Abstract
There is growing evidence that migratory species are particularly vulnerable to rapid environmental changes arising from human activity. Species are expected to vary in their capacity to respond to these changes: long-distance migrants and those lacking variability in migratory traits are probably at considerable disadvantage. The few studies that have assessed the degree of plasticity in behaviour of marine animals suggest that fidelity to non-breeding destinations is usually high. In the present study, we evaluated individual flexibility in migration strategy of a highly pelagic seabird, the Cory's shearwater Calonectris diomedea. Geolocation data from 72 different migrations, including 14 birds that were tracked for more than one non-breeding season, showed a remarkable capacity to change winter destinations between years. Although some birds exhibited high site fidelity, others shifted from the South to North Atlantic, from the western to eastern South Atlantic, and from the Atlantic to Indian Ocean. Individuals also showed flexibility in stopover behaviour and migratory schedule. Although their K-selected life-history strategy has the disadvantage that the chances of microevolution are slight if circumstances alter rapidly, these results suggest that Cory's shearwaters may be in a better position than many other long-distance migrants to face the consequences of a changing environment.
Keywords: behavioural plasticity, climate changes, Cory's shearwater, migration, non-breeding movements
1. Introduction
Migration is among the most spectacular and complex of all natural phenomena, is manifested in many different animal groups and is a key trait in a wide diversity of life-history strategies. In recent years, however, migratory behaviour has received attention for unfortunate reasons: it is becoming clear that migrant species are particularly affected by human-induced climate changes [1–3], and it seems that migration itself may be an endangered phenomenon [4,5].
There is strong evidence that recent climate changes may have already impacted migratory behaviour (reviewed by [6,7]). By far, the best-documented cases are shifts in phenology of several passerine birds [3,8,9], but other expected (in some cases, already verified) effects include changes in migration routes and wintering destinations [10,11], with consequent disruption of the complex patterns of temporal and spatial synchrony between predators and prey—the so-called match–mismatch events (reviewed by [12]) and even the disappearance of the migratory behaviour itself [6,13].
Under a scenario of rapid environmental changes, species will vary in their capacity to respond and adapt [5,8,11,14]. Although evolution through natural selection is likely to be the only mechanism that allows species to cope with extreme changes [15,16], range shifts and existing phenotypic plasticity per se may allow, within certain limits, the persistence of species and populations (even if in suboptimal circumstances), gaining time for selection to act, or for more favourable environmental conditions to be restored. Consequently, species lacking variability and plasticity in their migratory traits are expected to be at a considerable disadvantage [17] (but see also [18]). In order to assess how different species will cope with rapid environmental change, we need to know much more about behavioural plasticity in migratory behaviour [17,19]. Despite the growing number of theoretical studies [18,20,21], lack of empirical knowledge of major patterns and causes of variability in migratory behaviour for most species seriously handicaps an assessment of their capacity to adapt with sufficient speed in the face of major environmental change.
An increasing body of research has examined migratory fidelity of species in terrestrial environments (reviewed by [7,22]). In contrast, much less attention has been focused on marine vertebrates with only a handful of studies published to date, albeit on taxa as diverse as sharks [23], turtles [24], whales [25] and seabirds [26].
Here, we present the results of a three year study of the individual consistency and flexibility in annual schedules, stopover and wintering destinations of Cory's shearwaters Calonectris diomedea. This long-lived species represents a particularly good model for the study of individual plasticity under natural conditions. First, Cory's shearwater is a long-distance migrant, with a migratory pattern that can be followed using recently developed tracking technology [26–28]. In addition, adult survival rates in this species are strongly influenced by the environmental conditions during the non-breeding season [29] and therefore migratory behaviour probably plays a major role in its life history and demography. Finally, Cory's shearwater populations have a wide variety of potential wintering sites from which individuals may choose [28]. It is highly probable that the relative quality of each of these wintering quarters varies temporally as a consequence of physical forcing [30] and top-down processes, for example, fishing pressure [31]. This raises the question of whether Cory's shearwaters have enough plasticity in their migratory behaviour to quickly respond to such changes, which has important implications for their ability to adapt to longer term environmental shifts.
2. Material and methods
(a). Bird tracking
We tracked the migration of 57 individual Cory's shearwaters breeding at Selvagem Grande island (30°02′ N; 15°52′ W) using leg-mounted geolocators. These loggers (mk 7 model, weighting approx. 3.6 g, developed by British Antarctic Survey, Cambridge, UK) were deployed at the end of the breeding seasons of 2006, 2007 and 2008 (August/September), and recovered in the beginning of the following breeding seasons (April/June). Fourteen of these birds (eight males and six females, aged 14–27 years) were tracked more than once (three in 2006/2007 and 2008/2009, 10 in 2007/2008 and 2008/2009 and one bird during the three seasons). Over the three year study period, we gathered data from 72 complete migrations (2006/2007: n = 27; 2007/2008: n = 25; 2008/2009: n = 20; 39 males and 33 females). All study birds were adult and successful breeders. Individual quality of the birds tracked more than once was inferred from past reproductive history (following [32]) by calculating the proportion of years those individuals successfully raised a chick during a five year period (2005–2009).
