. B., Kou W., Harting M., del Rey A., Besedovsky H. O., Schedlowski M. 2004. Behavioural endocrine immune-conditioned response is induced by taste and superantigen pairing. Neuroscience 129, 555–562 10.1016/j.neuroscience.2004.08.033 (doi:10.1016/j.neuroscience.2004.08.033) [DOI] [PubMed] [Google Scholar]
- 35.Elmquist J. K., Ackermann M. R., Register K. B., Rimler R. B., Ross L. R., Jacobson C. D. 1993. Induction of Fos-like immunoreactivity in the rat brain following Pasteurella multocida endotoxin administration. Endocrinology 133, 3054–3057 10.1210/en.133.6.3054 (doi:10.1210/en.133.6.3054) [DOI] [PubMed] [Google Scholar]
- 36.Gaykema R. P., Chen C. C., Goehler L. E. 2007. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 1130, 130–145 10.1016/j.brainres.2006.10.084 (doi:10.1016/j.brainres.2006.10.084) [DOI] [PubMed] [Google Scholar]
- 37.Goehler L. E., Erisir A., Gaykema R. P. 2006. Neural–immune interface in the rat area postrema. Neuroscience 140, 1415–1434 10.1016/j.neuroscience.2006.03.048 (doi:10.1016/j.neuroscience.2006.03.048) [DOI] [PubMed] [Google Scholar]
- 38.Pacheco-López G., Espinosa E., Zamorano-Rojas H. M., Ramírez-Amaya V., Bermúdez-Rattoni F. 2002. Peripheral protein immunization induces rapid activation of the CNS, as measured by c-Fos expression. J. Neuroimmunol. 131, 50–59 10.1016/S0165-5728(02)00264-3 (doi:10.1016/S0165-5728(02)00264-3) [DOI] [PubMed] [Google Scholar]
- 39.Serrats J., Sawchenko P. E. 2009. How T-cell-dependent and -independent challenges access the brain: vascular and neural responses to bacterial lipopolysaccharide and staphylococcal enterotoxin B. Brain Behav. Immun. 23, 1038–1052 10.1016/j.bbi.2009.06.004 (doi:10.1016/j.bbi.2009.06.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan W., Janz L., Vriend C. Y., Sorensen C. M., Greenberg A. H., Nance D. M. 1993. Differential induction of c-Fos immunoreactivity in hypothalamus and brain stem nuclei following central and peripheral administration of endotoxin. Brain Res. Bull. 32, 581–587 10.1016/0361-9230(93)90158-8 (doi:10.1016/0361-9230(93)90158-8) [DOI] [PubMed] [Google Scholar]
- 41.Krügel U., Kittner H., Franke H., Illes P. 2003. Purinergic modulation of neuronal activity in the mesolimbic dopaminergic system in vivo. Synapse 47, 134–142 10.1002/syn.10162 (doi:10.1002/syn.10162) [DOI] [PubMed] [Google Scholar]
- 42.Bagaev V., Aleksandrov V. 2006. Visceral-related area in the rat insular cortex. Auton. Neurosci. 125, 16–21 10.1016/j.autneu.2006.01.006 (doi:10.1016/j.autneu.2006.01.006) [DOI] [PubMed] [Google Scholar]
- 43.Drewes A. M., Dimcevski G., Sami S. A., Funch-Jensen P., Huynh K. D., Le Pera D., Arendt-Nielsen L., Valeriani M. 2006. The ‘human visceral homunculus’ to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp. Brain Res. 174, 443–452 10.1007/s00221-006-0480-0 (doi:10.1007/s00221-006-0480-0) [DOI] [PubMed] [Google Scholar]
- 44.Sewards T. V. 2004. Dual separate pathways for sensory and hedonic aspects of taste. Brain Res. Bull. 62, 271–283 10.1016/j.brainresbull.2003.10.004 (doi:10.1016/j.brainresbull.2003.10.004) [DOI] [PubMed] [Google Scholar]
- 45.Pacheco-López G., Niemi M. B., Kou W., Harting M., Fandrey J., Schedlowski M. 2005. Neural substrates for behaviorally conditioned immunosuppression in the rat. J. Neurosci. 25, 2330–2337 10.1523/JNEUROSCI.4230-04.2005 (doi:10.1523/JNEUROSCI.4230-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramírez-Amaya V., Álvarez-Borda B., Ormsby C. E., Martínez R. D., Pérez-Montfort R., Bermúdez-Rattoni F. 1996. Insular cortex lesions impair the acquisition of conditioned immunosuppression. Brain Behav. Immun. 10, 103–114 10.1006/brbi.1996.0011 (doi:10.1006/brbi.1996.0011) [DOI] [PubMed] [Google Scholar]
