Abstract
Aggression is a behavioural strategy for securing resources (food, mates and territory) and its expression is strongly influenced by their presence and value. While it is known that resource holders are generally highly aggressive towards intruding consexuals and usually defeat them, the underlying neuronal mechanisms are not known. In a novel intruder–resident paradigm for field crickets (Gryllus bimaculatus), we show that otherwise submissive losers of a preceding aggressive encounter readily fight and often defeat aggressive winners after occupying an artificial shelter. This aggression enhancing effect first became evident after 2 min residency, and was maximal after 15 min, but absent 15 min after shelter removal. The residency effect was abolished following non-selective depletion of biogenic amines from the central nervous system using reserpine, or semi-selective depletion of octopamine and dopamine using α-methyl-tyrosine, but not following serotonin depletion using α-methyl-tryptophan. The residency effect was also abolished by the treatment with phentolamine, an α-adrenergic receptor antagonist, or epinastine, a highly selective octopamine receptor blocker, but not by propranolol, a ß-adrenergic receptor antagonist, or by yohimbine, an insect tyramine receptor blocker. We conclude that crickets evaluate residency as a rewarding experience that promotes aggressive motivation via a mechanism involving octopamine, the invertebrate analogue of noradrenaline.
Keywords: insects, biogenic amines, agonistic behaviour, pharmacology, intruder–resident paradigm, experience-dependent plasticity
1. Introduction
Intraspecific aggression is a complex social behavioural strategy, adapted for securing some limited resource at minimal cost. It is accordingly modified by numerous experiences. For example, owners of a territory are inclined to fight more ferociously and accordingly beat intruders. The physiological mechanisms that control how experiences, such as residency, influence aggressive behaviour are, however, largely unknown (review [1]).
In this paper, we describe a novel intruder–resident paradigm for the field cricket Gryllus bimaculatus (De Geer) and investigate whether biogenic amines mediate heightened aggressiveness of residents. In the field, crickets compete for natural shelters [2] and defend artificial shelters in the laboratory [2]. Their fighting behaviour is highly stereotyped, and results in winners that exhibit heighted aggression, and submissive losers that retreat upon confronting males, even several hours after social defeat [3,4]. We have shown that the experience of flying quickly restores the aggressiveness of losers [5] via a mechanism that depends on the biogenic amine octopamine, the invertebrate analogue of noradrenaline [3,6]. Using similar techniques, we investigate here whether pharmacological depletion of biogenic amines from the central nervous system and/or amine receptor antagonists influences the aggressiveness of submissive crickets towards aggressive intruders after residency in an artificial shelter. Our study suggests that the experience of occupancy activates the octopaminergic system, which enhances aggressiveness of the resident.
2. Material and methods
(a). Experimental animals
Mature adult male Mediterranean field crickets, G. bimaculatus de Geer were taken from a breeding stock maintained under constant standard conditions at Leipzig University (cf. [7]) and kept isolated for 24 h prior to all experiments. Control and test experiments were performed in parallel in the summer months, avoiding times when aggression tends to be depressed (just after mid-day and on generally dreary days).
(b). Intruder–resident paradigm
We tested the influence of residency on the aggressiveness of submissive crickets towards aggressive intruders. Fights were first staged in an initial fight between pairs of fight-inexperienced (naive) weight-matched crickets in a Perspex glass arena (16 × 9 cm) to generate aggressive winners and submissive losers (cf. [6]). The contestants were then divided by an opaque sliding door, and after a variable delay (0–15 min) an artificial shelter (2 × 4 × 7 cm half-cylinder with opaque rear panel) was placed with the loser, which they immediately occupied. The dividing door was then removed exactly 15 min after the initial fight and the interactions between the resident-loser and intruding-winner evaluated in the presence of the shelter (i.e. after various periods of residency, but at a constant time after losing). In another experimental series, resident-losers were first kept for a period in the absence of the shelter (1, 5 and 15 min), before removing the door to allow contact with the intruder.
(c). Pharmacological treatments
Unless stated otherwise, all drugs were obtained from Sigma (Deisenhofen, Germany). Amines were depleted from the cricket nervous system by injecting either the non-selective amine depleter reserpine, the octopamine/dopamine synthesis inhibitor α-methyl-p-tyrosine (AMT) or the serotonin synthesis inhibitor α-methyltryptophan (AMTP) into the thoracic cavity using a microsyringe (Hamilton, Bonaduz, Switzerland). The applied dosages and time required to achieve effective depletion have been previously established by immunocytochemical detection of octopamine, dopamine and serotonin [3]. We accordingly evaluated aggressive behaviour after the following treatment regimes. Reserpine: behavioural test 24–48 h after a single injection of 200 µg in 4 µl DMSO; AMT: 48 h after the last of two successive injections of 1.5 mg in 20 µl deionized water administered at 48 h intervals; AMTP: 48 h after the last of three successive injections of 1.0 mg in 40 µl deionized water administered at 48 h intervals.