(b). Analysis of location data
Geolocators provide two positions per day based on light levels, with an accuracy of approximately 186 ± 114 km [27]. Light data were analysed using TransEdit (to check for integrity of light curves and to fit dawn and dusk times) and Birdtrack software (to estimate the latitude from day length and longitude from the time of local mid-day relative to Greenwich Mean Time). We assumed a sun elevation angle of −4.5°, based on known positions obtained during ground-truthing of the loggers, carried out before and after each deployment. Unrealistic positions (those resulting from interference to light curves at dawn or dusk, or those around equinox periods [27]) were removed from the analyses. The final part of the return migration of many Cory's shearwaters coincides with spring equinox. Hence, the arrival dates at a colony were estimated on the basis of longitude data [33], which are not affected by the proximity to the equinox, and indicated clear (eastward) variation when birds were approaching the nesting island.
(c). Statistical procedures
One of the main aims of this study was to analyse the individual flexibility in choice of wintering areas. To define each wintering area, we generated 95 per cent kernel density maps (smoothing factor selected by least-square cross validation) based on all positions from the last or only non-breeding season in which each of the 57 study individuals was tracked. This was carried out in a Lambert azimuthal equal-area projection after smoothing all positions twice, in order to reduce the error associated with the geolocation method [27]. All disjunct or oceanographically distinct kernel areas were considered to be separate wintering regions (see §3 for further details). We were then able to assign one (or, in some cases, several) wintering areas to each individual. In order to assess whether the within-individual variation in wintering destinations was higher or lower than expected by chance, we applied an approach similar to niche overlap estimation [34]. We assumed as ‘resource availability’, the proportion of days spent by all individuals (n = 57) in each wintering area (analogous to the relative availability of resources in a niche overlap index). The level of wintering area overlap for individuals tracked in different non-breeding periods was then calculated (following procedures described in [35]), and compared with the distribution of overlaps between datasets from different individuals paired at random. This distribution was estimated through a Monte-Carlo randomization method (10 000 simulations). A similar randomization procedure was applied to compare the distances between the centroids of core winter distributions of the same individuals in different years (70% kernel densities), with those randomly paired datasets.
The existence of stopover sites was investigated using first-passage time (FPT) analysis [36]. This method allows the identification of areas of relatively intensive usage, by computing the amount of time required to cross a circle of a given radius, and has been widely used in studies of foraging ecology [37]. During migration, birds are expected to perform fast, directional movement; however, if they interrupt the journey for a few days, the FPT will increase in the area where this occurs. We first identified in the non-breeding movements of each bird, the spatial scale at which stopovers may occur (by varying the range of radius from 200 to 1200 km). Based on the distribution of FPT at each scale, we first checked for the existence of stopovers whenever the FPT was longer than 4 days at a 200 km scale, 8 days at a 500 km scale or 20 days at a 1100 km scale. Given that all the stopovers identified at larger scales were also identified at smaller ones, we defined as a stopover any position where FPT was longer than 4 days at a 200 km scale. We checked the validity of this new method by comparing the activity patterns of birds during stopovers (percentage of time spent on sea surface and number of landings per hour) with those outside stopovers (sensu [38]), using a bootstrap paired comparison design [39]. We were only able to analyse data during the southward migration, owing to the lack of latitudinal information during the return migration (see above). Activity patterns were derived from saltwater immersion data (wet/dry), registered by the geolocators with a 3 s precision.
Individual repeatability in migration timings was evaluated with intraclass correlation coefficients [40]. Oceanographic data (sea-surface temperature (SST) and chlorophyll a concentration; monthly averages with a 9 km resolution) during mid-winter (December and January, 2006–2009) were obtained from the SeaWiFS project (http://oceancolor.gsfc.nasa.gov/). Analyses were carried out using the R software, including the packages maptools, adehabitat, sp and proj4. Means are presented ± s.d. throughout.
3. Results
(a). Overall migration and wintering patterns
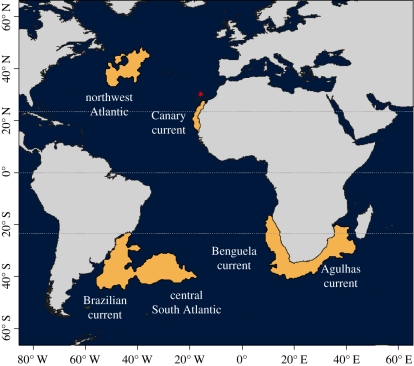
We identified six broad wintering areas of Cory's shearwaters from Selvagem Grande (figure 1): Benguela current (51% of days spent by the population in this area), Agulhas current (13%), central South Atlantic (16%), Brazilian current (8%), northwest Atlantic (9%) and Canary current (3%). The 95 per cent kernel did not clearly split the wintering areas of the Benguela and Agulhas currents (figure 1), but we considered these as separate destinations based on oceanography [41,42]. Merging these areas did not substantially change any of the analyses presented below.