- 47.Ramírez-Amaya V., Bermúdez-Rattoni F. 1999. Conditioned enhancement of antibody production is disrupted by insular cortex and amygdala but not hippocampal lesions. Brain Behav. Immun. 13, 46–60 10.1006/brbi.1998.0547 (doi:10.1006/brbi.1998.0547) [DOI] [PubMed] [Google Scholar]
- 48.Oberbeck R., Kromm A., Exton M. S., Schade U., Schedlowski M. 2003. Pavlovian conditioning of endotoxin-tolerance in rats. Brain Behav. Immun. 17, 20–27 10.1016/S0889-1591(02)00031-4 (doi:10.1016/S0889-1591(02)00031-4) [DOI] [PubMed] [Google Scholar]
- 49.Kittner H., Krügel U., Hoffmann E., Illes P. 2004. Modulation of feeding behaviour by blocking purinergic receptors in the rat nucleus accumbens: a combined microdialysis, electroencephalographic and behavioural study. Eur J Neurosci 19, 396–404 10.1111/j.0953-816X.2003.03090.x (doi:10.1111/j.0953-816X.2003.03090.x) [DOI] [PubMed] [Google Scholar]
- 50.Sewards T. V., Sewards M. A. 2001. Cortical association areas in the gustatory system. Neurosci. Biobehav. Rev. 25, 395–407 10.1016/S0149-7634(01)00021-5 (doi:10.1016/S0149-7634(01)00021-5) [DOI] [PubMed] [Google Scholar]
- 51.Pacheco-López G., Riether C., Doenlen R., Engler H., Niemi M. B., Engler A., Kavelaars A., Heijnen C. J., Schedlowski M. 2009. Calcineurin inhibition in splenocytes induced by pavlovian conditioning. FASEB J. 23, 1161–1167 10.1096/fj.08-115683 (doi:10.1096/fj.08-115683) [DOI] [PubMed] [Google Scholar]
- 52.Pacheco-López G., Niemi M. B., Kou W., Baum S., Hoffman M., Altenburger P., del Rey A., Besedovsky H. O., Schedlowski M. 2007. Central blockade of IL-1 does not impair taste-LPS associative learning. Neuroimmunomodulation 14, 150–156 [DOI] [PubMed] [Google Scholar]
- 53.Roth J., McClellan J. L., Kluger M. J., Zeisberger E. 1994. Attenuation of fever and release of cytokines after repeated injections of lipopolysaccharide in guinea-pigs. J. Physiol. 477, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saphier D., Abramsky O., Mor G., Ovadia H. 1987. A neurophysiological correlate of an immune response. Ann. N Y Acad. Sci. 496, 354–359 10.1111/j.1749-6632.1987.tb35787.x (doi:10.1111/j.1749-6632.1987.tb35787.x) [DOI] [PubMed] [Google Scholar]
- 55.Saphier D., Ovadia H., Abramsky O. 1990. Neural responses to antigenic challenges and immunomodulatory factors. Yale J. Biol. Med. 63, 109–119 [PMC free article] [PubMed] [Google Scholar]
- 56.Llewelyn M., Cohen J. 2002. Superantigens: microbial agents that corrupt immunity. Lancet Infect. Dis. 2, 156–162 10.1016/S1473-3099(02)00222-0 (doi:10.1016/S1473-3099(02)00222-0) [DOI] [PubMed] [Google Scholar]
- 57.Goehler L. E., Gaykema R. P., Hansen M. K., Kleiner J. L., Maier S. F., Watkins L. R. 2001. Staphylococcal enterotoxin B induces fever, brain c-Fos expression, and serum corticosterone in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1434–R1439 [DOI] [PubMed] [Google Scholar]
- 58.Nakano K., Suzuki S., Oh C. 1987. Significance of increased secretion of glucocorticoids in mice and rats injected with bacterial endotoxin. Brain Behav. Immun. 1, 159–172 10.1016/0889-1591(87)90018-3 (doi:10.1016/0889-1591(87)90018-3) [DOI] [PubMed] [Google Scholar]