In another experimental series, the crickets were injected with amine receptor antagonist (each 20 µl 20 mM−1 in 2% aqueous DMSO: for details see [6]): the ß-adrenoceptor blocker propranolol, the tyramine receptor blocker yohimbine, the α-adrenoceptor blocker phentolamine or selective octopamine receptor blocker epinastine (a generous gift from Boehringer Ingelheim, Germany). Control animals received vehicle only. The animals' behavioural performance was analysed 1–2 h after treatment with blockers.
(d). Data analysis
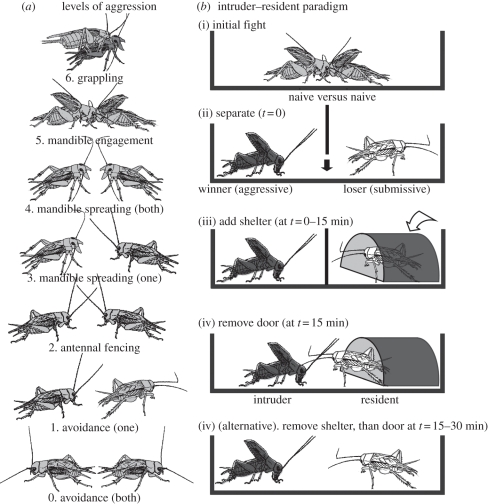
The intensity of aggressive interactions was scored on a scale of 0–6 (cf. [5] and figure 1) denoting the level to which a fight escalates before the winner is established by the retreat of one contestant: level 0, mutual avoidance without aggression; level 1, one cricket attacks, the other retreats; level 2, antennal fencing; level 3, mandible spreading by one cricket; level 4, mandible spreading by both crickets; level 5, mandible engagement and level 6, grappling, an all-out fight involving repeatedly engagements, biting and tossing. The fight can be concluded at any of the levels 2–6 by one opponent retreating. Fight duration, from first contact until conclusion, was measured to the nearest second with a stop-watch; the duration of any pauses that occasionally occurred when the animals lost contact was deducted.
Figure 1.
(a) Pictograms illustrating the escalating levels of aggression that characterize cricket fights (adopted from [6]): level 0 mutual avoidance, non-aggressive interaction. Level 1 pre-established dominance, one cricket attacks, the other retreats. This level is in accordance with the avoiding behaviour of losers. Level 2 antennal fencing, the two crickets fence with their antennae. Level 3 mandible spreading (unilateral), one cricket displays broadly spread mandibles. Level 4 mandible spreading (bilateral), both crickets display their spread mandibles. Level 5 mandible engagement, the mandibles of both contestants interlock. Level 6 grappling, all-out fighting involving repetitive biting, mandible pushing, and opponent flipping. (b) Pictograms illustrating the intruder–resident paradigm. (i) Contests were first staged between pairs of previously isolated, fight-inexperienced and weight-matched adult male crickets. (ii) For each pair, the resulting submissive loser was separated from the aggressive winner via an opaque door dividing the area. (iii) The loser was then provided with a shelter, either immediately (t = 0) or after a delay (up to 15 min), which it immediately occupied (control, no shelter). (iv) The dividing door was removed 15 min after separation in all cases and the interactions between resident-winners and intruding-winners evaluated. (iv) (alternative) In some experiments, the losers all experienced 15 min occupancy in the shelter, followed by a delay (0–15 min) before removing the door (t = 15–30 min).
The median and the interquartile range (i.q.r.) were calculated for non-parametric datasets; the significance of differences in the distributions was tested by the Mann–Whitney U-test for unpaired data and by the Wilcoxon signed-rank test for paired data; the χ2-test was employed for comparing relative frequencies (Prism 5, GraphPad Software Inc. La Jolla, CA, USA).
3. Results
(a). Intruder–resident paradigm
(i). Control
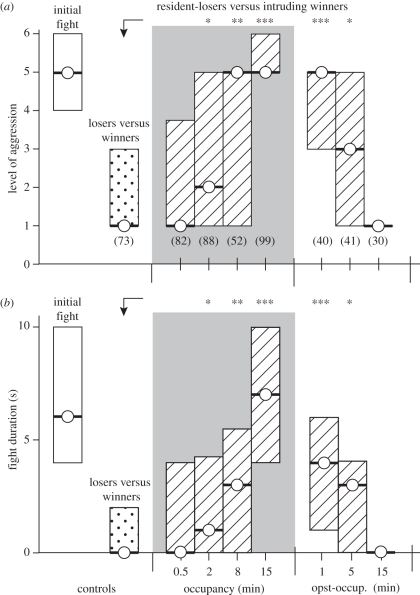
On meeting, inexperienced, weight-matched crickets usually fight aggressively (median level of aggression: 5, i.q.r. 4–6 n = 228; figure 2a, white bars). These fights are typically concluded after several seconds (median, 6 s, i.q.r. 4–10; figure 2b) by the retreat of one cricket, the loser. On subsequent contact with the winner, for example, 15 min after the initial fight, almost all losers fled immediately (71%; median level of aggression: 1, i.q.r. 1–3; median duration: 0 s i.q.r. 0–2, n = 73; significantly different to initial fights, Wilcoxon signed-rank test, p level < 0.001, p duration < 0.001; figure 2a,b stippled bars). Only a few encounters escalated to a physical level (14% equal or greater than level 5), rank reversals (loser wins) were observed only in 3 per cent of all cases. This depressed aggressiveness of subordinate crickets usually lasts several hours [5].