Figure 1.
Winter distribution of Cory's shearwaters from Selvagem Grande (95% kernel density maps, from 57 individual tracks). Red asterisk indicates the colony location.
Cory's shearwaters left the colony during the first fortnight of November (mean departure date: 5 November ± 14 days), and took 36 days to reach their first major wintering destination (mean arrival date: 11 December ± 17 days). Birds left their wintering areas around mid February (19 February ± 9 days) and arrived at Selvagem Grande three weeks later (14 March ± 11 days). There were no consistent differences in timing of these events between years or sexes (ANOVA; all p > 0.05).
(b). Wintering site fidelity
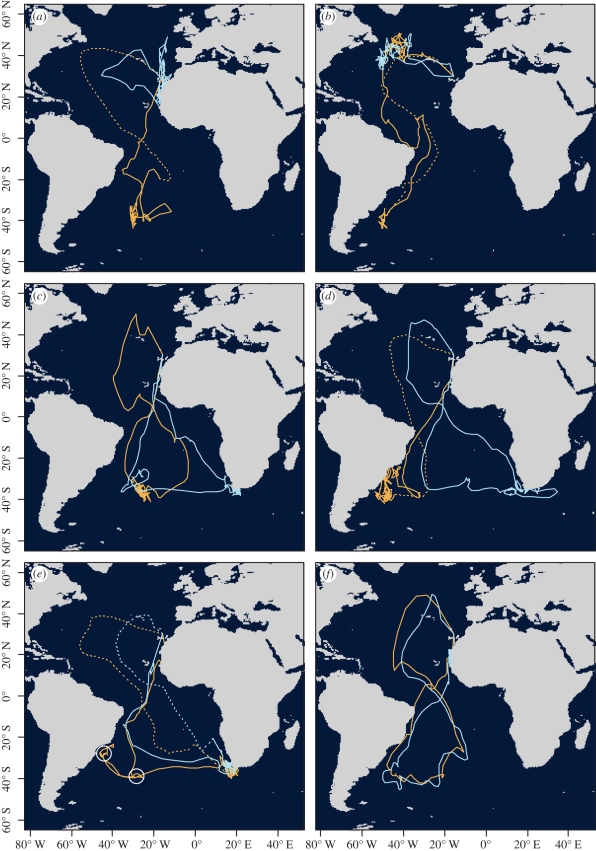
Five out of 14 individuals changed their main wintering areas in successive years. This includes two birds that switched from the South to North Atlantic (figure 2a,b), two from the western to eastern Atlantic (figure 2c,d) and one from the Benguela to Agulhas currents (not illustrated).
Figure 2.
Tracks of Cory's shearwaters in successive years (orange and light blue lines, respectively). Panels (a–d) represent individuals that changed their wintering destinations. Panels (e) and (f) exemplify individuals that were faithful to their wintering areas. White circles in panel (e) show the location of two stopovers detected by first-passage time analysis. Dashed lines represent hypothetical return paths of individuals for which no latitude data were available (see §2), based on longitude and mean travel speed (estimated only for mapping purposes).
The remaining nine individuals used the same wintering areas in different years: six travelled to the Benguela current (see figure 2e for an example), one to the central South Atlantic (figure 2f), one to the Brazilian current and the last bird to the Canary current.
We did not find any evidence of a relationship between the tendency of Cory's shearwaters to change their winter destination and their age (faithful birds were 16.3 ± 3.0 years old, on average, non-faithful were aged 18.6 ± 6.8 years, on their second trips), sex (one out of eight males and four out of six females changed destination) or individual quality (average quality index of faithful birds = 0.88 ± 0.11 and of non-faithful birds = 0.81 ± 0.20). Furthermore, there were no obvious relationships between the likelihood of a switch in migratory destination and changes in oceanographic conditions in the wintering areas: three birds abandoned areas where SST increased and two abandoned areas where it decreased; one individual moved from an area where chlorophyll a increased, one from an area where it remained constant and three from areas where it decreased. Similar mixed trends occurred in target areas.
Although one third of the study birds changed their main destination between years, overall, individuals tended to travel to the same area more often than expected by chance: the index of wintering area overlap between two non-breeding seasons of the same individual was significantly greater than the overlap between two randomly chosen individuals (5.15 ± 8.12 and 0.96 ± 0.57, respectively; p < 0.001). Similarly, the mean distance between the centroids of the wintering areas of the same individual was significantly shorter than the distance between those from randomly chosen pairs of birds (respectively, 1901 ± 2886 km and 3580 ± 790 km, p < 0.05).