- 59.Serrats J., Sawchenko P. E. 2006. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J. Comp. Neurol. 495, 236–254 10.1002/cne.20872 (doi:10.1002/cne.20872) [DOI] [PubMed] [Google Scholar]
- 60.Exton M. S., Bull D. F., King M. G. 1995. Behavioral conditioning of lipopolysaccharide-induced anorexia. Physiol. Behav. 57, 401–405 10.1016/0031-9384(94)00249-5 (doi:10.1016/0031-9384(94)00249-5) [DOI] [PubMed] [Google Scholar]
- 61.Janz L. J., Green-Johnson J., Murray L., Vriend C. Y., Nance D. M., Greenberg A. H., Dyck D. G. 1996. Pavlovian conditioning of LPS-induced responses: effects on corticosterone, splenic NE, and IL-2 production. Physiol. Behav. 59, 1103–1109 10.1016/0031-9384(95)02171-X (doi:10.1016/0031-9384(95)02171-X) [DOI] [PubMed] [Google Scholar]
- 62.Barnabi F., Cechetto D. F. 2001. Neurotransmitters in the thalamus relaying visceral input to the insular cortex in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1665–R1674 [DOI] [PubMed] [Google Scholar]
- 63.Cechetto D. F., Saper C. B. 1987. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J. Comp. Neurol. 262, 27–45 10.1002/cne.902620104 (doi:10.1002/cne.902620104) [DOI] [PubMed] [Google Scholar]
- 64.Miranda M. I., Ferreira G., Ramírez-Lugo L., Bermúdez-Rattoni F. 2002. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc. Natl Acad. Sci. USA 99, 11 417–11 422 10.1073/pnas.182200499 (doi:10.1073/pnas.182200499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang W., Koller L. D. 1998. Superantigen activation and kinetics of cytokines in the Long-Evans rat. Immunology 95, 331–338 10.1046/j.1365-2567.1998.00613.x (doi:10.1046/j.1365-2567.1998.00613.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banks W. A., Robinson S. M. 2010. Minimal penetration of lipopolysaccharide across the murine blood–brain barrier. Brain Behav. Immun. 24, 102–109 10.1016/j.bbi.2009.09.001 (doi:10.1016/j.bbi.2009.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bluthe R. M., et al. 1994. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C. R. Acad. Sci. III 317, 499–503 [PubMed] [Google Scholar]
- 68.Dantzer R. 1994. How do cytokines say hello to the brain? Neural versus humoral mediation. Eur. Cytokine Netw. 5, 271–273 [PubMed] [Google Scholar]
- 69.Elmquist J. K., Breder C. D., Sherin J. E., Scammell T. E., Hickey W. F., Dewitt D., Saper C. B. 1997. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J. Comp. Neurol. 381, 119–129 (doi:10.1002/(SICI)1096-9861(19970505)381:2<119::AID-CNE1>3.0.CO;2-6) [DOI] [PubMed] [Google Scholar]
- 70.Rivest S. 2001. How circulating cytokines trigger the neural circuits that control the hypothalamic–pituitary–adrenal axis. Psychoneuroendocrinology 26, 761–788 10.1016/S0306-4530(01)00064-6 (doi:10.1016/S0306-4530(01)00064-6) [DOI] [PubMed] [Google Scholar]
- 71.Schwartz G. J., Plata-Salaman C. R., Langhans W. 1997. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1β in rats. Am. J. Physiol. 273, R1193–R1198 [DOI] [PubMed] [Google Scholar]
- 72.Tracey K. J. 2007. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 10.1172/JCI30555 (doi:10.1172/JCI30555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hassert D. L., Miyashita T., Williams C. L. 2004. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav. Neurosci. 118, 79–88 10.1037/0735-7044.118.1.79 (doi:10.1037/0735-7044.118.1.79) [DOI] [PubMed] [Google Scholar]