Figure 2.
Bar graphs illustrating the impact of shelter occupancy on (a) level of aggression and (b) fight duration (circle/bold line, median; bar, i.q.r.). For paradigm see figure 1b. From left to right: White bar, initial fight between naive males, stippled bar—losers versus winners 15 min after the initial fight; hatched bars/grey background, resident-losers versus intruding-winners after increasingly longer periods of shelter occupancy (0.5, 2.0, 8.0 and 15 min); hatched bars/white background, resident-losers versus intruding-winners after 15 min shelter occupancy followed by 1, 5 or 15 min isolation in the absence of the shelter (post-occup.). n is given in parentheses beneath each column, excepting initial fight, which is pooled. Asterisks denote statistically significant differences (Mann–Whitney U-test; *p < 0.05; **p < 0.01; ***p < 0.001).
(ii). Effect of residency
Residency in an artificial shelter restored aggressive motivation of losers. As shown in figure 2, the effect of residency depends on occupancy time and is transient. Thus, losers that occupied the shelter for only 0.5 min retreated when subsequently confronted with the previous winner (median level 1, i.q.r. 1–3; median duration: 0 s i.q.r. 0–4, n = 82: no significant difference to control: U-test, p level = 0.35, p duration = 0.81, hatched bars/grey background). In contrast to this, losers that occupied the shelter for 15 min were highly aggressive towards intruding-winners (76% physical fighting; median level 5, i.q.r. 5–6; median duration 7 s i.q.r. 4–10, n = 99; significantly different to control-losers: U-tests, p level < 0.001, p duration < 0.001), and frequently even won the encounter (27% rank reversal, significantly different to control-losers: χ2-test, p < 0.001). Thus, residency restored the aggressiveness of losers to the same level as naive crickets (difference statistically non-significant: Wilcoxon signed-rank test, p = 0.17). Intermediate periods of residency (2 and 8 min) had corresponding intermediate influences (figure 2). Interestingly, the aggressiveness of resident-losers slowly dwindled after removal of the shelter (figure 2, post-occup.). For example, 1 min after removing the shelter, losers that had experienced 15 min residency were still highly aggressive towards the intruders (median level: 5, i.q.r. 3–5; median duration: 4 s i.q.r. 1–6, n = 40; both values significantly different to values for non-resident-losers versus intruders: U-tests, p level < 0.001, p duration < 0.001). In contrast to this, 15 min resident-losers were non-aggressive towards the intruding-winner 15 min after shelter removal (median level of aggression: 1, i.q.r. 1–1; median duration: 0 s i.q.r. 0–0, n = 30; neither value was significantly different from values of non-resident-losers versus intruders: U-tests, p level = 0.845, p duration = 0.466).
It should be noted that the above described effects of residency were only found for losers of a previous aggressive encounter, thus the aggressiveness of naive crickets was unchanged subsequent to 15 min occupancy in the artificial shelter (median level of aggression: 5, i.q.r. 3.75–6; median duration: 5.5 s i.q.r. 4–12, resident wins: 54%, n = 100; not significantly different from aggression of naive pairs: U-test, p level = 0.32, p duration = 0.59; data not shown).
(b). Amine depletion
Since octopamine is known to enhance the aggressiveness of subordinate crickets [3,6], we investigated whether this amine underlies the effect of residency. In a first series of experiments, we pretreated pairs of weight-matched, naive crickets with drugs at concentrations shown elsewhere to effectively deplete amines from the crickets nervous system (cf. [3]): the non-selective amine-depleting agent reserpine, the semi-selective octopamine-depleting agent AMT and the selective serotonin-depleting agent AMTP (controls received DMSO, the vehicle for reserpine).