(c). Individual consistency in migratory schedules
We found a significant between-year consistency in the chronology (departure dates from nesting and wintering areas and arrival dates at nesting and wintering areas) of individual migratory movements for birds that were faithful to their destinations (repeatability values higher than 0.51; all p < 0.05). We also found a significant repeatability in the departure dates from the colony for birds that changed their winter areas (r = 0.71; p < 0.05), but not in the timing of other events (all p > 0.05).
(d). Stopovers
The FPT analyses identified stopovers on 20 different journeys (27%; n = 72). Seventy per cent of these stopover areas coincided with known wintering areas—for example, five birds went to the northwest Atlantic before heading to the South Atlantic (example in figure 2b), and seven individuals stayed for a few days in the central South Atlantic en route to the Benguela/Agulhas region (figure 2c).
The activity patterns during stopovers differed clearly from those in the remaining migration days: in particular, birds showed a higher rate of landing on stopover days (table 1). The higher landing rate during stopovers may indicate that birds were actively fishing, and not just resting or waiting for a favourable wind.
Table 1.
Paired comparisons (using bootstrap methods) between the activity patterns during stopovers and during the remaining migration period of Cory's shearwaters (n = 17 individuals).
| stopovers | remaining migration period | paired comparison | |
|---|---|---|---|
| time spent on sea surface during the day (%) | 52.5 ± 4.5 | 33.5 ± 2.2 | p < 0.0001 |
| time spent on sea surface during the night (%) | 82.3 ± 2.5 | 54.7 ± 2.3 | p < 0.0001 |
| diurnal landing rate (number of landings per hour) | 5.7 ± 0.6 | 4.1 ± 0.3 | p < 0.01 |
| nocturnal landing rate (number of landings per hour) | 5.6 ± 1.2 | 3.2 ± 0.4 | p < 0.05 |
Of the 14 birds tracked twice, six had stopovers, but during only one of the journeys south, thus showing no repeated use of stopovers in successive trips. Three of these birds returned to the same wintering areas in successive years.
4. Discussion
(a). Wintering site selection: flexibility or fidelity?
This study documents remarkable individual flexibility in the choice of wintering sites by a migrant seabird, the Cory's shearwater. This was expressed not only in the relatively high percentage of birds that changed their wintering destination in successive years, but also in the variability and magnitude of the shifts. Some birds wintered more than 7000 km apart in different years, in distinct hemispheres. This is, to the best of our knowledge, the first report of a flexibility of such magnitude in the individual choices of a migratory bird, and is in striking contrast with results from the few previous studies of seabirds, which reported high winter site fidelity [26,43,44].
Despite the evident ability of Cory's shearwaters to vary their migratory destinations, birds choose the same areas more often than would be expected by chance, revealing an overall trend for fidelity. This combination of constancy and flexibility seems to apply to the migratory journey itself, and not just the choice of winter quarters. Stopovers were often made in areas used as wintering sites on other occasions, but, as in black-browed albatrosses Thalassarche melanophris [26], otherwise individuals did not show stopover site fidelity, despite a degree of within-individual consistency apparent in the use of migratory routes (figure 2e,f) and schedules. This suggests that each individual may have one (or maybe several) ‘preferred’ migratory strategies (in terms of route and wintering site), but maintains the capacity to choose alternatives.
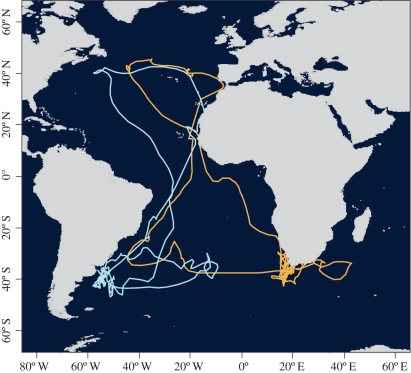
Cory's shearwaters have several and widespread potential wintering areas, as shown by this and other studies [28]. Knowledge of the conditions and foraging opportunities available at these alternative sites can be gathered throughout the life of an individual, particularly during the first years following fledging, as suggested for other seabirds [45,46]. Prior to the first breeding attempt (at around nine years old), Cory's shearwaters spend most of their time at sea [47]. Incidentally, the only young bird tracked by us (aged 4–5 years; age known because it was ringed as a chick) changed its main wintering area from one year to the next, performing an amazing journey of 108 000 km during which time it visited all the known wintering sites of the borealis subspecies (figure 3; cf. figure 1 and [28]).
Figure 3.