- 74.McGaugh J. L. 2006. Make mild moments memorable: add a little arousal. Trends Cogn. Sci. 10, 345–347 10.1016/j.tics.2006.06.001 (doi:10.1016/j.tics.2006.06.001) [DOI] [PubMed] [Google Scholar]
- 75.Grigor'ev V. A., Klimenko V. M. 1984. Space–time functional organization of the brain subcortical structures in the process of developing immune reactions. Fiziol. Zh. SSSR Im. I M Sechenova 70, 221–230 [PubMed] [Google Scholar]
- 76.Barneoud P., Neveu P. J., Vitiello S., Le Moal M. 1988. Early effects of right or left cerebral cortex ablation on mitogen-induced spleen lymphocyte DNA synthesis. Neurosci. Lett. 90, 302–307 10.1016/0304-3940(88)90206-6 (doi:10.1016/0304-3940(88)90206-6) [DOI] [PubMed] [Google Scholar]
- 77.Marcilhac A., Siaud P. 1996. Regulation of the adrenocorticotrophin response to stress by the central nucleus of the amygdala in rats depends upon the nature of the stressor. Exp Physiol 81, 1035–1038 [DOI] [PubMed] [Google Scholar]
- 78.Dimpfel W. 2005. Pharmacological modulation of cholinergic brain activity and its reflection in special EEG frequency ranges from various brain areas in the freely moving rat (Tele-Stereo-EEG). Eur. Neuropsychopharmacol. 15, 673–682 10.1016/j.euroneuro.2005.03.006 (doi:10.1016/j.euroneuro.2005.03.006) [DOI] [PubMed] [Google Scholar]
- 79.Ning G., Bai Y., Wang X., Yu F., Zheng X. 2005. New approaches to physiological study by telemetry technology. Conf. Proc. IEEE Eng. Med. Biol. Soc. 6, 6654–6657 [DOI] [PubMed] [Google Scholar]
- 80.Nava F., Carta G. 2000. Repeated lipopolysaccharide administration produces tolerance to anorexia and fever but not to inhibition of thirst in rat. Int. J. Immunopharmacol. 22, 943–953 10.1016/S0192-0561(00)00058-8 (doi:10.1016/S0192-0561(00)00058-8) [DOI] [PubMed] [Google Scholar]
- 81.Porter M. H., Arnold M., Langhans W. 1998. TNF-α tolerance blocks LPS-induced hypophagia but LPS tolerance fails to prevent TNF-α-induced hypophagia. Am. J. Physiol. 274, R741–R745 [DOI] [PubMed] [Google Scholar]
- 82.Urbach-Ross D., Crowell B., Kusnecov A. W. 2008. Relationship of varying patterns of cytokine production to the anorexic and neuroendocrine effects of repeated staphylococcal enterotoxin A exposure. J. Neuroimmunol. 196, 49–59 10.1016/j.jneuroim.2008.02.006 (doi:10.1016/j.jneuroim.2008.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosas-Ballina M., Tracey K. J. 2009. The neurology of the immune system: neural reflexes regulate immunity. Neuron 64, 28–32 10.1016/j.neuron.2009.09.039 (doi:10.1016/j.neuron.2009.09.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valles A., Marti O., Armario A. 2005. Mapping the areas sensitive to long-term endotoxin tolerance in the rat brain: a c-fos mRNA study. J. Neurochem. 93, 1177–1188 10.1111/j.1471-4159.2005.03100.x (doi:10.1111/j.1471-4159.2005.03100.x) [DOI] [PubMed] [Google Scholar]
- 85.Straub R. H., Cutolo M., Buttgereit F., Pongratz G. 2010. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J. Intern. Med. 267, 543–560 10.1111/j.1365-2796.2010.02218.x (doi:10.1111/j.1365-2796.2010.02218.x) [DOI] [PubMed] [Google Scholar]
- 86.Hotamisligil G. S., Erbay E. 2008. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 8, 923–934 10.1038/nri2449 (doi:10.1038/nri2449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schedlowski M., Pacheco-Lopez G. 2010. The learned immune response: Pavlov and beyond. Brain Behav. Immun. 24, 176–185 10.1016/j.bbi.2009.08.007 (doi:10.1016/j.bbi.2009.08.007) [DOI] [PubMed] [Google Scholar]