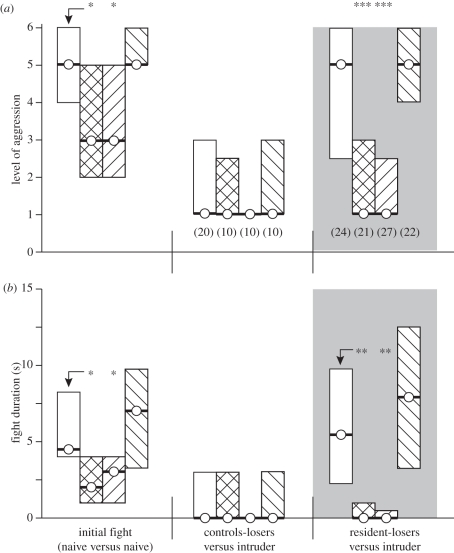
As shown previously (cf. [3]) and in figure 3, amine-depleted crickets express normal fighting behaviour, although the aggressiveness and the fight duration are somewhat less for reserpine and AMT-treated crickets (reserpine: median level 3, i.q.r. 2–5; median duration 4 s i.q.r. 1–6, n = 31; significantly different to vehicle-control, U-test, p level = 0.013, p duration = 0.012; AMT: median level 3, i.q.r. 2–5; median duration: 4 s i.q.r. 1–6, n = 37; significantly different to vehicle-control, U-tests, p level = 0.013, p duration = 0.014) but unchanged in serotonin-depleted crickets (median level 5, i.q.r. 5–6; median duration 4 s i.q.r. 1–6, n = 32; not significantly different to vehicle-control: U-tests, p level = 0.31, p duration = 0.40). Irrespective of the pharmacological treatment, all encounters produced clear winners and losers, and the latter exhibited depressed aggressiveness when tested 15 min after the initial encounter (figure 3). The residency effect was not altered by serotonin depletion. Following 15 min residency, the AMTP-treated losers escalated to the same level and fought as long as vehicle-treated losers against the intruding-winners (U-tests, p level = 0.63, p duration = 0.45). By contrast, residency failed to enhance the aggressiveness of reserpine- and AMT-treated crickets. In both groups, the fight level and the duration were not significantly different to the corresponding loser-groups without shelter (U-tests, reserpine p level = 0.81, p duration = 0.91; AMT p level = 0.41, p duration = 0.63). Furthermore, reserpine- and AMT-treated resident-losers were significantly less aggressive than at their initial encounter (Wilcoxon signed-rank tests, reserpine p level = 0.023, p duration = 0.020; AMT p level = 0.0016, p duration = 0.0014), indicating that defeat still suppresses aggressiveness in animals lacking octopamine.
Figure 3.
Bar graphs illustrating the influence of amine depletion on (a) level of aggression and (b) fight duration after residency (circle/bold line, median; bar, i.q.r.). The crickets were treated prior to the initial fight with either vehicle (white bar), reserpine (cross-hatched bar), AMT (upwards-hatched bar) or AMTP (downwards-hatched bar). The aggressiveness of treated animals was evaluated in an initial fight (naive versus naive) and in a second contact 15 min later before which the losers remained in the arena either without a shelter (control-loser versus intruder) or with a shelter that they always occupied (resident-loser versus intruder, grey background). n is given in parentheses beneath each column, excepting initial fight, which is pooled. Asterisks denote statistically significant differences (for unpaired datasets: Mann–Whitney U-test).
(c). Amine receptor antagonists
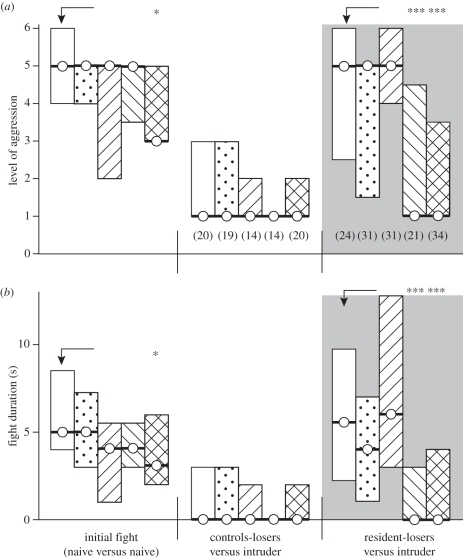
The effect of residency was abolished by antagonists, which selectively block octopamine receptors. In our experiments, crickets were pretreated with either propranolol, a vertebrate β-adrenoceptor blocker with low affinity for insect octopamine receptors, yohimbine, a vertebrate α-adrenoceptor- and potent insect tyramine receptor blocker (cf. [8,9]), phentolamine, a vertebrate α-adrenoceptor blocker that also blocks octopamine receptors and finally epinastine, a highly selective octopamine receptor blocker. Controls received vehicle only (2% aqueous DMSO). After treatment, all groups exhibited normal fighting behaviour. However, in contrast to all other groups, the level and the duration of aggressive interaction between epinastine-treated crickets were reduced (U-tests, p level = 0.014, p duration = 0.046). Irrespective of treatment, all interactions produced clear winners and losers, whereby the losers of all groups, which were not offered a shelter, retreated from the previous winners when tested 15 min after the initial fight (figure 4). Furthermore, the enhancing effect of residency (15 min) on aggressiveness was clearly evident in propranolol-treated losers, which fought as aggressively as vehicle-treated resident-losers against intruding-winners (median level 5, i.q.r. 1.5–5, median duration: 4 s i.q.r. 1–7, n = 32; neither value significantly different to vehicle, U-test, p level = 0.086, p duration = 0.21). Similarly, yohimbine also had no influence on the effectiveness of residency on loser aggression (median level 5, i.q.r. 4–6; median duration 6 s i.q.r. 3–13, n = 28; both values significantly different to corresponding losers without a shelter, U-tests, p level < 0.001, p duration < 0.001; not significantly different to initial fights: Wilcoxon signed-rank tests, p level = 0.18, p duration = 0.10). In contrast to this, phentolamine and epinastine both blocked the effect of residency, since these groups of losers mostly