Journey of an immature (4–5 years old) Cory's shearwater tracked in two consecutive years (orange and light blue correspond to first and second years, respectively). Note that this individual visited each of the six wintering areas used by the study population.
(b). Challenges in a changing environment: is the Cory's shearwater a winner or a loser?
Despite the growing attention given to the impact of climate changes on bird migration [17], very few studies have focused on marine taxa (but see [10]). Global warming is predicted to cause an overall decline in marine primary productivity, but with contrasting impacts in different regions [30,48]. Another potential consequence is the increase in extreme weather events, such as storms and hurricanes [49], and of other extreme phenomena, such as harmful algal blooms [50]. As a result, the quality of wintering areas (in terms of both prey abundance and weather conditions), as well as its predictability, will probably decrease, which may be exacerbated by the additional effects of fisheries [51]. Therefore, the long-term survival of marine migrants will depend not only on how they can cope with global directional changes, but also with their responsiveness to increasing variability and unpredictability in oceanic environments [19].
Ostensibly, the degree of individual flexibility in the migratory strategies displayed by Cory's shearwaters suggests a good capacity to deal with the unpredictability of resources [52]. However, the extent to which such plasticity represents an adaptive advantage in a changing environment (i.e. if it constitutes an adaptive plasticity) depends on its relevance to the individual absolute fitness [18], and whether it can be translated to real population adaptability via microevolution [16]. The choice of wintering habitat certainly has the potential to influence the chances of overwinter survival [29], and perhaps of future breeding success through carry-over effects [53]. On the other hand, the K-selected life-history strategies of the majority of seabird species (high longevity, low fecundity and long deferred breeding) are likely to prevent microevolution from keeping pace with rapid climate change [19]. Furthermore, the adaptive advantage of plasticity in a scenario of increasing environmental stochasticity strongly depends on the reliability of the cues used for optimal behavioural decisions [18]. Climate change can reduce this reliability, resulting in maladaptive behaviours and, consequently, leading the most plastic populations to an ‘evolutionary trap’ [18,54]. More research and larger sample sizes will be needed to identify the drivers of changes in migratory choices of shearwaters, and the reliability of the cues in a climate change scenario.
The ability of individual shearwaters to spend the winter either in the Northern or in the Southern Hemisphere, and still return in sufficient time to engage in the next breeding attempt, suggests that these seabirds are not dependent on a particular photoperiodic cue to directly trigger spring migratory behaviour. Such dependence may be one of the major factors preventing other long-distance migrants from successfully adjusting their migratory schedules to changes in spring phenology of resources, resulting in seasonal mismatches [55]. This does not mean, however, that Cory's shearwaters do not rely on photoperiodic cues to synchronize their circannual rhythms; they may simply adjust their internal clock at a different time of the year [56].
There was a significant repeatability in individual departure dates from the colony area. The remaining migration timings (arrival and departure from wintering sites, and arrival at the colony) were only consistent among individuals that were faithful to their wintering destinations. Individual consistency in timing of events during the return migration has also been reported in species that routinely show high wintering site fidelity [26]. Low repeatability among birds with variable strategies suggests that the phenotypic variation in migratory schedules is mainly owing to environmental factors [17]. On the other hand, the observed lack of repeatability in some timings can be due to adaptive plasticity, adjusting to wintering site location, and not necessarily to random variation [57], which means that there may still be some genetic basis (maybe defining reaction norms) on which natural selection can potentially act [57].
(c). Conclusions
While only few still dispute that predicted climate changes will have profound effects on biodiversity and ecosystems, there is, as yet, considerable debate on the extent to which individual species will be able to cope with a changing global environment and, particularly, on the role that phenotypic plasticity per se can play in the adjustment process. Nevertheless, within limits, it seems likely that behavioural plasticity will be more advantageous than inflexibility under a global change scenario [8]. Thus, Cory's shearwater may be in a better position than other long-distance migrants with relatively inflexible migratory strategies. Whether Cory's shearwaters are unusual in this respect (as appears to be the case based on the previous work), or whether future analyses will reveal that flexibility in migration strategies is a more general trait of marine migratory fauna is something that repeated tracking of individuals in coming years should clarify. In this context, the causes and fitness consequences of consistency and flexibility of migratory behaviour constitute a promising field of research, with potentially major implications for conservation.
Acknowledgements
Parque Natural da Madeira, and particularly Paulo Oliveira, Dília Menezes and Carolina Santos provided permissions to carry out the work and, together with the wardens at the Nature Reserve where this study took place, gave important logistical support. Rafael Matias, Miguel Lecoq, Rui Rebelo, Filipe Moniz, Teresa Catry and others helped with fieldwork. This study is an output from a project on the ecology in Cory's shearwaters (PDCT/MAR/ PTDC/MAR/71927/2006) supported by Fundação para a Ciência e a Tecnologia (FCT—Portugal) and FEDER and further support was received through Programa Plurianual (UI&D 331/94). M. Dias and H. Alonso benefited from fellowships from FCT (BPD/46827/08 and BD/47055/2008, respectively). This study represents a contribution to the British Antarctic Survey Ecosystems programme.