retreated upon confronting winners, without displaying aggression (phentolamine: median level 1, i.q.r. 1–4.5, median duration 0 s, i.q.r. 0–3, n = 23; epinastine: median level 1, i.q.r. 1–3.5; median duration: 0 s i.q.r. 0–4, n = 35). These interactions between phentolamine and epinastine treated loser residents were not more aggressive than those between the corresponding loser-groups without shelter (U-tests, phentolamine p level = 0.12, p duration = 0.19; epinastine p level = 0.76, p duration = 0.26) and significantly less aggressive than those of vehicle-treated resident-losers (U-tests: all tested differences significant p < 0.001; figure 4) and their own initial fights (Wilcoxon signed-rank test, phentolamine p level = 0.017, p duration = 0.040; epinastine p level = 0.0011, p duration = 0.0011).
Figure 4.
Bar graphs illustrating the influence of amine receptor blockers on (a) level of aggression and (b) fight duration after residency (circle/bold line, median; bar, i.q.r.). The crickets were treated prior to the initial fight with either vehicle (white bar), propranolol (stippled bar), yohimbine (upwards-hatched bar), phentolamine (downwards-hatched bar) or epinastine (cross-hatched bar). The aggressiveness of treated animals was evaluated in an initial fight (naive versus naive) and in a second contact 15 min later before which the losers remained in the area without a shelter (control-loser versus intruder) or with a shelter that they always occupied (resident-loser versus intruder, grey background). n is given in parentheses beneath each column, excepting initial fight, which is pooled. Asterisks denote statistically significant differences (for unpaired datasets: Mann–Whitney U-test).
4. Discussion
As in many other animal species, male crickets normally behave submissively towards conspecific males for a long period after suffering social defeat [4,5,10]. In a novel intruder–resident paradigm, we show here that otherwise submissive losers of a preceding aggressive encounter readily fight and often even defeat aggressive winners after a short period of residency in an artificial shelter (figure 2). In the field, natural shelters are valuable assets for attracting females, which mate preferentially with burrow owners, and males accordingly compete aggressively for shelter acquisition [2,11]. Regardless of species, residents usually defeat intruders [12], but it is hotly debated how this is controlled (e.g. [13]).
A shelter may in itself be perceived as a strong agonistic signal by the intruder, since intrinsically aggressive males can be expected to accumulate as residents and accordingly win more often [12,14]. In our paradigm, however, the resident was initially subordinate, but nonetheless fought aggressively and even won almost a third of all fights against otherwise dominant intruders. Furthermore, this residency effect was still evident even shortly after removing the shelter (figure 2), so that the intruder had no indication of the opponent's status. This also illustrates that crickets, in the forced fight paradigm at least, do not behave fully bourgeois (cf. [15]), i.e. they do not only fight for the possession of an actual benefit. In contrast to losers, residency had no significant influence on the aggressiveness of fight-inexperienced crickets. This is probably due partly to these crickets being already aggressive, and partly to influences of other asymmetries, such as body size, which can out-weight the effect of residency in crayfish [16] and mice [17]. We conclude that in the absence of other disparities, the experience of residency increases aggressive motivation, and accordingly the resource holding potential (cf. [18]) of resident crickets.
Since the residency effect was only evident after an occupancy period of at least 2 min, became maximal after 15 min and waned 15 min after removing the shelter (figure 2), it is very unlikely that the initial sensory experience of shelter acquisition per se is sufficient to restore an aggressive state. Increased aggressiveness with prolonged residency or territoriality is known in many animal species (e.g. lobsters: [19]) and is thought to reflect the increase in value of the resource with time as the animal gathers more information on it and invests increasingly more in it [20]. On the other hand, the transient nature of the residency effect in crickets suggests that the underlying mechanism involves temporal changes in some neurochemical mediator. We have previously shown that the experience of flying in the laboratory also causes a transient enhancement of aggression in crickets and that this is mediated by the biogenic amine octopamine [3,6]. Similarly, data presented here suggest that the shelter-residence effect is also mediated by this well-known invertebrate neuromodulator.