References
- 1.Both C., Bouwhuis S., Lessells C., Visser M. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83 10.1038/nature04539 (doi:10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 2.Sanderson F., Donald P., Pain D., Burfield I., van Bommel F. 2006. Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131, 93–105 10.1016/j.biocon.2006.02.008 (doi:10.1016/j.biocon.2006.02.008) [DOI] [Google Scholar]
- 3.Gordo O. 2007. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Clim. Res. 35, 37–58 10.3354/cr00713 (doi:10.3354/cr00713) [DOI] [Google Scholar]
- 4.Brower L., Malcolm S. 1991. Animal migrations: endangered phenomena. Am. Zool. 31, 265–276 10.1093/icb/31.1.265 (doi:10.1093/icb/31.1.265) [DOI] [Google Scholar]
- 5.Wilcove D., Wikelski M. 2008. Going, going, gone: is animal migration disappearing? PLoS Biol. 6, e188. 10.1371/journal.pbio.0060188 (doi:10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiedler W. 2003. Recent changes in migration behaviour of birds: a compilation of field observations and ringing data. In Avian migration (eds Berthold P., Gwinner E., Sonnenschein E.), pp. 21–38 Berlin, Germany: Springer [Google Scholar]
- 7.Newton I. 2008. The migration ecology of birds. London, UK: Academic Press [Google Scholar]
- 8.Bradley N., Leopold A., Ross J., Huffaker W. 1999. Phenological changes reflect climate change in Wisconsin. Proc. Natl Acad. Sci. USA 96, 9701–9704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonzén N., Hedenström A., Lundberg P. 2007. Climate change and the optimal arrival of migratory birds. Proc. R. Soc. B 274, 269–274 10.1098/rspb.2006.3719 (doi:10.1098/rspb.2006.3719) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veit R., Pyle P., McGowan J. 1996. Ocean warming and long-term change in pelagic bird abundance within the California current system. Mar. Ecol. Prog. Ser. 139, 11–18 10.3354/meps139011 (doi:10.3354/meps139011) [DOI] [Google Scholar]
- 11.Sutherland W. 1998. Evidence for flexibility and constraint in migration systems. J. Avian Biol. 29, 441–446 [Google Scholar]
- 12.Durant J., Hjermann D., Ottersen G., Stenseth N. 2007. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283 10.3354/cr033271 (doi:10.3354/cr033271) [DOI] [Google Scholar]
- 13.Vliet J., Musters C., Keurs W. 2009. Changes in migration behaviour of blackbirds Turdus merula from The Netherlands. Bird Stud. 56, 276–281 10.1080/00063650902792148 (doi:10.1080/00063650902792148) [DOI] [Google Scholar]
- 14.Hamer K. 2010. The search for winners and losers in a sea of climate change. Ibis 152, 3–5 10.1111/j.1474-919X.2009.00995.x (doi:10.1111/j.1474-919X.2009.00995.x) [DOI] [Google Scholar]
- 15.Gienapp P., Leimu R., Merilä J. 2007. Responses to climate change in avian migration time—microevolution versus phenotypic plasticity. Clim. Res. 35, 25–35 10.3354/cr00712 (doi:10.3354/cr00712) [DOI] [Google Scholar]
- 16.Visser M. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 10.1098/rspb.2007.0997 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulido F. 2007. Phenotypic changes in spring arrival: evolution, phenotypic plasticity, effects of weather and condition. Clim. Res. 35, 5–23 10.3354/cr00711 (doi:10.3354/cr00711) [DOI] [Google Scholar]
- 18.Reed T., Waples R., Schindler D., Hard J., Kinnison M. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400 10.1098/rspb.2010.0771 (doi:10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grémillet D., Boulinier T. 2009. Spatial ecology and conservation of seabirds facing global climate change: a review. Mar. Ecol. Prog. Ser. 391, 121–137 10.3354/meps08212 (doi:10.3354/meps08212) [DOI] [Google Scholar]
- 20.Chevin L.-M., Lande R. 2010. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150 10.1111/j.1558-5646.2009.00875.x (doi:10.1111/j.1558-5646.2009.00875.x) [DOI] [PubMed] [Google Scholar]
- 21.Chevin L.-M., Lande R., Mace G. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catry P., Encarnação V., Araújo A., Fearon P., Fearon A., Armelin M., Delaloye P. 2004. Are long-distance migrant passerines faithful to their stopover sites? J. Avian Biol. 35, 170–181 10.1111/j.0908-8857.2004.03112.x (doi:10.1111/j.0908-8857.2004.03112.x) [DOI] [Google Scholar]
- 23.Jorgensen S., et al. 2010. Philopatry and migration of Pacific white sharks. Proc. R. Soc. B 277, 679–688 10.1098/rspb.2009.1155 (doi:10.1098/rspb.2009.1155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broderick A., Coyne M., Fuller W., Glen F., Godley B. 2007. Fidelity and over-wintering of sea turtles. Proc. R. Soc. B 274, 1533–1538 10.1098/rspb.2007.0211 (doi:10.1098/rspb.2007.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calambokidis J., et al. 2001. Movements and population structure of humpback whales in the North Pacific. Mar. Mammal Sci. 17, 769–794 10.1111/j.1748-7692.2001.tb01298.x (doi:10.1111/j.1748-7692.2001.tb01298.x) [DOI] [Google Scholar]
- 26.Phillips R., Silk J., Croxall J., Afanasyev V., Bennett V. 2005. Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology 86, 2386–2396 10.1890/04-1885 (doi:10.1890/04-1885) [DOI] [Google Scholar]
- 27.Phillips R., Silk J., Croxall J., Afanasyev V., Briggs D. 2004. Accuracy of geolocation estimates for flying seabirds. Mar. Ecol. Prog. Ser. 266, 265–272 10.3354/meps266265 (doi:10.3354/meps266265) [DOI] [Google Scholar]
- 28.González-Solís J., Croxall J., Oro D., Ruiz X. 2007. Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front. Ecol. Environ. 5, 297–301 10.1890/1540-9295(2007)5[297:TMAMIT]2.0.CO;2 (doi:10.1890/1540-9295(2007)5[297:TMAMIT]2.0.CO;2) [DOI] [Google Scholar]
- 29.Jenouvrier S., Thibault J.-C., Viallefont A., Vidal P., Ristow D., Mougin J.-L., Brichetti P., Borg J., Bretagnolle V. 2009. Global climate patterns explain range-wide synchronicity in survival of a migratory seabird. Glob. Change Biol. 15, 268–279 10.1111/j.1365-2486.2008.01715.x (doi:10.1111/j.1365-2486.2008.01715.x) [DOI] [Google Scholar]
- 30.Behrenfeld M., et al. 2006. Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 10.1038/nature05317 (doi:10.1038/nature05317) [DOI] [PubMed] [Google Scholar]
- 31.Pichegru L., Ryan P., Bohec C., van der Lingen C., Navarro R., Petersen S., Lewis S., van der Westhuizen J., Grémillet D. 2009. Overlap between vulnerable top predators and fisheries in the Benguela upwelling system: implications for marine protected areas. Mar. Ecol. Prog. Ser. 391, 199–208 10.3354/meps08283 (doi:10.3354/meps08283) [DOI] [Google Scholar]
- 32.Cobley N., Croxall J., Prince P. 1998. Individual quality and reproductive performance in the grey-headed albatross Diomedea chrysostoma. Ibis 140, 315–322 10.1111/j.1474-919X.1998.tb04395.x (doi:10.1111/j.1474-919X.1998.tb04395.x) [DOI] [Google Scholar]
- 33.Catry I., Dias M. P., Catry T., Afanasyev V., Fox J., Franco A., Sutherland W. In press Individual variation in migratory movements and winter behaviour of Iberian lesser kestrels Falco naumanni revealed by geolocators. Ibis. 10.1111/j.1474-919X.2010.01073.x (doi:10.1111/j.1474-919X.2010.01073.x) [DOI] [Google Scholar]
- 34.Hulbert S. 1978. The measurement of niche overlap and some relatives. Ecology 59, 67–77 10.2307/1936632 (doi:10.2307/1936632) [DOI] [Google Scholar]
- 35.Ludwing J., Reynolds J. 1988. Statistical ecology. A primer on methods and computing. New York, NY: Wiley-Interscience [Google Scholar]
- 36.Fauchald P., Tveraa T. 2003. Using first-passage time in the analysis of area-restricted search and habitat selection. Ecology 84, 282–288 10.1890/0012-9658(2003)084[0282:UFPTIT]2.0.CO;2 (doi:10.1890/0012-9658(2003)084[0282:UFPTIT]2.0.CO;2) [DOI] [Google Scholar]
- 37.Pinaud D., Weimerskirch H. 2005. Scale-dependent habitat use in a long-ranging central place predator. J.Anim. Ecol. 74, 852–863 10.1111/j.1365-2656.2005.00984.x (doi:10.1111/j.1365-2656.2005.00984.x) [DOI] [Google Scholar]