The effect of shelter-residency was abolished following either non-selective depletion of amines with reserpine, or semi-selective depletion of octopamine and dopamine with AMT, but not by treatment with the selective serotonin-depleting agent AMTP. Similarly, serotonin appears to have no clear effect on shelter competition in lobsters [21]. Furthermore, since insect octopamine receptors are pharmacologically similar to vertebrate α-adrenoceptors [22], our finding that the shelter-residency effect was blocked by the α-adrenergic receptor antagonist phentolamine, but not by the ß-adrenergic receptor antagonist propranolol implicates the involvement of octopamine rather than dopamine. Verifying this, the residency effect was most effectively blocked by epinastine (figure 4), a highly selective octopamine receptor antagonist [23]. We can also discount the involvement of octopamine's precursor tyramine, which is now recognized as a bona fide neurotransmitter in insects [24,25], since the high-affinity tyramine receptor blocker yohimbine [9] failed to abolish the enhancing effect of residency on aggression. The actions of octopamine are thought to be mainly mediated by G-protein coupled receptors which, depending on the receptor subtype, lead to increased levels of the second messenger cyclic AMP or calcium mobilization. However, our experiments allow no insight into which of these pathways is involved since it is not yet feasible to distinguish between octopamine receptor subtypes in whole tissue pharmacological studies (see also [22]). We can nonetheless conclude that the experience of shelter-residency in crickets enhances aggressive motivation via activation of the octopaminergic system.
Interestingly, the resident crickets slowly reverted to the submissive state some 15 min after removing the shelter. Thus, residency can temporarily over-ride the effect of losing, but not wipe it out and reset aggression to a default condition. Hence, there must be two, opposing control systems, one activated by residency and involving octopamine that transiently promotes aggressive motivation, and a second determined by the losing experience that suppresses it over a longer time scale.
The seemingly paradoxical question posed by our studies is how experiences as diverse as flying [6] and shelter-residency (this paper), which represent two extremes of the locomotory and energy expenditure spectrum, can both lead to activation of the octopaminergic system promoting aggressive motivation? Activation of the insect octopaminergic system is generally thought to prepare the animal for a period of prolonged activity or to assist the animal in recovering from a period of increased energy demand [26]. It occurs during flying [27] and under stressful conditions, during which the ratio of octopamine increases relative to its precursor tyramine in identified neurons [25]. However, residency can hardly be regarded as a strenuous or stressful condition. Alternatively, the enhanced aggressiveness that follows a short period of isolation in a dark shelter may be due to sensory deprivation. We have shown that crickets deprived of sensory inputs from the opponent fight more aggressively ([4]; see [28] for a similar effect in crayfish) and social isolation is a prerequisite for aggressive behaviour to become overt (cf. [29]). However, although octopamine reduces some effects of social deprivation in carpenter ants [30], isolation is accompanied by a reduction in octopamine levels in crickets [29]. A far more attractive hypothesis is that residency may represent a positive, rewarding experience that triggers octopamine release. Indeed, octopamine is known to convey reward signals in appetitive learning paradigms in both honeybees Apis mellifera [31] and crickets [32]. In honeybees, reinforcement in reward learning can be mediated by a single neuron (VUMmx1, [33]), which is member of the well-known group of octopaminergic dorsal and ventral unpaired median neurons (DUM/VUM cells) [34,35]. Interestingly, cells of this type reside in the same brain region that houses neurons required for the expression of aggression in Drosophila [36]. Clearly, what we now need to know is whether and which octopaminergic neurons in crickets are necessary and sufficient to enhance aggression and under which circumstances such octopaminergic modulation takes place.
Acknowledgements
The experiments complied with the Principles of Laboratory Animal Care and the German Law on the Protection of Animals (Deutsches Tierschutzgesetz).
We thank our colleagues Hans-Joachim Pflüger (FU-Berlin) and Bertram Gerber (University of Leipzig) as well as two anonymous referees for helpful suggestions on this manuscript. This project was supported in its final stages by the Deutsche Forschungsgemeinschaft (STE 714/4-1).