- 38.Guilford T., Meade J., Willis J., Phillips R. A., Boyle D., Roberts S., Collett M., Freeman R., Perrins C. 2009. Migration and stopover in a small pelagic seabird, the Manx shearwater Puffinus puffinus: insights from machine learning. Proc. R. Soc. B 276, 1215–1223 10.1098/rspb.2008.1577 (doi:10.1098/rspb.2008.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manly B. 1998. randomization, bootstrap and Monte Carlo methods in biology, 2nd edn. London, UK: Chapman & Hall [Google Scholar]
- 40.Lessells C., Boag P. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- 41.Grémillet D., et al. 2008. Spatial match–mismatch in the Benguela upwelling zone: should we expect chlorophyll and sea-surface temperature to predict marine predator distributions? J. Appl. Ecol. 45, 610–621 10.1111/j.1365-2664.2007.01447.x (doi:10.1111/j.1365-2664.2007.01447.x) [DOI] [Google Scholar]
- 42.Hutchings L., et al. 2009. The Benguela Current: an ecosystem of four components. Prog. Oceanogr. 83, 15–32 10.1016/j.pocean.2009.07.046 (doi:10.1016/j.pocean.2009.07.046) [DOI] [Google Scholar]
- 43.Croxall J., Silk J., Phillips R., Afanasyev V., Briggs D. 2005. Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science 307, 249–250 10.1126/science.1106042 (doi:10.1126/science.1106042) [DOI] [PubMed] [Google Scholar]
- 44.Hatch S., Gill V., Mulcahy D. 2010. Individual and colony-specific wintering areas of Pacific northern fulmars (Fulmarus glacialis). Can. J. Fish. Aquat. Sci. 67, 386–400 10.1139/F09-184 (doi:10.1139/F09-184) [DOI] [Google Scholar]
- 45.Baker R. 1980. The significance of the lesser black-backed gull to models of bird migration. Bird Stud. 27, 41–50 10.1080/00063658009476655 (doi:10.1080/00063658009476655) [DOI] [Google Scholar]
- 46.Åkesson S., Weimerskirch H. 2005. Albatross long-distance navigation: comparing adults and juveniles. J. Navigation 58, 365–373 10.1017/S0373463305003401 (doi:10.1017/S0373463305003401) [DOI] [Google Scholar]
- 47.Mougin J.-L., Jouanin C., Roux F. 2000. Démographie du puffin cendré Calonectris diomedea de Selvagem Grande. Rev. Ecol. Terre Vie 55, 275–290 [Google Scholar]
- 48.Toggweiler J., Russell J. 2008. Ocean circulation in a warming climate. Nature 451, 286–288 10.1038/nature06590 (doi:10.1038/nature06590) [DOI] [PubMed] [Google Scholar]
- 49.Alley R., et al. 2003. Abrupt climate change. Science 299, 2005–2010 10.1126/science.1081056 (doi:10.1126/science.1081056) [DOI] [PubMed] [Google Scholar]
- 50.Hallegraeff G. 2010. Ocean climate change, phytoplankton community responses, and harmful algal blooms: a formidable predictive challenge. J. Phycol. 46, 220–235 10.1111/j.1529-8817.2010.00815.x (doi:10.1111/j.1529-8817.2010.00815.x) [DOI] [Google Scholar]
- 51.Cury P., Shin Y.-J., Planque B., Durant J., Fromentin J.-M., Kramer-Schadt S., Stenseth N., Travers M., Volker Grimm V. 2008. Ecosystem oceanography for global change in fisheries. Trends Ecol. Evol. 23, 338–346 10.1016/j.tree.2008.02.005 (doi:10.1016/j.tree.2008.02.005) [DOI] [PubMed] [Google Scholar]
- 52.Weimerskirch H. 2007. Are seabirds foraging for unpredictable resources? Deep-Sea Res. II 54, 211–223 10.1016/j.dsr2.2006.11.013 (doi:10.1016/j.dsr2.2006.11.013) [DOI] [Google Scholar]
- 53.Norris D. 2005. Carry-over effects and habitat quality in migratory populations. Oikos 109, 178–186 10.1111/j.0030-1299.2005.13671.x (doi:10.1111/j.0030-1299.2005.13671.x) [DOI] [Google Scholar]
- 54.Schalaepfer M., Runge M. C., Sherman P. W. 2002. Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480 10.1016/S0169-5347(02)02580-6 (doi:10.1016/S0169-5347(02)02580-6) [DOI] [Google Scholar]
- 55.Both C., Visser M. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 10.1038/35077063 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- 56.Gwinner E. 1977. Circannual rhythms in bird migration. Ann. Rev. Ecol. Syst. 8, 381–405 10.1146/annurev.es.08.110177.002121 (doi:10.1146/annurev.es.08.110177.002121) [DOI] [Google Scholar]
- 57.Naya D. 2010. Why may repeatability of highly flexible traits say little about their evolutionary potential? Open Ecol. J. 3, 26–28 10.2174/1874213001003010026 (doi:10.2174/1874213001003010026) [DOI] [Google Scholar]