References
- 1.Hsu Y. Y., Earley R. L., Wolf L. L. 2006. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74 10.1017/S146479310500686X (doi:10.1017/S146479310500686X) [DOI] [PubMed] [Google Scholar]
- 2.Simmons L. W. 1986. Inter-male competition and mating success in the field cricket, Gryllus bimaculatus (de Geer). Anim. Behav. 34, 567–579 10.1016/S0003-3472(86)80126-9 (doi:10.1016/S0003-3472(86)80126-9) [DOI] [Google Scholar]
- 3.Stevenson P. A., Hofmann H. A., Schoch K., Schildberger K. 2000. The fight and flight responses of crickets depleted of biogenic amines. J. Neurobiol. 43, 107–120 (doi:10.1002/(SICI)1097-4695(200005)43:2<107::AID-NEU1>3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- 4.Rillich J., Stevenson P. A., Schildberger K. 2007. Assessment strategy of fighting crickets revealed by manipulating information exchange. Anim. Behav. 74, 823–836 10.1016/j.anbehav.2006.11.022 (doi:10.1016/j.anbehav.2006.11.022) [DOI] [Google Scholar]
- 5.Hofmann H. A., Stevenson P. A. 2000. Flight restores fight in crickets. Nature 403, 613. 10.1038/35001137 (doi:10.1038/35001137) [DOI] [PubMed] [Google Scholar]
- 6.Stevenson P. A., Dyakonova V., Rillich J., Schildberger K. 2005. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431–1441 10.1523/JNEUROSCI.4258-04.2005 (doi:10.1523/JNEUROSCI.4258-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staudacher E., Schildberger K. 1998. Gating of sensory responses of descending brain neurones during walking in crickets. J. Exp. Biol. 201, 559–572 [DOI] [PubMed] [Google Scholar]
- 8.Evans P. D., Robb S. 1993. Octopamine receptor subtypes and their modes of action (review). Neurochem. Res. 18, 869–874 10.1007/BF00998270 (doi:10.1007/BF00998270) [DOI] [PubMed] [Google Scholar]
- 9.Roeder T. 2005. Tyramine and octopamine: ruling behaviour and metabolism. Annu. Rev. Entomol. 50, 447–477 10.1146/annurev.ento.50.071803.130404 (doi:10.1146/annurev.ento.50.071803.130404) [DOI] [PubMed] [Google Scholar]
- 10.Hsu Y., Wolf L. L. 2001. The winner and loser effect: what fighting behaviours are influenced? Anim. Behav. 61, 777–786 10.1006/anbe.2000.1650 (doi:10.1006/anbe.2000.1650) [DOI] [Google Scholar]
- 11.Alexander R. D. 1961. Aggressiveness, territoriality and sexual behaviour in field crickets (Orthoptera: Gryllidae). Behaviour 17, 130–223 10.1163/156853961X00042 (doi:10.1163/156853961X00042) [DOI] [Google Scholar]
- 12.Leimar O., Enquist M. 1984. Effects of asymmetries in owner–intruder conflicts. J. Theor. Biol. 111, 475–492 10.1016/S0022-5193(84)80235-0 (doi:10.1016/S0022-5193(84)80235-0) [DOI] [Google Scholar]
- 13.Kemp D. J., Wiklund C. 2004. Residency effects in animal contests. Proc. R. Soc. Lond. B 271, 1707–1711 10.1098/rspb.2004.2775 (doi:10.1098/rspb.2004.2775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitehouse M. E. A., Jaffe K. 1996. Ant wars: combat strategies, territory and nest defence in the leaf-cutting ant Atta laevigata. Anim. Behav. 51, 1207–1217 10.1006/anbe.1996.0126 (doi:10.1006/anbe.1996.0126) [DOI] [Google Scholar]
- 15.Maynard-Smith J., Parker G. A. 1976. The logic of asymmetric contests. Anim. Behav. 24, 159–175 [Google Scholar]
- 16.Gali-Muhtasib H. U., Reichman J., Smith C. 1998. The significance of isolation and body size on the aggression and dominance of the crayfish Orconectes nais. Trans. Kansas Acad. Sci. 101, 11–16 10.2307/3628171 (doi:10.2307/3628171) [DOI] [Google Scholar]
- 17.Hilakivi-Clarke L. A., Lister R. G. 1992. The role of body weight in resident-intruder aggression. Aggress. Behav. 18, 281–287 (doi:10.1002/1098-2337(1992)18:4<281::AID-AB2480180404>3.0.CO;2-2) [DOI] [Google Scholar]
- 18.Parker G. A. 1974. Assessment strategy and the evolution of fighting behaviour. J. Theor. Biol. 47, 223–243 10.1016/0022-5193(74)90111-8 (doi:10.1016/0022-5193(74)90111-8) [DOI] [PubMed] [Google Scholar]
- 19.Cromarty S. I., Mello J., Kass-Simon G. 1999. Time in residence affects escape and agonistic behavior in adult male American lobsters. Biol. Bull. 196, 105–112 10.2307/1543172 (doi:10.2307/1543172) [DOI] [PubMed] [Google Scholar]
- 20.Bradbury W. J., Vehrencamp L. 1998. Principles of animal communication, pp. 771–782 Sunderland, MA: Sinauer [Google Scholar]
- 21.Peeke H. V. S., Blank G. S., Figler M. H., Chang E. S. 2000. Effects of exogenous serotonin on a motor behavior and shelter competition in juvenile lobsters (Homarus americanus). J. Comp. Physiol. A 186, 575–582 10.1007/s003590000113 (doi:10.1007/s003590000113) [DOI] [PubMed] [Google Scholar]
- 22.Evans P. D., Maqueira B. 2005. Insect octopamine receptors: a new classification scheme based on studies of cloned Drosophila G-protein coupled receptors. Invert. Neurosci. 5, 111–118 10.1007/s10158-005-0001-z (doi:10.1007/s10158-005-0001-z) [DOI] [PubMed] [Google Scholar]
- 23.Roeder T., Degen J., Gewecke M. 1998. Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur. J. Pharmacol. 349, 171–177 10.1016/S0014-2999(98)00192-7 (doi:10.1016/S0014-2999(98)00192-7) [DOI] [PubMed] [Google Scholar]
- 24.Roeder T., Seifert M., Kaehler C., Gewecke M. 2003. Tyramine and octopamine: antagonistic modulators of behavior and metabolism. Arch. Insect Biochem. Physiol. 54, 1–13 10.1002/arch.10102 (doi:10.1002/arch.10102) [DOI] [PubMed] [Google Scholar]
- 25.Kononenko N. L., Wolfenberg H., Pflüger J. 2009. Tyramine as an independent transmitter and a precursor of octopamine in the locust central nervous system: an immunocytochemical study. J. Comp. Neurol. 512, 433–452 10.1002/cne.21911 (doi:10.1002/cne.21911) [DOI] [PubMed] [Google Scholar]
- 26.Verlinden H., Vleugels R., Marchal E., Badisco L., Pflüger H.-J., Blenau W., Broeck J. V. 2010. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 56, 854–867 10.1016/j.jinsphys.2010.05.018 (doi:10.1016/j.jinsphys.2010.05.018) [DOI] [PubMed] [Google Scholar]
- 27.Adamo S. A., Linn C. E., Hoy R. R. 1995. The role of neurohormonal octopamine during ‘fight or flight’ behaviour in the field cricket Gryllus bimaculatus. J. Exp. Biol. 198, 1691–1700 [DOI] [PubMed] [Google Scholar]
- 28.Bergman D. A., Moore P. A. 2003. Field observations of intraspecific agonistic behavior of two crayfish species, Orconectes rusticus and Orconectes virilis, in different habitats. Biol. Bull. 205, 26–35 10.2307/1543442 (doi:10.2307/1543442) [DOI] [PubMed] [Google Scholar]
- 29.Iba M., Nagao T., Urano A. 1995. Effect of population density on growth, behavior and level of biogenic amines in the cricket, Gryllus bimaculatus. Zool. Sci. 12, 695–702 10.2108/zsj.12.695 (doi:10.2108/zsj.12.695) [DOI] [Google Scholar]
- 30.Boulay R., Hefetz A., Soroker V., Lenoir A. 2000. Camponotus fellah colony integration: worker individuality necessitates frequent hydrocarbon exchanges. Anim. Behav. 59, 1127–1133 10.1006/anbe.2000.1408 (doi:10.1006/anbe.2000.1408) [DOI] [PubMed] [Google Scholar]
- 31.Hammer M., Menzel R. 1995. Learning and memory in the honeybee. J. Neurosci. 15, 1617–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unoki S., Matsumoto Y., Mizunami M. 2005. Participation of octopaminergic reward system and dopaminergic punishment system in insect olfactory learning revealed by pharmacological study. Eur. J. Neurosci. 22, 1409–1416 10.1111/j.1460-9568.2005.04318.x (doi:10.1111/j.1460-9568.2005.04318.x) [DOI] [PubMed] [Google Scholar]
- 33.Hammer M. 1993. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honeybees. Nature 366, 59–63 10.1038/366059a0 (doi:10.1038/366059a0) [DOI] [PubMed] [Google Scholar]
- 34.Stevenson P. A., Spörhase-Eichmann U. 1995. Review: Localization of octopaminergic neurones in insects. Comp. Biochem. Physiol. B 110, 203–215 [DOI] [PubMed] [Google Scholar]
- 35.Schröter U., Malun D., Menzel R. 2007. Innervation pattern of suboesophageal VUM neurons in the honeybee brain. Cell Tissue Res. 326, 647–667 [DOI] [PubMed] [Google Scholar]
- 36.Zhou C., Rao Y., Rao Y. 2008. A subset of octopaminergic neurones are important for Drosophila aggression. Nat. Neurosci. 11, 1059–1061 10.1038/nn.2164 (doi:10.1038/nn.2164) [DOI] [PubMed] [Google Scholar]